TiO2/Arabic Gum for Degradation of Pollutants in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TiO2/Arabic Gum
2.3. Physico-Chemical Characterization
2.4. Photocatalytic Performance
2.5. Reuse Test ans Photostability of Phocatalystic
3. Results and Discussion
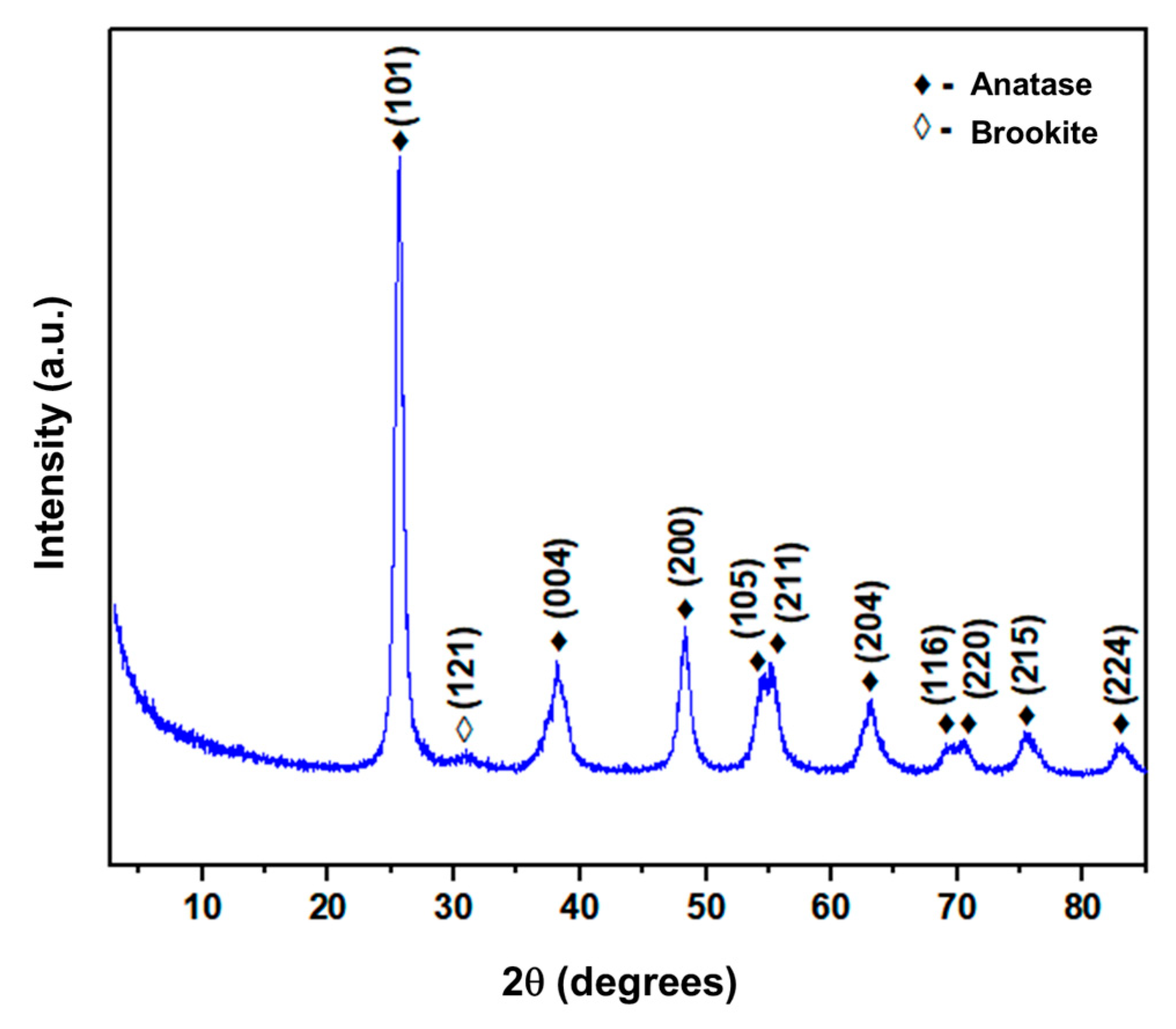
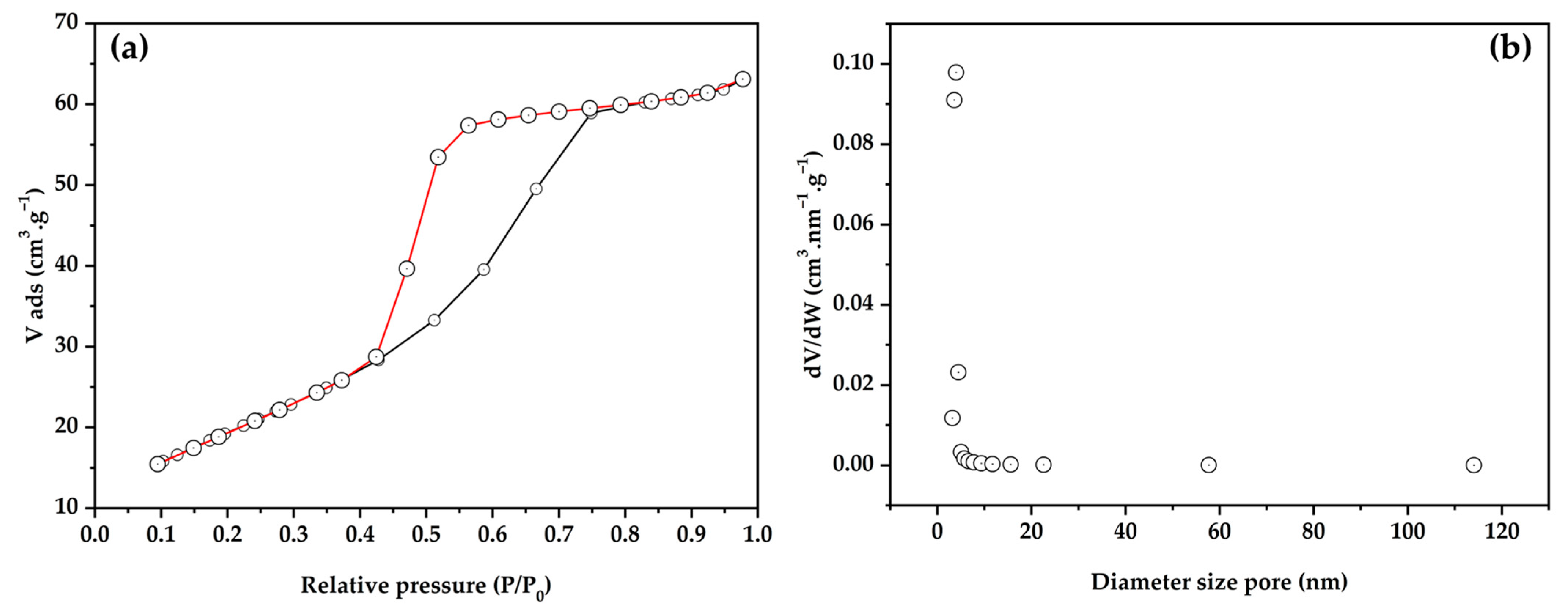
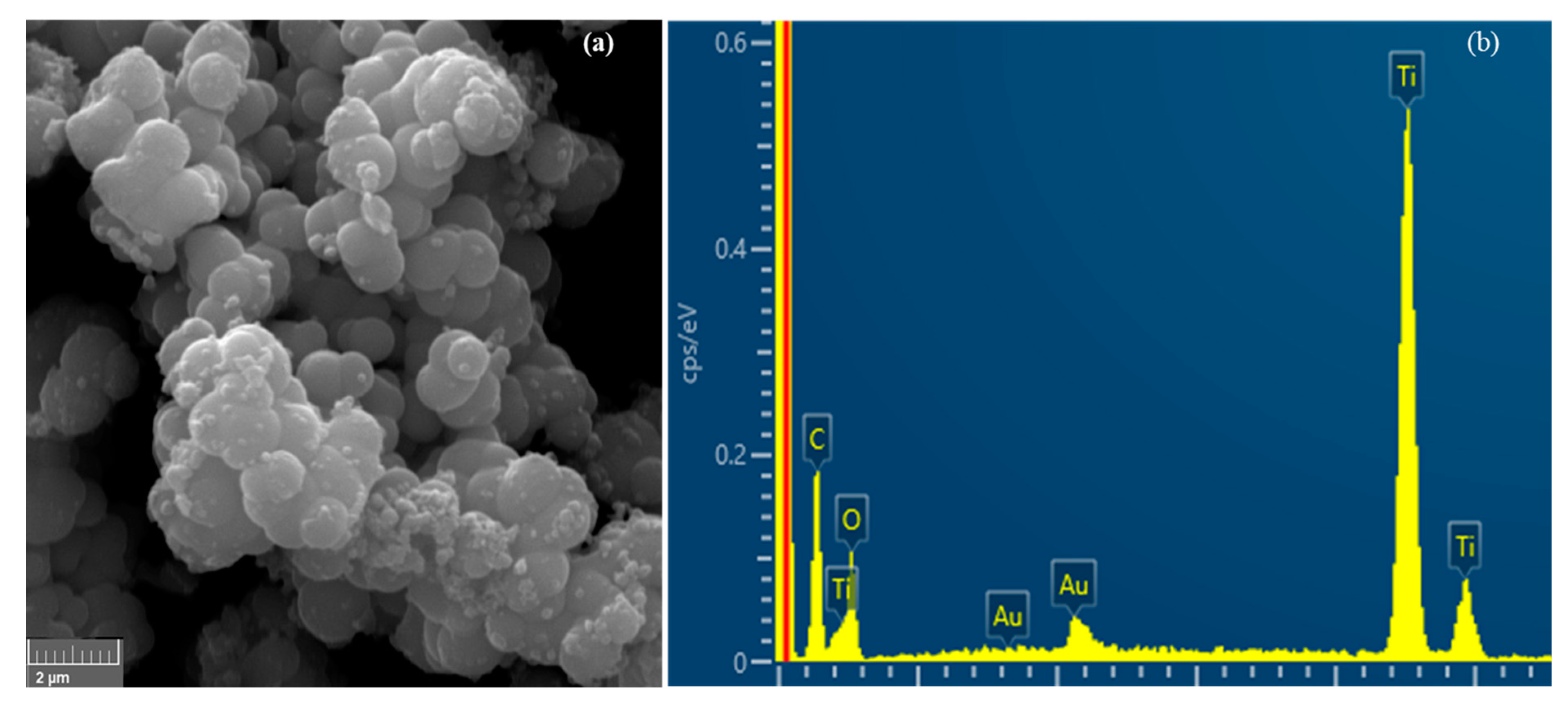
3.1. Characterization
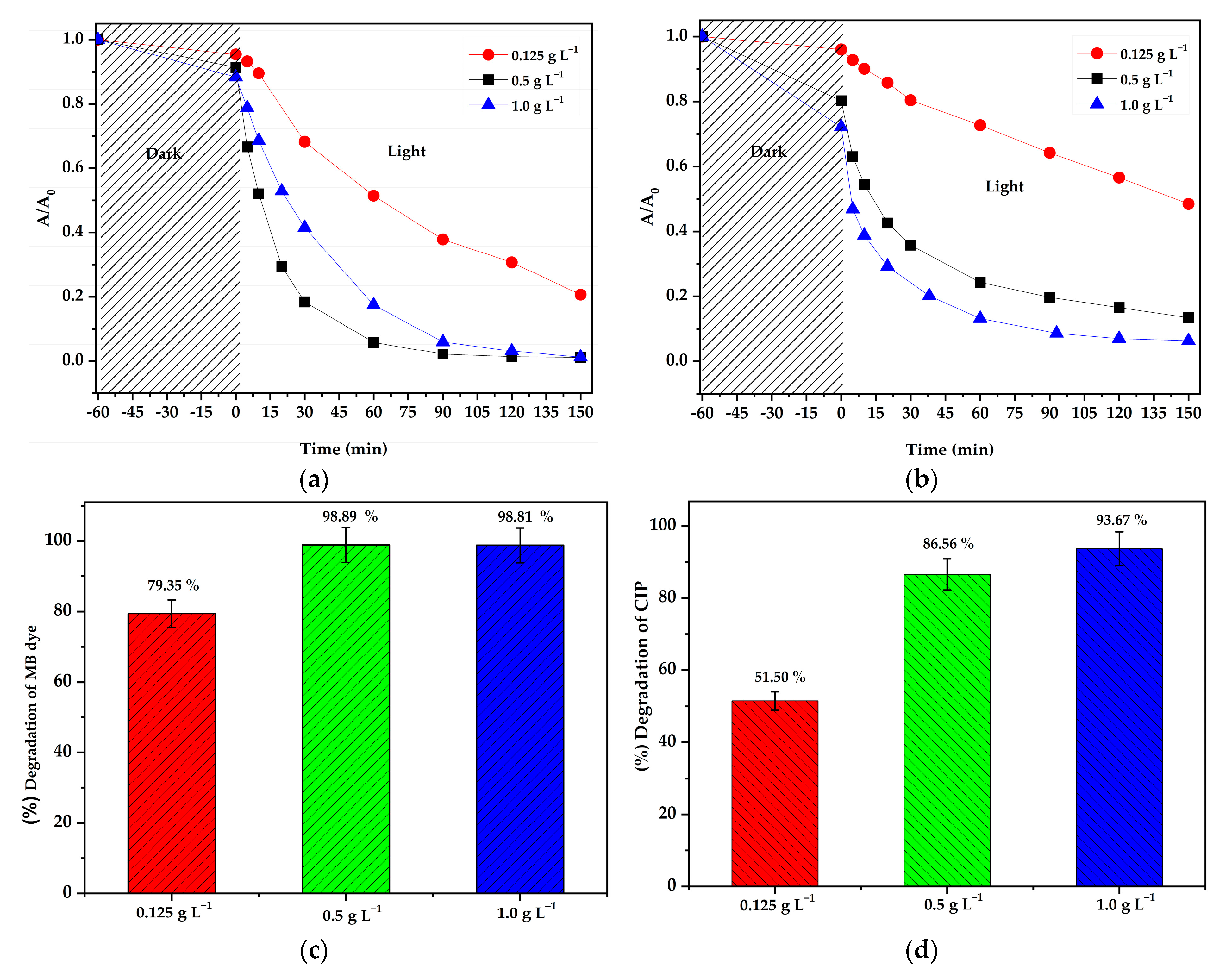
3.2. Photocatalytic Activities
3.3. Recycling and Stability of Photocatalytic
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Popescu, V.; Corina, I.; Angel, J.; Martin, S.; Kanhaiya, K.; Florea, D.; Gavril, G.; Tusa, M.I.; Pacioglu, O.; Popa, L.I.; et al. Network Analytics for Drug Repurposing in COVID-19. Brief. Bioinform. 2022, 23, bbab490. [Google Scholar]
- Saha, S.; Kumar, A.; Sekhar, S.; Chatterjee, P.; Nasipuri, M.; Bose, D.; Basu, S. Drug Repurposing for COVID-19 Using Computational Screening: Is Fostamatinib/R406 a Potential Candidate ? Methods 2023, 203, 564–574. [Google Scholar] [CrossRef]
- Bhatia, A. Role of Drugs in COVID 19 Patient: A Review. J. Pharm. Res. Int. 2021, 33, 99–105. [Google Scholar] [CrossRef]
- Gwenzi, W.; Selvasembian, R.; Offiong, N.A.O.; Mahmoud, A.E.D.; Sanganyado, E.; Mal, J. COVID-19 Drugs in Aquatic Systems: A Review. Environ. Chem. Lett. 2022, 20, 1275–1294. [Google Scholar] [CrossRef]
- Seethalakshmi, P.S.; Charity, O.J.; Giakoumis, T.; Kiran, G.S.; Sriskandan, S.; Voulvoulis, N.; Selvin, J. Delineating the Impact of COVID-19 on Antimicrobial Resistance: An Indian Perspective. Sci. Total Environ. 2022, 818, 151702. [Google Scholar] [CrossRef]
- Diaz-Camal, N.; Cardoso-Vera, J.D.; Islas-Flores, H.; Gómez-Oliván, L.M.; Mejía-García, A. Consumption and Ocurrence of Antidepressants (SSRIs) in Pre- and Post-COVID-19 Pandemic, Their Environmental Impact and Innovative Removal Methods: A Review. Sci. Total Environ. 2022, 829, 154656. [Google Scholar] [CrossRef]
- Wojcieszyńska, D.; Guzik, H.; Guzik, U. Non-Steroidal Anti-Inflammatory Drugs in the Era of the Covid-19 Pandemic in the Context of the Human and the Environment. Sci. Total Environ. 2022, 834, 155317. [Google Scholar] [CrossRef]
- Morales-Paredes, C.A.; Rodríguez-Díaz, J.M.; Boluda-Botella, N. Pharmaceutical Compounds Used in the COVID-19 Pandemic: A Review of Their Presence in Water and Treatment Techniques for Their Elimination. Sci. Total Environ. 2022, 814, 152691. [Google Scholar] [CrossRef]
- Morin, N.; Eric, C.; Marc, L.; Ana, F.; Lado, R. Removal of Emerging Contaminants from Wastewater Using Advanced Treatments. A Review. Environ. Chem. Lett. 2022, 20, 1333–1375. [Google Scholar]
- Pereira, A.; Silva, L.; Laranjeiro, C.; Pena, A. Assessment of Human Pharmaceuticals in Drinking Water Catchments, Tap and Drinking Fountain Waters. Appl. Sci. 2021, 11, 7062. [Google Scholar] [CrossRef]
- Nieto-Juárez, J.I.; Torres-Palma, R.A.; Botero-Coy, A.M.; Hernández, F. Pharmaceuticals and Environmental Risk Assessment in Municipal Wastewater Treatment Plants and Rivers from Peru. Environ. Int. 2021, 155, 106674. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Varela Della Giustina, S.; Llorca, M.; Barceló, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic Residues in Final Effluents of European Wastewater Treatment Plants and Their Impact on the Aquatic Environment. Environ. Int. 2020, 140, 105733. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, D.; Shah, M.; Yadav, A.; Panchal, J. A Critical Review on Emerging Contaminants: Origin, Discernment, and Remedies. Sustain. Water Resour. Manag. 2023, 9, 69. [Google Scholar] [CrossRef]
- Kumar, M.; Das, N.; Tripathi, S.; Verma, A.; Jha, P.K.; Bhattacharya, P.; Mahlknecht, J. Global Co-Occurrences of Multi-(Emerging)-Contaminants in the Hotspots of Arsenic Polluted Groundwater: A Pattern of Menace. Curr. Opin. Environ. Sci. Heal. 2023, 34, 100483. [Google Scholar] [CrossRef]
- Puri, M.; Gandhi, K.; Kumar, M.S. Emerging Environmental Contaminants: A Global Perspective on Policies and Regulations. J. Environ. Manag. 2023, 332, 117344. [Google Scholar] [CrossRef]
- Chopra, L. Photocatalytic Activity of Zinc Oxide for Dye and Drug Degradation: A Review. Mater. Today Proc. 2022, 52, 1653–1656. [Google Scholar] [CrossRef]
- Li, L.; Liu, J.; Zeng, J.; Li, J.; Liu, Y.; Sun, X.; Xu, L.; Li, L. Complete Degradation and Detoxification of Ciprofloxacin by a Micro-/Nanostructured Biogenic Mn Oxide Composite from a Highly Active Mn(2+)-Oxidizing Pseudomonas Strain. Nanomaterials 2021, 11, 1660. [Google Scholar] [CrossRef]
- Martins, P.; Kappert, S.; Le, H.N.; Sebastian, V.; Kühn, K.; Alves, M.; Pereira, L.; Cuniberti, G.; Melle-Franco, M.; Lanceros-Méndez, S. Enhanced Photocatalytic Activity of Au/TiO2 Nanoparticles against Ciprofloxacin. Catalysts 2020, 10, 234. [Google Scholar] [CrossRef]
- Girardi, C.; Greve, J.; Lamshöft, M.; Fetzer, I.; Miltner, A.; Schäffer, A.; Kästner, M. Biodegradation of Ciprofloxacin in Water and Soil and Its Effects on the Microbial Communities. J. Hazard. Mater. 2011, 198, 22–30. [Google Scholar] [CrossRef]
- Kumar, A.; Rana, A.; Sharma, G.; Naushad, M.; Dhiman, P.; Kumari, A.; Stadler, F.J. Recent Advances in Nano-Fenton Catalytic Degradation of Emerging Pharmaceutical Contaminants. J. Mol. Liq. 2019, 290, 111177. [Google Scholar] [CrossRef]
- Jallouli, N.; Pastrana-Martínez, L.M.; Ribeiro, A.R.; Moreira, N.F.F.; Faria, J.L.; Hentati, O.; Silva, A.M.T.; Ksibi, M. Heterogeneous Photocatalytic Degradation of Ibuprofen in Ultrapure Water, Municipal and Pharmaceutical Industry Wastewaters Using a TiO2/UV-LED System. Chem. Eng. J. 2018, 334, 976–984. [Google Scholar] [CrossRef]
- Song, C.; Shang, C.; Li, S.; Wang, W.; Qi, M.; Chen, J.; Liu, H. Efficient Visible-Light-Responsive Ag3PO4/g-C3N4/Hydroxyapatite Photocatalyst (from Oyster Shells) for the Degradation of Methylene Blue: Preparation, Properties and Mechanism. Catalysts 2022, 12, 115. [Google Scholar] [CrossRef]
- Pereira Rocha, R.L.; Silva, T.L.; Araujo, F.P.; Vieira, E.G.; Honório, L.M.; Furtini, M.B.; da Fonseca, M.G.; da Silva-Filho, E.C.; Osajima, J.A. Gallium-Containing Hydroxyapatite as a Promising Material for Photocatalytic Performance. Minerals 2021, 11, 1347. [Google Scholar] [CrossRef]
- Araujo, F.P.; Honorio, L.M.C.; Lima, I.S.; Trigueiro, P.; Almeida, L.C.; Fechine, P.B.A.; Santos, F.E.P.; Peña-Garcia, R.; Silva-Filho, E.C.; Osajima, J.A. New Composite TiO2/Naturals Gums for High Efficiency in Photodiscoloration Process. Ceram. Int. 2020, 46, 15534–15543. [Google Scholar] [CrossRef]
- Waghchaure, R.H.; Adole, V.A.; Jagdale, B.S. Photocatalytic Degradation of Methylene Blue, Rhodamine B, Methyl Orange and Eriochrome Black T Dyes by Modified ZnO Nanocatalysts: A Concise Review. Inorg. Chem. Commun. 2022, 143, 109764. [Google Scholar] [CrossRef]
- Freitas, W.A.; Soares, B.E.C.F.; Rodrigues, M.S.; Trigueiro, P.; Honorio, L.M.C.; Peña-Garcia, R.; Alcântara, A.C.S.; Silva-Filho, E.C.; Fonseca, M.G.; Furtini, M.B.; et al. Facile Synthesis of ZnO-Clay Minerals Composites Using an Ultrasonic Approach for Photocatalytic Performance. J. Photochem. Photobiol. A Chem. 2022, 429, 113934. [Google Scholar] [CrossRef]
- Machín, A.; Soto-Vázquez, L.; García, D.; Cotto, M.C.; Ortiz, D.; Berríos-Rolón, P.J.; Fontánez, K.; Resto, E.; Morant, C.; Petrescu, F.; et al. Photodegradation of Ciprofloxacin and Levofloxacin by Au@ZnONPs-MoS2-RGO Nanocomposites. Catalysts 2023, 13, 538. [Google Scholar] [CrossRef]
- Raja, A.; Rajasekaran, P.; Selvakumar, K.; Arunpandian, M.; Kaviyarasu, K.; Asath Bahadur, S.; Swaminathan, M. Visible Active Reduced Graphene Oxide-BiVO4-ZnO Ternary Photocatalyst for Efficient Removal of Ciprofloxacin. Sep. Purif. Technol. 2020, 233, 115996. [Google Scholar] [CrossRef]
- Van Thuan, D.; Nguyen, T.B.H.; Pham, T.H.; Kim, J.; Hien Chu, T.T.; Nguyen, M.V.; Nguyen, K.D.; Al-Onazi, W.A.; Elshikh, M.S. Photodegradation of Ciprofloxacin Antibiotic in Water by Using ZnO-Doped g-C(3)N(4) Photocatalyst. Chemosphere 2022, 308, 136408. [Google Scholar] [CrossRef]
- Damacena, D.H.; Macedo, V.H.; Silva, A.S.; Honorio, C.; Silva-Filho, L.M.; Osajima, E.C. Ag@ZnO-Saponite Nanocomposite for Photodegradation of 2 Ciprofloxacin. Chem. Proc 2021, 3, 1–5. [Google Scholar]
- Bai, L.; Dong, X.; Wang, F.; Ding, X.; Diao, Z.; Chen, D. A Review on the Removal of Phthalate Acid Esters in Wastewater Treatment Plants: From the Conventional Wastewater Treatment to Combined Processes. Environ. Sci. Pollut. Res. 2022, 29, 51339–51353. [Google Scholar] [CrossRef]
- Shewa, W.A.; Dagnew, M. Revisiting Chemically Enhanced Primary Treatment of Wastewater: A Review. Sustainability 2020, 12, 5928. [Google Scholar]
- Sylwan, I.; Thorin, E. Removal of Heavy Metals during Primary Treatment of Municipal Wastewater and Possibilities of Enhanced Removal: A Review. Water 2021, 13, 1121. [Google Scholar]
- Krzeminski, P.; Concetta, M.; Karaolia, P.; Langenhoff, A.; Almeida, C.M.R.; Felis, E.; Gritten, F.; Rasmus, H.; Fernandes, T.; Manaia, C.M.; et al. Science of the Total Environment Performance of Secondary Wastewater Treatment Methods for the Removal of Contaminants of Emerging Concern Implicated in Crop Uptake and Antibiotic Resistance Spread: A Review. Sci. Total Environ. 2019, 648, 1052–1081. [Google Scholar] [CrossRef] [PubMed]
- Angeles, L.F.; Mullen, R.A.; Huang, I.J.; Wilson, C.; Khunjar, W.; Sirotkin, H.I.; McElroy, A.E.; Aga, D.S. Assessing Pharmaceutical Removal and Reduction in Toxicity Provided by Advanced Wastewater Treatment Systems. Environ. Sci. Water Res. Technol. 2020, 6, 62–77. [Google Scholar] [CrossRef]
- Parimelazhagan, V.; Natarajan, K.; Shanbhag, S.; Madivada, S.; Kumar, H.S. Effective Adsorptive Removal of Coomassie Violet Dye from Aqueous Solutions Using Green Synthesized Zinc Hydroxide Nanoparticles Prepared from Calotropis Gigantea Leaf Extract. ChemEngineering 2023, 7, 31. [Google Scholar] [CrossRef]
- Vairavel, P.; Rampal, N. Continuous Fixed-Bed Column Study for Removal of Congo Red Dye from Aqueous Solutions Using Nelumbo Nucifera Leaf Adsorbent. Int. J. Environ. Anal. Chem. 2021, 1–20. [Google Scholar] [CrossRef]
- Parimelazhagan, V.; Yashwath, P.; Arukkani Pushparajan, D.; Carpenter, J. Rapid Removal of Toxic Remazol Brilliant Blue-R Dye from Aqueous Solutions Using Juglans Nigra Shell Biomass Activated Carbon as Potential Adsorbent: Optimization, Isotherm, Kinetic, and Thermodynamic Investigation. Int. J. Mol. Sci. 2022, 23, 12484. [Google Scholar] [CrossRef]
- Tomaz, A.T.; Barthus, R.C.; Costa, C.R.; Ribeiro, J. Decontamination of Wastewater Containing Organic Pollutants: A Review. Rev. Virtual Química. 2022, 15, 183–199. [Google Scholar] [CrossRef]
- Eniola, J.O.; Kumar, R.; Barakat, M.A.; Rashid, J. A Review on Conventional and Advanced Hybrid Technologies for Pharmaceutical Wastewater Treatment. J. Clean. Prod. 2022, 356, 131826. [Google Scholar] [CrossRef]
- Pal, S.; Ahamed, Z.; Pal, P. Removal of Antibiotics and Pharmaceutically Active Compounds from Water Environment: Experiments towards Industrial Scale Up. Sep. Purif. Technol. 2023, 295, 121249. [Google Scholar] [CrossRef]
- Soliman, A.I.A.; Abdel-Wahab, A.M.A.; Abdelhamid, H.N. Hierarchical Porous Zeolitic Imidazolate Frameworks (ZIF-8) and ZnO@N-Doped Carbon for Selective Adsorption and Photocatalytic Degradation of Organic Pollutants. RSC Adv. 2022, 12, 7075–7084. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, A.B.A.; El-Bery, H.M.; Abdelhamid, H.N.; El-Gyar, S.A. ZIF-67 and Cobalt-Based@heteroatom-Doped Carbon Nanomaterials for Hydrogen Production and Dyes Removal via Adsorption and Catalytic Degradation. J. Environ. Chem. Eng. 2022, 10, 108848. [Google Scholar] [CrossRef]
- Sun, J.; Mu, Q.; Kimura, H.; Murugadoss, V.; He, M.; Du, W.; Hou, C. Oxidative Degradation of Phenols and Substituted Phenols in the Water and Atmosphere: A Review. Adv. Compos. Hybrid Mater. 2022, 5, 627–640. [Google Scholar] [CrossRef]
- Zeshan, M.; Bhatti, I.A.; Mohsin, M.; Iqbal, M.; Amjed, N.; Nisar, J.; AlMasoud, N.; Alomar, T.S. Remediation of Pesticides Using TiO2 Based Photocatalytic Strategies: A Review. Chemosphere 2022, 300, 134525. [Google Scholar] [CrossRef]
- Sendão, R.M.S.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Photocatalytic Removal of Pharmaceutical Water Pollutants by TiO2—Carbon Dots Nanocomposites: A Review. Chemosphere 2022, 301, 134731. [Google Scholar] [CrossRef]
- Nur, A.S.M.; Sultana, M.; Mondal, A.; Islam, S.; Robel, F.N.; Islam, A.; Sumi, M.S.A. A Review on the Development of Elemental and Codoped TiO2 Photocatalysts for Enhanced Dye Degradation under UV–Vis Irradiation. J. Water Process Eng. 2022, 47, 102728. [Google Scholar] [CrossRef]
- Verma, V.; Al-Dossari, M.; Singh, J.; Rawat, M.; Kordy, M.G.M.; Shaban, M. A Review on Green Synthesis of TiO2 NPs: Photocatalysis and Antimicrobial Applications. Polymers 2022, 14, 1444. [Google Scholar] [CrossRef]
- Manickam, A.; Selvakumaran, D.; Narendran, K.; Abdul Razack, S.; Selvakumar, S.; Krishnamurthy, B. Fabrication of Gum Acacia Protected Zinc Oxide Nanoparticles for UV Assisted Photocatalysis of Methyl Green Textile Dye. Chem. Phys. Lett. 2022, 800, 139662. [Google Scholar] [CrossRef]
- Saranya, S.K.S.K.S.; Padil, V.V.T.; Senan, C.; Pilankatta, R.; Saranya, S.K.K.; George, B.; Wacławek, S.; Černík, M. Green Synthesis of High Temperature Stable Anatase Titanium Dioxide Nanoparticles Using Gum Kondagogu: Characterization and Solar Driven Photocatalytic Degradation of Organic Dye. Nanomaterials 2018, 8, 1002. [Google Scholar] [CrossRef]
- Alwared, A.I.; Mohammed, N.A.; Al-Musawi, T.J.; Mohammed, A.A. Solar-Induced Photocatalytic Degradation of Reactive Red and Turquoise Dyes Using a Titanium Oxide/Xanthan Gum Composite. Sustainability 2023, 15, 10815. [Google Scholar] [CrossRef]
- Inamuddin. Xanthan Gum/Titanium Dioxide Nanocomposite for Photocatalytic Degradation of Methyl Orange Dye. Int. J. Biol. Macromol. 2019, 121, 1046–1053. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D.; Taneja, S. Exudate Gums: Chemistry, Properties and Food Applications—A Review. J. Sci. Food Agric. 2020, 100, 2828–2835. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Nigen, M.; Mejia Tamayo, V.; Doco, T.; Williams, P.; Amine, C.; Renard, D. Acacia Gum: History of the Future. Food Hydrocoll. 2018, 78, 140–160. [Google Scholar] [CrossRef]
- Lopes, A.C.B.; Araujo, F.P.; Morais, A.I.S.; de Lima, I.S.; Honorio, L.M.C.; Almeida, L.C.; Garcia, R.P.; Silva, E.C.; Furtini, M.B.; Osajima, J.A. TiO2/Karaya Composite for Photoinactivation of Bacteria. Materials 2022, 15, 4559. [Google Scholar] [CrossRef]
- Parvathiraja, C.; Katheria, S.; Siddiqui, M.R.; Wabaidur, S.M.; Islam, M.A.; Lai, W.C. Activated Carbon-Loaded Titanium Dioxide Nanoparticles and Their Photocatalytic and Antibacterial Investigations. Catalysts 2022, 12, 834. [Google Scholar] [CrossRef]
- Miditana, S.R.; Tirukkovalluri, S.R.; Raju, I.M. Synthesis and Antibacterial Activity of Transition Metal (Ni/Mn) Co-Doped TiO2 Nanophotocatalyst on Different Pathogens under Visible Light Irradiation. Nanosyst. Chem. Math. 2022, 13, 104–114. [Google Scholar] [CrossRef]
- Ashfaq, A.; Ikram, M.; Haider, A.; Ul-Hamid, A.; Shahzadi, I.; Haider, J. Nitrogen and Carbon Nitride-Doped TiO2 for Multiple Catalysis and Its Antimicrobial Activity. Nanoscale Res. Lett. 2021, 16, 119. [Google Scholar] [CrossRef]
- Helmy, E.T.; Abouellef, E.M.; Soliman, U.A.; Pan, J.H. Novel Green Synthesis of S-Doped TiO2 Nanoparticles Using Malva Parviflora Plant Extract and Their Photocatalytic, Antimicrobial and Antioxidant Activities under Sunlight Illumination. Chemosphere 2021, 271, 129524. [Google Scholar] [CrossRef]
- Priyadarshini, S.; Mainal, A.; Sonsudin, F.; Yahya, R.; Alyousef, A.A.; Mohammed, A. Biosynthesis of TiO2 Nanoparticles and Their Superior Antibacterial Effect against Human Nosocomial Bacterial Pathogens. Res. Chem. Intermed. 2020, 46, 1077–1089. [Google Scholar] [CrossRef]
- Ngoepe, N.M.; Mathipa, M.M.; Hintsho-Mbita, N.C. Biosynthesis of Titanium Dioxide Nanoparticles for the Photodegradation of Dyes and Removal of Bacteria. Optik 2020, 224, 165728. [Google Scholar] [CrossRef]
- Sanitnon, P.; Chiarakorn, S.; Chawengkijwanich, C.; Chuangchote, S.; Pongprayoon, T. Synergistic Effects of Zirconium and Silver Co-Dopants in TiO2 Nanoparticles for Photocatalytic Degradation of an Organic Dye and Antibacterial Activity. J. Aust. Ceram. Soc. 2020, 56, 579–590. [Google Scholar] [CrossRef]
- Desai, K.R.; Alone, S.T.; Wadgane, S.R.; Shirsath, S.E.; Batoo, K.M.; Imran, A.; Raslan, E.H.; Hadi, M.; Ijaz, M.F.; Kadam, R.H. X-Ray Diffraction Based Williamson–Hall Analysis and Rietveld Refinement for Strain Mechanism in Mg–Mn Co-Substituted CdFe2O4 Nanoparticles. Phys. B Condens. Matter 2021, 614, 413054. [Google Scholar] [CrossRef]
- Ajoyan, Z.; Copeman, C.; Bicalho, H.A.; Do, J.L.; Te, T.; Romero, J.; Howarth, A.J. A Simple Method for Teaching Bragg’s Law in an Undergraduate Teaching Laboratory with the Use of Metal-Organic Frameworks. J. Chem. Educ. 2023, 100, 1990–1996. [Google Scholar] [CrossRef]
- Ahmad, I.; Zou, Y.; Yan, J.; Liu, Y.; Shukrullah, S.; Naz, M.Y.; Hussain, H.; Khan, W.Q.; Khalid, N.R. Semiconductor Photocatalysts: A Critical Review Highlighting the Various Strategies to Boost the Photocatalytic Performances for Diverse Applications. Adv. Colloid Interface Sci. 2023, 311, 102830. [Google Scholar] [CrossRef]
- Yang, M.; Ma, G.; Yang, H.; Xiaoqiang, Z.; Yang, W.; Hou, H. Advanced Strategies for Promoting the Photocatalytic Performance of FeVO4 Based Photocatalysts: A Review of Recent Progress. J. Alloys Compd. 2023, 941. [Google Scholar] [CrossRef]
- Osajima, J.A.; Sá, A.S.; Feitosa, R.P.; Furtini, M.B.; Honorio, L.M.C.; Fonseca, M.G.; Trigueiro, P.; Caregnato, P.; Triboni, E.R.; Silva-Filho, E.C. Improved Remediation of Contaminated Water Using ZnO Systems via Chemical Treatment: Applications, Implications and Toxicological Mitigation. Sustain. Water Resour. Manag. 2023, 9, 42. [Google Scholar] [CrossRef]
- Sá, A.S.; Feitosa, R.P.; Honório, L.; Peña-Garcia, R.; Almeida, L.C.; Dias, J.S.; Brazuna, L.P.; Tabuti, T.G.; Triboni, E.R.; Osajima, J.A.; et al. A Brief Photocatalytic Study of Zno Containing Cerium towards Ibuprofen Degradation. Materials 2021, 14, 5891. [Google Scholar] [CrossRef]
- Lafjah, M.; Mayoufi, A.; Schaal, E.; Djafri, F.; Bengueddach, A.; Keller, N.; Keller, V. TiO2 Nanorods for Gas Phase Photocatalytic Applications. Catal. Today 2014, 235, 193–200. [Google Scholar] [CrossRef]
- Ates, A. Activity and Stability of TiO2 Samples with Different Phase Compositions in the Decomposition of Formaldehyde in SCW. Int. J. Hydrogen Energy 2021, 46, 1842–1856. [Google Scholar] [CrossRef]
- Shi, J.W.; Zheng, J.T.; Wu, P. Preparation, Characterization and Photocatalytic Activities of Holmium-Doped Titanium Dioxide Nanoparticles. J. Hazard. Mater. 2009, 161, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Kang, J.M.; Lee, J.E.; Kim, K.S.; Kim, K.H.; Cho, M.; Lee, S.G. Effects of Calcination Temperature on the Phase Composition, Photocatalytic Degradation, and Virucidal Activities of TiO2Nanoparticles. ACS Omega 2021, 6, 10668–10678. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.B.T.; Ha, T.T.L.; Do Nguyen, T.; Le, H.N.; Ha-Thuc, C.N.; Nguyen, T.M.L.; Perre, P.; Nguyen, D.M. Effectiveness of Photocatalysis of MMT-Supported TiO2 and TiO2 Nanotubes for Rhodamine B Degradation. Chemosphere 2021, 280, 130802. [Google Scholar] [CrossRef] [PubMed]
- Vakhrushev, A.Y.; Boitsova, T.B. TiO2 and TiO2/Ag Nanofibers: Template Synthesis, Structure, and Photocatalytic Properties. J. Porous Mater. 2021, 28, 1023–1030. [Google Scholar] [CrossRef]
- Abdelnasser, S.; Al Sakkaf, R.; Palmisano, G. Environmental and Energy Applications of TiO2 photoanodes Modified with Alkali Metals and Polymers. J. Environ. Chem. Eng. 2021, 9, 104873. [Google Scholar] [CrossRef]
- Marinho, J.Z.; Nascimento, L.L.; Santos, A.L.R.; Faria, A.M.; Machado, A.E.H.; Patrocinio, A.O.T. On the Influence of Hydrothermal Treatment PH on the Performance of Bi2WO6 as Photocatalyst in the Glycerol Photoreforming. Photochem. Photobiol. Sci. 2022, 21, 1659–1675. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic Degradation of Organic Pollutants Using TiO2-Based Photocatalysts: A Review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Rauf, M.A.; Meetani, M.A.; Hisaindee, S. An Overview on the Photocatalytic Degradation of Azo Dyes in the Presence of TiO2 Doped with Selective Transition Metals. Desalination 2011, 276, 13–27. [Google Scholar] [CrossRef]
- Honorio, L.M.C.; Trigueiro, P.A.; Viana, B.C.; Ribeiro, A.B.; Osajima, J.A. Nanostructured Materials for the Photocatalytic Degradation of Organic Pollutants in Water BT. In Nanostructured Materials for Treating Aquatic Pollution; Gonçalves, G.A.B., Marques, P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 65–90. ISBN 978-3-030-33745-2. [Google Scholar]
- Yang, Z.; Yan, J.; Jiabiao, L.; Hui, X.; She, X.; Li, H. G-C3N4/TiO2 Nanocomposites for Degradation of Ciprofloxacin under Visible Light Irradiation. ChemistrySelect 2016, 1, 5679–5685. [Google Scholar] [CrossRef]
- Parmar, N.; Srivastava, J.K. Degradation of Pharmaceutical Antibiotic (Ciprofloxacin) by Photocatalysis Process Using Sol-Gel Based Titanium Dioxide Nanoparticles. Int. J. Chem. React. Eng. 2021, 19, 929–938. [Google Scholar] [CrossRef]
- Park, Y.; Kim, S.; Kim, J.; Khan, S.; Han, C. UV/TiO2 Photocatalysis as an Efficient Livestock Wastewater Quaternary Treatment for Antibiotics Removal. Water 2022, 14, 958. [Google Scholar] [CrossRef]
- Bennemla, M.; Bouafia-Chergui, S.; Amrane, A.; Chabani, M. The Photocatalytic Degradation Kinetics of the Anti-Inflammatory Drug Ibuprofen in Aqueous Solution under UV/TiO2 System and Neural Networks Modeling. Int. J. Chem. React. Eng. 2022, 20, 1149–1161. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic Activity Improvement and Application of UV-TiO2 Photocatalysis in Textile Wastewater Treatment: A Review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Peiris, S.; de Silva, H.B.; Ranasinghe, K.N.; Bandara, S.V.; Perera, I.R. Recent Development and Future Prospects of TiO2 Photocatalysis. J. Chinese Chem. Soc. 2021, 68, 738–769. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Green, M.; Just, M.; Li, Y.Y.; Chen, X. Titanium Dioxide Nanomaterials for Photocatalysis. J. Phys. D Appl. Phys. 2017, 50, 193003. [Google Scholar] [CrossRef]
- Kutuzova, A.; Dontsova, T.; Kwapinski, W. Application of TiO2-based Photocatalysts to Antibiotics Degradation: Cases of Sulfamethoxazole, Trimethoprim and Ciprofloxacin. Catalysts 2021, 11, 728. [Google Scholar] [CrossRef]
- Magaña-López, R.; Zaragoza-Sánchez, P.I.; Jiménez-Cisneros, B.E.; Chávez-Mejía, A.C. The Use of TiO2 as a Disinfectant in Water Sanitation Applications. Water 2021, 13, 1641. [Google Scholar] [CrossRef]
- Denisov, N.; Yoo, J.E.; Schmuki, P. Effect of Different Hole Scavengers on the Photoelectrochemical Properties and Photocatalytic Hydrogen Evolution Performance of Pristine and Pt-Decorated TiO2 Nanotubes. Electrochim. Acta 2019, 319, 61–71. [Google Scholar] [CrossRef]
- Tumbelaka, R.M.; Istiqomah, N.I.; Kato, T.; Oshima, D.; Suharyadi, E. High Reusability of Green-Synthesized Fe3O4/TiO2 Photocatalyst Nanoparticles for Efficient Degradation of Methylene Blue Dye. Mater. Today Commun. 2022, 33, 104450. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Van Tran, T.T.; Tran, M.L.; Juang, R.S. Facile Synthesis of Reusable Ag/TiO2 Composites for Efficient Removal of Antibiotic Oxytetracycline under UV and Solar Light Irradiation. J. Taiwan Inst. Chem. Eng. 2023, 145, 104825. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Fu, C.C.; Juang, R.S. Degradation of Methylene Blue and Methyl Orange by Palladium-Doped TiO2 Photocatalysis for Water Reuse: Efficiency and Degradation Pathways. J. Clean. Prod. 2018, 202, 413–427. [Google Scholar] [CrossRef]


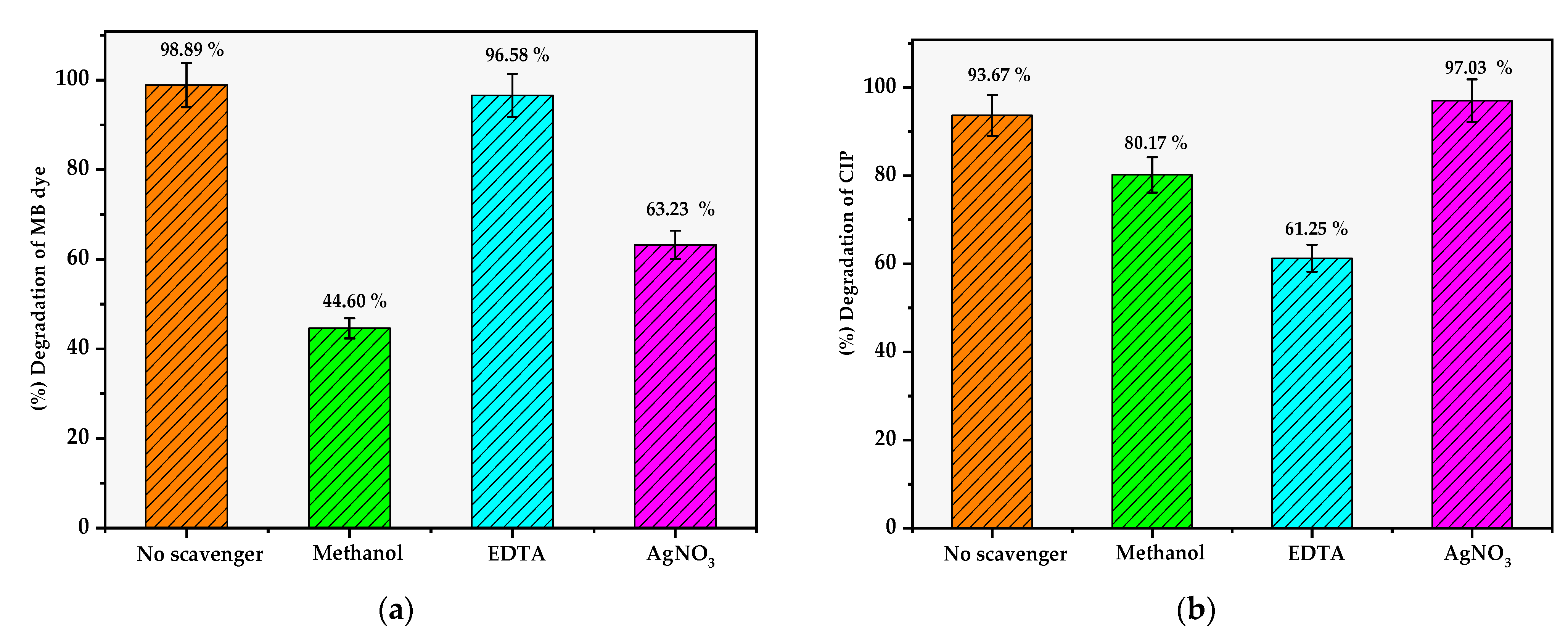
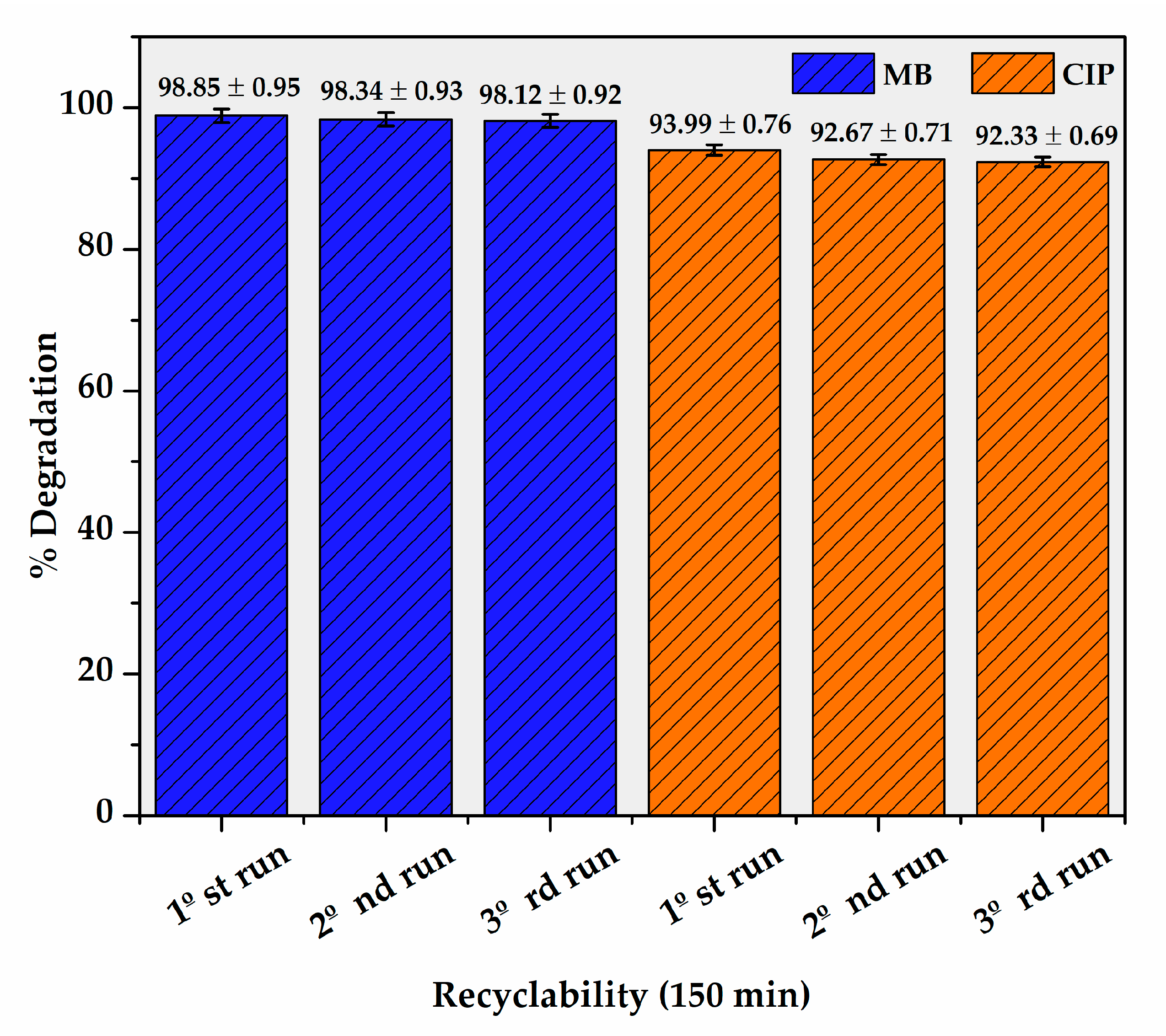
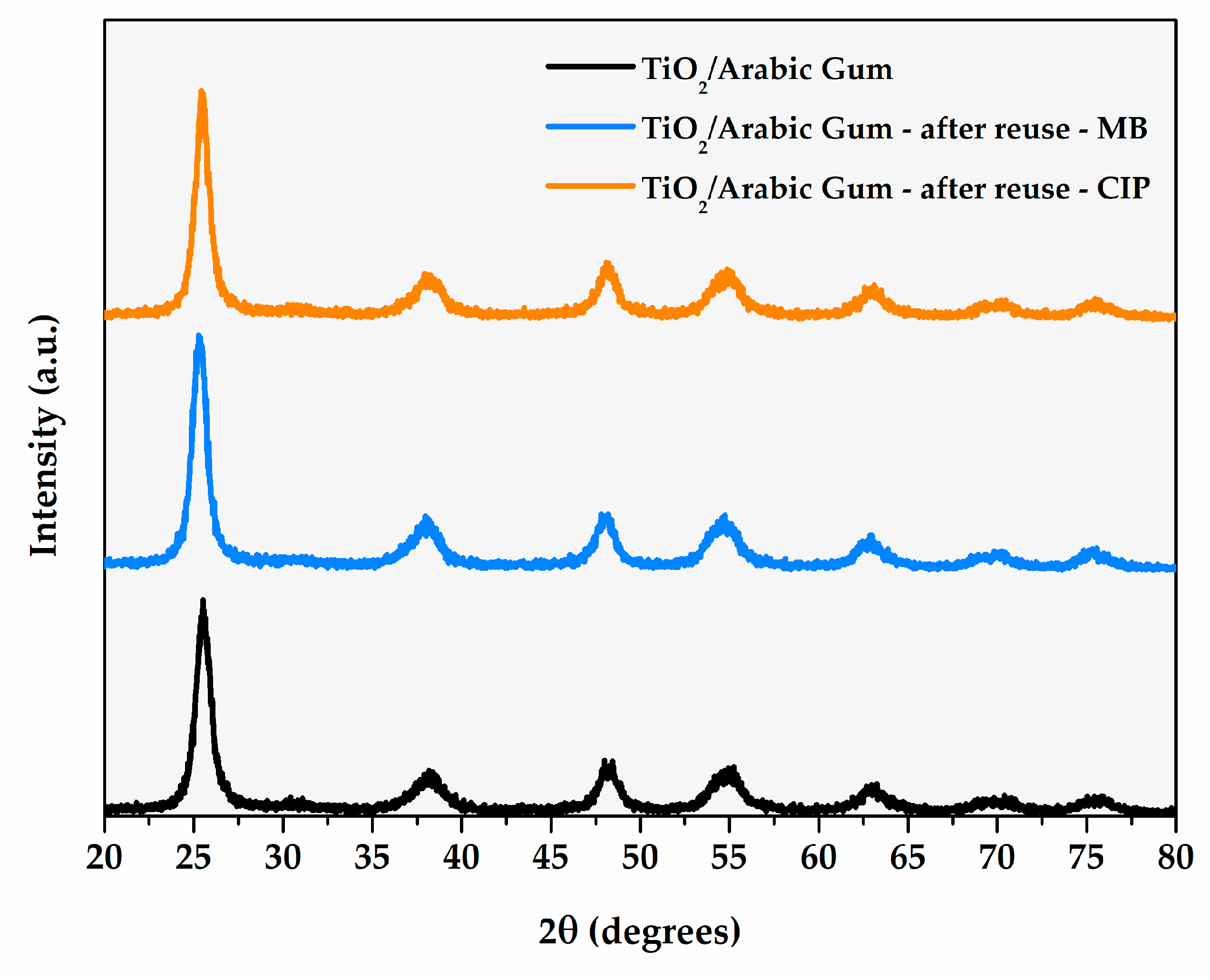
| Photocatalyst | % Degradation of Ciprofloxacin | Ref. |
|---|---|---|
| ZnO-clay minerals | ZnO-Hal = 83% ZnO-Pal = 85% ZnO-Pal/Hal = 91% | [26] |
| Au@ZnONPs-MoS2-rGO | 1%Au@ZnONPs-3%MoS 2-1%rGO = 96% 5%Au@ZnONPs-3%MoS 2-1%rGO = 99.8%. | [27] |
| rGO-BiVO4-ZnO | 98.4% | [28] |
| ZnO-doped g-C3N4 | 93.8% under pH 8.0 | [29] |
| Ag@ZnO-saponite | 90% | [30] |
| Sample Phases | N° JCPDS | N° CIF |
|---|---|---|
| TiO2–anatase | 00-021-1272 | 5000223 |
| TiO2–rutile | 00-021-1276 | 8104269 |
| TiO2–brookite | 00-016-0617 | 9015662 |
| Ti–titanium | 00-044-1294 | 9012924 |
| Phase | Phase Percentage (%wt) |
|---|---|
| TiO2–anatase | 89.6 |
| TiO2–rutile | 0 |
| TiO2–brookite | 10.4 |
| Ti–titanium | 0 |
| Parameter | Experimental | JCPDS | CIF |
|---|---|---|---|
| a | 3.7679 Å | 3.7852 Å | 3.7892 Å |
| c | 9.0537 Å | 9.5139 Å | 9.5370 Å |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, A.; Araújo, F.P.; Damasceno, D.; Honório, L.M.C.; Morais, A.I.S.; Almeida, L.C.; Garcia, R.P.; Silva-Filho, E.C.; Furtini, M.B.; Osajima, J.A. TiO2/Arabic Gum for Degradation of Pollutants in Water. Sustainability 2023, 15, 15768. https://doi.org/10.3390/su152215768
Lopes A, Araújo FP, Damasceno D, Honório LMC, Morais AIS, Almeida LC, Garcia RP, Silva-Filho EC, Furtini MB, Osajima JA. TiO2/Arabic Gum for Degradation of Pollutants in Water. Sustainability. 2023; 15(22):15768. https://doi.org/10.3390/su152215768
Chicago/Turabian StyleLopes, Anderson, Francisca P. Araújo, Dihego Damasceno, Luzia M. C. Honório, Alan I. S. Morais, Luciano C. Almeida, Ramón Peña Garcia, Edson C. Silva-Filho, Marcelo B. Furtini, and Josy A. Osajima. 2023. "TiO2/Arabic Gum for Degradation of Pollutants in Water" Sustainability 15, no. 22: 15768. https://doi.org/10.3390/su152215768
APA StyleLopes, A., Araújo, F. P., Damasceno, D., Honório, L. M. C., Morais, A. I. S., Almeida, L. C., Garcia, R. P., Silva-Filho, E. C., Furtini, M. B., & Osajima, J. A. (2023). TiO2/Arabic Gum for Degradation of Pollutants in Water. Sustainability, 15(22), 15768. https://doi.org/10.3390/su152215768













