Enhanced Sewage Sludge Disintegration and Nutrients Release by Catalytic Microbubbles Ozonation Using Sewage Sludge-Based Char as Catalyst
Abstract
:1. Introduction
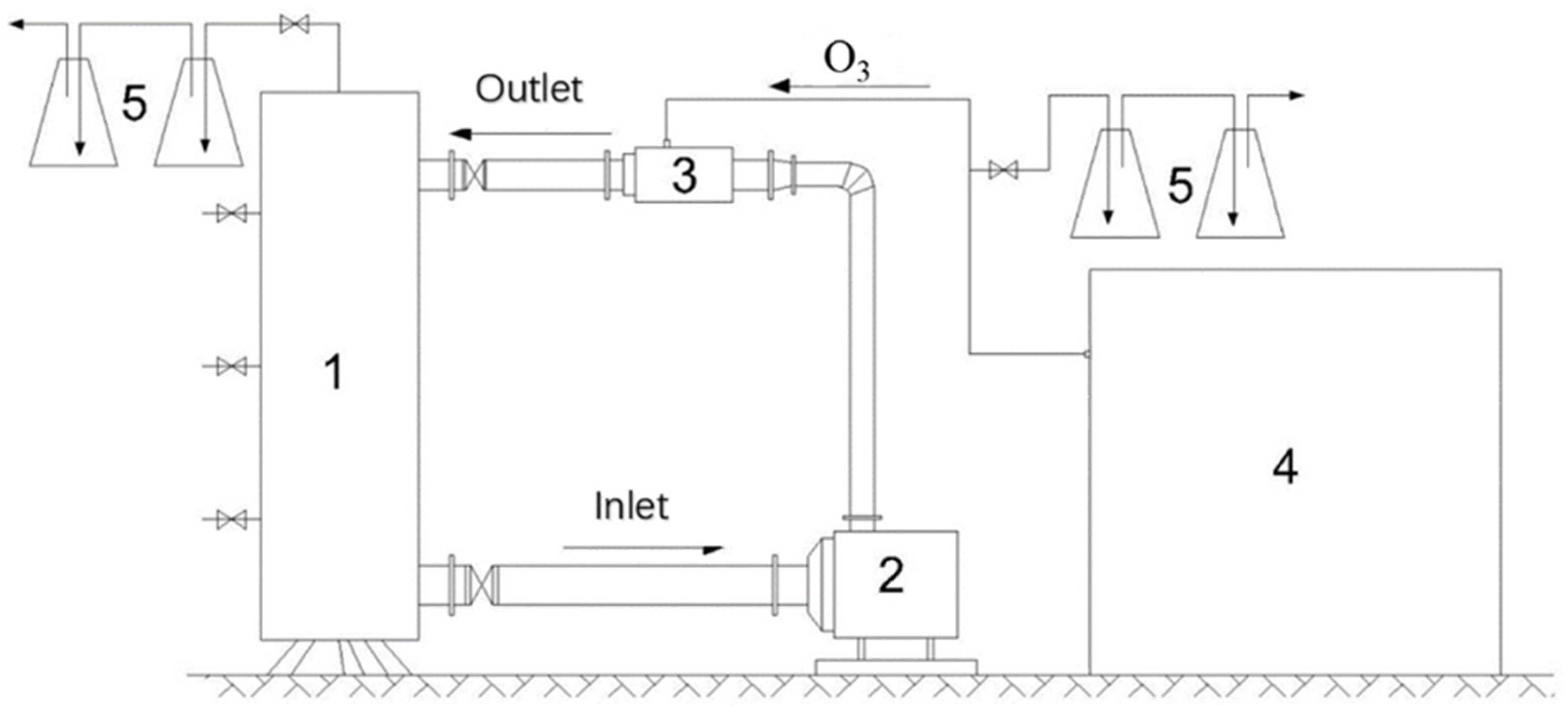
2. Materials and Methods
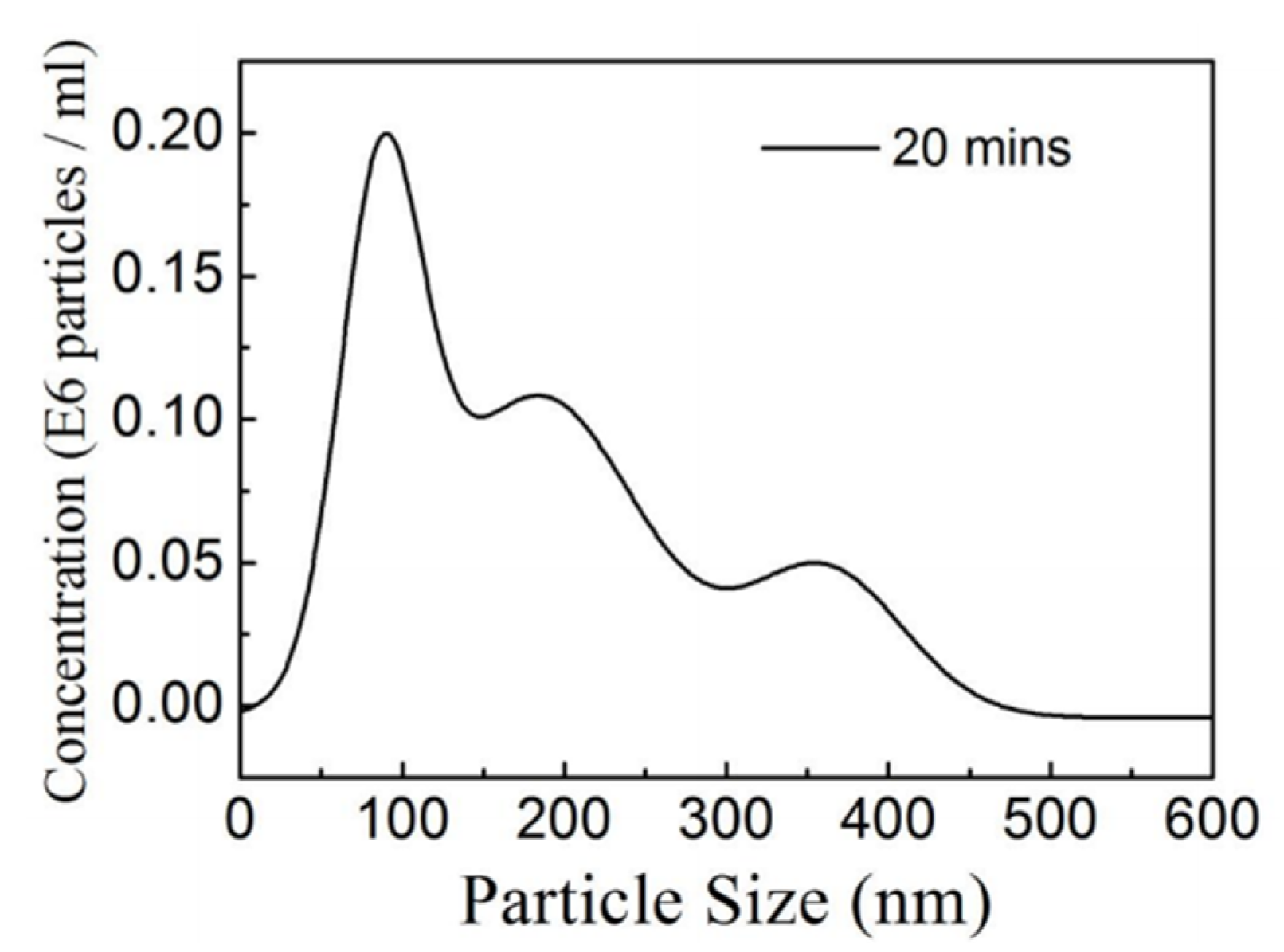
3. Results
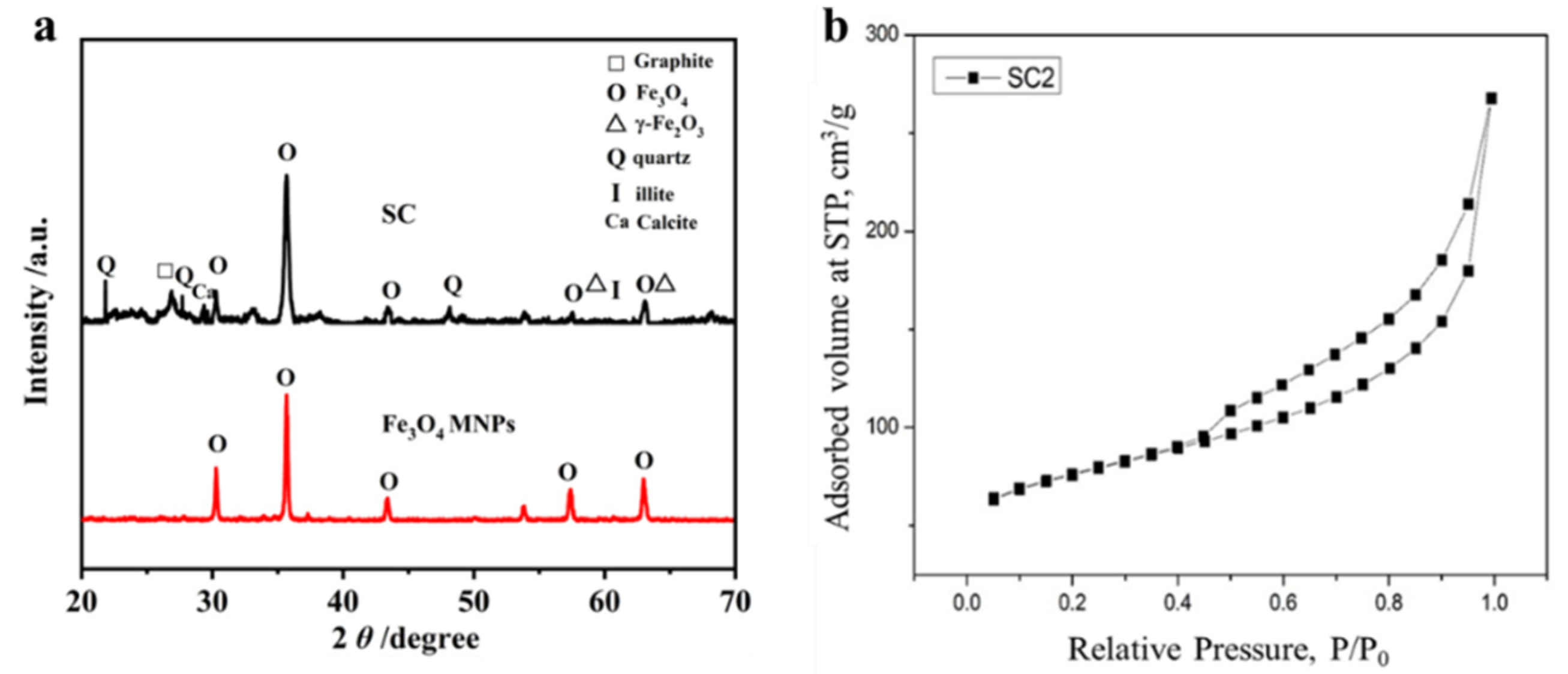
3.1. Characterizations of SC
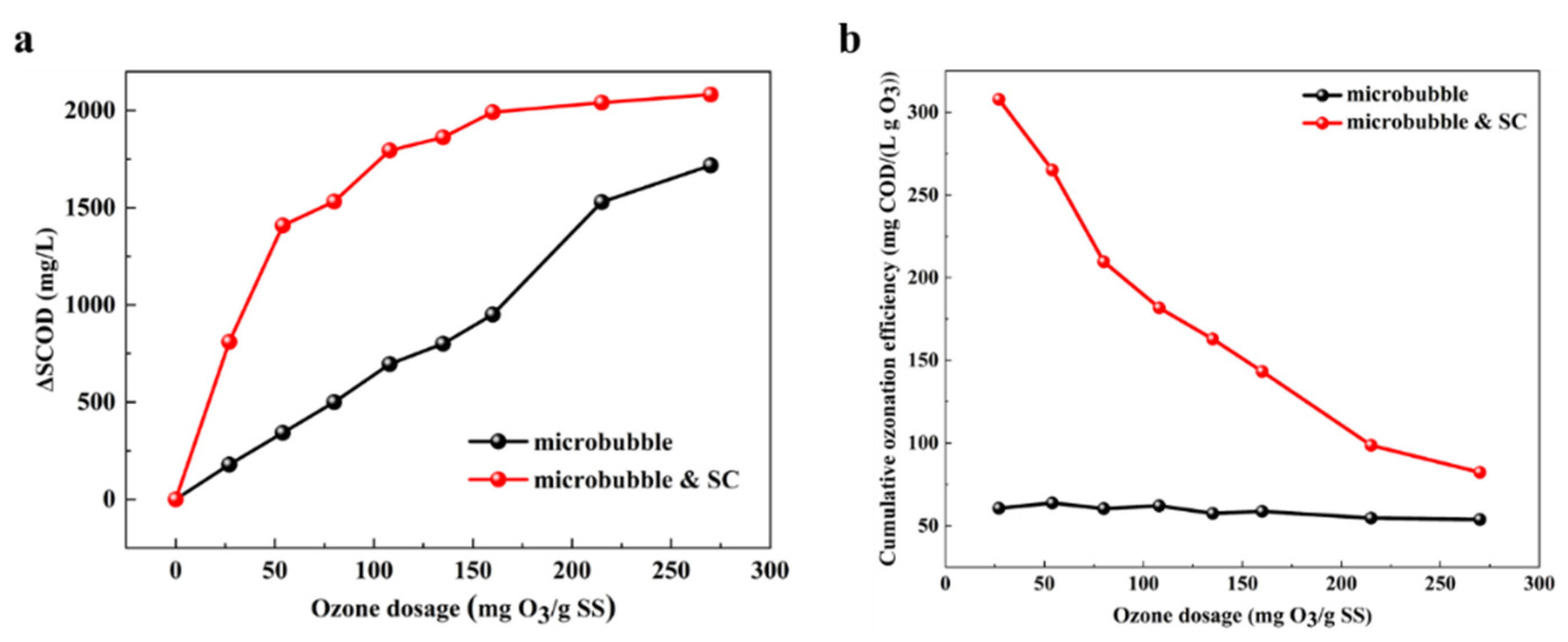
3.2. Performance of Catalytic Ozonation with SC on Sludge Disintegration
3.3. Comparative Performance of Phosphorus and Nitrogen Release by Microbubble Ozonation with and without SC Addition
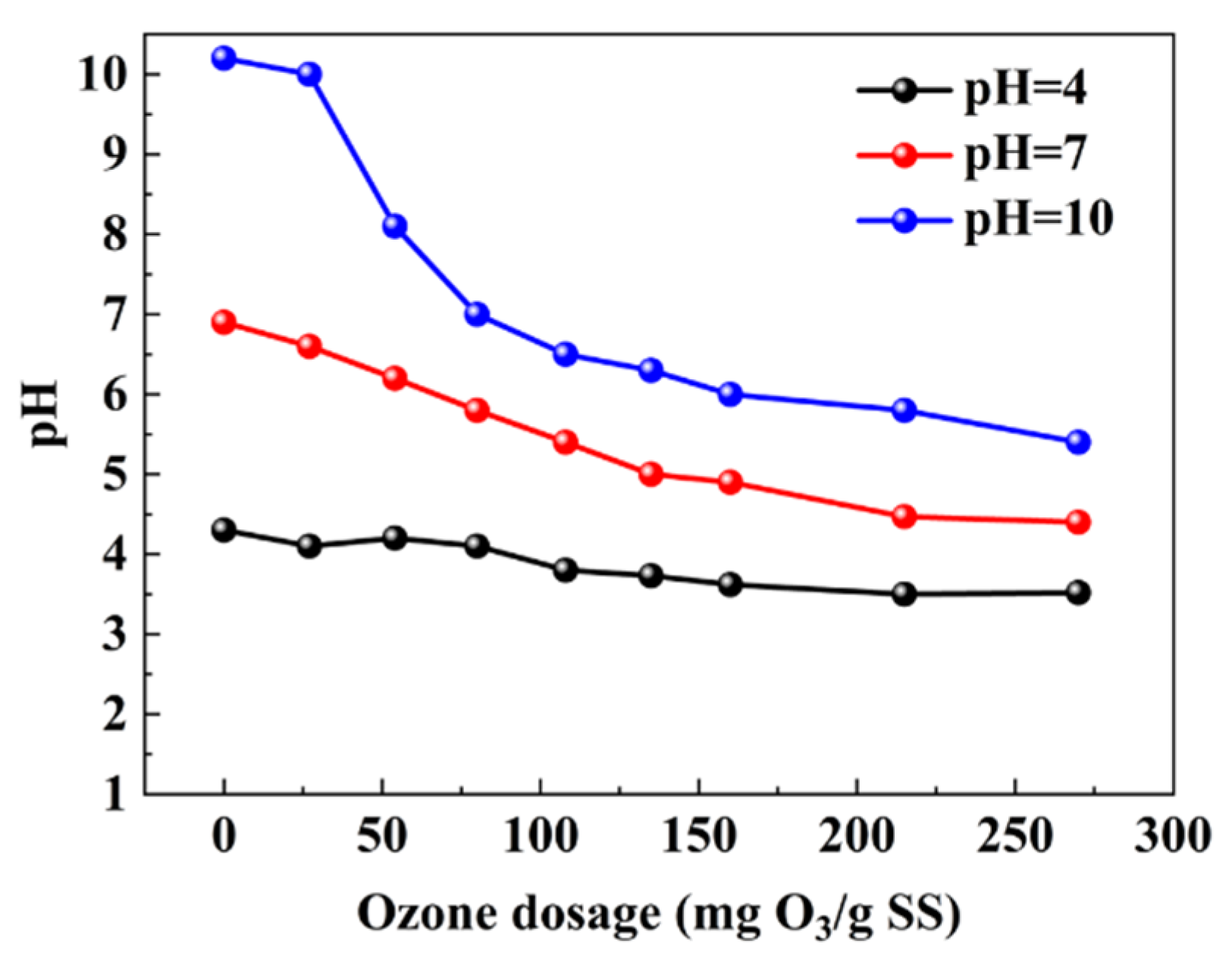
3.4. Effect of pH on the Performance of SC Catalyzed Microbubble Sludge Ozonation
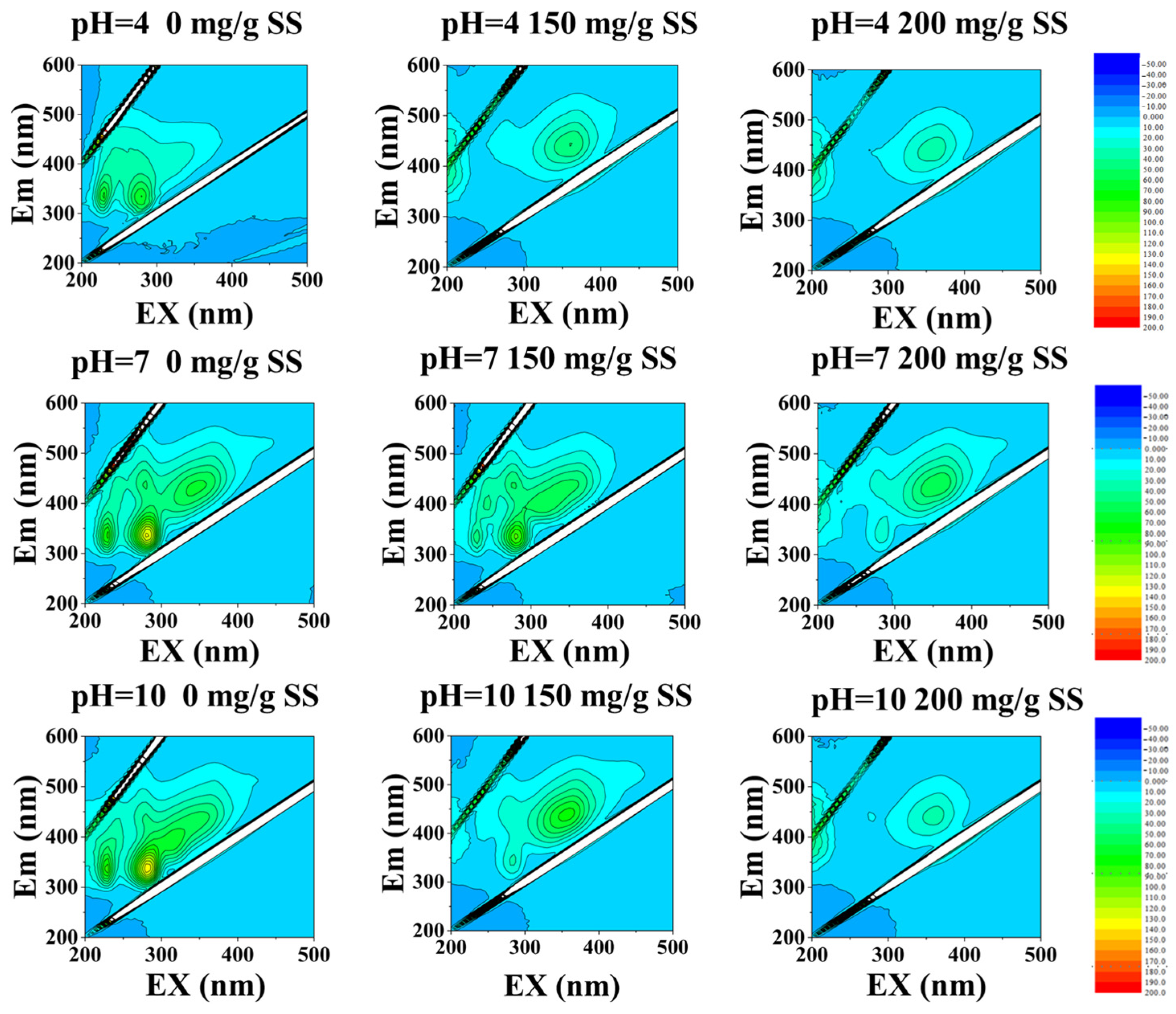
3.5. Mechanism Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, J.; Liu, Y.; Yan, Y.; Wan, J.; Liu, F. Promotion of sludge process reduction using low-intensity ultrasonic treatment. J. Clean. Prod. 2021, 325, 129289. [Google Scholar] [CrossRef]
- Sengar, A.; Vijayanandan, A. Effects of pharmaceuticals on membrane bioreactor: Review on membrane fouling mechanisms and fouling control strategies. Sci. Total Environ. 2022, 808, 152132. [Google Scholar] [CrossRef]
- Shrestha, B.; Hernandez, R.; Fortela, D.L.B.; Sharp, W.; Chistoserdov, A.; Gang, D.; Revellame, E.; Holmes, W.; Zappi, M.E. A review of pretreatment methods to enhance solids reduction during anaerobic digestion of municipal wastewater sludges and the resulting digester performance: Implications to future urban biorefineries. Appl. Sci. 2020, 10, 9141. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, P.; Yang, J.; Chen, Y. Ultrasonic reduction of excess sludge from the activated sludge system. J. Hazard. Mater. 2007, 145, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhu, N.; Song, L. Conditioning of sewage sludge with electrolysis: Effectiveness and optimizing study to improve dewaterability. Bioresour. Technol. 2010, 101, 4285–4290. [Google Scholar] [CrossRef]
- Wang, Y.W.; Xiao, Q.C.; Liu, J.B.; Yan, H.; Wei, Y.S. Pilot-scale study of sludge pretreatment by microwave and sludge reduction based on lysis-cryptic growth. Bioresour. Technol. 2015, 190, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.C.; Li, Y.Y.; Bi, Q.; Hu, Y.Y. Reduction of excess sludge production in sequencing batch reactor (SBR) by lysis-cryptic growth using homogenization disruption. Bioresour. Technol. 2013, 134, 43–50. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, D.; Zhang, G.; Ren, Z.; Zhu, Y. Critical review on ultrasound lysis-cryptic growth for sludge reduction. J. Environ. Chem. Eng. 2021, 9, 106263. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Tian, Y.; Li, N.; Kong, L.; Sun, L.; Yu, M.; Zuo, W. Changes of physicochemical properties of sewage sludge during ozonation treatment: Correlation to sludge dewaterability. Chem. Eng. J. 2016, 301, 238–248. [Google Scholar] [CrossRef]
- Feng, L.; Chen, Y.; Chen, X.; Duan, X.; Xie, J.; Chen, Y. Anaerobic accumulation of short-chain fatty acids from algae enhanced by damaging cell structure and promoting hydrolase activity. Bioresour. Technol. 2018, 250, 777–783. [Google Scholar] [CrossRef]
- Zhou, J.; You, X.; Niu, B.; Yang, X.; Gong, L.; Zhou, Y.; Wang, J.; Zhang, H. Enhancement of methanogenic activity in anaerobic digestion of high solids sludge by nano zero-valent iron. Sci. Total Environ. 2020, 703, 135532. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, P.; Zhang, G.; Cheng, R. Enhancement of ultrasonic disintegration of sewage sludge by aeration. J. Environ. Sci. 2016, 42, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Ghafarzadeh, M.; Abedini, R.; Rajabi, R. Optimization of ultrasonic waves application in municipal wastewater sludge treatment using response surface method. J. Clean. Prod. 2017, 150, 361–370. [Google Scholar] [CrossRef]
- Horttanainen, M.; Deviatkin, I.; Havukainen, J. Nitrogen release from mechanically dewatered sewage sludge during thermal drying and potential for recovery. J. Clean. Prod. 2017, 142, 1819–1826. [Google Scholar] [CrossRef]
- Zubrowska-Sudol, M.; Walczak, J. Effects of mechanical disintegration of activated sludge on the activity of nitrifying and denitrifying bacteria and phosphorus accumulating organisms. Water Res. 2014, 61, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Sun, F.; Zhou, Y. Insights into anaerobic transformation of key dissolved organic matters produced by thermal hydrolysis sludge pretreatment. Bioresour. Technol. 2018, 266, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dong, B.; Dai, X.; Dai, L. Enhancement of sludge dewaterability via the thermal hydrolysis anaerobic digestion mechanism based on moisture and organic matter interactions. Sci. Total Environ. 2021, 798, 149229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yang, J.; Liu, H.; Zhang, J. Sludge ozonation: Disintegration, supernatant changes and mechanisms. Bioresour. Technol. 2009, 100, 1505–1509. [Google Scholar] [CrossRef]
- Alfonso-Muniozguren, P.; Lee, J.; Bussemaker, M.; Chadeesingh, R.; Jones, C.; Oakley, D.; Saroj, D. A combined activated sludge-filtration-ozonation process for abattoir wastewater treatment. J. Water Process Eng. 2018, 25, 157–163. [Google Scholar] [CrossRef]
- Otieno, B.; Apollo, S.; Kabuba, J.; Naidoo, B.; Simate, G.; Ochieng, A. Ozonolysis pre-treatment of waste activated sludge for solubilization and biodegradability enhancement. J. Environ. Chem. Eng. 2019, 7, 102945. [Google Scholar] [CrossRef]
- Hodaei, M.; Ghasemi, S.; Khosravi, A.; Vossoughi, M. Effect of the ozonation pretreatment on biogas production from waste activated sludge of tehran wastewater treatment plant. Biomass Bioenergy 2021, 152, 106198. [Google Scholar] [CrossRef]
- Parandoush, S.; Mokhtarani, N. Reducing excess sludge volume in sequencing batch reactor by integrating ultrasonic waves and ozonation. J. Environ. Manag. 2022, 317, 115405. [Google Scholar] [CrossRef] [PubMed]
- Salsabil, M.R.; Laurent, J.; Casellas, M.; Dagot, C. Techno-economic evaluation of thermal treatment, ozonation and sonication for the reduction of wastewater biomass volume before aerobic or anaerobic digestion. J. Hazard. Mater. 2010, 174, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.D.; Bian, C.; Yuan, H.P.; Zhu, N.W. An in-depth study on the deep-dewatering mechanism of waste activated sludge by ozonation pre-oxidation and chitosan re-flocculation conditioning. Sci. Total Environ. 2020, 714, 136627. [Google Scholar] [CrossRef]
- Kianmehr, P.; Parker, W.; Seto, P. An evaluation of protocols for characterization of ozone impacts on WAS properties and digestibility. Bioresour. Technol. 2010, 101, 8565–8572. [Google Scholar] [CrossRef] [PubMed]
- Braguglia, C.M.; Gianico, A.; Mininni, G. Comparison between ozone and ultrasound disintegration on sludge anaerobic digestion. J. Environ. Manag. 2012, 95, S139–S143. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.-B.; Yan, S.-T.; Xing, X.-H.; Yu, A.-F.; Sun, X.-L.; Jurcik, B. Enhanced sludge solubilization by microbubble ozonation. Chemosphere 2008, 72, 205–212. [Google Scholar] [CrossRef]
- Wen, H.; Gu, L.; Yu, H.; Qiao, X.; Zhang, D.; Ye, J. Radical assisted iron impregnation on preparing sewage sludge derived Fe/carbon as highly stable catalyst for heterogeneous Fenton reaction. Chem. Eng. J. 2018, 352, 837–846. [Google Scholar] [CrossRef]
- Wang, X.; Gu, L.; Zhou, P.; Zhu, N.; Li, C.; Tao, H.; Wen, H.; Zhang, D. Pyrolytic temperature dependent conversion of sewage sludge to carbon catalyst and their performance in persulfate degradation of 2-Naphthol. Chem. Eng. J. 2017, 324, 203–215. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G.; Fu, B. Conversion of sewage sludge into environmental catalyst and microbial fuel cell electrode material: A review. Sci. Total Environ. 2019, 666, 525–539. [Google Scholar] [CrossRef]
- Wen, G.; Pan, Z.-H.; Ma, J.; Liu, Z.-Q.; Zhao, L.; Li, J.-J. Reuse of sewage sludge as a catalyst in ozonation—Efficiency for the removal of oxalic acid and the control of bromate formation. J. Hazard. Mater. 2012, 239, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Xiong, Y.; Tian, S.; Kong, L.; Descorme, C. Catalytic wet air oxidation of 2-chlorophenol over sewage sludge-derived carbon-based catalysts. J. Hazard. Mater. 2014, 276, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.-C.; Jiang, J.; Huang, G.-X.; Yu, H.-Q. Sludge biochar-based catalysts for improved pollutant degradation by activating peroxymonosulfate. J. Mater. Chem. A 2018, 6, 8978–8985. [Google Scholar] [CrossRef]
- He, D.-Q.; Wang, L.-F.; Jiang, H.; Yu, H.-Q. A Fenton-like process for the enhanced activated sludge dewatering. Chem. Eng. J. 2015, 272, 128–134. [Google Scholar] [CrossRef]
- Shih, Y.-J.; Hsu, C.-H. Kinetics and highly selective N2 conversion of direct electrochemical ammonia oxidation in an undivided cell using NiCo oxide nanoparticle as the anode and metallic Cu/Ni foam as the cathode. Chem. Eng. J. 2021, 409, 128024. [Google Scholar] [CrossRef]
- Lv, Z.; Hao, L.; Yao, Z.; Li, W.; Robertson, A.W.; Sun, Z. Rigorous Assessment of Cl−-Based Anolytes on Electrochemical Ammonia Synthesis. Adv. Sci. 2022, 9, 2204205. [Google Scholar] [CrossRef]
- Song, Q.; Li, M.; Wang, L.; Ma, X.; Liu, F.; Liu, X. Mechanism and optimization of electrochemical system for simultaneous removal of nitrate and ammonia. J. Hazard. Mater. 2019, 363, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Hu, J.; Liu, B.; Wang, D.; Wu, L.; Xiao, K.; Liang, S.; Hou, H.; Yang, J. In situ generation of zero valent iron for enhanced hydroxyl radical oxidation in an electrooxidation system for sewage sludge dewatering. Water Res. 2018, 145, 162–171. [Google Scholar] [CrossRef]
- Ernst, M.; Lurot, F.; Schrotter, J.C. Catalytic ozonation of refractory organic model compounds in aqueous solution by aluminum oxide. Appl. Catal. B 2004, 47, 15–25. [Google Scholar] [CrossRef]
- Xu, L.; Liu, W.; Li, X.; Rashid, S.; Shen, C.; Wen, Y. Fabrication of MnOx heterogeneous catalysts from wet sludge for degradation of azo dyes by activated peroxymonosulfate. RSC Adv. 2015, 5, 12248–12256. [Google Scholar] [CrossRef]




| Parameters | Data (mg/L) |
|---|---|
| Mixed liquid suspended solids (MLSS) | 4500–6500 |
| Mixed liquid volatile suspended solids (MLVSS) | 3000–3500 |
| pH | 6.5–7.5 |
| Soluble chemical oxygen demand (SCOD) | 40–250 |
| TP | 1.5–6.0 |
| TN | 4.5–10.3 |
| NH4+-N | 2.0–6.2 |
| NO3−-N | 0.12–0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, G.; Li, Y.; Ma, Y.; Han, X.; Zhou, X. Enhanced Sewage Sludge Disintegration and Nutrients Release by Catalytic Microbubbles Ozonation Using Sewage Sludge-Based Char as Catalyst. Sustainability 2023, 15, 1641. https://doi.org/10.3390/su15021641
Zhang X, Li G, Li Y, Ma Y, Han X, Zhou X. Enhanced Sewage Sludge Disintegration and Nutrients Release by Catalytic Microbubbles Ozonation Using Sewage Sludge-Based Char as Catalyst. Sustainability. 2023; 15(2):1641. https://doi.org/10.3390/su15021641
Chicago/Turabian StyleZhang, Xin, Guangming Li, Yijing Li, Yan Ma, Xiaomeng Han, and Xinyu Zhou. 2023. "Enhanced Sewage Sludge Disintegration and Nutrients Release by Catalytic Microbubbles Ozonation Using Sewage Sludge-Based Char as Catalyst" Sustainability 15, no. 2: 1641. https://doi.org/10.3390/su15021641





