Cobalt Ferrite (CoFe2O4) Spinel as a New Efficient Magnetic Heterogeneous Fenton-like Catalyst for Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of the Cobalt Ferrite (CoFe2O4) Spinel Catalyst
2.2. Remazol RR Dye Degradation
2.3. Catalyst Reusability
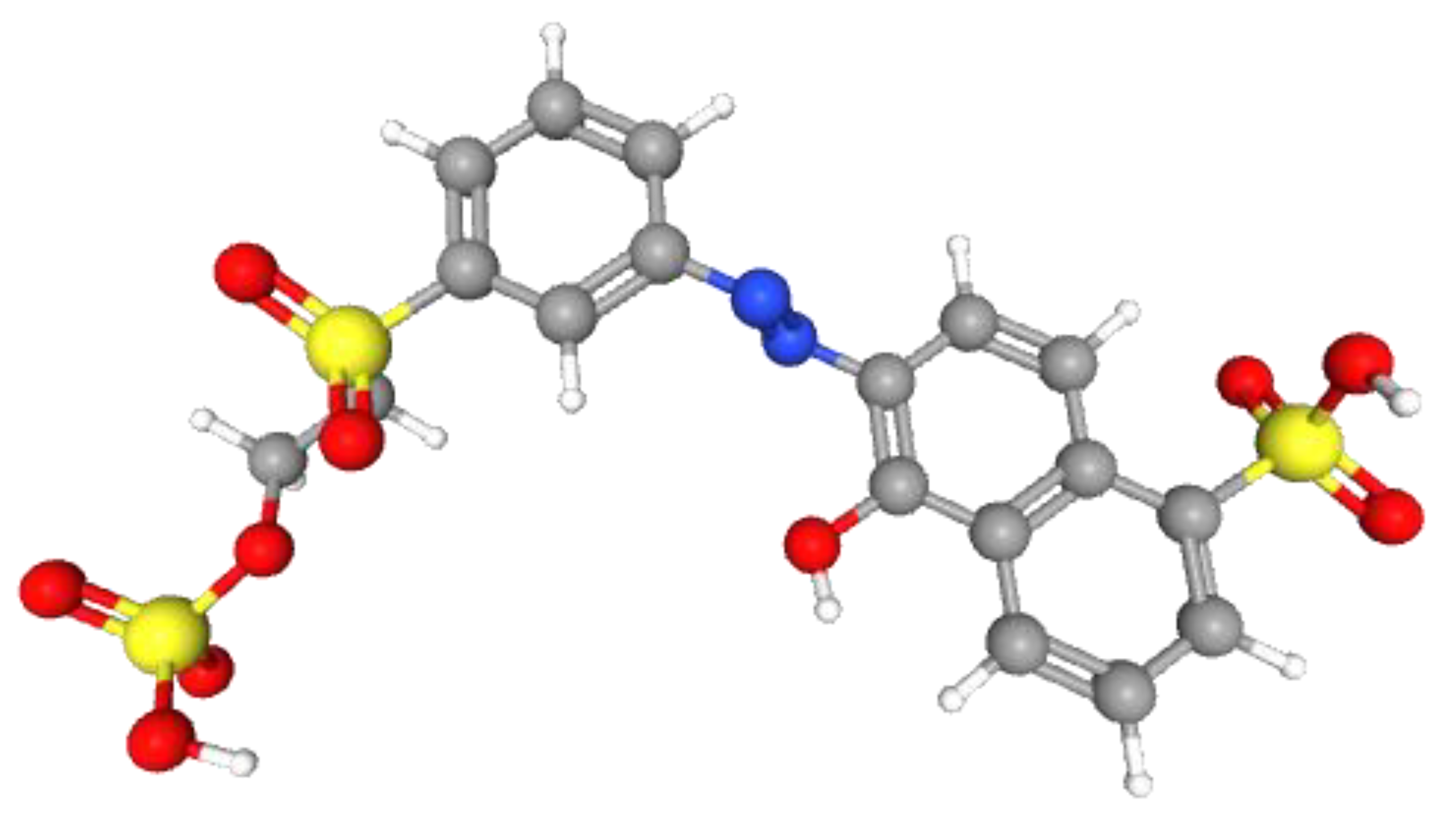
2.4. RR Dye Degradation Pathway
3. Results and Discussion
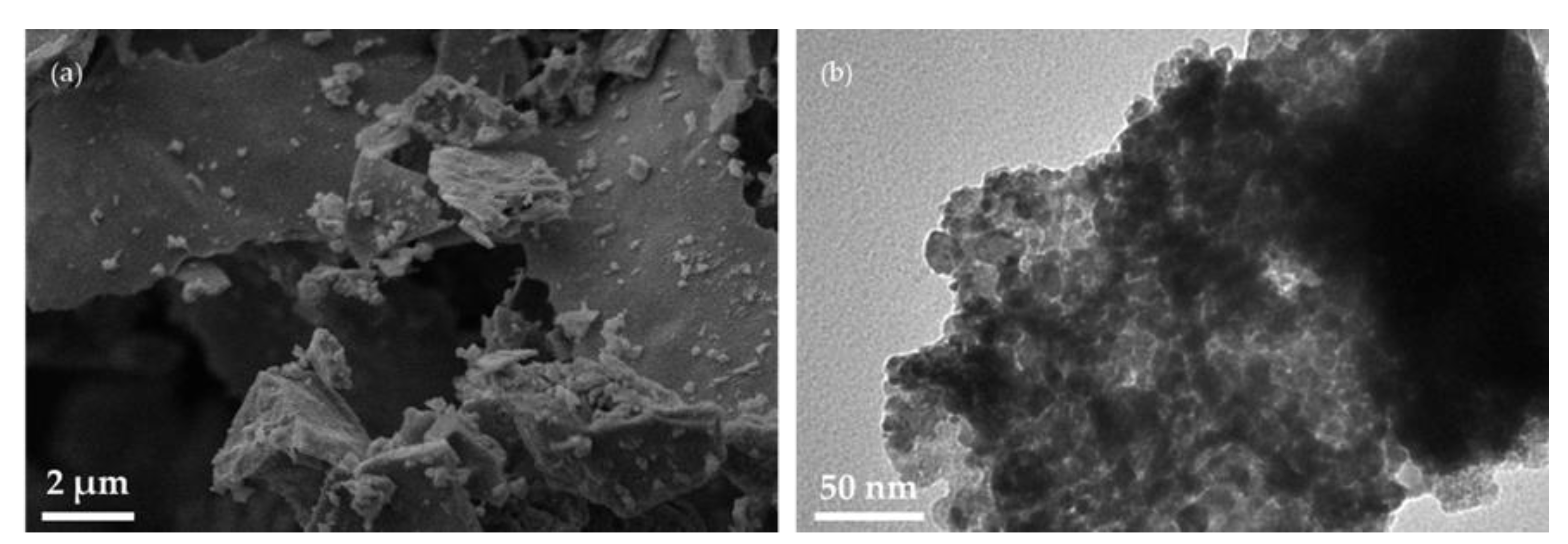
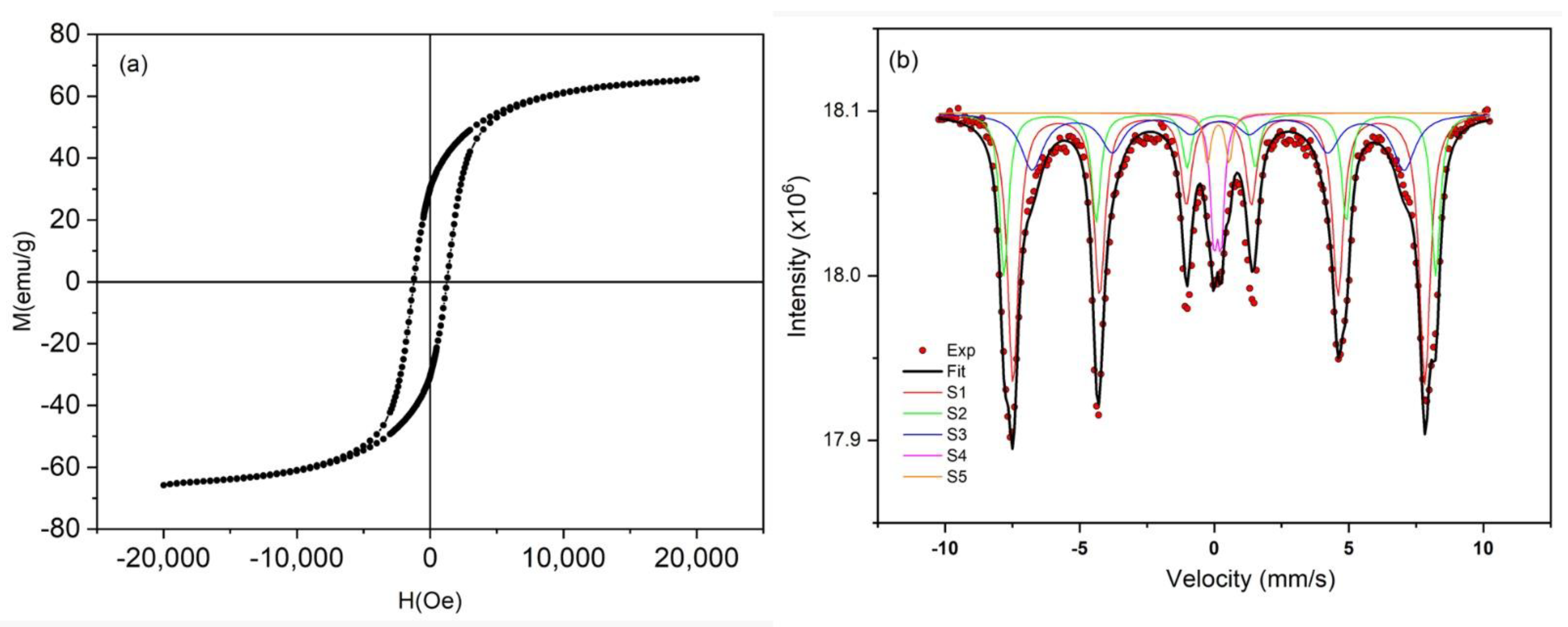
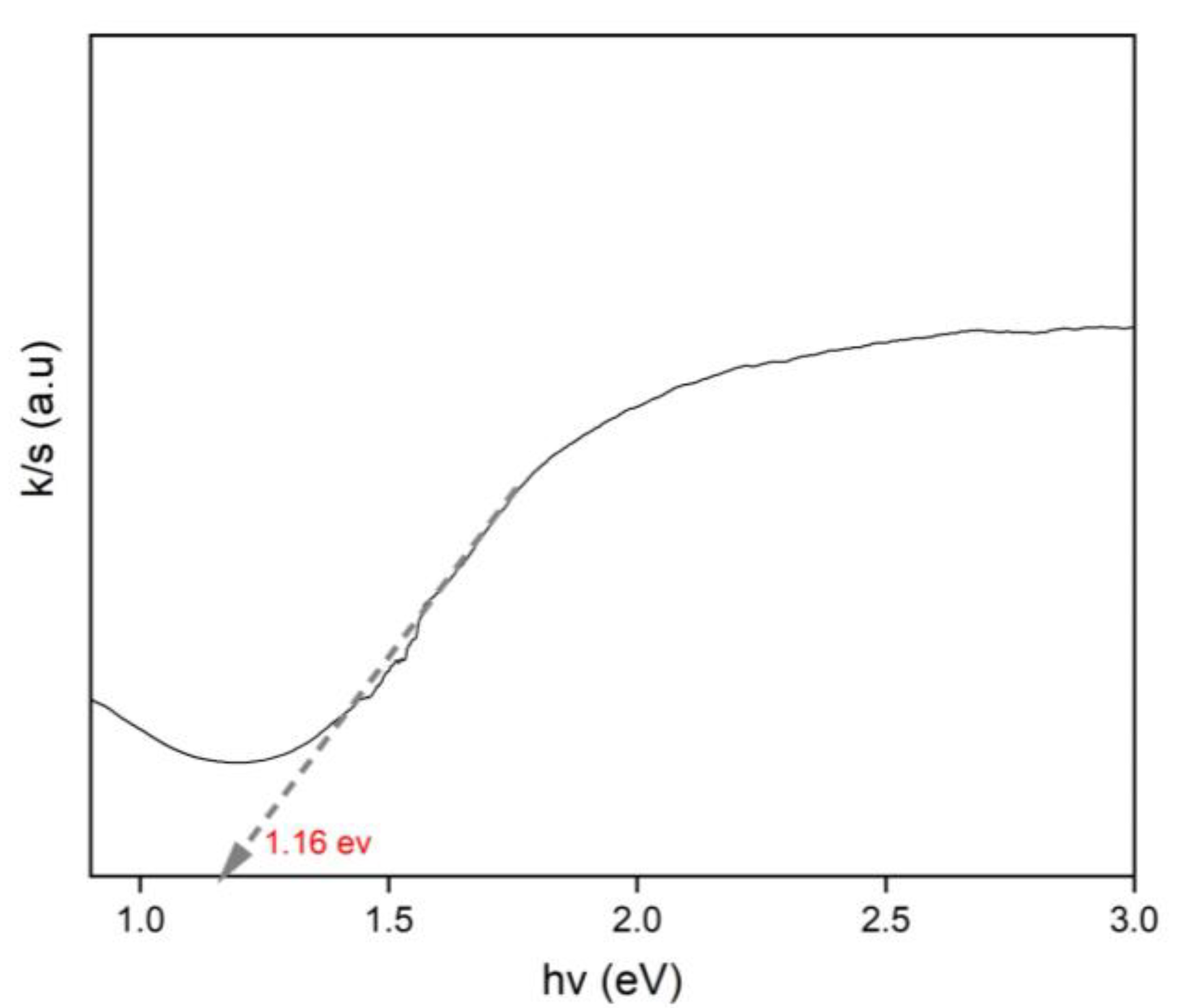
3.1. Characterization of CoFe2O4 Nanoparticles
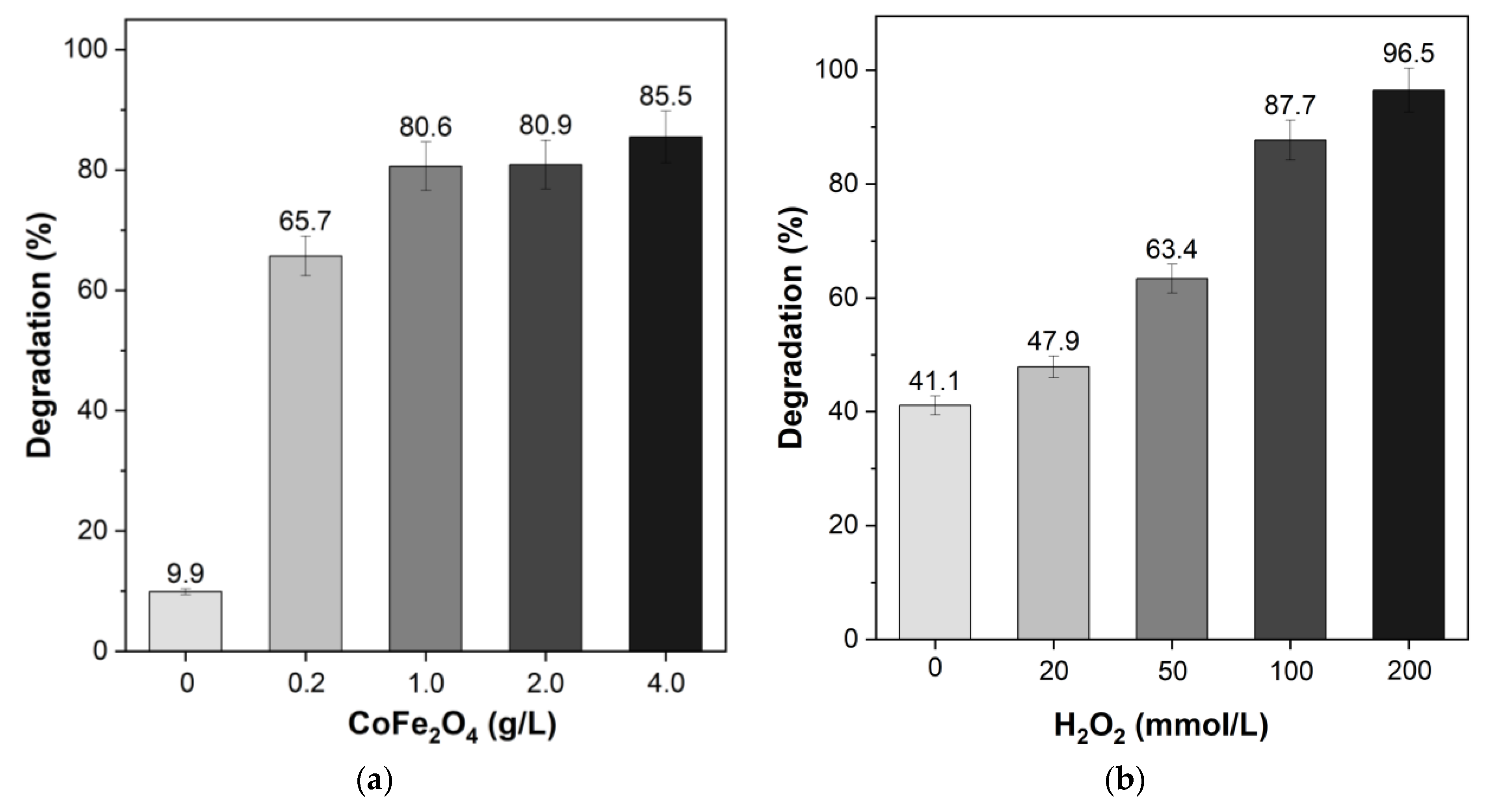
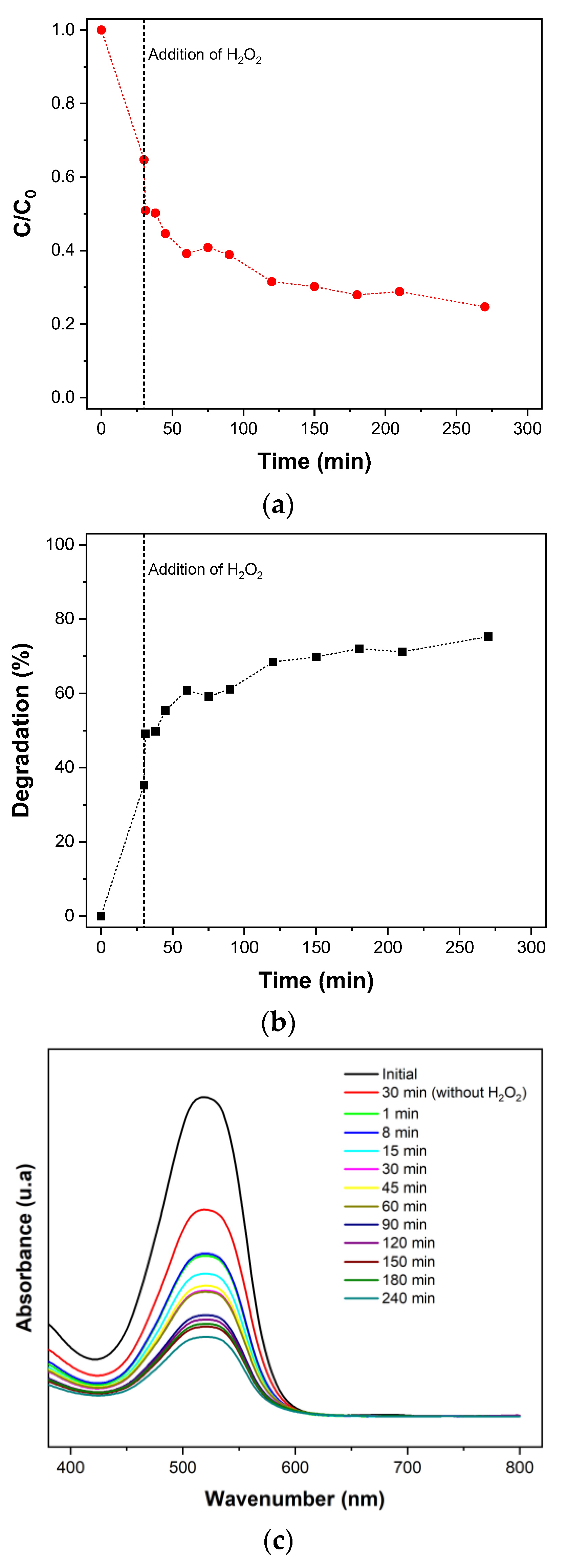
3.2. Remazol RR Dye Degradation
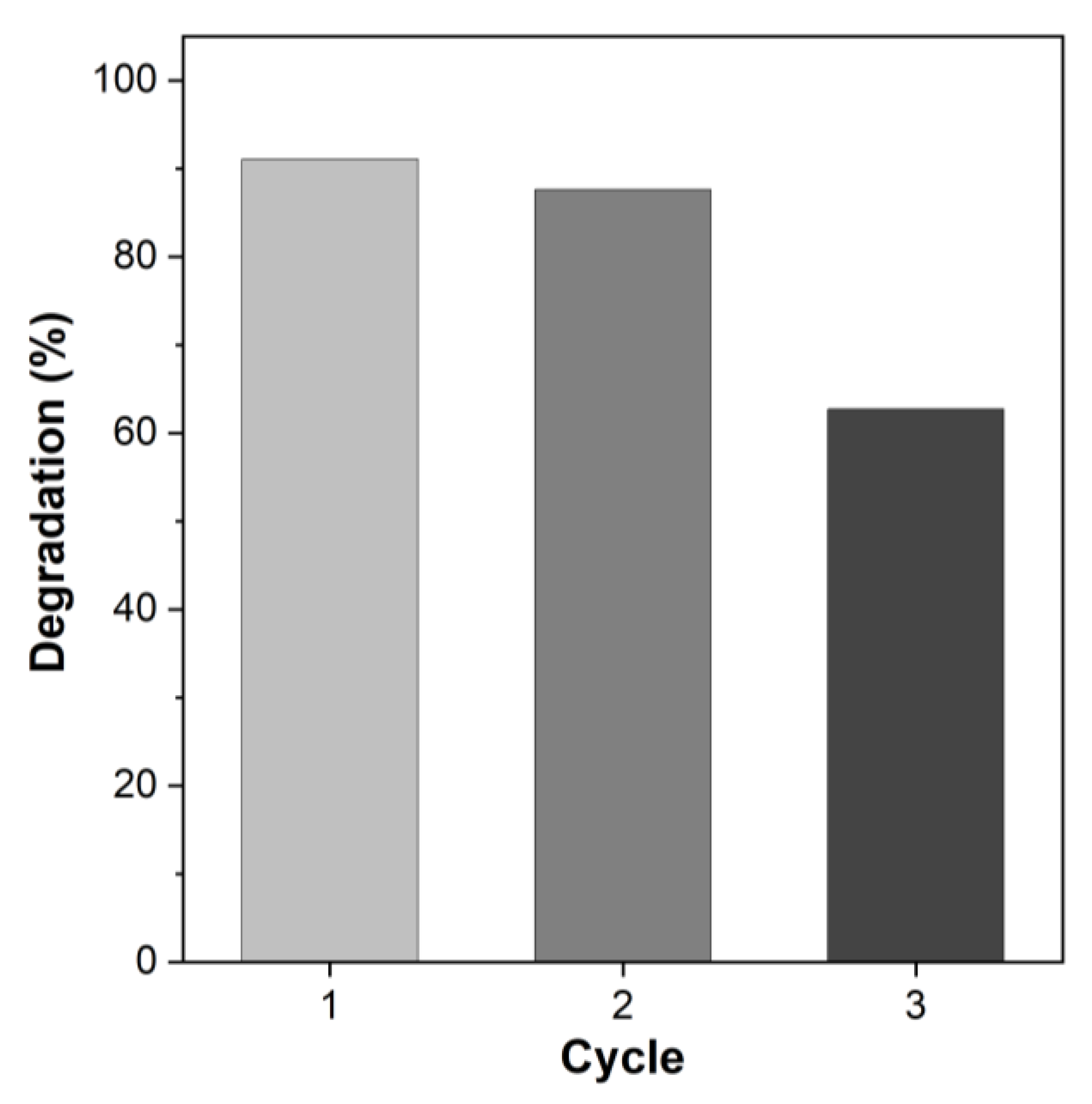
3.3. Catalyst Reusability
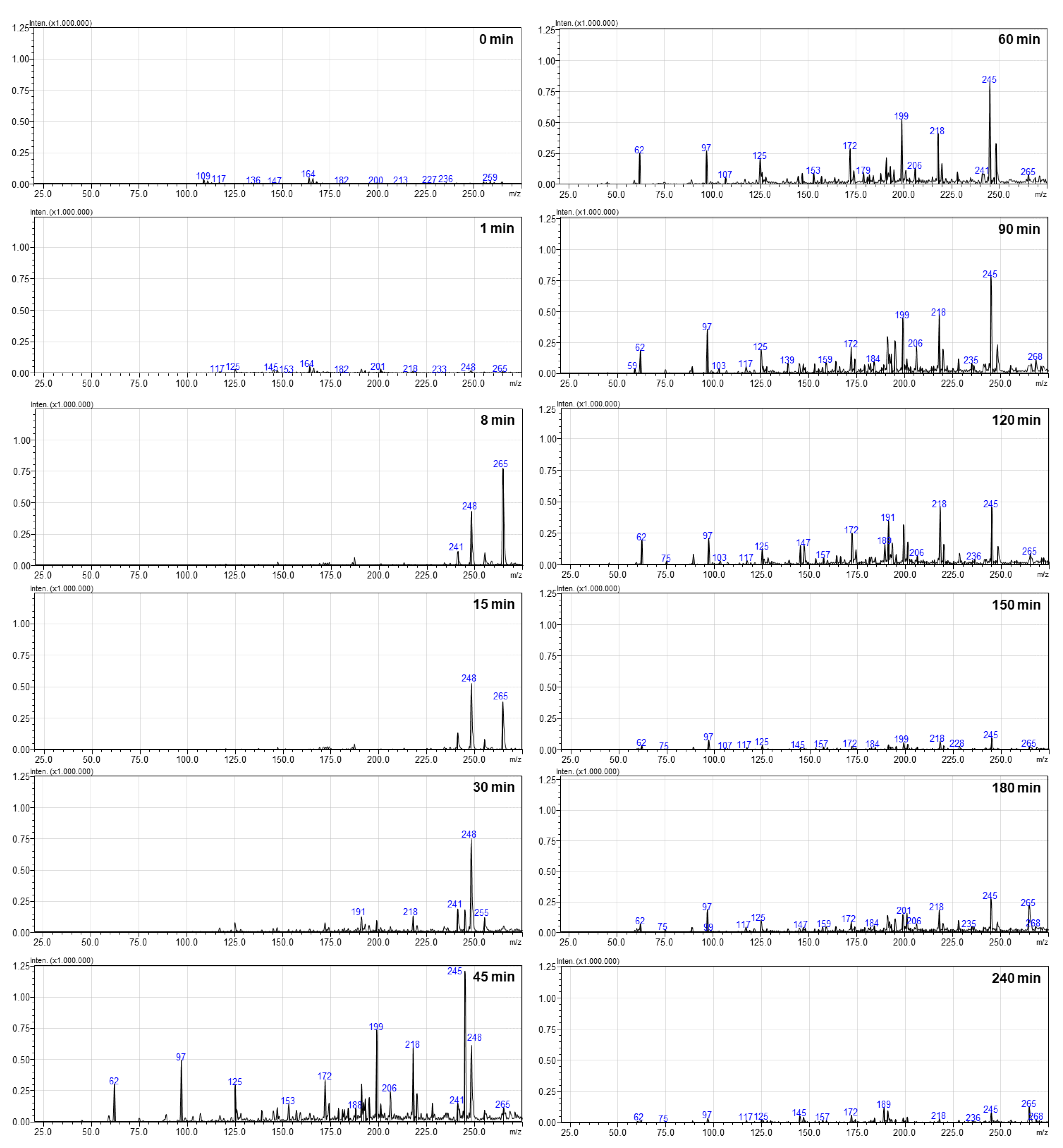
3.4. RR Dye Degradation Pathway
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wermuth, T.B.; Arcaro, S.; Venturini, J.; Ribeiro, T.M.H.; Rodriguez, A.d.A.L.; Machado, E.L.; de Oliveira, T.F.; de Oliveira, S.E.F.; Baibich, M.N.; Bergmann, C.P. Microwave-synthesized KNbO3 perovskites: Photocatalytic pathway on the degradation of rhodamine B. Ceram. Int. 2019, 45, 24137–24145. [Google Scholar] [CrossRef]
- Anton, D.C.; Debrassi, A.; Buzzi, F.d.C.; Magro, J.D.; Scapinello, J.; Nedelko, N.; Ślawska-Waniewska, A.; Dłużewski, P.; Rodrigues, C.A. Effect of microwave radiation on the adsorption of the dye Remazol Red 198 (RR198) by O-carboxymethylchitosan-N-lauryl/F2O3 magnetic nanoparticles. Process. Saf. Environ. Prot. 2016, 102, 392–402. [Google Scholar] [CrossRef]
- Medina-Zazueta, L.; Miranda-Castro, F.C.; Romo-Garcia, F.; Martínez-Gil, M.; Esparza-Ponce, H.E.; Encinas-Basurto, D.; Ibarra, J. Development of Sustainable Magnetic Biosorbent Using Aqueous Leaf Extract of Vallesia glabra for Methylene Blue Removal from Wastewater. Sustainability 2023, 15, 4586. [Google Scholar] [CrossRef]
- Das, K.C.; Das, B.; Dhar, S.S. Effective Catalytic Degradation of Organic Dyes by Nickel Supported on Hydroxyapatite-Encapsulated Cobalt Ferrite (Ni/HAP/CoFe2O4) Magnetic Novel Nanocomposite. Water Air Soil Pollut. 2020, 231, 43. [Google Scholar] [CrossRef]
- Lapertot, M.; Pulgarín, C.; Fernández-Ibáñez, P.; Maldonado, M.I.; Pérez-Estrada, L.; Oller, I.; Gernjak, W.; Malato, S. Enhancing biodegradability of priority substances (pesticides) by solar photo-Fenton. Water Res. 2006, 40, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Dai, X.; Wang, H.; Wang, Z.; Li, Z.; Chen, Y.; Luo, Y.; Ouyang, D. Metronidazole Degradation by UV and UV/H2O2 Advanced Oxidation Processes: Kinetics, Mechanisms, and Effects of Natural Water Matrices. Int. J. Environ. Res. Public Health 2022, 19, 12354. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Chai, L.; Tang, C.; Li, B.; Yang, Z. Comparison of the degradation of molecular and ionic ibuprofen in a UV/H2O2 system. Water Sci. Technol. 2018, 77, 2174–2183. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S.; Sánchez Pérez, J.A. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination—A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Nunes, M.I. Recent trends and developments in Fenton processes for industrial wastewater treatment—A critical review. Environ. Res. 2021, 197, 110957. [Google Scholar] [CrossRef]
- Ziembowicz, S.; Kida, M. Limitations and future directions of application of the Fenton-like process in micropollutants degradation in water and wastewater treatment: A critical review. Chemosphere 2022, 296, 134041. [Google Scholar] [CrossRef]
- Su, R.; Xie, C.; Alhassan, S.I.; Huang, S.; Chen, R.; Xiang, S.; Wang, Z.; Huang, L. Oxygen Reduction Reaction in the Field of Water Environment for Application of Nanomaterials. Nanomaterials 2020, 10, 1719. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liu, S.; Huo, X.; Cheng, F.; Zhang, M.; Guo, M. Facile and large-scale fabrication of (Mg,Ni)(Fe,Al)2O4 heterogeneous photo-Fenton-like catalyst from saprolite laterite ore for effective removal of organic contaminants. J. Hazard. Mater. 2020, 392, 122295. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Kang, S.; Xie, X.; Liao, C.; Duan, X.; Dionysiou, D.D. Efficient degradation of bisphenol A in water by heterogeneous activation of peroxymonosulfate using highly active cobalt ferrite nanoparticles. J. Hazard. Mater. 2020, 399, 122979. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Hao, S.; Cao, H. Solvothermal synthesis of magnetic CoFe2O4/rGO nanocomposites for highly efficient dye removal in wastewater. RSC Adv. 2017, 7, 4062–4069. [Google Scholar] [CrossRef]
- Nadeem, N.; Yaseen, M.; Rehan, Z.A.; Zahid, M.; Shakoor, R.A.; Jilani, A.; Iqbal, J.; Rasul, S.; Shahid, I. Coal fly ash supported CoFe2O4 nanocomposites: Synergetic Fenton-like and photocatalytic degradation of methylene blue. Environ. Res. 2022, 206, 112280. [Google Scholar] [CrossRef]
- Cai, C.; Duan, X.; Xie, X.; Kang, S.; Liao, C.; Dong, J.; Liu, Y.; Xiang, S.; Dionysiou, D.D. Efficient degradation of clofibric acid by heterogeneous catalytic ozonation using CoFe2O4 catalyst in water. J. Hazard. Mater. 2021, 410, 124604. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Shyichuk, A.; Danyliuk, N.; Naushad, M.; Kotsyubynsky, V.; Boychuk, V. Cobalt ferrite as an electromagnetically boosted metal oxide hetero-Fenton catalyst for water treatment. Chemosphere 2023, 326, 138364. [Google Scholar] [CrossRef]
- Hassani, A.; Çelikdağ, G.; Eghbali, P.; Sevim, M.; Karaca, S.; Metin, Ö. Heterogeneous sono-Fenton-like process using magnetic cobalt ferrite-reduced graphene oxide (CoFe2O4-rGO) nanocomposite for the removal of organic dyes from aqueous solution. Ultrason. Sonochemistry 2018, 40, 841–852. [Google Scholar] [CrossRef]
- Al-Harbi, L.; Darwish, M.S. Functionalized iron oxide nanoparticles: Synthesis through ultrasonic-assisted co-precipitation and performance as hyperthermic agents for biomedical applications. Heliyon 2022, 8, e09654. [Google Scholar] [CrossRef]
- Xu, Z.; Xie, M.; Ben, Y.; Shen, J.; Qi, F.; Chen, Z. Efficiency and mechanism of atenolol decomposition in Co-FeOOH catalytic ozonation. J. Hazard. Mater. 2019, 365, 146–154. [Google Scholar] [CrossRef]
- Moitra, D.; Chandel, M.; Ghosh, B.K.; Jani, R.K.; Patra, M.K.; Vadera, S.R.; Ghosh, N.N. A simple ‘in situ’ co-precipitation method for the preparation of multifunctional CoFe2O4–reduced graphene oxide nanocomposites: Excellent microwave absorber and highly efficient magnetically separable recyclable photocatalyst for dye degradation. RSC Adv. 2016, 6, 76759–76772. [Google Scholar] [CrossRef]
- Gan, L.; Shang, S.; Yuen, C.W.M.; Jiang, S.-X.; Hu, E. Hydrothermal synthesis of magnetic CoFe2O4/graphene nanocomposites with improved photocatalytic activity. Appl. Surf. Sci. 2015, 351, 140–147. [Google Scholar] [CrossRef]
- De Souza, B.G.; Figueira, G.; Carvalho, M.H.; Alcaraz-Gonzalez, V.; Saldaña-Flores, K.E.; Godinho, M.; de Oliveira, A.J.; Kiminami, R.H.; Ruotolo, L.A.; Urquieta-González, E.A. A novel synthesis route to obtain magnetic nanocrystalline cobalt ferrite with photo-Fenton activity. Mater. Chem. Phys. 2020, 257, 123741. [Google Scholar] [CrossRef]
- Guan, D.; Xu, H.; Zhang, Q.; Huang, Y.; Shi, C.; Chang, Y.; Xu, X.; Tang, J.; Gu, Y.; Pao, C.; et al. Identifying A Universal Activity Descriptor and a Unifying Mechanism Concept on Perovskite Oxides for Green Hydrogen Production. Adv. Mater. 2023, e2305074. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y.; Xu, H.; Guan, D.; Hu, Z.; Jing, C.; Sha, Y.; Gu, Y.; Huang, Y.; Chang, Y.; et al. Combined Corner-Sharing and Edge-Sharing Networks in Hybrid Nanocomposite with Unusual Lattice-Oxygen Activation for Efficient Water Oxidation. Adv. Funct. Mater. 2022, 32, 2207618. [Google Scholar] [CrossRef]
- Guan, D.; Zhong, J.; Xu, H.; Huang, Y.-C.; Hu, Z.; Chen, B.; Zhang, Y.; Ni, M.; Xu, X.; Zhou, W.; et al. A universal chemical-induced tensile strain tuning strategy to boost oxygen-evolving electrocatalysis on perovskite oxides. Appl. Phys. Rev. 2022, 9, 011422. [Google Scholar] [CrossRef]
- Hemasankari, S.; Priyadharshini, S.; Thangaraju, D.; Sathiyanarayanamoorthi, V.; Al Sdran, N.; Shkir, M. Effect of neodymium (Nd) doping on the photocatalytic organic dye degradation performance of sol-gel synthesized CoFe2O4 self-assembled microstructures. Phys. B Condens. Matter 2023, 660, 414870. [Google Scholar] [CrossRef]
- Thakur, P.; Thakur, P.; Kishore, K.; Singh, M.; Sharma, S.; Sharma, P.; Sharma, P.; Lal, M. Structural, morphological, and magnetic properties of CoFe2O4 nano-ferrites synthesized via Co-precipitation route. Mater. Today Proc. 2023, 13, 189. [Google Scholar] [CrossRef]
- Nicolini, J.L.; Chavarriaga, E.A.; Lopera, A.; Wermuth, T.B.; García, C.; Alarcón, J.; Viegas, A.C.; Vasconcellos, M.A.Z.; Montedo, O.R.K.; Bergmann, C.P.; et al. One-step CoFe2O4 gel combustion synthesis using tris(hydroxymethyl)aminomethane (TRIS) as alternative fuel: Control of oxidiser-to-fuel molar ratio for tuning its structural, magnetic, and optical properties. J. Magn. Magn. Mater. 2022, 563, 169923. [Google Scholar] [CrossRef]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution Combustion Synthesis of Nanoscale Materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef]
- ENovitskaya, E.; Kelly, J.P.; Bhaduri, S.; Graeve, O.A. A review of solution combustion synthesis: An analysis of parameters controlling powder characteristics. Int. Mater. Rev. 2020, 66, 188–214. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachrichten Von Der Ges. Der Wiss. Zu Göttingen Math. Phys. Kl. 1918, 1918, 98–100. [Google Scholar]
- Kubelka, P.; Munk, F. Ein Beitrag Zur Optik Der Farbanstriche. Z. Für Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Aksu, Z.; Tezer, S. Biosorption of reactive dyes on the green alga Chlorella vulgaris. Process. Biochem. 2005, 40, 1347–1361. [Google Scholar] [CrossRef]
- Akyol, A.; Yatmaz, H.; Bayramoglu, M. Photocatalytic decolorization of Remazol Red RR in aqueous ZnO suspensions. Appl. Catal. B Environ. 2004, 54, 19–24. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information, PubChem Compound Summary for CID 29646. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/29646#section=3D-Conformer (accessed on 6 August 2023).
- Vilar, V.J.; Botelho, C.M.; Boaventura, R.A. Equilibrium and kinetic modelling of Cd(II) biosorption by algae Gelidium and agar extraction algal waste. Water Res. 2006, 40, 291–302. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie der sogenannten Adsorption gelöster Stoffe. Z. Für Chem. Und Ind. Der Kolloide 1907, 2, 15. [Google Scholar] [CrossRef]
- Darvishi, M.; Hasani, S.; Mashreghi, A.; Rezvan, M.T.; Ziarati, A. Application of the full factorial design to improving the properties of CoFe2O4 nanoparticles by simultaneously adding apple cider vinegar and agarose. Mater. Sci. Eng. B 2023, 297, 116754. [Google Scholar] [CrossRef]
- Gatchakayala, N.B.; Dachuru, R.S.R. Synthesis of CoFe2O4 Nanomaterials via Sol–Gel Auto-Combustion Method using Different Chelating Agent, and Its Effects on Magnetic and Dielectric Properties. Phys. Status Solidi (B) 2023, 260, 2300010. [Google Scholar] [CrossRef]
- Benali, A.; Saher, L.; Bejar, M.; Dhahri, E.; Graca, M.F.P.; Valente, M.A.; Sanguino, P.; Helguero, L.A.; Bachari, K.; Silva, A.M.S.; et al. CoFe2O4 spinel ferrite studies on permanent magnet application and cytotoxic effects on breast and prostate cancer cell lines. J. Mater. Sci. Mater. Electron. 2023, 34, 53. [Google Scholar] [CrossRef]
- Yousefi, M.; Manouchehri, S.; Arab, A.; Mozaffari, M.; Amiri, G.R.; Amighian, J. Preparation of cobalt–zinc ferrite (Co0.8Zn0.2Fe2O4) nanopowder via combustion method and investigation of its magnetic properties. Mater. Res. Bull. 2010, 45, 1792–1795. [Google Scholar] [CrossRef]
- Chavarriaga, E.; Lopera, A.; Franco, V.; Bergmann, C.; Alarcón, J. Gel combustion synthesis and magnetic properties of CoFe2O4, ZnFe2O4, and MgFe2O4 using 6-aminohexanoic acid as a new fuel. J. Magn. Magn. Mater. 2020, 497, 166054. [Google Scholar] [CrossRef]
- Chavarriaga, E.; Lopera, A.A.; Wermuth, T.B.; Arcaro, S.; Bezzon, V.D.; García, C.; Alarcón, J.; Ramirez, J.G.; Moreno, R.; Bergmann, C.P. Influence of caffeine and citrulline on magnetic properties when used as new fuels in the synthesis of CoFe2O4 nanoparticles by gel combustion. J. Magn. Magn. Mater. 2022, 560, 169632. [Google Scholar] [CrossRef]
- Wermuth, T.B.; Venturini, J.; Guaglianoni, W.C.; Tonelli, A.M.; Chavarriaga, E.; Arcaro, S.; Baibich, M.N.; Bergmann, C.P. Enhancement of magnetic and dielectric properties of KNbO3–CoFe2O4 multiferroic composites via thermal treatment. Ceram. Int. 2021, 47, 4874–4883. [Google Scholar] [CrossRef]
- Caldeira, L.E.; Erhardt, C.S.; Mariosi, F.R.; Venturini, J.; Zampiva, R.Y.S.; Montedo, O.R.K.; Arcaro, S.; Bergmann, C.P.; Bragança, S.R. Correlation of synthesis parameters to the structural and magnetic properties of spinel cobalt ferrites (CoFe2O4)—An experimental and statistical study. J. Magn. Magn. Mater. 2022, 550, 169128. [Google Scholar] [CrossRef]
- Babu, D.R.; Venkatesan, K. Synthesis of nanophasic CoFe2O4 powder by self-igniting solution combustion method using mix up fuels. J. Cryst. Growth 2017, 468, 179–184. [Google Scholar] [CrossRef]
- Sontu, U.B.; Yelasani, V.; Musugu, V.R.R. Structural, electrical and magnetic characteristics of nickel substituted cobalt ferrite nano particles, synthesized by self combustion method. J. Magn. Magn. Mater. 2015, 374, 376–380. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, T.; Wang, J.; Zhao, R.; Ma, Y.; Wang, F.; Xu, X. Influence of Ce-Mn co-doping on the structure and magnetic properties of cobalt ferrites. J. Alloys Compd. 2022, 929, 167256. [Google Scholar] [CrossRef]
- Aslibeiki, B.; Eskandarzadeh, N.; Jalili, H.; Varzaneh, A.G.; Kameli, P.; Orue, I.; Chernenko, V.; Hajalilou, A.; Ferreira, L.; Cruz, M. Magnetic hyperthermia properties of CoFe2O4 nanoparticles: Effect of polymer coating and interparticle interactions. Ceram. Int. 2022, 48, 27995–28005. [Google Scholar] [CrossRef]
- Jamir, M.; Borgohain, C.; Borah, J. Influence of structure and magnetic properties of surface modified nanoparticles for hyperthermia application. Phys. B Condens. Matter 2023, 648, 414405. [Google Scholar] [CrossRef]
- Badizi, A.M.; Maleki, H. One-step combustion synthesis of SiO2–CoFe2O4 nanocomposites and characterization for structural, thermal, optical and magnetic properties. Mater. Sci. Semicond. Process. 2021, 124, 105594. [Google Scholar] [CrossRef]
- Lahmar, H.; Benamira, M.; Messaadia, L.; Hamdi, M.; Avramova, I.; Trari, M. Synthesis, physical and photo-electrochemical properties of Gd2CuO4. J. Alloys Compd. 2020, 816, 152629. [Google Scholar] [CrossRef]
- Sheshmani, S.; Falahat, B.; Nikmaram, F.R. Preparation of magnetic graphene oxide-ferrite nanocomposites for oxidative decomposition of Remazol Black B. Int. J. Biol. Macromol. 2017, 97, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Liu, Y.; Li, X.; Sun, T.; Xu, Y. Enhanced heterogeneous Fenton-like degradation of methylene blue by reduced CuFe2O4. RSC Adv. 2018, 8, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Thy, L.T.M.; Tuyen, N.N.K.; Viet, N.D.; Huong, L.M.; Tinh, N.T.; Lin, T.H.; Son, N.T.; Oanh, D.T.Y.; Phong, M.T.; Hieu, N.H. Nickel ferrite nanoparticles-doped graphene oxide as a heterogeneous Fenton catalyst: Synthesis, characterization, and catalytic application. Vietnam. J. Chem. 2022, 60, 532–539. [Google Scholar]
- Tony, M.A.; Eltabey, M.M. End-of-life waste criteria: Synthesis and utilization of Mn–Zn ferrite nanoparticles as a superparamagnetic photocatalyst for synergistic wastewater remediation. Appl. Water Sci. 2022, 12, 21. [Google Scholar] [CrossRef]
- Rezgui, S.; Díez, A.M.; Monser, L.; Adhoum, N.; Pazos, M.; Sanromán, M.A. ZnFe2O4-chitosan magnetic beads for the removal of chlordimeform by photo-Fenton process under UVC irradiation. J. Environ. Manag. 2021, 283, 111987. [Google Scholar] [CrossRef]
- Chan, K.H.; Chu, W. The dose and ratio effects of Fe(II) and H2O2 in Fenton’s process on the removal of atrazine. Environ. Technol. 2003, 24, 703–710. [Google Scholar] [CrossRef]
- USP Technologies, Fentons Reagent General Chemistry Using H2O2. Available online: https://www.h2o2.com/industrial/fentons-reagent.aspx?pid=143&name=General-Chemistry-of-Fenton-s-Reagent (accessed on 30 August 2023).
- Mei, Y.; Qi, Y.; Li, J.; Deng, X.; Ma, S.; Yao, T.; Wu, J. Construction of yolk/shell Fe3O4@MgSiO3 nanoreactor for enhanced Fenton-like reaction via spatial separation of adsorption sites and activation sites. J. Taiwan Inst. Chem. Eng. 2020, 113, 363–371. [Google Scholar] [CrossRef]
- Wu, Q.; Siddique, M.S.; Yu, W. Iron-nickel bimetallic metal-organic frameworks as bifunctional Fenton-like catalysts for enhanced adsorption and degradation of organic contaminants under visible light: Kinetics and mechanistic studies. J. Hazard. Mater. 2021, 401, 123261. [Google Scholar] [CrossRef]
- Usman, M.; Monfort, O.; Gowrisankaran, S.; Hameed, B.H.; Hanna, K.; Al-Abri, M. Dual functional materials capable of integrating adsorption and Fenton-based oxidation processes for highly efficient removal of pharmaceutical contaminants. J. Water Process. Eng. 2023, 52, 103566. [Google Scholar] [CrossRef]
- Chen, F.; Shen, X.; Wang, Y.; Zhang, J. CeO2/H2O2 system catalytic oxidation mechanism study via a kinetics investigation to the degradation of acid orange 7. Appl. Catal. B Environ. 2012, 121–122, 223–229. [Google Scholar] [CrossRef]
- Cai, W.; Chen, F.; Shen, X.; Chen, L.; Zhang, J. Enhanced catalytic degradation of AO7 in the CeO2–H2O2 system with Fe3+ doping. Appl. Catal. B Environ. 2010, 101, 160–168. [Google Scholar] [CrossRef]
- Zang, C.; Yu, K.; Hu, S.; Chen, F. Adsorption-depended Fenton-like reaction kinetics in CeO2-H2O2 system for salicylic acid degradation. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 553, 456–463. [Google Scholar] [CrossRef]
- Behnajady, M.; Modirshahla, N.; Ghanbary, F. A kinetic model for the decolorization of C.I. Acid Yellow 23 by Fenton process. J. Hazard. Mater. 2007, 148, 98–102. [Google Scholar] [CrossRef]
- Cruz, D.R.; de Jesus, G.K.; Santos, C.A.; Silva, W.R.; Wisniewski, A.; Cunha, G.C.; Romão, L.P. Magnetic nanostructured material as heterogeneous catalyst for degradation of AB210 dye in tannery wastewater by electro-Fenton process. Chemosphere 2021, 280, 130675. [Google Scholar] [CrossRef]
- Nadeem, N.; Abbas, Q.; Yaseen, M.; Jilani, A.; Zahid, M.; Iqbal, J.; Murtaza, A.; Janczarek, M.; Jesionowski, T. Coal fly ash-based copper ferrite nanocomposites as potential heterogeneous photocatalysts for wastewater remediation. Appl. Surf. Sci. 2021, 565, 150542. [Google Scholar] [CrossRef]
- Zhu, P.; Nair, A.S.; Shengjie, P.; Shengyuan, Y.; Ramakrishna, S. Facile Fabrication of TiO2–Graphene Composite with Enhanced Photovoltaic and Photocatalytic Properties by Electrospinning. ACS Appl. Mater. Interfaces 2012, 4, 581–585. [Google Scholar] [CrossRef]
- Ahmad, F.; Liu, X.; Zhou, Y.; Yao, H. An in vivo evaluation of acute toxicity of cobalt ferrite (CoFe2O4) nanoparticles in larval-embryo Zebrafish (Danio rerio). Aquat. Toxicol. 2015, 166, 21–28. [Google Scholar] [CrossRef]
- Valério, A.; Sárria, M.P.; Rodriguez-Lorenzo, L.; Hotza, D.; Espiña, B.; González, S.Y.G. Are TiO2 nanoparticles safe for photocatalysis in aqueous media? Nanoscale Adv. 2020, 2, 4951–4960. [Google Scholar] [CrossRef]
- Waghmode, T.R.; Kurade, M.B.; Kabra, A.N.; Govindwar, S.P. Degradation of Remazol Red dye by Galactomyces geotrichum MTCC 1360 leading to increased iron uptake in Sorghum vulgare and Phaseolus mungo from soil. Biotechnol. Bioprocess Eng. 2012, 17, 117–126. [Google Scholar] [CrossRef]
- Mustafa, G.; Zahid, M.T.; Kurade, M.B.; Patil, S.M.; Shakoori, F.R.; Shafiq, Z.; Ihsan, S.; Ahn, Y.; Khan, A.A.; Gacem, A.; et al. Molecular characterization of azoreductase and its potential for the decolorization of Remazol Red R and Acid Blue 29. Environ. Pollut. 2023, 335, 122253. [Google Scholar] [CrossRef]
- Thanavel, M.; Kadam, S.K.; Biradar, S.P.; Govindwar, S.P.; Jeon, B.-H.; Sadasivam, S.K. Combined biological and advanced oxidation process for decolorization of textile dyes. SN Appl. Sci. 2019, 1, 97. [Google Scholar] [CrossRef]
- Jadhav, S.B.; Phugare, S.S.; Patil, P.S.; Jadhav, J.P. Biochemical degradation pathway of textile dye Remazol red and subsequent toxicological evaluation by cytotoxicity, genotoxicity and oxidative stress studies. Int. Biodeterior. Biodegradation 2011, 65, 733–743. [Google Scholar] [CrossRef]
- Osma, J.F.; Toca-Herrera, J.L.; Rodríguez-Couto, S. Transformation pathway of Remazol Brilliant Blue R by immobilised laccase. Bioresour. Technol. 2010, 101, 8509–8514. [Google Scholar] [CrossRef]



| Fuel | Temperature (°C) | Reference |
|---|---|---|
| Citric acid | 800 | [39] |
| Tartaric acid, citric acid, and oxalic acid | 500 | [40] |
| Glycine or citric acid | 700 | [41] |
| Urea | 700 | [42] |
| 6-aminohexanoic acid | 230 | [43] |
| Caffeine and citrulline | 200–250 | [44] |
| Tris with Ψ = 0.8 | 140 | [29] |
| Structural and Morphological Properties | |
|---|---|
| Lattice parameter (Å) | 8.3850 |
| Crystallite size (nm) | 35 |
| Surface area (m2/g) | 59 |
| Magnetic properties | |
| Remnant magnetization (Mr) (emu/g) | 30.4 |
| Coercivity (Hc) (Oe) | 1243 |
| Quadrature (S–Mr/Ms) | 0.46 |
| Saturation magnetization (Ms) (emu/g) | 65.7 |
| Parameters | Values |
|---|---|
| m | 28 ± 3 |
| b | 1.24 ± 0.02 |
| S2R | 190.93 |
| R2 | 0.998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cechinel, M.A.P.; Nicolini, J.L.; Tápia, P.M.; Miranda, E.A.C.; Eller, S.; de Oliveira, T.F.; Raupp-Pereira, F.; Montedo, O.R.K.; Wermuth, T.B.; Arcaro, S. Cobalt Ferrite (CoFe2O4) Spinel as a New Efficient Magnetic Heterogeneous Fenton-like Catalyst for Wastewater Treatment. Sustainability 2023, 15, 15183. https://doi.org/10.3390/su152015183
Cechinel MAP, Nicolini JL, Tápia PM, Miranda EAC, Eller S, de Oliveira TF, Raupp-Pereira F, Montedo ORK, Wermuth TB, Arcaro S. Cobalt Ferrite (CoFe2O4) Spinel as a New Efficient Magnetic Heterogeneous Fenton-like Catalyst for Wastewater Treatment. Sustainability. 2023; 15(20):15183. https://doi.org/10.3390/su152015183
Chicago/Turabian StyleCechinel, Maria Alice Prado, João Lucas Nicolini, Pedro Monteiro Tápia, Edgar Andrés Chavarriaga Miranda, Sarah Eller, Tiago Franco de Oliveira, Fabiano Raupp-Pereira, Oscar Rubem Klegues Montedo, Tiago Bender Wermuth, and Sabrina Arcaro. 2023. "Cobalt Ferrite (CoFe2O4) Spinel as a New Efficient Magnetic Heterogeneous Fenton-like Catalyst for Wastewater Treatment" Sustainability 15, no. 20: 15183. https://doi.org/10.3390/su152015183
APA StyleCechinel, M. A. P., Nicolini, J. L., Tápia, P. M., Miranda, E. A. C., Eller, S., de Oliveira, T. F., Raupp-Pereira, F., Montedo, O. R. K., Wermuth, T. B., & Arcaro, S. (2023). Cobalt Ferrite (CoFe2O4) Spinel as a New Efficient Magnetic Heterogeneous Fenton-like Catalyst for Wastewater Treatment. Sustainability, 15(20), 15183. https://doi.org/10.3390/su152015183










