Structural Heterogeneity of European Beech (Fagus sylvatica L.) Stands at Its Northernmost Limits
Abstract
:1. Introduction
2. Materials and Methods
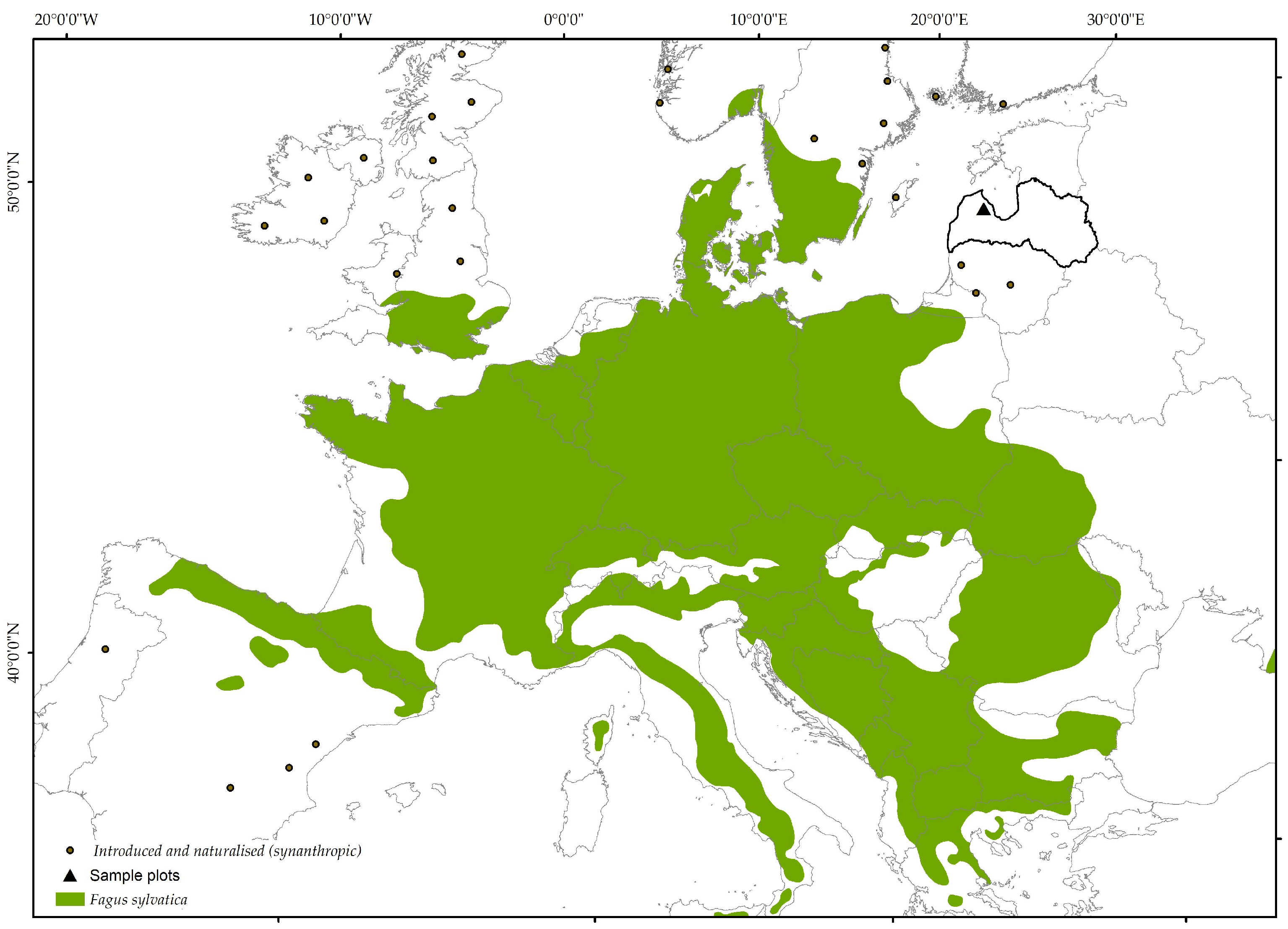
2.1. Study Area
2.2. Sampling and Measurements
2.3. Data Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Strømme, C.B.; Schmidt, E.; Olsen, J.E.; Nybakken, L. Climatic Effects on Bud Break and Frost Tolerance in the Northernmost Populations of Beech (Fagus sylvatica) in Europe. Trees 2019, 33, 79–89. [Google Scholar] [CrossRef]
- Stojnić, S.; Orlović, S.; Miljković, D.; Galić, Z.; Kebert, M.; von Wuehlisch, G. Provenance Plasticity of European Beech Leaf Traits under Differing Environmental Conditions at Two Serbian Common Garden Sites. Eur. J. Res. 2015, 134, 1109–1125. [Google Scholar] [CrossRef]
- Pretzsch, H.; Biber, P.; Schütze, G.; Bielak, K. Changes of Forest Stand Dynamics in Europe. Facts from Long-Term Observational Plots and Their Relevance for Forest Ecology and Management. For. Ecol. Manag. 2014, 316, 65–77. [Google Scholar] [CrossRef]
- Pavlović, L.; Stojanović, D.; Mladenović, E.; Lakićević, M.; Orlović, S. Potential Elevation Shift of the European Beech Stands (Fagus sylvatica L.) in Serbia. Front. Plant Sci. 2019, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Geßler, A.; Keitel, C.; Kreuzwieser, J.; Matyssek, R.; Seiler, W.; Rennenberg, H. Potential Risks for European Beech (Fagus sylvatica L.) in a Changing Climate. Trees 2007, 21, 1–11. [Google Scholar] [CrossRef]
- Augustynczik, A.L.D.; Yousefpour, R. Assessing the Synergistic Value of Ecosystem Services in European Beech Forests. Ecosyst. Serv. 2021, 49, 101264. [Google Scholar] [CrossRef]
- Pretzsch, H.; Biber, P.; Uhl, E.; Dauber, E. Long-Term Stand Dynamics of Managed Spruce-Fir-Beech Mountain Forests in Central Europe: Structure, Productivity and Regeneration Success. For. Int. J. For. Res. 2015, 88, 407–428. [Google Scholar] [CrossRef]
- Brunet, J.; Fritz, Ö.; Richnau, G. Biodiversity in European Beech Forests—A Review with Recommendations for Sustainable Forest Management. Ecol. Bull. 2010, 53, 77–94. [Google Scholar]
- Kramer, K.; Degen, B.; Buschbom, J.; Hickler, T.; Thuiller, W.; Sykes, M.T.; de Winter, W. Modelling Exploration of the Future of European Beech (Fagus sylvatica L.) under Climate Change—Range, Abundance, Genetic Diversity and Adaptive Response. For. Ecol. Manag. 2010, 259, 2213–2222. [Google Scholar] [CrossRef]
- Jansone, D.; Diena, L.; Rieksts-Riekstiņš, R.; Jansons, A. Stem Quality of European Beech in Latvia and Its Effect on Tree and Stand Monetary Value. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2021, 75, 292–298. [Google Scholar] [CrossRef]
- Augustaitis, A.; Kliučius, A.; Marozas, V.; Pilkauskas, M.; Augustaitiene, I.; Vitas, A.; Staszewski, T.; Jansons, A.; Dreimanis, A. Sensitivity of European Beech Trees to Unfavorable Environmental Factors on the Edge and Outside of Their Distribution Range in Northeastern Europe. IForest 2016, 9, 259–269. [Google Scholar] [CrossRef]
- Saltré, F.; Saint-Amant, R.; Gritti, E.S.; Brewer, S.; Gaucherel, C.; Davis, B.A.S.; Chuine, I. Climate or Migration: What Limited European Beech Post-Glacial Colonization? Ecol. Biogeogr. 2013, 22, 1217–1227. [Google Scholar] [CrossRef]
- Kramer, K.; Leinonen, I.; Loustau, D. The Importance of Phenology for the Evaluation of Impact of Climate Change on Growth of Boreal, Temperate and Mediterranean Forests Ecosystems: An Overview. Int. J. Biometeorol. 2000, 44, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Buras, A.; Menzel, A. Projecting Tree Species Composition Changes of European Forests for 2061–2090 under RCP 4.5 and RCP 8.5 Scenarios. Front. Plant Sci. 2018, 9, 986. [Google Scholar] [CrossRef]
- Bolte, A.; Czajkowski, T.; Kompa, T. The North-Eastern Distribution Range of European Beech—A Review. Forestry 2007, 80, 413–429. [Google Scholar] [CrossRef]
- Puriņa, L.; Matisons, R.; Jansons, Ā.; Šēnhofa, S. Survival of European Beech in the Central Part of Latvia 33 Years since the Plantation. Silva Fenn. 2016, 50, 1656. [Google Scholar] [CrossRef]
- Karse, V. Analysis of European beech Fagus sylvatica L. stand structure at forest research stations Šķēdes forest district. Ph.D. Thesis, Latvia University of Life Sciences and Technologies, Jelgava, Latvia, 2019. [Google Scholar]
- Knuff, A.K.; Staab, M.; Frey, J.; Dormann, C.F.; Asbeck, T.; Klein, A.M. Insect Abundance in Managed Forests Benefits from Multi-Layered Vegetation. Basic Appl. Ecol. 2020, 48, 124–135. [Google Scholar] [CrossRef]
- Kovács, B.; Tinya, F.; Ódor, P. Stand Structural Drivers of Microclimate in Mature Temperate Mixed Forests. Agric. For. Meteorol. 2017, 234–235, 11–21. [Google Scholar] [CrossRef]
- Pretzsch, H. The Elasticity of Growth in Pure and Mixed Stands of Norway Spruce (Picea Abies [L.] Karst.) and Common Beech (Fagus sylvatica L.). J. For. Sci. 2003, 49, 491–501. [Google Scholar] [CrossRef]
- Ehbrecht, M.; Seidel, D.; Annighöfer, P.; Kreft, H.; Köhler, M.; Zemp, D.C.; Puettmann, K.; Nilus, R.; Babweteera, F.; Willim, K.; et al. Global Patterns and Climatic Controls of Forest Structural Complexity. Nat. Commun. 2021, 12, 519. [Google Scholar] [CrossRef]
- Winter, S.; Möller, G.C. Microhabitats in Lowland Beech Forests as Monitoring Tool for Nature Conservation. For. Ecol. Manag. 2008, 255, 1251–1261. [Google Scholar] [CrossRef]
- Haq, S.M.; Waheed, M.; Khoja, A.A.; Amjad, M.S.; Bussmann, R.W.; Ali, K.; Jones, D.A. Measuring Forest Health at Stand Level: A Multi-Indicator Evaluation for Use in Adaptive Management and Policy. Ecol. Indic. 2023, 150, 110225. [Google Scholar] [CrossRef]
- Ammer, C.; Bickel, E.; Kölling, C. Converting Norway Spruce Stands with Beech—A Review of Arguments and Techniques. Austrian J. For. Sci. 2008, 125, 3–26. [Google Scholar]
- Matisons, R.; Šņepsts, G.; Puriņa, L.; Donis, J.; Jansons, Ā. Dominant Height Growth of European Beech at the Northeasternmost Stands in Europe. Silva Fenn. 2018, 52, 7818. [Google Scholar] [CrossRef]
- Farahat, E.; Linderholm, H.W. Growth–Climate Relationship of European Beech at Its Northern Distribution Limit. Eur. J. For. Res. 2018, 137, 619–629. [Google Scholar] [CrossRef]
- Cavin, L.; Jump, A.S. Highest Drought Sensitivity and Lowest Resistance to Growth Suppression Are Found in the Range Core of the Tree Fagus sylvatica L. Not the Equatorial Range Edge. Glob. Chang. Biol. 2017, 23, 362–379. [Google Scholar] [CrossRef]
- Harris, I.; Osborn, T.J.; Jones, P.; Lister, D. Version 4 of the CRU TS Monthly High-Resolution Gridded Multivariate Climate Dataset. Sci. Data 2020, 7, 109. [Google Scholar] [CrossRef]
- Dreimanis, A. Dižskābaržu Mežaudžu Ražība Šķēdes Novadā; Productivity of Beech Stands in Skede Forest District. LLU Proc. 2001, 16, 94–100. [Google Scholar]
- Larrieu, L.; Paillet, Y.; Winter, S.; Bütler, R.; Kraus, D.; Krumm, F.; Lachat, T.; Michel, A.K.; Regnery, B.; Vandekerkhove, K. Tree Related Microhabitats in Temperate and Mediterranean European Forests: A Hierarchical Typology for Inventory Standardization. Ecol. Indic. 2018, 84, 194–207. [Google Scholar] [CrossRef]
- Reventlow, D.O.J.; Nord-Larsen, T.; Skovsgaard, J.P. Pre-Commercial Thinning in Naturally Regenerated Stands of European Beech (Fagus sylvatica L.): Effects of Thinning Pattern, Stand Density and Pruning on Tree Growth and Stem Quality. Forestry 2019, 92, 120–132. [Google Scholar] [CrossRef]
- Pommerening, A.; Uria-Diez, J. Do Large Forest Trees Tend towards High Species Mingling? For. Int. J. For. Res. 2017, 42, 139–147. [Google Scholar] [CrossRef]
- Gadow, K.V.; Zhang, C.Y.; Wehenkel, C.; Pommerening, A.; Corral-Rivas, J.; Korol, M.; Myklush, S.; Hui, G.Y.; Kiviste, A.; Zhao, X.H. Forest Structure and Diversity. In Continuous Cover Forestry. Managing Forest Ecosystems; Pukkala, T., von Gadow, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 23, pp. 29–83. [Google Scholar] [CrossRef]
- Pommerening, A.; Stoyan, D. Edge-Correction Needs in Estimating Indices of Spatial Forest Structure. Can. J. For. Res. 2006, 36, 1723–1739. [Google Scholar] [CrossRef]
- Aguirre, O.; Hui, G.; Von Gadow, K.; Jiménez, J. An Analysis of Spatial Forest Structure Using Neighbourhood-Based Variables. For. Ecol. Manag. 2003, 183, 137–145. [Google Scholar] [CrossRef]
- Hui, G.Y.; Gadow, K.V. Das Winkelmass—Herleitung des optimalen Standardwinkels. Allg. Forst Und Jagdztg. 2002, 173, 173–177. [Google Scholar]
- Põldveer, E.; Korjus, H.; Kiviste, A.; Kangur, A.; Paluots, T.; Laarmann, D. Assessment of Spatial Stand Structure of Hemiboreal Conifer Dominated Forests According to Different Levels of Naturalness. Ecol. Indic. 2020, 110, 105944. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language AND Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2002. Available online: https://www.R-project.org/ (accessed on 5 January 2022).
- Lebourgeois, F.; Eberlé, P.; Mérian, P.; Seynave, I. Social Status-Mediated Tree-Ring Responses to Climate of Abies Alba and Fagus sylvatica Shift in Importance with Increasing Stand Basal Area. For. Ecol. Manag. 2014, 328, 209–218. [Google Scholar] [CrossRef]
- von Oheimb, G.; Westphal, C.; Tempel, H.; Härdtle, W. Structural Pattern of a Near-Natural Beech Forest (Fagus sylvatica) (Serrahn, North-East Germany). For. Ecol. Manag. 2005, 212, 253–263. [Google Scholar] [CrossRef]
- Dubois, H.; Verkasalo, E.; Claessens, H. Potential of Birch (Betula pendula Roth and B. Pubescens Ehrh.) for Forestry and Forest-Based Industry Sector within the Changing Climatic and Socio-Economic Context Ofwestern Europe. Forests 2020, 11, 336. [Google Scholar] [CrossRef]
- Vaníček, L. Beech Provenance Trail in Sweden-Growth and Timber Quality Evaluation. Master’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2021. [Google Scholar]
- Câmpu, V.R.; Dumitrache, R. Frost-Crack Frequency in Beech Stands. Bull. Transilv. Univ. Bras. II For Wood Ind. Agric. Food Eng. 2013, 6, 9–14. [Google Scholar]
- Fritz, Ö.; Heilmann-Clausen, J. Rot Holes Create Key Microhabitats for Epiphytic Lichens and Bryophytes on Beech (Fagus sylvatica). Biol. Conserv. 2010, 143, 1008–1016. [Google Scholar] [CrossRef]
- Larrieu, L.; Cabanettes, A. Species, Live Status, and Diameter Are Important Tree Features for Diversity and Abundance of Tree Microhabitats in Subnatural Montane Beech-Fir Forests. Can. J. For. Res. 2012, 42, 1433–1445. [Google Scholar] [CrossRef]
- Mania, P.; Tomczak, A. Properties of Oak Roundwood with and without Frost Cracks. Forests 2020, 11, 538. [Google Scholar] [CrossRef]
- Colin, F.; Sanjines, A.; Fortin, M.; Bontemps, J.D.; Nicolini, E. Fagus sylvatica Trunk Epicormics in Relation to Primary and Secondary Growth. Ann. Bot. 2012, 110, 995–1005. [Google Scholar] [CrossRef]
- Bütler, R.; Lachat, T.; Krumm, F.; Kraus, D.; Larrieu, L. Field Guide to Tree-Related Microhabitats. In Descriptions and Size Limits for Their Inventory; Swiss Federal Institute for Forest, Snow and Landscape Research WSL: Davos Dorf, Switzerland, 2020; p. 59. [Google Scholar]
- Zeller, L.; Pretzsch, H. Effect of Forest Structure on Stand Productivity in Central European Forests Depends on Developmental Stage and Tree Species Diversity. For. Ecol. Manag. 2019, 434, 193–204. [Google Scholar] [CrossRef]


| Stand No. | Area, ha | Density, Trees ha−1 | Stand Age | DBH, cm | H, m | Basal Area, m2 ha−1 |
|---|---|---|---|---|---|---|
| 14_3 | 2.2 | 630 ± 71 | 69 | 27.6 ± 2.2 | 27.9 ± 1.7 | 41.4 ± 7.0 |
| 19_2 | 0.2 | 1520 ± 255 | 59 | 24.2 ± 1.8 | 22.0 ± 1.0 | 84.6 ± 10.2 |
| 21_16 | 1.7 | 570 ± 156 | 44 | 23.4 ± 3.4 | 23.7 ± 0.9 | 31.4 ± 6.7 |
| 21_30 | 0.4 | 1480 ± 57 | 65 | 19.4 ± 1.2 | 25.4 ± 1.7 | 49.9 ± 2.8 |
| 21_42 | 4.1 | 930 ± 71 | 44 | 20.0 ± 1.5 | 22.6 ± 0.6 | 35.1 ± 6.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansone, D.; Matisons, R.; Kārše, V.; Bāders, E.; Kaupe, D.; Jansons, Ā. Structural Heterogeneity of European Beech (Fagus sylvatica L.) Stands at Its Northernmost Limits. Sustainability 2023, 15, 14681. https://doi.org/10.3390/su152014681
Jansone D, Matisons R, Kārše V, Bāders E, Kaupe D, Jansons Ā. Structural Heterogeneity of European Beech (Fagus sylvatica L.) Stands at Its Northernmost Limits. Sustainability. 2023; 15(20):14681. https://doi.org/10.3390/su152014681
Chicago/Turabian StyleJansone, Diāna, Roberts Matisons, Viesturs Kārše, Endijs Bāders, Dārta Kaupe, and Āris Jansons. 2023. "Structural Heterogeneity of European Beech (Fagus sylvatica L.) Stands at Its Northernmost Limits" Sustainability 15, no. 20: 14681. https://doi.org/10.3390/su152014681







