Investigation of Thermochemical Properties and Pyrolysis of Barley Waste as a Source for Renewable Energy
Abstract
1. Introduction
2. Experimental
2.1. Sample Collection and Preparation
2.2. Biomass Characterization
2.3. Proximate Analysis
2.4. Ultimate Analysis
2.5. Morphology and Elemental Analysis (SEM/EDX)
2.6. Calorific Value/Heating Value
2.7. Fourier Transform Infrared (FTIR) Spectroscopy
2.8. Thermogravimetric Analysis (TGA) and Derivative Thermogravimetry (DTG)
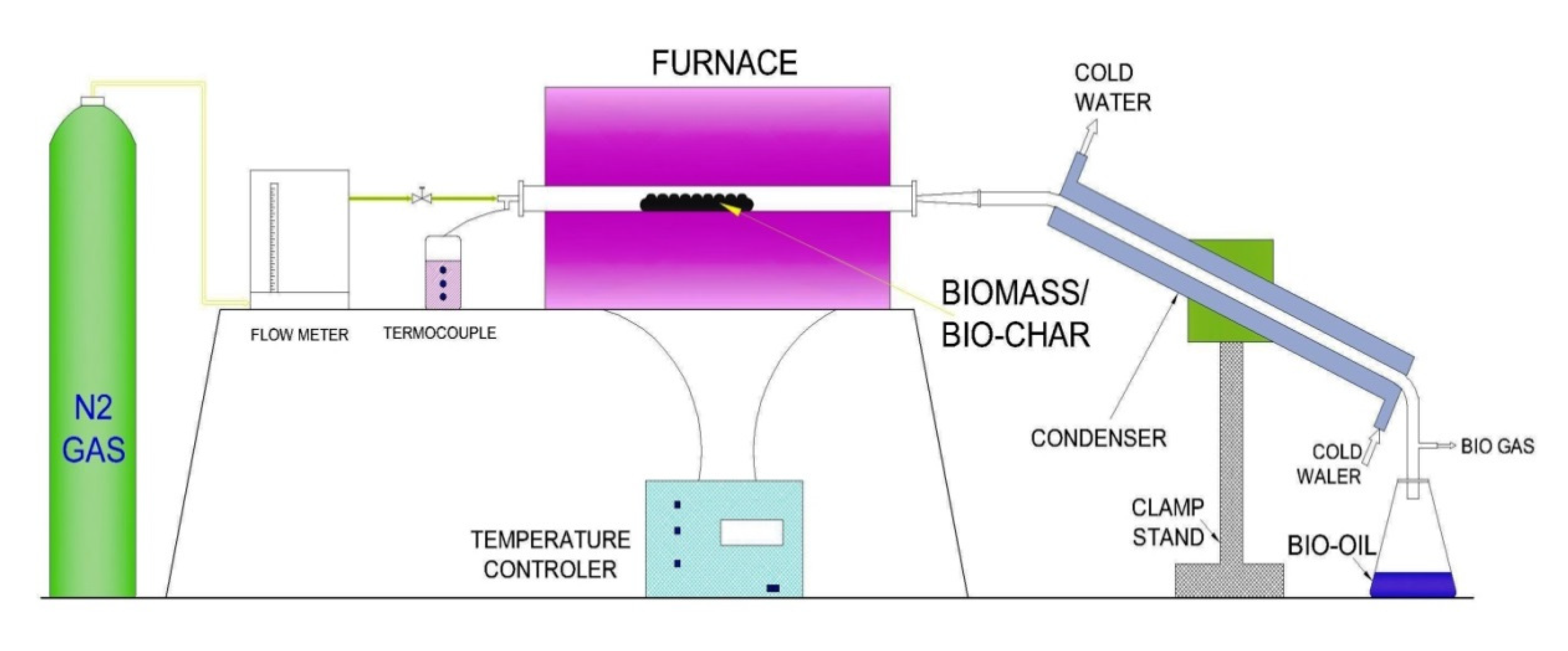
2.9. Pyrolysis (Fixed-Bed)
3. Results and Discussion
3.1. Proximate Analysis
3.2. Ultimate Analysis
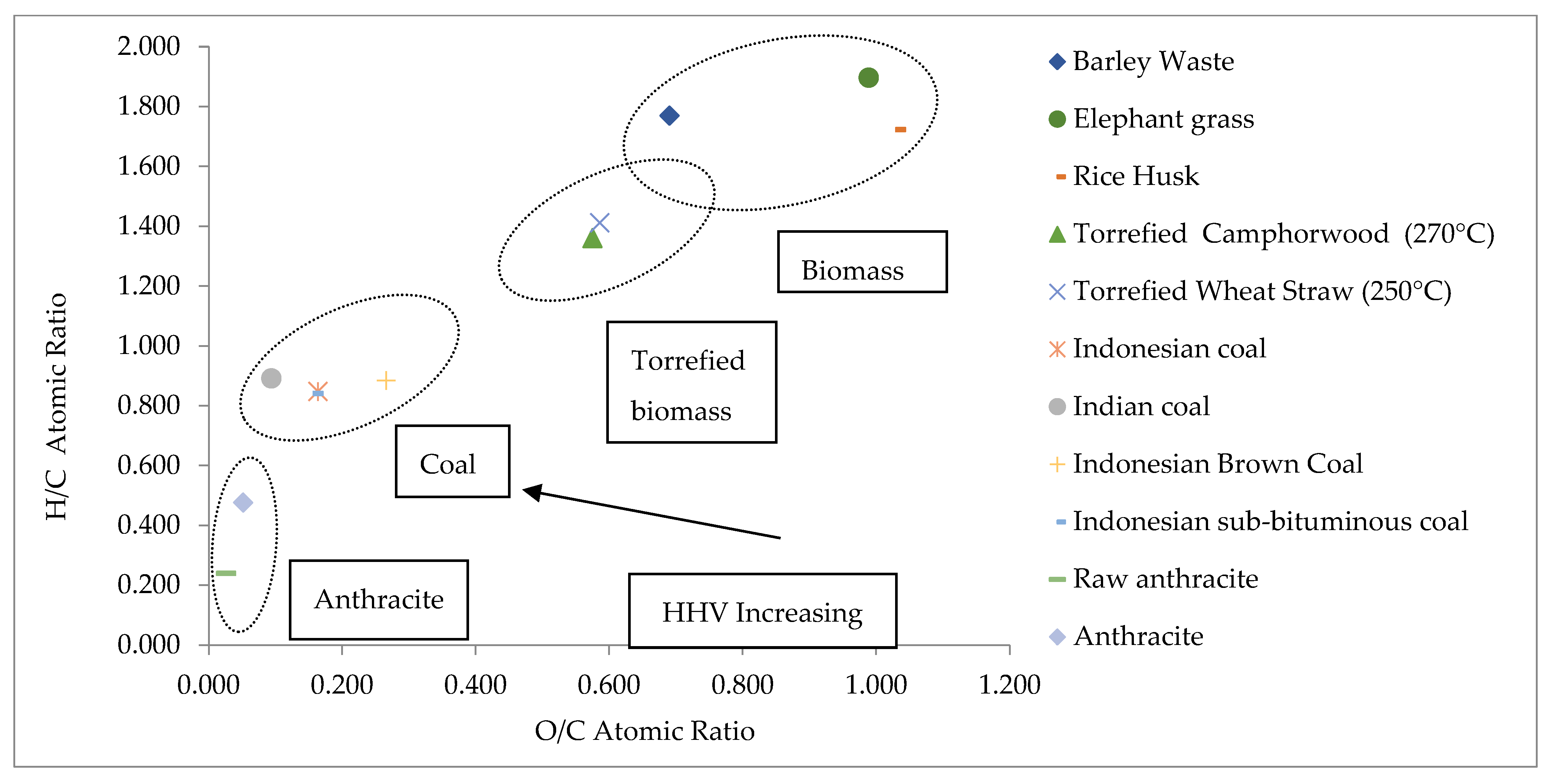
Van Krevelen Diagram
3.3. Calorific Value/Heating Value
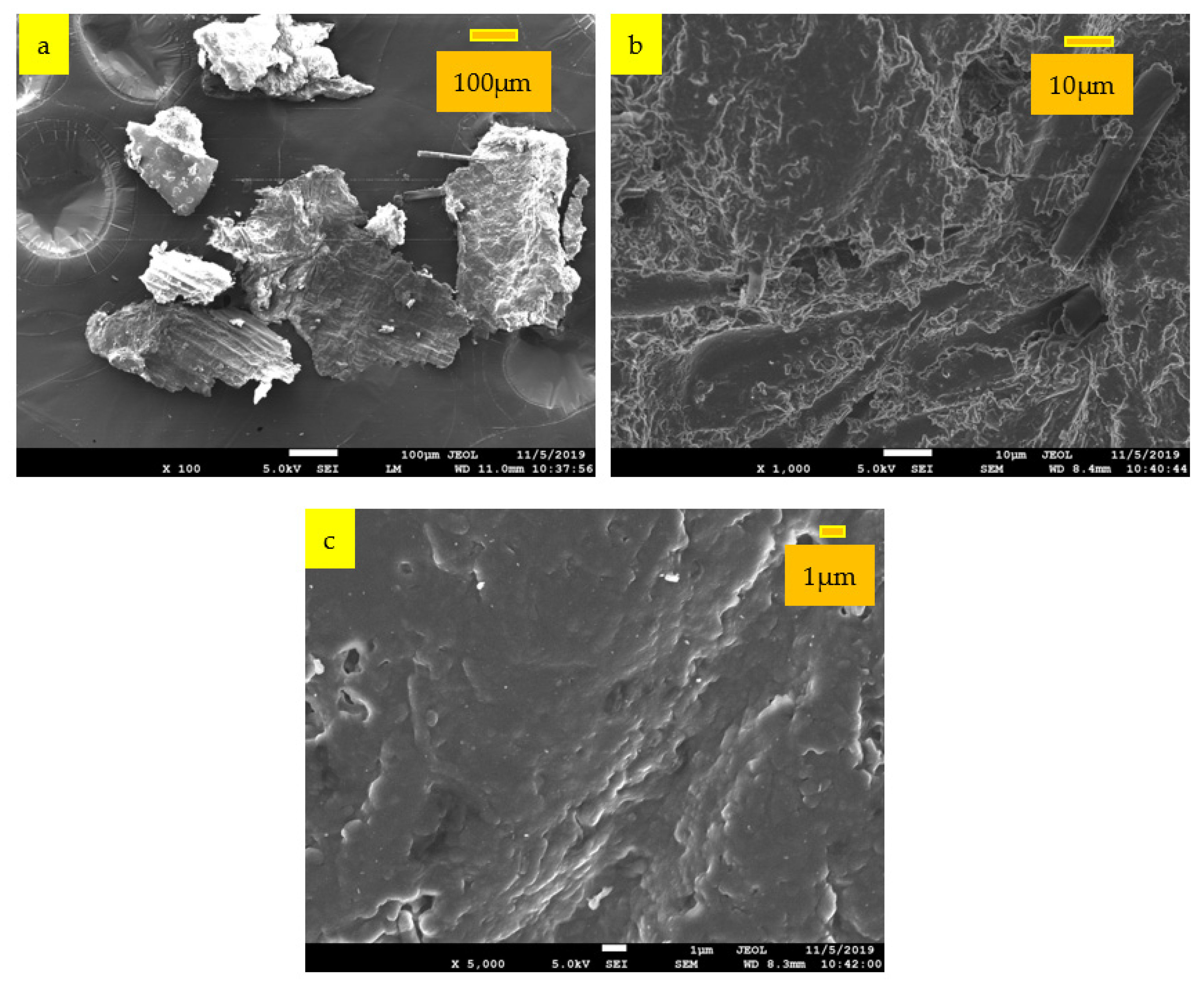
3.4. Scanning Electron Microscope (SEM)
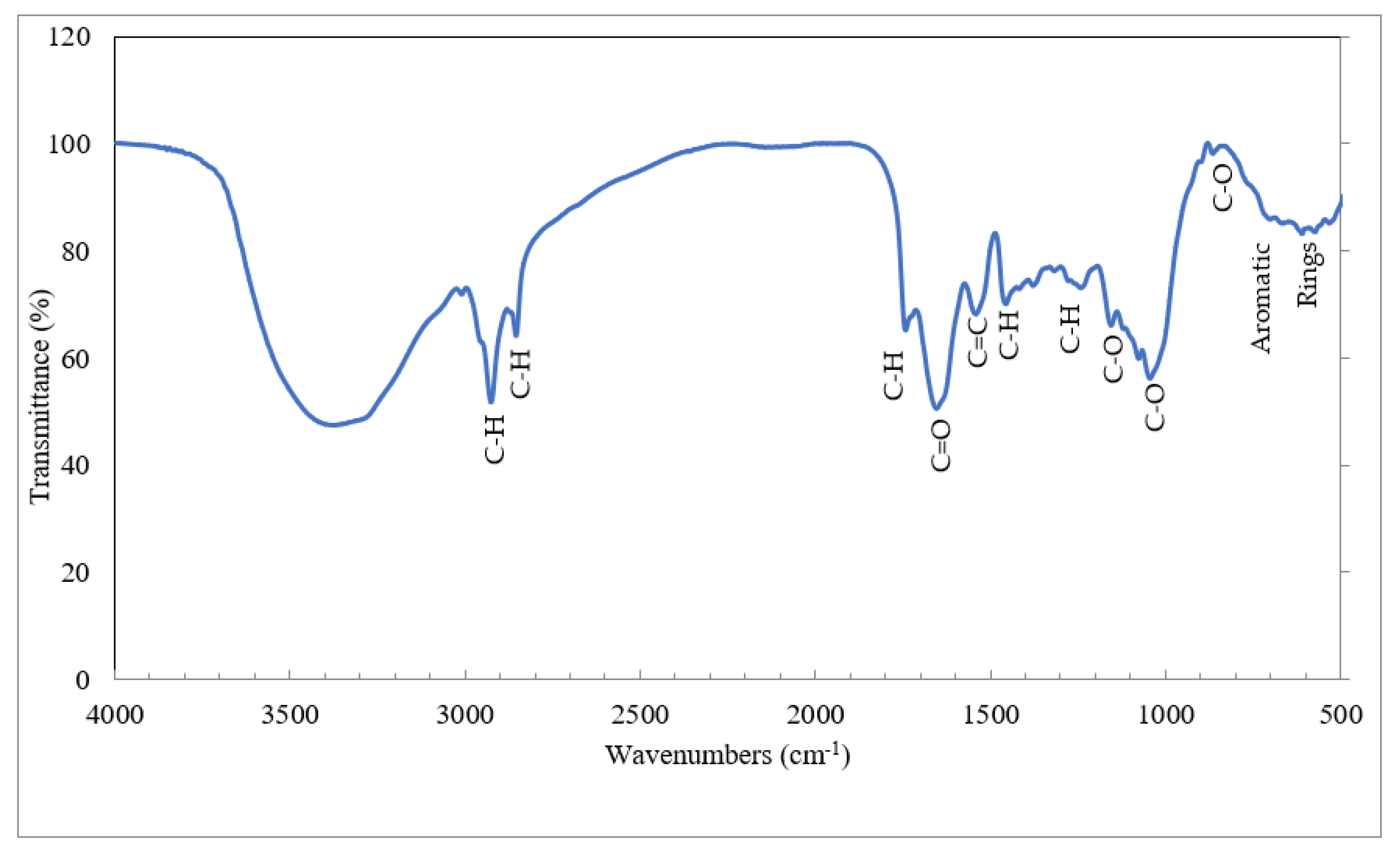
3.5. Fourier Transforms Infrared Spectroscopy
| Functional Groups | Wave Number (cm−1) | Reference |
|---|---|---|
| C-H stretching in aliphatic creation | 2931, 2860 | [62] |
| C-H stretching in cellulose and hemicellulose | 1749 | [61] |
| C=O Bending of hemicelluloses | 1668 | [62] |
| Aromatic C=C ring stretching | 1558 | [65] |
| Aliphatic C-H deformation | 1467 | [64] |
| C-H deformation in cellulose and hemicellulose | 1396 | [62] |
| C-O stretching in lignin and xylan | 1271 | [62] |
| C-O stretching vibration in cellulose and hemicelluloses | 1089, 1056 | [4] |
| C-O stretching in cellulose | 912 | [64] |
| Aromatic rings | 734–612 | [66] |
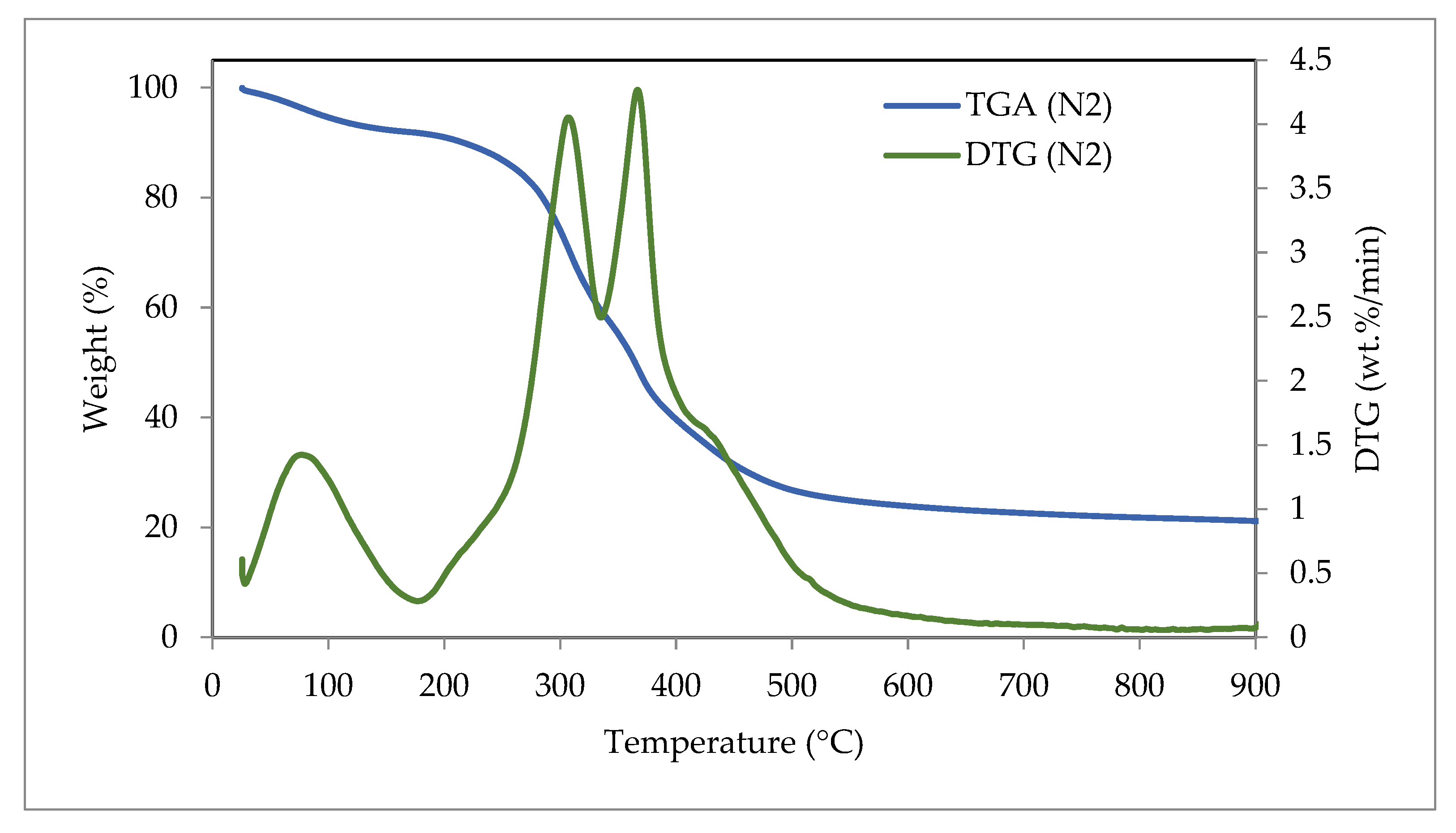
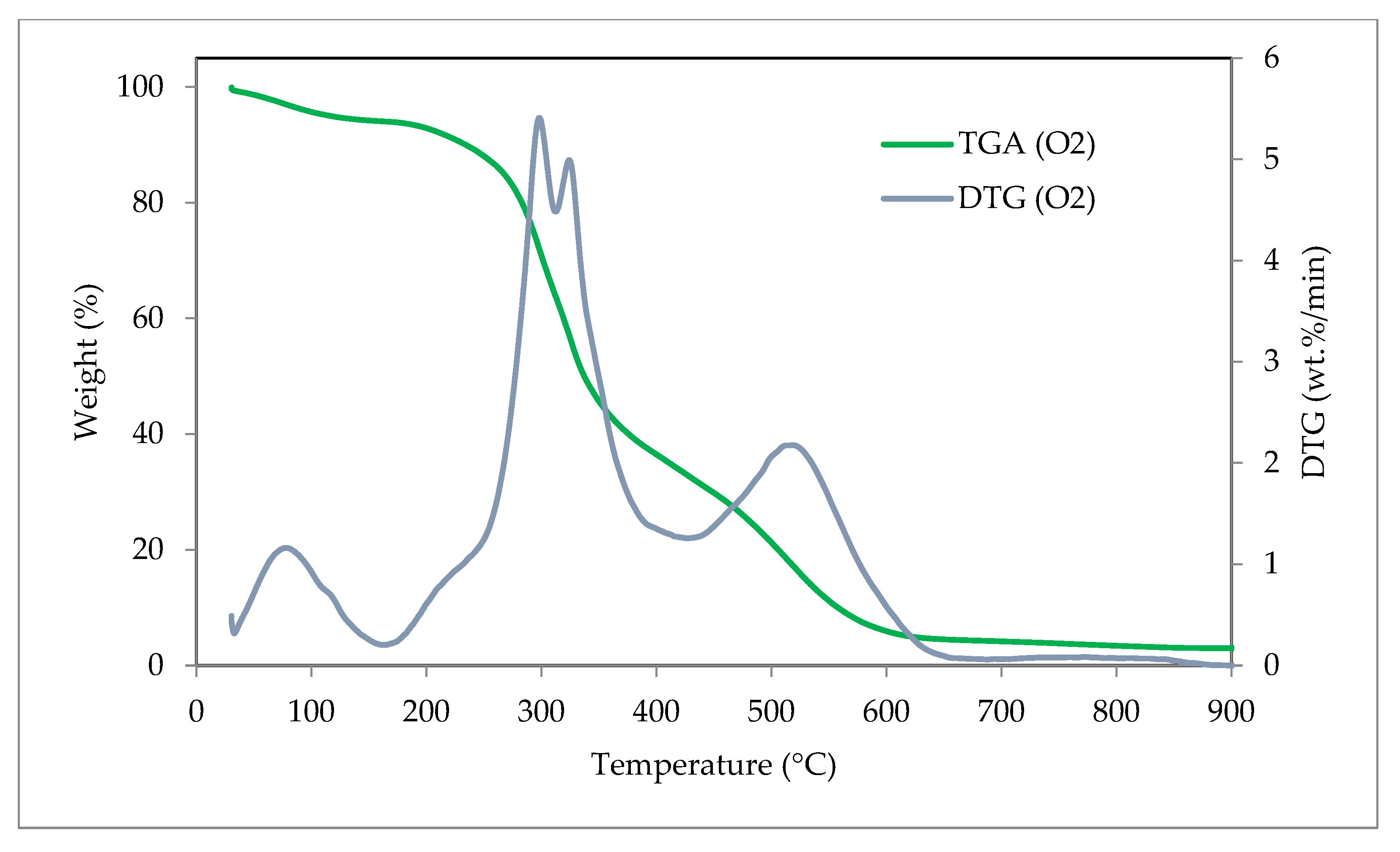
3.6. Thermogravimetric Analysis (TGA) and Derivative Thermogravimetry (DTG)
3.7. Pyrolysis Yields
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, A.; Abu Bakar, M.S.; Azad, A.K.; Sukri, R.S.; Mahlia, T.M.I. Potential thermochemical conversion of bioenergy from Acacia species in Brunei Darussalam: A review. Renew. Sustain. Energy Rev. 2018, 82, 3060–3076. [Google Scholar] [CrossRef]
- Kabeyi, M.J.B.; Olanrewaju, O.A. Sustainable Energy Transition for Renewable and Low Carbon Grid Electricity Generation and Supply. Front. Energy Res. 2022, 9, 1–45. [Google Scholar] [CrossRef]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Reza, M.S.; Ahmed, A.; Caesarendra, W.; Abu Bakar, M.S.; Shams, S.; Saidur, R.; Aslfattahi, N.; Azad, A.K. Acacia Holosericea: An Invasive Species for Bio-char, Bio-oil and Biogas Production. Bioengineering 2019, 6, 33. [Google Scholar] [CrossRef]
- Roy, H.; Alam, S.R.; Bin-Masud, R.; Prantika, T.R.; Pervez, M.N.; Islam, M.S.; Naddeo, V. A Review on Characteristics, Techniques, and Waste-to-Energy Aspects of Municipal Solid Waste Management: Bangladesh Perspective. Sustainability 2022, 14, 10265. [Google Scholar] [CrossRef]
- Siddiqua, A.; Hahladakis, J.N.; Al-Attiya, W.A.K.A. An overview of the environmental pollution and health effects associated with waste landfilling and open dumping. Environ. Sci. Pollut. Res. 2022, 29, 58514–58536. [Google Scholar] [CrossRef]
- Islam, M.S.; Kwak, J.H.; Nzediegwu, C.; Wang, S.; Palansuriya, K.; Kwon, E.E.; Naeth, M.A.; El-Din, M.G.; Ok, Y.S.; Chang, S.X. Biochar heavy metal removal in aqueous solution depends on feedstock type and pyrolysis purging gas. Environ. Pollut. 2021, 281, 117094. [Google Scholar] [CrossRef]
- Jha, S.; Nanda, S.; Acharya, B.; Dalai, A.K. A Review of Thermochemical Conversion of Waste Biomass to Biofuels. Energies 2022, 15, 6352. [Google Scholar] [CrossRef]
- Halder, P.; Azad, A.K. Recent trends and challenges of algal biofuel conversion technologies. In Advanced Biofuels: Applications, Technologies and Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2019; pp. 169–179. ISBN 9780081027912. [Google Scholar]
- Zhang, L.; Xu, C.C.; Champagne, P. Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers. Manag. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Zaman, C.Z.; Pal, K.; Yehye, W.A.; Sagadevan, S.; Shah, S.T.; Adebisi, G.A.; Marliana, E.; Rafique, R.F.; Johan, R. Bin Pyrolysis: A Sustainable Way to Generate Energy from Waste. In Pyrolysis; InTech: London, UK, 2017; pp. 1–35. [Google Scholar]
- Wang, C.; Sun, R.; Huang, R. Highly dispersed iron-doped biochar derived from sawdust for Fenton-like degradation of toxic dyes. J. Clean. Prod. 2021, 297, 126681. [Google Scholar] [CrossRef]
- Luo, D.; Wang, L.; Nan, H.; Cao, Y.; Wang, H.; Kumar, T.V.; Wang, C. Phosphorus adsorption by functionalized biochar: A review. Environ. Chem. Lett. 2022, 2022, 1–28. [Google Scholar] [CrossRef]
- Wang, C.; Huang, R.; Sun, R.; Yang, J.; Sillanpää, M. A review on persulfates activation by functional biochar for organic contaminants removal: Synthesis, characterizations, radical determination, and mechanism. J. Environ. Chem. Eng. 2021, 9, 106267. [Google Scholar] [CrossRef]
- Reza, M.S.; Azad, A.K.; Bakar, M.S.A.; Karim, M.R.; Sharifpur, M.; Taweekun, J. Evaluation of Thermochemical Characteristics and Pyrolysis of Fish Processing Waste for Renewable Energy Feedstock. Sustainability 2022, 14, 1203. [Google Scholar] [CrossRef]
- Reza, M.S.; Yun, C.S.; Afroze, S.; Radenahmad, N.; Bakar, M.S.A.; Saidur, R.; Taweekun, J.; Azad, A.K. Preparation of activated carbon from biomass and its’ applications in water and gas purification, a review. Arab J. Basic Appl. Sci. 2020, 27, 208–238. [Google Scholar] [CrossRef]
- Mussatto, S.I. Brewer’s spent grain: A valuable feedstock for industrial applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef]
- Fărcaş, A.C.; Socaci, S.A.; Mudura, E.; Dulf, F.V.; Vodnar, D.C.; Tofană, M.; Salanță, L.C.; Fărcaş, A.C.; Socaci, S.A.; Mudura, E.; et al. Exploitation of Brewing Industry Wastes to Produce Functional Ingredients. In Brewing Technology; IntechOpen: London, UK, 2017. [Google Scholar]
- Nigam, P.S. An overview: Recycling of solid barley waste generated as a by-product in distillery and brewery. Waste Manag. 2017, 62, 255–261. [Google Scholar] [CrossRef]
- Cai, J.; He, Y.; Yu, X.; Banks, S.W.; Yang, Y.; Zhang, X.; Yu, Y.; Liu, R.; Bridgwater, A.V. Review of physicochemical properties and analytical characterization of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2017, 76, 309–322. [Google Scholar] [CrossRef]
- Detailed Overview (ASTM International). Available online: https://www.astm.org/about/overview/detailed-overview.html (accessed on 31 December 2022).
- ASTM D 3173-11; Standard Test Method for Moisture in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM D 3175-07; Standard Test Method for Volatile Matter in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2007.
- ASTM D3174-04; Standard Test Method for Ash in the Analysis Sample of Coal and Coke from Coal. ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM D5468-02; Standard Test Method for Gross Calorific and Ash Value of Waste Materials. ASTM International: West Conshohocken, PA, USA, 2007.
- Samuel, O.O.; Olugbenga, E.A. Estimation and assesment of free swelling index and some petro-graphic properties from chemical analysis of coals across river niger. Pet. Coal 2017, 59, 273–287. [Google Scholar]
- Gaber, A.; Saif, H.; Ali, M.R.O. C Yields. Bioresour. Bioprocess. 2020. preprint. [Google Scholar]
- Collazzo, G.C.; Broetto, C.C.; Perondi, D.; Junges, J.; Dettmer, A.; Dornelles Filho, A.A.; Foletto, E.L.; Godinho, M. A detailed non-isothermal kinetic study of elephant grass pyrolysis from different models. Appl. Therm. Eng. 2017, 110, 1200–1211. [Google Scholar] [CrossRef]
- Promdee, K.; Vitidsant, T. Preparation of Biofuel by Pyrolysis of Plant Matter in a Continuous Reactor. Theor. Exp. Chem. 2013, 49, 126–129. [Google Scholar] [CrossRef]
- Imam, T.; Capareda, S. Characterization of bio-oil, syn-gas and bio-char from switchgrass pyrolysis at various temperatures. J. Anal. Appl. Pyrolysis 2012, 93, 170–177. [Google Scholar] [CrossRef]
- Kenney, K.L.; Smith, W.A.; Gresham, G.L.; Westover, T.L. Understanding biomass feedstock variability. Biofuels 2013, 4, 111–127. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, S.V.; Vassileva, C.G.; Vassilev, V.S. Advantages and disadvantages of composition and properties of biomass in comparison with coal: An overview. Fuel 2015, 158, 330–350. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Li, J.; Su, L.; Zuo, J.; Komarneni, S.; Wang, Y. Improving the aromatic production in catalytic fast pyrolysis of cellulose by co-feeding low-density polyethylene. Appl. Catal. A Gen. 2013, 455, 114–121. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Lim, X.Y.; Ani, F.N.; Jusoh, A. Fruit waste as feedstock for recovery by pyrolysis technique. Int. Biodeterior. Biodegrad. 2016, 113, 325–333. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Kent Hoekman, S.; Balasubramanian, R. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Gondek, K.; Jewiarz, M.; Dziedzic, K. Assessment of energy parameters of biomass and biochars, leachability of heavy metals and phytotoxicity of their ashes. J. Mater. Cycles Waste Manag. 2019, 21, 786–800. [Google Scholar] [CrossRef]
- Kwak, J.H.; Islam, M.S.; Wang, S.; Messele, S.A.; Naeth, M.A.; El-Din, M.G.; Chang, S.X. Biochar properties and lead(II) adsorption capacity depend on feedstock type, pyrolysis temperature, and steam activation. Chemosphere 2019, 231, 393–404. [Google Scholar] [CrossRef]
- Sadiku, N.A.; Oluyege, A.O.; Sadiku, I.B. Analysis of the Calorific and Fuel Value Index of Bamboo as a Source of Renewable Biomass Feedstock for Enegry Generation in Nigeria. Lignocellulose 2016, 5, 34–49. [Google Scholar]
- Aup-Ngoen, K.; Noipitak, M. Effect of carbon-rich biochar on mechanical properties of PLA-biochar composites. Sustain. Chem. Pharm. 2020, 15, 100204. [Google Scholar] [CrossRef]
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Abu Bakar, M. Catalytic Intermediate Pyrolysis of Brunei Rice Husk for Bio-Oil Production. Ph.D. Thesis, Aston University, Birmingham, UK, 2013. [Google Scholar]
- Reza, M.S.; Islam, S.N.; Afroze, S.; Bakar, M.S.A.; Sukri, R.S.; Rahman, S.; Azad, A.K. Evaluation of the bioenergy potential of invasive Pennisetum purpureum through pyrolysis and thermogravimetric analysis. Energy Ecol. Environ. 2020, 5, 118–133. [Google Scholar] [CrossRef]
- Abu Bakar, M.S.; Titiloye, J.O. Catalytic pyrolysis of rice husk for bio-oil production. J. Anal. Appl. Pyrolysis 2013, 103, 362–368. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z. Sorption of naphthalene and 1-naphthol by biochars of orange peels with different pyrolytic temperatures. Chemosphere 2009, 76, 127–133. [Google Scholar] [CrossRef]
- Molino, A.; Nanna, F.; Ding, Y.; Bikson, B.; Braccio, G. Biomethane production by anaerobic digestion of organic waste. Fuel 2013, 103, 1003–1009. [Google Scholar] [CrossRef]
- Gravalos, I.; Xyradakis, P.; Kateris, D.; Gialamas, T.; Bartzialis, D.; Giannoulis, K. An Experimental Determination of Gross Calorific Value of Different Agroforestry Species and Bio-Based Industry Residues. Nat. Resour. 2016, 07, 57–68. [Google Scholar] [CrossRef]
- Hidayat, S.; Abu Bakar, M.S.; Yang, Y.; Phusunti, N.; Bridgwater, A.V. Characterisation and Py-GC/MS analysis of Imperata Cylindrica as potential biomass for bio-oil production in Brunei Darussalam. J. Anal. Appl. Pyrolysis 2018, 134, 510–519. [Google Scholar] [CrossRef]
- Wang, W.-C.; Lee, A.-C. Thermochemical Processing of Miscanthus through Fluidized-Bed Fast Pyrolysis: A Parametric Study. Chem. Eng. Technol. 2018, 41, 1737–1745. [Google Scholar] [CrossRef]
- Mehmood, M.A.; Ye, G.; Luo, H.; Liu, C.; Malik, S.; Afzal, I.; Xu, J.; Ahmad, M.S. Pyrolysis and kinetic analyses of Camel grass (Cymbopogon schoenanthus) for bioenergy. Bioresour. Technol. 2017, 228, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Moreno, J.M.; Callejón-Ferre, A.J.; Pérez-Alonso, J.; Velázquez-Martí, B. A review of the mathematical models for predicting the heating value of biomass materials. Renew. Sustain. Energy Rev. 2012, 16, 3065–3083. [Google Scholar] [CrossRef]
- Huang, C.; Han, L.; Yang, Z.; Liu, X. Ultimate analysis and heating value prediction of straw by near infrared spectroscopy. Waste Manag. 2009, 29, 1793–1797. [Google Scholar] [CrossRef] [PubMed]
- Okolodkov, Y.B.; Huerta-Quintanilla, D.A. A low voltage scanning electron microscopy study of the theca of marine dinoflagellates. J. Adv. Microsc. Res. 2012, 7, 170–175. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Rajulu, A.V. Extraction and characterization of cellulose single fi bers from native african napier grass. Carbohydr. Polym. 2018, 188, 85–91. [Google Scholar]
- Blesa, M.J.; Fierro, V.; Miranda, J.L.; Moliner, R.; Palacios, J.M. Effect of the pyrolysis process on the physicochemical and mechanical properties of smokeless fuel briquettes. Fuel Process. Technol. 2001, 74, 1–17. [Google Scholar] [CrossRef]
- Newbury, D.E.; Ritchie, N.W.M. Performing elemental microanalysis with high accuracy and high precision by scanning electron microscopy/silicon drift detector energy-dispersive X-ray spectrometry (SEM/SDD-EDS). J. Mater. Sci. 2015, 50, 493–518. [Google Scholar] [CrossRef]
- Hossain, N.; Zaini, J.; Mahlia, T.M.I.; Azad, A.K. Elemental, morphological and thermal analysis of mixed microalgae species from drain water. Renew. Energy 2019, 131, 617–624. [Google Scholar] [CrossRef]
- Smith, F.A.; White, J.W.C. Modern calibration of phytolith carbon isotope signatures for C 3/C4 paleograssland reconstruction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 207, 277–304. [Google Scholar] [CrossRef]
- Özçimen, D.; Ersoy-Meriçboyu, A. Characterization of biochar and bio-oil samples obtained from carbonization of various biomass materials. Renew. Energy 2010, 35, 1319–1324. [Google Scholar] [CrossRef]
- Chem.libretexts.org Infrared Spectroscopy Absorption Table—Chemistry LibreTexts. Available online: https://chem.libretexts.org/Ancillary_Materials/Reference/Reference_Tables/Spectroscopic_Parameters/Infrared_Spectroscopy_Absorption_Table (accessed on 8 July 2019).
- Akhtar, N.; Goyal, D.; Goyal, A. Physico-chemical characteristics of leaf litter biomass to delineate the chemistries involved in biofuel production. J. Taiwan Inst. Chem. Eng. 2016, 62, 239–246. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Traoré, M.; Kaal, J.; Cortizas, A.M. Application of FTIR spectroscopy to the characterization of archeological wood. SAA 2015, 153, 63–70. [Google Scholar] [CrossRef]
- El-Hendawy, A.-N.A. Variation in the FTIR spectra of a biomass under impregnation, carbonization and oxidation conditions. J. Anal. Appl. Pyrolysis 2006, 75, 159–166. [Google Scholar] [CrossRef]
- Ahmed, A.; Hidayat, S.; Abu Bakar, M.S.; Azad, A.K.; Sukri, R.S.; Phusunti, N. Thermochemical characterisation of Acacia auriculiformis tree parts via proximate, ultimate, TGA, DTG, calorific value and FTIR spectroscopy analyses to evaluate their potential as a biofuel resource. Biofuels 2018, 7269, 9–20. [Google Scholar] [CrossRef]
- Baloch, H.A.; Nizamuddin, S.; Siddiqui, M.T.H.; Riaz, S.; Jatoi, A.S.; Dumbre, D.K.; Mubarak, N.M.; Srinivasan, M.P.; Griffin, G.J. Recent advances in production and upgrading of bio-oil from biomass: A critical overview. J. Environ. Chem. Eng. 2018, 6, 5101–5118. [Google Scholar] [CrossRef]
- Mabuda, A.I.; Mamphweli, N.S.; Meyer, E.L. Model free kinetic analysis of biomass/sorbent blends for gasification purposes. Renew. Sustain. Energy Rev. 2016, 53, 1656–1664. [Google Scholar] [CrossRef]
- Naik, S.; Goud, V.V.; Rout, P.K.; Jacobson, K.; Dalai, A.K. Characterization of Canadian biomass for alternative renewable biofuel. Renew. Energy 2010, 35, 1624–1631. [Google Scholar] [CrossRef]
- Park, Y.H.; Kim, J.; Kim, S.S.; Park, Y.K. Pyrolysis characteristics and kinetics of oak trees using thermogravimetric analyzer and micro-tubing reactor. Bioresour. Technol. 2009, 100, 400–405. [Google Scholar] [CrossRef]
- Titiloye, J.O.; Abu Bakar, M.S.; Odetoye, T.E. Thermochemical characterisation of agricultural wastes from West Africa. Ind. Crops Prod. 2013, 47, 199–203. [Google Scholar] [CrossRef]
- Choudhury, N.D.; Chutia, R.S.; Bhaskar, T.; Kataki, R. Pyrolysis of jute dust: Effect of reaction parameters and analysis of products. J. Mater. Cycles Waste Manag. 2014, 16, 449–459. [Google Scholar] [CrossRef]
- Brownsort, P. Biomass Pyrolysis Processes: Performance Parameters and Their Influence on Biochar System Benefits. Master’s Thesis, University of Edinburgh, Edinburgh, UK, 2009. [Google Scholar]
- Aqsha, A.; Tijani, M.M.; Moghtaderi, B.; Mahinpey, N. Catalytic pyrolysis of straw biomasses (wheat, flax, oat and barley) and the comparison of their product yields. J. Anal. Appl. Pyrolysis 2017, 125, 201–208. [Google Scholar] [CrossRef]
- Ferreira, S.D.; Manera, C.; Silvestre, W.P.; Pauletti, G.F.; Altafini, C.R.; Godinho, M. Use of Biochar Produced from Elephant Grass by Pyrolysis in a Screw Reactor as a Soil Amendment. Waste Biomass Valorization 2019, 10, 3089–3100. [Google Scholar] [CrossRef]
- Kang, Z.; Jia, X.; Zhang, Y.; Kang, X.; Ge, M.; Liu, D.; Wang, C.; He, Z. A Review on Application of Biochar in the Removal of Pharmaceutical Pollutants through Adsorption and Persulfate-Based AOPs. Sustainability 2022, 14, 10128. [Google Scholar] [CrossRef]
- Wang, C.; Luo, D.; Zhang, X.; Huang, R.; Cao, Y.; Liu, G.; Zhang, Y.; Wang, H. Biochar-based slow-release of fertilizers for sustainable agriculture: A mini review. Environ. Sci. Ecotechnology 2022, 10, 100167. [Google Scholar] [CrossRef]
- Quan, C.; Gao, N.; Song, Q. Pyrolysis of biomass components in a TGA and a fixed-bed reactor: Thermochemical behaviors, kinetics, and product characterization. J. Anal. Appl. Pyrolysis 2016, 121, 84–92. [Google Scholar] [CrossRef]
- Reza, M.S.; Afroze, S.; Bakar, M.S.A.; Saidur, R.; Aslfattahi, N.; Taweekun, J.; Azad, A.K. Biochar characterization of invasive Pennisetum purpureum grass: Effect of pyrolysis temperature. Biochar 2020, 2, 239–251. [Google Scholar] [CrossRef]
- Lin, B.-J.; Chen, W.-H. Sugarcane Bagasse Pyrolysis in a Carbon Dioxide Atmosphere with Conventional and Microwave-Assisted Heating. Front. Energy Res. 2015, 3, 4. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, X.; Li, W.; Shi, Y. Preparation and Characterization of Bio-Oil from Biomass. In Progress in Biomass and Bioenergy Production; Shaukat, S., Ed.; InTech: Shanghai, China, 2011; pp. 197–222. ISBN 978-953-307-491-7. [Google Scholar]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Encinar, J.; González, J.; González, J. Fixed-bed pyrolysis of Cynara cardunculus L. Product yields and compositions. Fuel Process. Technol. 2000, 68, 209–222. [Google Scholar] [CrossRef]
- Guedes, R.E.; Luna, A.S.; Torres, A.R. Operating parameters for bio-oil production in biomass pyrolysis: A review. J. Anal. Appl. Pyrolysis 2018, 129, 134–149. [Google Scholar] [CrossRef]
- Mullen, C.A.; Boateng, A.A.; Hicks, K.B.; Goldberg, N.M.; Moreau, R.A. Analysis and Comparison of Bio-Oil Produced by Fast Pyrolysis from Three Barley Biomass/Byproduct Streams. Energy and Fuels 2009, 24, 699–706. [Google Scholar] [CrossRef]



| Biomass Sample | MC (wt.%) | VM (wt.%) | FC (wt.%) | AC (wt.%) | Reference |
|---|---|---|---|---|---|
| Barley waste | 5.43 | 73.41 | 18.15 | 3.01 | This Study |
| Elephant grass | 9.80 | 69.20 | 7.70 | 13.30 | [28] |
| Imperata cylindrica | 9.30 | 64.30 | 16.10 | 10.30 | [29] |
| Switchgrass | 8.40 | 84.20 | 11.90 | 3.90 | [30] |
| Sample | C (wt.%) | H (wt.%) | N (wt.%) | S (wt.%) | O (wt.%) | H/C | O/C | Ref. |
|---|---|---|---|---|---|---|---|---|
| Barley Waste | 46.04 | 6.84 | 3.89 | 0.91 | 42.32 | 1.770 | 0.690 | This Study |
| Elephant grass | 39.63 | 6.31 | 1.70 | 0.20 | 52.16 | 1.897 | 0.988 | [4,43] |
| Rice husk | 39.48 | 5.71 | 0.665 | <0.10 | 54.12 | 1.723 | 1.029 | |
| Torrefied camphorwood | 53.05 | 6.06 | 0.23 | -- | 40.60 | 1.361 | 0.575 | |
| Torrefied rape straw | 48.86 | 5.79 | -- | -- | 38.09 | 1.412 | 0.585 | |
| Indonesian coal | 75.47 | 5.37 | 1.10 | -- | 16.40 | 0.848 | 0.163 | |
| Indian coal | 44.50 | 3.33 | 1.02 | -- | 5.55 | 0.892 | 0.094 | |
| Indonesian brown Coal | 69.20 | 5.14 | 0.89 | 0.28 | 24.49 | 0.885 | 0.266 | |
| Indonesian sub-bituminous coal | 37.95 | 2.68 | 0.69 | 0.31 | 7.91 | 0.841 | 0.157 | |
| Raw anthracite | 90.60 | -- | -- | -- | 3.10 | 0.240 | 0.025 | |
| Anthracite | 72.49 | 2.90 | 0.94 | 0.39 | 4.96 | 0.477 | 0.051 |
| Biomass | HHV (MJ/kg) | LHV * (MJ/kg) | Reference |
|---|---|---|---|
| Barley waste | 20.06 | 18.44 | This Study |
| Imperata cylindrica | 18.39 | 16.99 | [49] |
| Miscanthus floridulus | 11.05 | 9.67 | [50] |
| Camel grass | 15.00 | 13.40 | [51] |
| Element | Wt. (%) | Atomic (%) |
|---|---|---|
| Carbon (C) | 67.20 | 73.96 |
| Oxygen (O) | 30.08 | 24.85 |
| Magnesium (Mg) | 0.12 | 0.07 |
| Aluminum (Al) | 0.38 | 0.19 |
| Silicon (Si) | 0.24 | 0.11 |
| Phosphorus (P) | 1.00 | 0.43 |
| Sulfur (S) | 0.62 | 0.26 |
| Calcium (Ca) | 0.38 | 0.13 |
| TGA and DTG Conditions | Weight Loss (%) | Residue (%) | DTG Peak Temperature (°C) | DTG Peak (wt.%/min) | ||
|---|---|---|---|---|---|---|
| Stage I 40 to 200 (°C) | Stage II 200 to 450 (°C) | Stage III 450 to 900 (°C) | ||||
| Pyrolysis (N2) | 9.02 | 59.54 | 10.29 | 21.15 | 366 | 4.27 |
| Combustion (O2) | 7.11 | 63.03 | 26.85 | 3.01 | 298 | 5.41 |
| Pyrolysis Temperature (°C) | Biochar (wt.%) | Bio-Oil (wt.%) | Syngas 1 (wt.%) |
|---|---|---|---|
| 400 | 38.57 | 33.28 | 28.15 |
| 500 | 34.04 | 36.79 | 29.17 |
| 600 | 30.61 | 29.25 | 40.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reza, M.S.; Taweekun, J.; Afroze, S.; Siddique, S.A.; Islam, M.S.; Wang, C.; Azad, A.K. Investigation of Thermochemical Properties and Pyrolysis of Barley Waste as a Source for Renewable Energy. Sustainability 2023, 15, 1643. https://doi.org/10.3390/su15021643
Reza MS, Taweekun J, Afroze S, Siddique SA, Islam MS, Wang C, Azad AK. Investigation of Thermochemical Properties and Pyrolysis of Barley Waste as a Source for Renewable Energy. Sustainability. 2023; 15(2):1643. https://doi.org/10.3390/su15021643
Chicago/Turabian StyleReza, Md Sumon, Juntakan Taweekun, Shammya Afroze, Shohel Ahmed Siddique, Md. Shahinoor Islam, Chongqing Wang, and Abul K. Azad. 2023. "Investigation of Thermochemical Properties and Pyrolysis of Barley Waste as a Source for Renewable Energy" Sustainability 15, no. 2: 1643. https://doi.org/10.3390/su15021643
APA StyleReza, M. S., Taweekun, J., Afroze, S., Siddique, S. A., Islam, M. S., Wang, C., & Azad, A. K. (2023). Investigation of Thermochemical Properties and Pyrolysis of Barley Waste as a Source for Renewable Energy. Sustainability, 15(2), 1643. https://doi.org/10.3390/su15021643










