Exploring the Potential of Utilizing Aquatic Macrophytes for Enhanced Phytoremediation of Zinc in Artificial Wastewater: Characteristics and Parameter Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Plants
2.3. Preparation of Zn Stock Solution and Sodium Chloride Stock Solution
2.4. Phytoremediation of Zn
2.5. Plants Characterization
2.6. Analysis of Liquid Samples
3. Results and Discussion
3.1. Characterization of Water Lettuce and Water Hyacinth before and after Phytoremediation of Zn
3.2. Parameter Effect
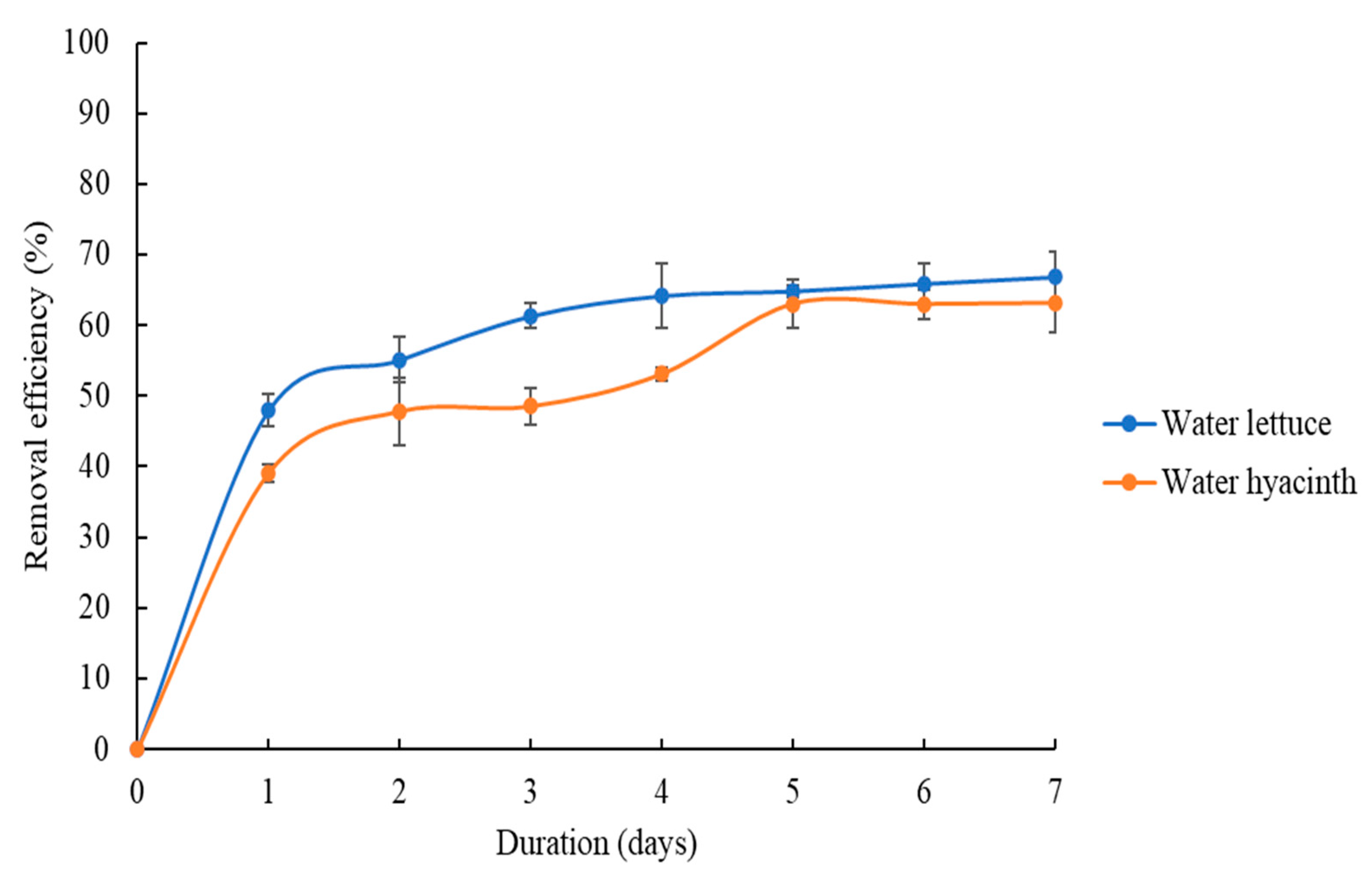
3.2.1. Effect of Duration of Phytoremediation
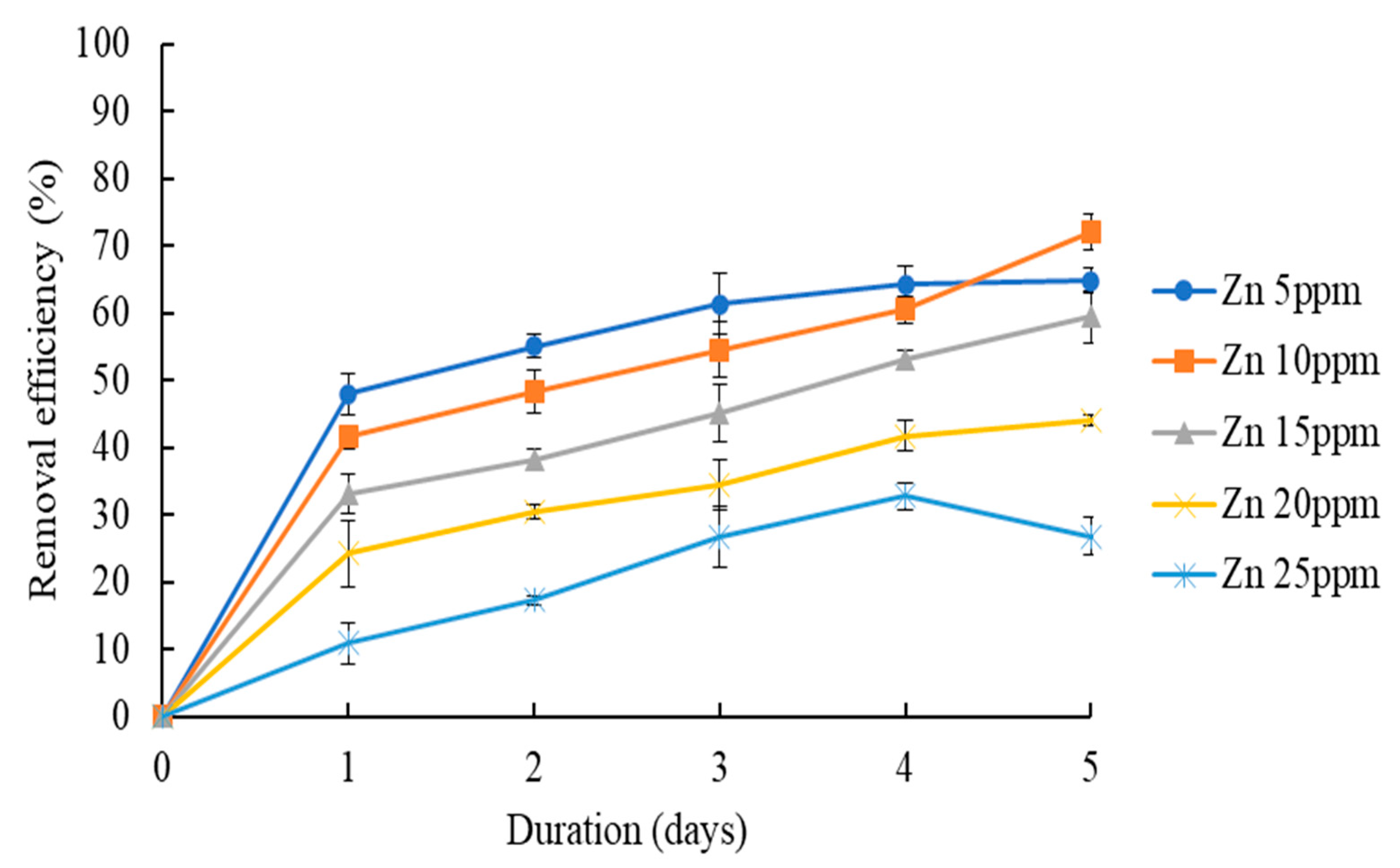
3.2.2. Effect of Zn Concentration
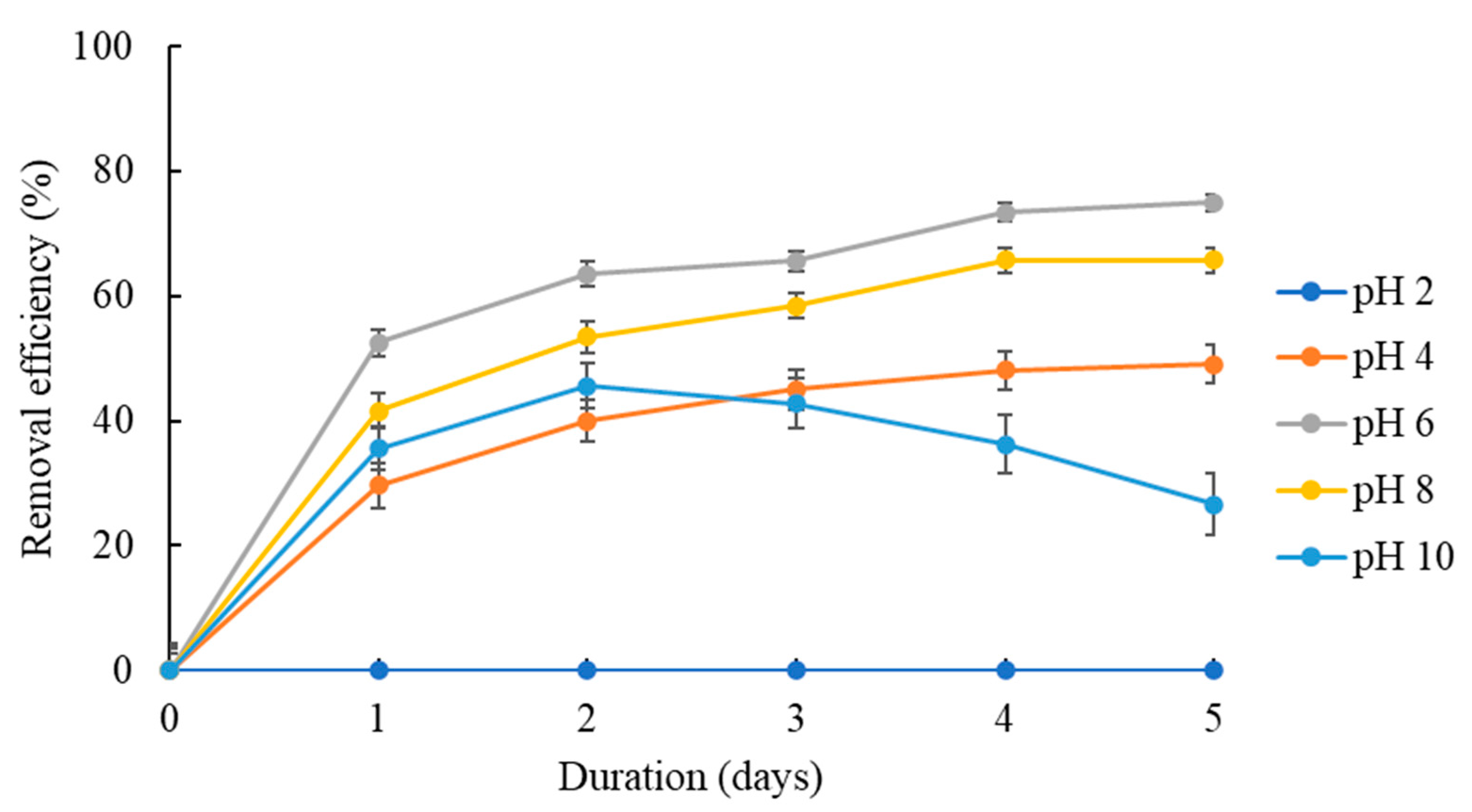
3.2.3. Effect of pH Solution
3.2.4. Effect of Salinity Concentration
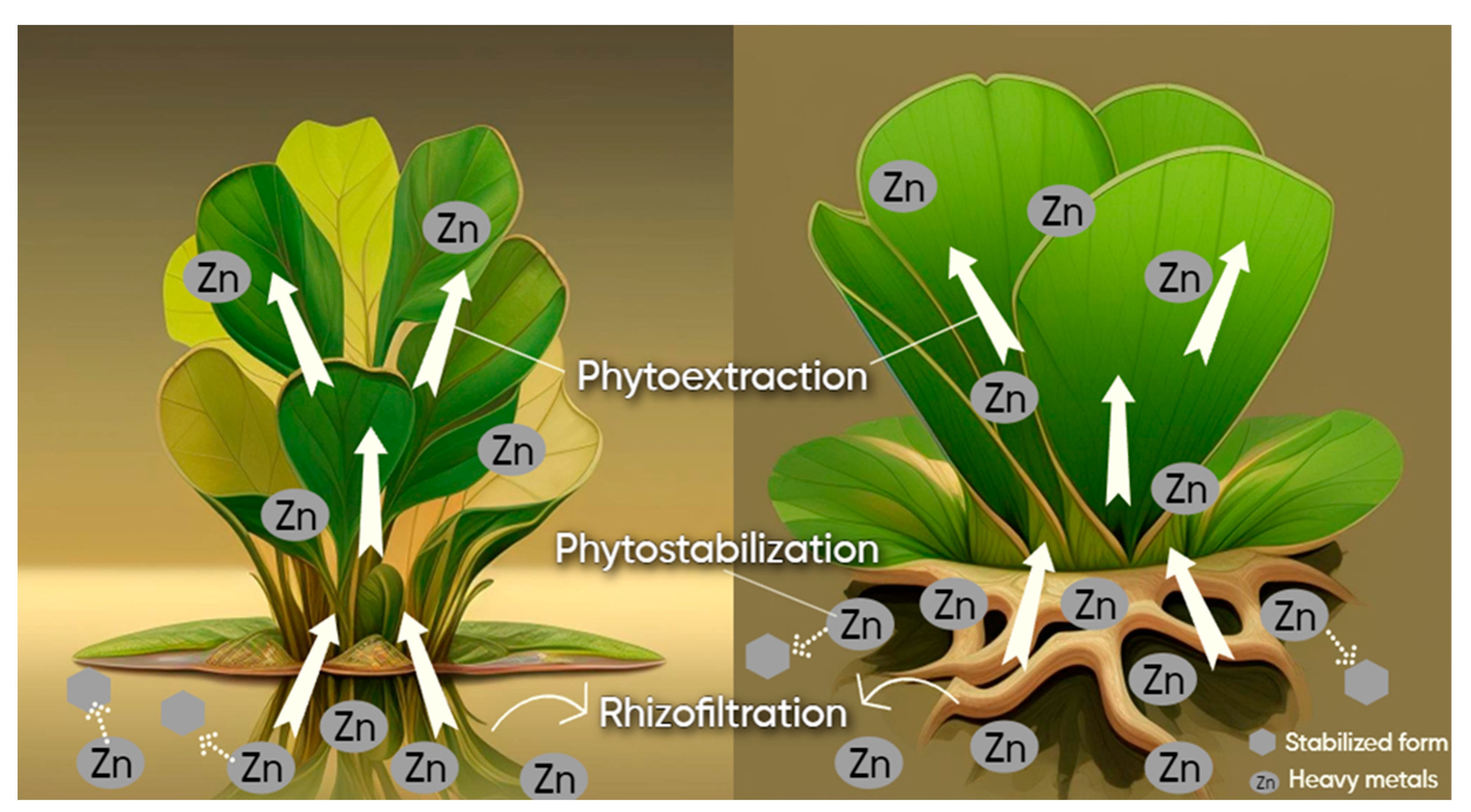
3.3. Possible Phytoremediation Strategies of Zn
3.4. Comparative Results and Literature Review
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Luo, P.; Zha, X.; Xu, C.; Kang, S.; Zhou, M.; Nover, D.; Wang, Y. Overview Assessment of Risk Evaluation and Treatment Technologies for Heavy Metal Pollution of Water and Soil. J. Clean. Prod. 2022, 379, 134043. [Google Scholar] [CrossRef]
- EPA Potential Well Water Contaminants and Their Impacts. Available online: https://www.epa.gov/privatewells/potential-well-water-contaminants-and-their-impacts#:~:text=Heavymetalscancontami-nate%20private,damage%2Canemia%2Candcancer (accessed on 18 June 2023).
- Raj, A.R.A.; Mylsamy, P.; Sivasankar, V.; Kumar, B.S.; Omine, K.; Sunitha, T.G. Heavy Metal Pollution of River Water and Eco-Friendly Remediation Using Potent Microalgal Species. Water Sci. Eng. 2023. [Google Scholar] [CrossRef]
- Jyoti, D.; Sinha, R.; Faggio, C. Advances in Biological Methods for the Sequestration of Heavy Metals from Water Bodies: A Review. Environ. Toxicol. Pharmacol. 2022, 94, 103927. [Google Scholar] [CrossRef]
- Barakat, M.A. New Trends in Removing Heavy Metals from Industrial Wastewater. Arab. J. Chem. 2019, 4, 361–377. [Google Scholar] [CrossRef]
- Abdel-Raouf, M.S.; Abdul-Raheim, A.R.M. Removal of Heavy Metals from Industrial Waste Water by Biomass-Based Materials: A Review. J. Pollut. Eff. Control 2016, 5, 15–19. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Yang, Y.; Li, S.; Wang, T.; Oleksak, P.; Chrienova, Z.; Wu, Q.; Nepovimova, E.; Zhang, X.; et al. Phytoremediation of Heavy Metal Pollution: Hotspots and Future Prospects. Ecotoxicol. Environ. Saf. 2022, 234, 113403. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.M.; Belal, M.; Safiur, R.; Moshiur, R.; Shafiuddin, A.; Ahmed, R.; Monjurul, H.; Xiao, J.B. Phytoremediation of Toxic Metals: A Sustainable Green Solution for Clean Environment. Appl. Sci. 2021, 11, 3–48. [Google Scholar]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Singh, J.; Kumar, P. Heavy Metal Uptake by Water Lettuce (Pistia Stratiotes l.) from Paper Mill Effluent (Pme): Experimental and Prediction Modeling Studies. Environ. Sci. Pollut. Res. 2019, 26, 14400–14413. [Google Scholar] [CrossRef]
- Sundarakumar, M.; Sankar, S.; Pandey, D.; Pandey, B.; George, A.; Karki, B.; Dadeech, P. An Approach to Improve the Water Quality on Industrial Effluent by Phytoremediation with Water Hyacinth (Eichhornia Crassipes). Bioeng. Bioinf. 2021, 1, 1–8. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, H.; Feng, H.; Li, W. Competitive Sorption of Heavy Metals by Water Hyacinth Roots. Environ. Pollut. 2016, 219, 837–845. [Google Scholar] [CrossRef]
- Lamine, S.; Saunders, I. Retraction:Phytoremediation of Heavy-Metals-Contaminated Soils: A Short-Term Trial Involving Two Willow Species from Gloucester WillowBank in the UK. Minerals 2023, 13, 2023. [Google Scholar] [CrossRef]
- Usman, K.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H. The Assessment of Cadmium, Chromium, Copper, and Nickel Tolerance and Bioaccumulation by Shrub Plant Tetraena Qataranse. Sci. Rep. 2019, 9, 5658. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Benítez, J.J.; Domínguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared and Raman Spectroscopic Features of Plant Cuticles: A Review. Front. Plant Sci. 2014, 5, 305. [Google Scholar] [CrossRef]
- Saralegui, A.B.; Willson, V.; Piol, M.N.; Boeykens, S.P. Macrophyte Biomass Productivity for Heavy Metal Adsorption. J. Environ. Manage. 2021, 289, 112398. [Google Scholar] [CrossRef]
- EPA Secondary Drinking Water Standards: Guidance for Nuisance Chemicals. Available online: https://www.epa.gov/sdwa/secondary-drinking-water-standards-guidance-nuisance-chemicals (accessed on 1 September 2023).
- DOE Environmental Quality (Industrial Effluent) Regulations 2009. Available online: https://www.doe.gov.my/wp-content/uploads/2021/08/Environmental_Quality_Industrial_Effluent_Regulations_2009_-_P.U.A_434-2009.pdf (accessed on 1 September 2023).
- Ministry of Health Malaysia National Water Quality Standards for Malaysia. Available online: https://www.doe.gov.my/wp-content/uploads/2021/11/Standard-Kualiti-Air-Kebangsaan.pdf (accessed on 1 September 2023).
- Romero-Guzmán, E.T.; Reyes-Gutiérrez, L.R.; Marín-Allende, M.J.; González-Acevedo, Z.I.; Olguín-Gutiérrez, M.T. Physicochemical Properties of Non-Living Water Hyacinth (Eichhornia Crassipes) and Lesser Duckweed (Lemna Minor) and Their Influence on the As(V) Adsorption Processes. Chem. Ecol. 2019, 29, 459–475. [Google Scholar] [CrossRef]
- Zhou, J.M.; Jiang, Z.C.; Qin, X.Q.; Zhang, L.K.; Huang, Q.B.; Xu, G.L.; Dionysiou, D.D. Efficiency of Pb, Zn, Cd, and Mn Removal from Karst Water by Eichhornia Crassipes. Int. J. Environ. Res. Public Health 2020, 17, 5329. [Google Scholar] [CrossRef]
- Rodrigues, A.C.D.; do Amaral Sobrinho, N.M.B.; dos Santos, F.S.; dos Santos, A.M.; Pereira, A.C.C.; Lima, E.S.A. Biosorption of Toxic Metals by Water Lettuce (Pistia Stratiotes) Biomass. Water. Air. Soil Pollut. 2017, 228, 156. [Google Scholar] [CrossRef]
- Chieng, H.I.; Priyantha, N.; Linda, B.L. Effective Adsorption of Toxic Brilliant Green from Aqueous Solution Using Peat of Brunei Darussalam: Isotherm, Thermodynamics, Kinetics and Regeneration Studies. RSC Adv. 2015, 1, 34603–34615. [Google Scholar] [CrossRef]
- Kaitlyn, E. The 6 Essential Nutrients For Healthy Plants. Available online: https://www.holganix.com/blog/the-6-essential-nutrients-for-healthy-plants (accessed on 28 August 2023).
- Sukarni, S.; Zakaria, Y.; Sumarli, S.; Wulandari, R.; Ayu Permanasari, A.; Suhermanto, M. Physical and Chemical Properties of Water Hyacinth (Eichhornia Crassipes) as a Sustainable Biofuel Feedstock. IOP Conf. Ser. Mater. Sci. Eng. 2019, 515, 12070. [Google Scholar] [CrossRef]
- Nayanathara, O.S.; Bindu, A. Effectiveness of Water Hyacinth and Water Lettuce for the Treatment of Greywater—A Review. Int. J. Innov. Res. Sci. Eng. 2019, 3, 349–355. [Google Scholar]
- Rodrigues, A.C.D.; de Rocha, M.V.C.; Lima, E.S.A.; de Pinho, C.F.; dos Santos, A.M.; dos Santos, F.S.; Sobrinho, N.M.B.A. do Potential of Water Lettuce (Pistia Stratiotes L.) for Phytoremediation: Physiological Responses and Kinetics of Zinc Uptake. Int. J. Phytoremediation 2020, 22, 1019–1027. [Google Scholar] [CrossRef]
- Gupta, P.; Roy, S.; Mahindrakar, A. Treatment of Water Using Water Hyacinth, Water Lettuce and Vetiver Grass—A Review. Resour. Environ. 2018, 2, 202–215. [Google Scholar] [CrossRef]
- Mohamed, O.I.; Soeprobowati, T.R.; Sudarno, I. The Ability of Aquatic Weed Water Hyacinth and Water Lettuce To Reduce Heavy Metal Manganese (Mn), Zinc (Zn) and Iron (Fe). J. Pure Appl. Chem. 2015, 1, 101–113. [Google Scholar]
- Jayasri, M.; Suthindhiran, K. Effect of Zinc and Lead on the Physiological and Biochemical Properties of Aquatic Plant Lemna Minor: Its Potential Role in Phytoremediation. Appl. Water Sci. 2017, 7, 1247–1253. [Google Scholar] [CrossRef]
- Shingadgaon, S..; Chavan, B. Zinc Uptake Potential in Water Lettuce (Pistia Stratiotes, L.). Int. J. Sci. Res. 2018, 7, 1497–1504. [Google Scholar] [CrossRef]
- Dutta, S.; Mitra, M.; Agarwal, P.; Mahapatra, K.; De, S.; Sett, U.; Roy, S. Oxidative and Genotoxic Damages in Plants in Response to Heavy Metal Stress and Maintenance of Genome Stability. Plant Signal. Behav. 2018, 13, e1460048. [Google Scholar] [CrossRef]
- DalCorso, G.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Heavy Metal Pollutions: State of the Art and Innovation in Phytoremediation. Int. J. Mol. Sci. 2019, 20, 3412. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.K.; Tripathi, B.D. Accumulation of Chromium and Zinc from Aqueous Solutions Using Water Hyacinth (Eichhornia Crassipes). J. Hazard. Mater. 2019, 164, 1059–1063. [Google Scholar] [CrossRef]
- Ministry of Environment and Water Malaysia Sewage and Industrial Effluent Discharge Standards. Available online: http://www.water-treatment.com.cn/resources/discharge-standards/malaysia.htm (accessed on 4 August 2021).
- Nasir, M.; Nur, M.; Pandiangan, D.; Mambu, S.M.; Fauziah, S.; Raya, I.; Fudholi, A.; Irfandi, R. Phytoremediation Study of Water Hyacinth (E Ichhornia Crassipes ) on Zinc Metal Ion (Zn2+). Int. J. Des. Nat. Ecodynamics 2022, 17, 417–422. [Google Scholar] [CrossRef]
- Swarnalatha, K.; Radhakrishnan, B. Studies on Removal of Zinc and Chromium from Aqueous Solutions Using Water Hyacinth. Pollution 2015, 1, 193–202. [Google Scholar]
- Emilia, G.; Andrada, M.; Petru, I. Removal of Zinc Ions as Zinc Chloride Complexes from Strongly Acidic Aqueous Solutions by Ionic Exchange. Cent. Eur. J. Chem. 2014, 12, 821–828. [Google Scholar] [CrossRef]
- Sadeghzadeh, B. A Review of Zinc Nutrition and Plant Breeding. J. Soil Sci. Plant Nutr. 2019, 13, 905–927. [Google Scholar] [CrossRef]
- Sena, P.H.; Martin, C.; Albant, S.; Edmond, A.; Martin, P.A. Influence of PH on Water Hyacinth Ponds Treating and Recycling Wastewater. J. Water Resour. Prot. 2022, 14, 86–99. [Google Scholar]
- Wang, X.; Sun, R.; Tian, Y.; Guo, K.; Sun, H.; Liu, X.; Chu, H.; Liua, B. Long-Term Phytoremediation of Coastal Saline Soil Reveals Plant Species-Specific Patterns of Microbial Community Recruitment. mSystems 2020, 5, 10-1128. [Google Scholar] [CrossRef]
- Liu, J.; Fu, C.; Li, G.; Nauman Khan, M.; Wu, H. Ros Homeostasis and Plant Salt Tolerance: Plant Nanobiotechnology Updates. Sustainability 2021, 13, 3552. [Google Scholar] [CrossRef]
- Hongqiao, L.; Suyama, A.; Mitani-ueno, N.; Hell, R. A Low Level of NaCl Stimulates Plant Growth by Improving Carbon and Sulfur Assimilation in Arabidopsis Thaliana. Plants 2021, 10, 2138. [Google Scholar] [CrossRef]
- Litalien, A.A.S.; Rutter, A.; Zeeb, B.A.; Litalien, A.A.S.; Rutter, A.; The, B.A.Z. The Impact of Soil Chloride Concentration and Salt Type on the Excretions of Four Recretohalophytes with Different Excretion Mechanisms. Int. J. Phytoremediation 2020, 22, 1122–1128. [Google Scholar] [CrossRef]
- Filipović, L.; Romić, M.; Romić, D.; Filipović, V.; Ondrašek, G. Organic Matter and Salinity Modify Cadmium Soil (Phyto)Availability. Ecotoxicol. Environ. Saf. 2018, 147, 824–831. [Google Scholar] [CrossRef]
- Tandon, K.; John, M.; Heuss-Aßbichler, S.; Schaller, V. Influence of Salinity and Pb on the Precipitation of Zn in a Model System. Minerals 2018, 8, 43. [Google Scholar] [CrossRef]
- Woraharn, S.; Meeinkuirt, W.; Phusantisampan, T.; Chayapan, P. Rhizofiltration of Cadmium and Zinc in Hydroponic Systems Rhizofiltration of Cadmium and Zinc in Hydroponic Systems. Water Air Soil Pollut. 2021, 232, 204. [Google Scholar] [CrossRef]
- Yanitch, A.; Kadri, H.; Frenette-dussault, C.; Joly, S.; Pitre, F.E.; Labrecque, M. A Four-Year Phytoremediation Trial to Decontaminate Soil Polluted by Wood Preservatives: Phytoextraction of Arsenic, Chromium, Copper, Dioxins and Furans. Int. J. Phytoremediation 2020, 22, 1505–1514. [Google Scholar] [CrossRef]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of Heavy Metals: A Promising Tool for Clean-up of Polluted Environment. Front. Plant Sci. 2018, 9, 1476. [Google Scholar] [CrossRef]
- Sharma, J.; Kumar, N.; Singh, N.; Santal, A. Phytoremediation Technologies and Their Mechanism for Removal of Heavy Metal from Contaminated Soil: An Approach for a Sustainable Environment. Front. Plant Sci. 2023, 14, 1076876. [Google Scholar] [CrossRef]
- Nascimento, C.; Biondi, C.M.; da Silva, F.B.V.; Lima, L.H.V. Using Plants to Remediate or Manage Metal-Polluted Soils: An Overview on the Current State of Phytotechnologies. Agronomy 2021, 43. [Google Scholar] [CrossRef]
- Shehata, S.M.; Badawy, R.K.; Aboulsoud, Y.I.E. Phytoremediation of Some Heavy Metals in Contaminated Soil. Bull. Natl. Res. Cent. 2019, 43, 1–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, J.; Cao, W.; Qin, M.; Song, B. Influence of Biochar and Fulvic Acid on the Ryegrass-Based Phytoremediation of Sediments Contaminated with Multiple Heavy Metals. J. Environ. Chem. Eng. 2023, 11, 109446. [Google Scholar] [CrossRef]
- Njoku, K.L.; Nwani, S.O. Phytoremediation of Heavy Metals Contaminated Soil Samples Obtained from Mechanic Workshop and Dumpsite Using Amaranthus Spinosus. Sci. African 2022, 17, e01278. [Google Scholar] [CrossRef]
- Durante-Yánez, E.V.; Martínez-Macea, M.A.; Enamorado-Montes, G.; Caballero, E.C.; Marrugo-Negrete, J. Phytoremediation of Soils Contaminated with Heavy Metals from Gold Mining Activities Using Clidemia Sericea D. Don. Plants 2022, 11, 597. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Gao, S.; Zhang, Z.; Huang, L. Effect of Humic Acid on Phytoremediation of Heavy Metal Contaminated Sediment. J. Hazard. Mater. Adv. 2023, 9, 100235. [Google Scholar] [CrossRef]
- Singh, K.; Tripathi, S.; Chandra, R. Bacterial Assisted Phytoremediation of Heavy Metals and Organic Pollutants by Cannabis Sativa as Accumulator Plants Growing on Distillery Sludge for Ecorestoration of Polluted Site. J. Environ. Manage. 2023, 332, 117294. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tian, Y.; Wang, B.; Peng, R.; Xu, J.; Fu, X.; Han, H.; Wang, L.; Zhang, W.; Deng, Y.; et al. Enhanced Phytoremediation of Selenium Using Genetically Engineered Rice Plants. J. Plant Physiol. 2022, 271, 153665. [Google Scholar] [CrossRef] [PubMed]





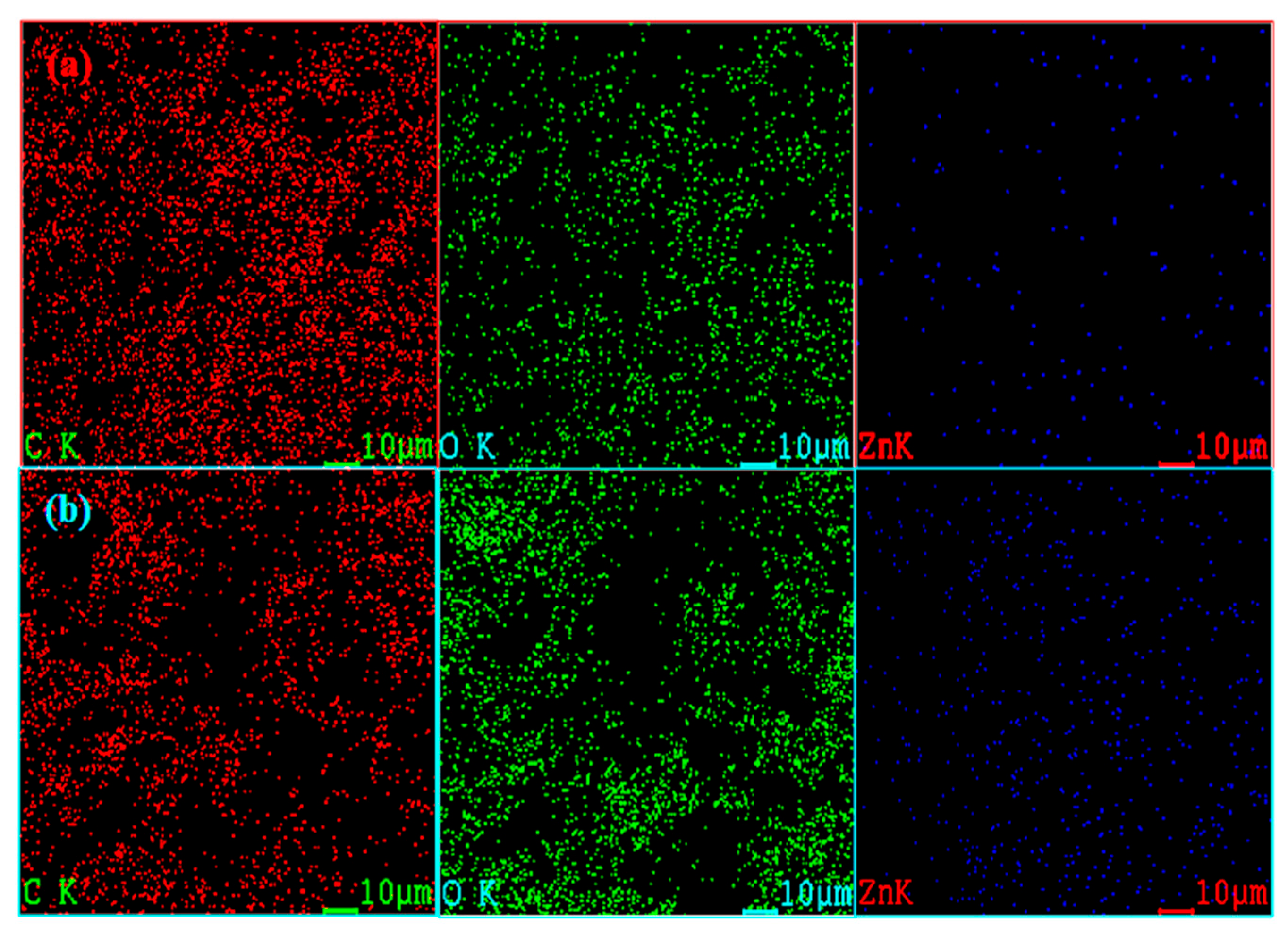




| Plant Sample | Elemental Atomic Percent (%) | ||||||
|---|---|---|---|---|---|---|---|
| C | O | Zn | Na | Ca | K | ||
| Water lettuce | Leaf (without Zn) | 43.3 | 33.7 | - | - | 16.2 | 6.8 |
| Leaf (with Zn) | 57.1 | 41.5 | 1.4 | - | - | - | |
| Roots (without Zn) | 44.4 | 35.7 | - | 4.1 | 7.6 | 8.2 | |
| Roots (with Zn) | 40.6 | 52.1 | 7.3 | - | - | - | |
| Water hyacinth | Leaf (without Zn) | 36.2 | 37.2 | - | - | 18.4 | 8.2 |
| Leaf (with Zn) | 77.1 | 20.7 | 2.2 | - | - | - | |
| Roots (without Zn) | 48.7 | 46.8 | - | 1.6 | - | 2.9 | |
| Roots (with Zn) | 46.8 | 43.8 | 9.4 | - | - | - | |
| Plants Used | Targeted Heavy Metals | Phytoremediation Duration | Removal Efficiency | Reference |
|---|---|---|---|---|
| Hibiscus cannabinus L. (Kenaf) and Linum usitatissimum L. (Flax) | Cr, Co, Cd and Mn | 8 weeks | Cr—34% Co—36.5% Mn—17.1% Cd—12% Mn—45.2% Cr—21.2% Co—17% Cd—9.4% | [52] |
| Clidemia sericea D. Don (Michelang) | Cd, Hg and Pb | 12 weeks | Cd—49.2% Hg—18.4% Pb—32.3% | [55] |
| Lolium perenne L. (Ryegrass) | Cd, Pb and Zn | 30 days | Cd—47.8% Pb—37.2% Zn—42.5% | [53] |
| Typha orientalis (Bulrush) | Cd, Cu, Ni and Pb | 60 days | Cd—80% Cu—60% Ni—68.9% Pb— 8.6% | [56] |
| Cannabis sativa (Indian hemp) | Cu, Fe, Mn, Ni, Pb and Zn | 60 days | Cu—75.9% Fe—88.6% Mn—70.8% Ni—78.7% Pb—83.9% Zn—39% | [57] |
| Amaranthus spinosus (Spiny pigweed) | Cd, Cu, Pb and Zn | 12 weeks | Cd—50.5% Cu—49% Pb—43.3% Zn—47.4% | [54] |
| Pistia stratiotes (water lettuce) and Eichhornia crassipes (water hyacinth) | Zn | 5 days | Zn—80.1% Zn—88% | Current work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, H.W.; Pang, Y.L.; Lim, S.; Chong, W.C.; Lai, C.W.; Abdullah, A.Z. Exploring the Potential of Utilizing Aquatic Macrophytes for Enhanced Phytoremediation of Zinc in Artificial Wastewater: Characteristics and Parameter Studies. Sustainability 2023, 15, 15170. https://doi.org/10.3390/su152015170
Tan HW, Pang YL, Lim S, Chong WC, Lai CW, Abdullah AZ. Exploring the Potential of Utilizing Aquatic Macrophytes for Enhanced Phytoremediation of Zinc in Artificial Wastewater: Characteristics and Parameter Studies. Sustainability. 2023; 15(20):15170. https://doi.org/10.3390/su152015170
Chicago/Turabian StyleTan, Hui Wun, Yean Ling Pang, Steven Lim, Woon Chan Chong, Chin Wei Lai, and Ahmad Zuhairi Abdullah. 2023. "Exploring the Potential of Utilizing Aquatic Macrophytes for Enhanced Phytoremediation of Zinc in Artificial Wastewater: Characteristics and Parameter Studies" Sustainability 15, no. 20: 15170. https://doi.org/10.3390/su152015170
APA StyleTan, H. W., Pang, Y. L., Lim, S., Chong, W. C., Lai, C. W., & Abdullah, A. Z. (2023). Exploring the Potential of Utilizing Aquatic Macrophytes for Enhanced Phytoremediation of Zinc in Artificial Wastewater: Characteristics and Parameter Studies. Sustainability, 15(20), 15170. https://doi.org/10.3390/su152015170









