Carbon Stock and CO2 Fluxes in Various Land Covers in Karang Gading and Langkat Timur Laut Wildlife Reserve, North Sumatra, Indonesia
Abstract
:1. Introduction
2. Materials and Methods
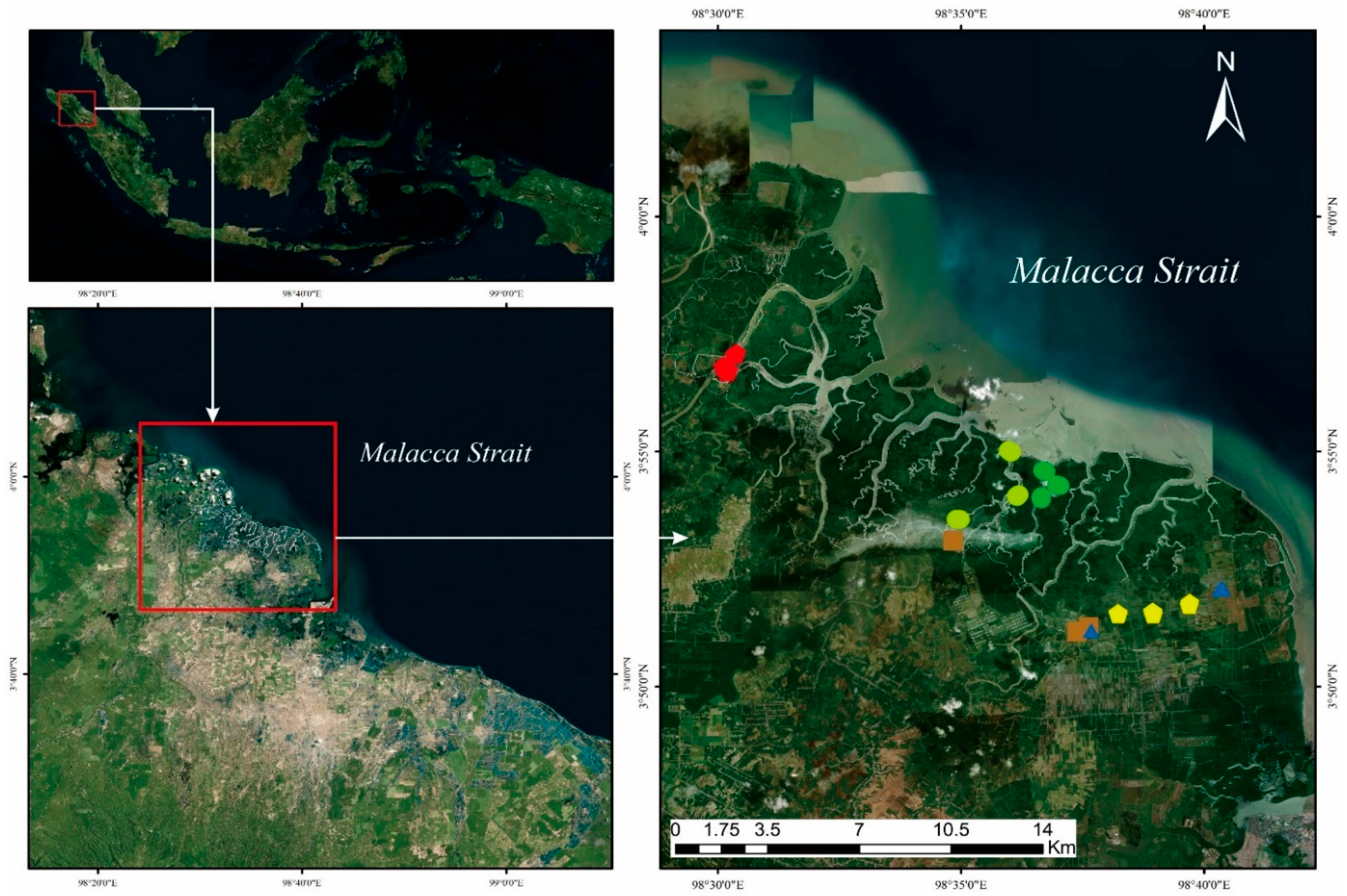
2.1. Materials
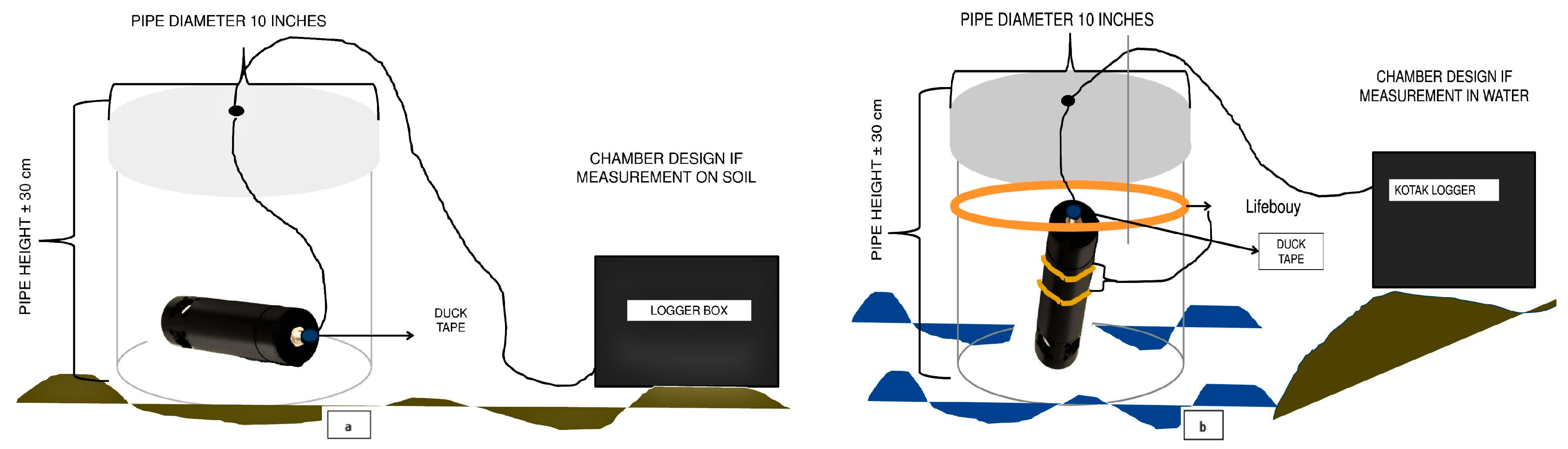
2.2. Soil Sampling and Biomass Assessment
| Above-Ground Biomass (kg) | Below-Ground Biomass (kg) |
|---|---|
| A. marina | A. marina |
| Wtop = 0.308DBH2.11, r2 = 0.97, n = 22, | WR = 1.28DBH 1.17, r2 = 0.80, n = 14, |
| Dmax = 35 cm [21] | Dmax = 35 cm, [21] |
| Rhizophora spp. Wtop = 0.128DBH2.60, r2 = 0.92, n = 9, Dmax = 32 cm [22] Wtop = 0.105DBH2.68, r2 = 0.99, n = 23, Dmax = 25 cm [23] Rhizophora apiculata Wtop = 0.235DBH2.42, r2 = 0.98, n = 57, Dmax = 28 cm [22] Bruguiera gymnorrhiza Wtop = 0.186DBH2.31, r2 = 0.99, n = 17, Dmax = 25 cm [23] Bruguiera gymnorrhiza Wtop = 0.186DBH2.31, r2 = 0.99, n = 17, Dmax = 25 cm [23] Bruguiera parviflora Wtop = 0.168DBH2.42, r2 = 0.99, Dmax = 25 cm, n = 16 [23] Xylocarpus grnatum Wtop = 0.0823DBH2.59, r2 = 0.99, n = 15, Dmax = 25 cm [23] Common equation: Wtop = 0.251 pD2.46, r2 = 0.98, n = 104, Dmax = 49 cm [24] | Bruguiera spp. WR = 0.0188 (D2H)0.909, n = 11, Dmax = 33 cm cf, H = D/(0.025D + 0.583) [25] R. apiculata WR = 0,00698DBH2.61, r2 = 0.99, n = 11, Dmax = 28 cm [22] R. stylosa WR = 0.261DBH1.86, r2 = 0.92, n = 5, Dmax = 15 cm [25] Rhizophora spp. WR = 0.00974 (D2H)1.05, r2: undefined, n = 16, D max = 40 cm [22] Xylocarpus granatum WR = 0.145DBH2.55, r2 = 0.99, n = 6, Dmax = 8 cm [26] Bruguiera exaristata WR = 0.302DBH2.15, r2 = 0.88, n = 9, Dmax = 10 cm [21] Common equation: WR = 0.199 p 0.899 D2.22, r2 = 0.95, n =26, Dmax = 45 cm [24] |
2.3. CO2 Flux Calculation
2.4. Data Analysis
2.5. Analysis of CO2 Flux in Different Land Cover Types
2.5.1. Analysis of CO2 Flux in Rainy and Dry Seasons
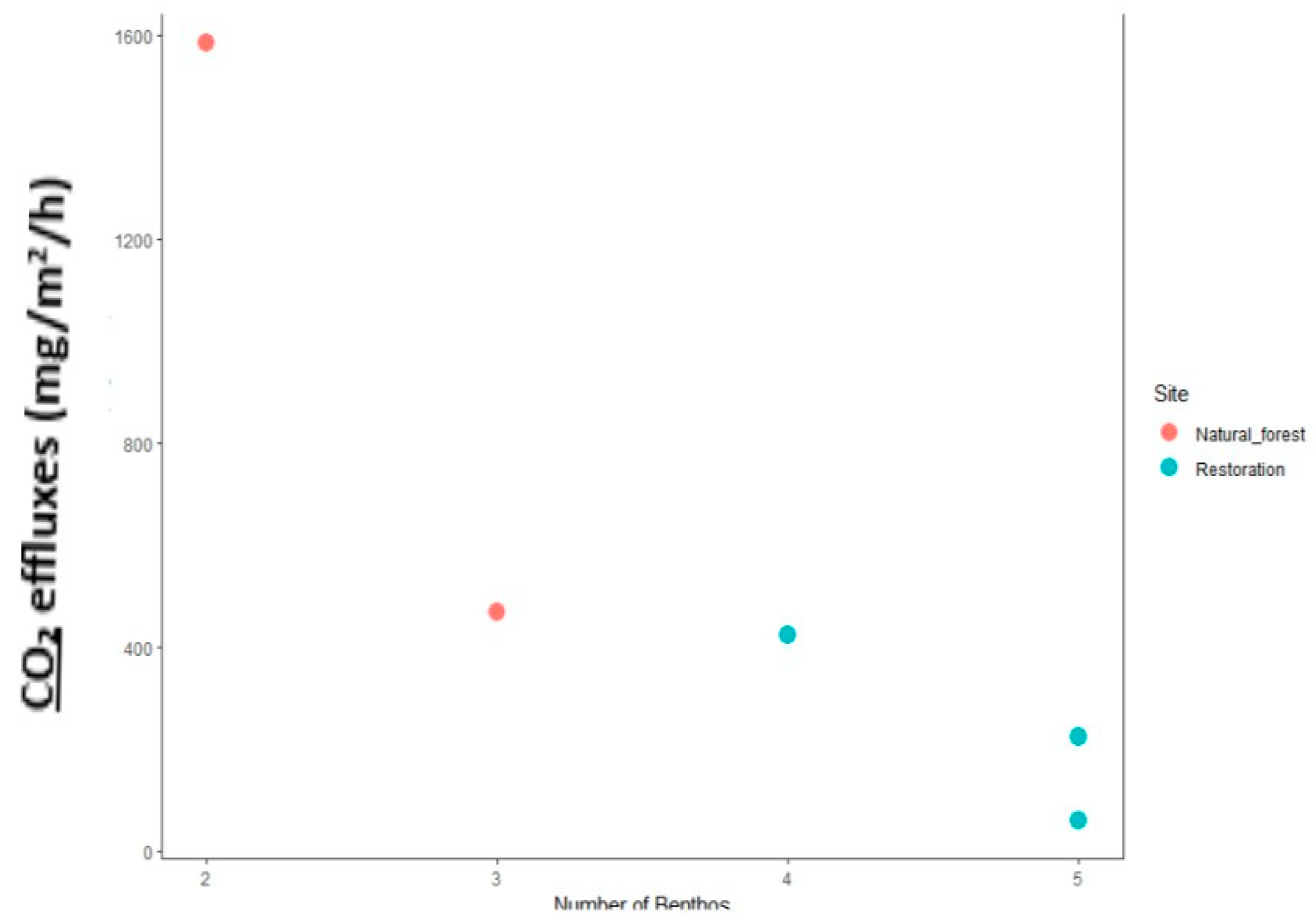
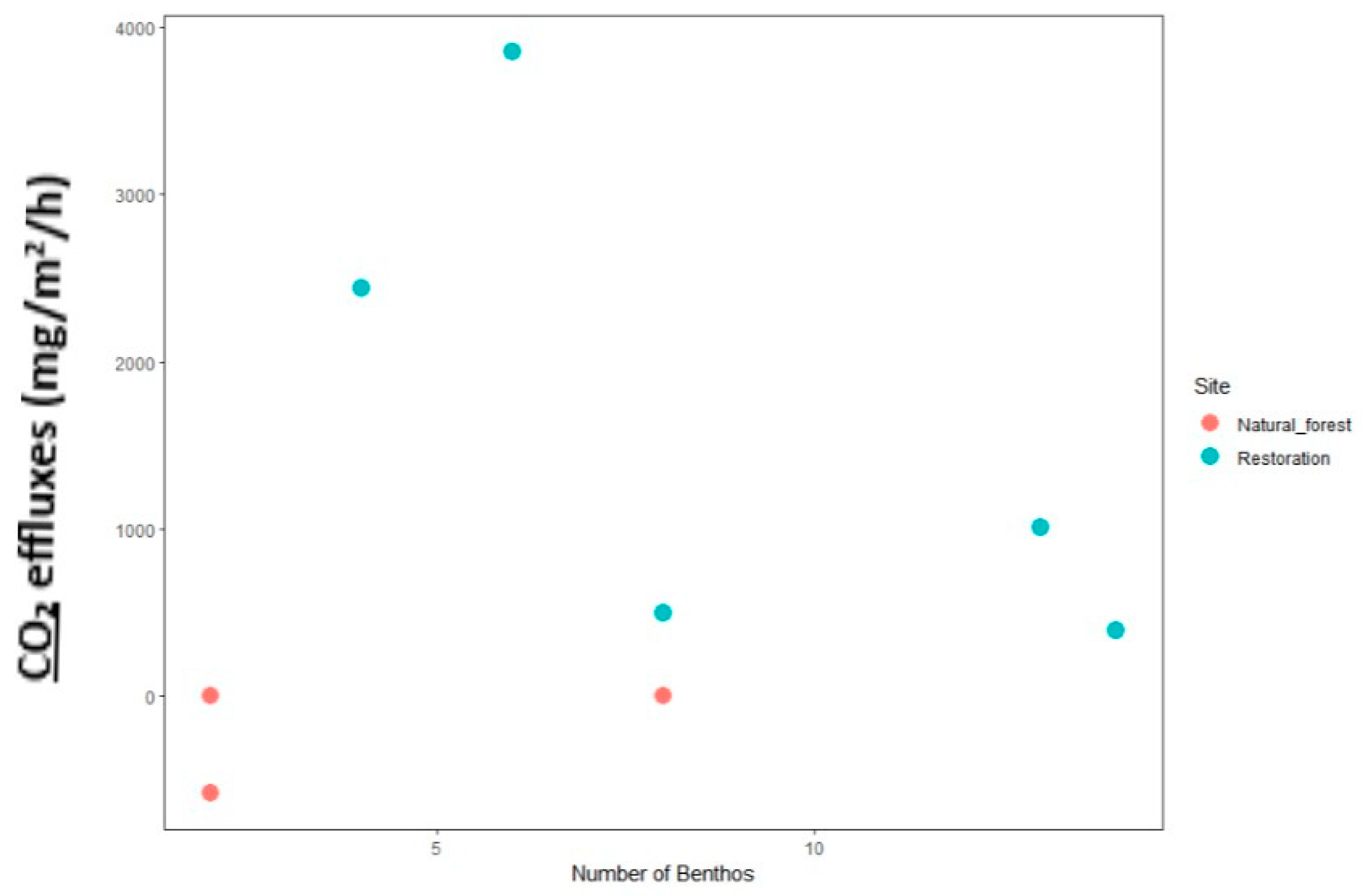
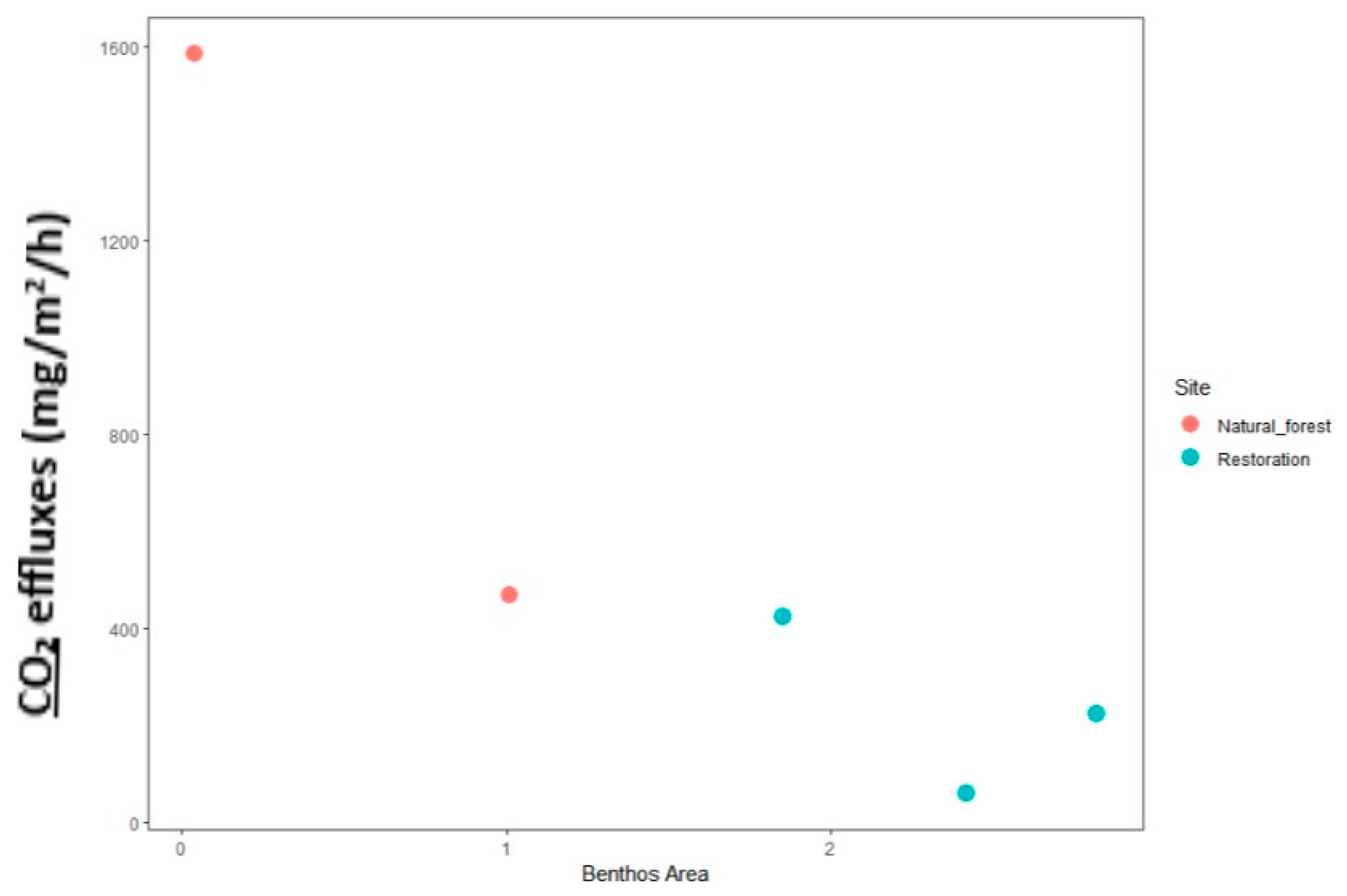
2.5.2. Analysis of the Relationship of Macrozoobenthos with CO2 Flux
3. Results
3.1. Standing Structure
3.2. Standing Structure, Average Diameter, and Basal Area
3.3. Vegetation and Soil Carbon Stocks in Mangrove Forests and Various Land Covers
3.4. Content of CO2 Flux Values in Various Land Covers
3.5. Analysis of the Effect of CO2 Flux on Various Land Covers
3.6. Correlation Analysis of Macrozoobenthos with CO2 Flux
3.6.1. Number of Macrozoobenthos Holes and Their Impact on CO2 Flux Rate
3.6.2. Macrozoobenthos and Their Impact on CO2 Flux Levels in a Given Area
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sidik, F.; Supriyanto, B.; Krisnawati, H.; Muttaqin, M.Z. Mangrove conservation for climate change mitigation in Indonesia. WIREs Clim. Chang. 2018, 9, e529. [Google Scholar] [CrossRef]
- Murdiyarso, D.; Purbopuspito, J.; Kauffman, J.B.; Warren, M.W.; Sasmito, S.D.; Donato, D.C.; Manuri, S.; Krisnawati, H.; Taberima, S.; Kurnianto, S. The potential of Indonesian mangrove forests for global climate change mitigation. Nat. Clim. Chang. 2015, 5, 1089–1092. [Google Scholar] [CrossRef]
- BBKSDA Sumatera Utara: Kawasa Suaka Margasatwa Karang Gading Langkat Timut Laut Sumatera Utara. Available online: https://bbksdasumaterautara.id/sm-karang-gadinglangkat-timur-laut/ (accessed on 12 August 2023).
- Basyuni, M.; Putri, L.A.P.; Murni, M.B. Implication of Land-Use and Land-Cover Change into Carbon Dioxide Emissions in Karang Gading and Langkat Timur Wildlife Reserve, North Sumatra, Indonesia. J. Manaj. Hutan Trop. 2015, 21, 25–35. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Barr, J.G.; Engel, V.; Smith, T.J.; Fuentes, J.D. Hurricane disturbance and recovery of energy balance, CO2 fluxes and canopy structure in a mangrove forest of the Florida Everglades. Agric. For. Meteorol. 2012, 153, 54–66. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon sequestration in mangrove forests. Carbon Manag. 2012, 3, 313–322. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Donato, D.C. Protocols for the Measurement, Monitoring and Reporting of Structure, Biomass and Carbon Stocks in Mangrove Forests; CFIOR: Bogor, Indonesia, 2012. [Google Scholar] [CrossRef]
- Mahesh, K.S.; Astley, H.; Smith, P.; Ghoshal, N. Soil CO2-C Flux and Carbon stock in the Dry Tropics: Impact of Land-Use Change Involving Bioenergy Crop Plantation. Biomass Bioenergy 2015, 83, 123–130. [Google Scholar]
- Cherubini, F.; Peters, G.P.; Berntsen, T.; Strømman, A.H.; Hertwich, E. CO2 emissions from biomass combustion for bioenergy: Atmospheric decay and contribution to global warming. GCB Bioenergy 2011, 3, 413–426. [Google Scholar] [CrossRef]
- Chau, T.T.T.; Gehlen, M.; Chevallier, F. A seamless ensemble-based reconstruction of surface ocean pCO2 and air–sea CO2 fluxes over the global coastal and open oceans. Biogeosciences 2022, 19, 1087–1109. [Google Scholar] [CrossRef]
- Huang, W.J.; Cai, W.J.; Wang, Y.; Lohrenz, S.E.; Murrell, M.C. The carbon dioxide system on the Mississippi River-dominated continental shelf in the northern Gulf of Mexico: 1. Distribution and air-sea CO2 flux. J. Geophys. Res. Ocean. 2015, 120, 1429–1445. [Google Scholar] [CrossRef]
- Harper, C.W.; Blair, J.M.; Fay, P.A.; Knapp, A.K.; Carlisle, J.D. Increased rainfall variability and reduced rainfall amount decreases soil CO2 flux in a grassland ecosystem. Glob. Chang. Biol. 2005, 11, 322–334. [Google Scholar] [CrossRef]
- William, F.L.; Thomas, W.; Julia, P.; Robbie, A.; Monica, C.; Jos, G.J.O.; Dominik, W.; Giulio, M.; Alaa, A.K.; Jo, H.; et al. A Review of Trends and Drivers of Greenhouse Gas Emissions by Sector from 1990 to 2018. Environ. Res. Lett. 2021, 16, 073005. [Google Scholar]
- Alongi, D.M.; Murdiyarso, D.; Fourqurean, J.W.; Kauffman, J.B.; Hutahaean, A.; Crooks, S.; Lovelock, C.E.; Howard, J.; Herr, D.; Fortes, M. Indonesia’s blue carbon: A globally significant and vulnerable sink for seagrass and mangrove carbon. Wetl. Ecol. Manag. 2016, 24, 3–13. [Google Scholar] [CrossRef]
- Slamet, N.S.; Dargusch, P.; Aziz, A.A.; Wadley, D. Mangrove vulnerability and potential carbon stock loss from land reclamation in Jakarta Bay, Indonesia. Ocean Coast. Manag. 2020, 195, 105283. [Google Scholar] [CrossRef]
- Borges, A.V.; Djenidi, S.; Lacroix, G.; Théate, J.; Delille, B.; Frankignoulle, M. Atmospheric CO2 flux from mangrove surrounding waters. Geophys. Res. Lett. 2003, 30, 1558. [Google Scholar] [CrossRef]
- Komiyama, A.; Ong, J.E.; Poungparn, S. Allometry, biomass, and productivity of mangrove forests: A review. Aquat. Bot. 2008, 89, 128–137. [Google Scholar] [CrossRef]
- Dewi, S. Carbon Footprintnof Indonesian Palm Oil Production: A Pilot Study; World Agroforestry Centre (ICRAF), SEA Regional Office: Malang, Indonesia, 2009. [Google Scholar]
- Ketterings, Q.M.; Coe, R.; van Noordwijk, M.; Palm, C.A. Reducing uncertainty in the use of allometric biomass equations for predicting above-ground tree biomass in mixed secondary forests. For. Ecol. Manag. 2001, 146, 199–209. [Google Scholar] [CrossRef]
- Comley, B.W.T.; McGuinness, K.A. Above- and below-ground biomass, and allometry of four common northern Australian mangroves. Aust. J. Bot. 2005, 53, 431–436. [Google Scholar] [CrossRef]
- Ong, J.E.; Gong, W.K.; Wong, C.H. Allometry and partitioning of the mangrove, Rhizophora apiculata. For. Ecol. Manag. 2004, 188, 395–408. [Google Scholar] [CrossRef]
- Clough, B.F.; Scott, K. Allometric relationships for estimating above-ground biomass in six mangrove species. For. Ecol. Manag. 1989, 27, 117–127. [Google Scholar] [CrossRef]
- Komiyama, A.; Poungparn, S.; Kato, S. Common allometric equations for estimating the tree weight of mangroves. J. Trop. Ecol. 2005, 21, 471–477. [Google Scholar] [CrossRef]
- Tamai, S.; Nakasuga, T.; Tabuchi, R.; Ogino, K. Standing biomass of mangrove forests in southern Thailand. J. Jpn. For. Soc. 1986, 68, 384–388. [Google Scholar]
- Poungparn, S.; Komiyama, A.; Intana, V.; Piriyaota, S.; Sangtiean, T.; Tanapermpool, P.; Patanaponpaiboon, P.; Kato, S. A Quantitative Analysis on the Root System of a Mangrove, Xylocarpus granatum Koenig. Tropics 2002, 12, 35–42. [Google Scholar] [CrossRef]
- Rochette, P.; Ellert, B.; Gregorich, E.G.; Desjardins, R.L.; Pattey, E.; Lessard, R.; Johnson, B.G. Description of a dynamic closed chamber for measuring soil respiration and its comparison with other techniques. Can. J. Soil Sci. 1997, 77, 195–203. [Google Scholar] [CrossRef]
- Sasmito, S.; Arriyadi, D.; Bimantara, Y.; Amelia, R.; Saragi-Sasmito, M.; Darusman, T.; Basyuni, M.; Maher, D.; Hutley, L.; Murdiyarso, D. CO2 and CH4 Effluxes Across Six Land Uses in Coastal Wetlands of North Sumatra. In EGU General Assembly Conference Abstracts; European Geosciences Union: Munich, Germany, 2022; p. EGU22-13486. [Google Scholar]
- Kaye, J.P.; McCulley, R.L.; Burke, I.C. Carbon fluxes, nitrogen cycling, and soil microbial communities in adjacent urban, native and agricultural ecosystems. Glob. Chang. Biol. 2005, 11, 575–587. [Google Scholar] [CrossRef]
- Milodowski, D.T.; Coomes, D.A.; Swinfield, T.; Jucker, T.; Riutta, T.; Malhi, Y.; Svátek, M.; Kvasnica, J.; Burslem, D.F.; Ewers, R.M.; et al. The impact of log-ging on vertical canopy structure across a gradient of tropical forest degradation intensity in Borneo. J. Appl. Ecol. 2021, 58, 1764–1775. [Google Scholar] [CrossRef]
- Bai, J.; Meng, Y.; Gou, R.; Lyu, J.; Dai, Z.; Diao, X.; Zhang, H.; Luo, Y.; Zhu, X.; Lin, G. Mangrove diversity enhances plant biomass produc-tion and carbon stock in Hainan Island, China. Funct. Ecol. 2021, 35, 774–786. [Google Scholar] [CrossRef]
- Xiong, Y.; Cakir, R.; Phan, S.M.; Ola, A.; Krauss, K.W.; Lovelock, C.E. Global patterns of tree stem growth and stand aboveground wood production in mangrove forests. For. Ecol. Manag. 2019, 444, 382–392. [Google Scholar] [CrossRef]
- Rao, K.; Ramanathan, A.; Raju, N.J. Assessment of Blue Carbon Stock of Coringa Mangroves: Climate Change Perspective. J. Clim. Chang. 2022, 8, 41–58. [Google Scholar] [CrossRef]
- Chen, G.; Shen, H.; Cao, J.; Zhang, W. The Influence of Tree Species on Carbon stock in Northern China. For. Chron. 2016, 92, 3. [Google Scholar] [CrossRef]
- Krauss, K.W.; McKee, K.L.; Lovelock, C.E.; Cahoon, D.R.; Saintilan, N.; Reef, R.; Chen, L. How mangrove forests adjust to rising sea level. New Phytol. 2014, 202, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, M.U. The temperature dependence of soil organic matter decomposition, and the effect of global warming on soil organic C storage. Soil Biol. Biochem. 1995, 27, 753–760. [Google Scholar] [CrossRef]
- Maza, M.; Lara, J.L.; Losada, I.J. Predicting the evolution of coastal protection service with mangrove forest age. Coast. Eng. 2021, 168, 103922. [Google Scholar] [CrossRef]
- Yitaferu, B.; Abewa, A.; Amare, T. Expansion of Eucalyptus Woodlots in the Fertile Soils of the Highlands of Ethiopia: Could It Be a Treat on Future Cropland Use? J. Agric. Sci. 2013, 5, 97. [Google Scholar] [CrossRef]
- Alongi, D.M.; Mukhopadhyay, S.K. Contribution of mangroves to coastal carbon cycling in low latitude seas. Agric. For. Meteorol. 2015, 213, 266–272. [Google Scholar] [CrossRef]
- Widyati, E.; Nuroniah, H.S.; Tata, H.L.; Mindawati, N.; Lisnawati, Y.; Darwo; Abdulah, L.; Lelana, N.E.; Mawazin; Octavia, D.; et al. Soil Degradation Due to Conversion from Natural to Plantation Forests in Indonesia. Forests 2022, 13, 1913. [Google Scholar] [CrossRef]
- Liddicoat, C.; Schapel, A.; Davenport, D.; Dwyer, E. Soil Carbon and Climate Change; Sustainable Systems Group, Agriculture, Food and Wine, Primary Industries and Resources: Adelaide, Australia, 2010. [Google Scholar]
- Rahajoe, J.S.; Alhamd, L.; Sundari, S.; Handayani, D. Stok Karbon dan Biomasa Beberapa Komoditas Tanaman Pertanian Di Bodogol-Taman Nasional Gunung Gede Pangrango—Jawa Barat. J. Biol. Indones. 2016, 12, 203–210. [Google Scholar]
- Roose, E.J.; Lal, R.; Feller, C.; Barthes, B.; Stewart, B.A. Advances in Soil Science: Soil Erosion and Carbon Dynamics; CRC Press, Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2005; p. 352. [Google Scholar]
- Stiglitz, R.; Mikhailova, E.; Post, C.; Schlautman, M.; Sharp, J. Using an inexpensive color sensor for rapid assess-ment of soil organic carbon. Geoderma 2017, 286, 98–103. [Google Scholar] [CrossRef]
- Tono, S.; Wawan; Amri, A.I. CO2 Flux in Various Conditions of Peat Swamp Forest in The Concession Area of PT. Diamond Raya Timber District of Rokan Hilir, Bangko, Universitas Riau. J. Online Mhs. Fak. Pertan. Univ. Riau 2014, 1, 1–10. [Google Scholar]
- Chen, G.C.; Tam, N.F.; Ye, Y. Spatial and seasonal variations of atmospheric N2O and CO2 fluxes from a subtropical mangrove swamp and their relationships with soil characteristics. Soil Biol. Biochem. 2012, 48, 175–181. [Google Scholar] [CrossRef]
- Min, S.; Rulík, M. Comparison of Carbon Dioxide (CO2) Fluxes Between Conventional and Conserved Irrigated Rice Paddy Fields in Myanmar. Sustainability 2020, 12, 5798. [Google Scholar] [CrossRef]
- Jennerjahn, T.C. Relevance and magnitude of ‘Blue Carbon’ storage in mangrove sediments: Carbon accumulation rates vs. stocks, sources vs. sinks. Estuar. Coast. Shelf Sci. 2020, 247, 107027. [Google Scholar] [CrossRef]
- Wang, G.; Guan, D.; Peart, M.; Chen, Y.; Peng, Y. Ecosystem carbon stocks of mangrove forest in Yingluo Bay, Guangdong Province of South China. For. Ecol. Manag. 2013, 310, 539–546. [Google Scholar] [CrossRef]
- Nam, V.N.; Sasmito, S.D.; Murdiyarso, D.; Purbopuspito, J.; MacKenzie, R.A. Carbon stocks in artificially and naturally regenerated mangrove ecosystems in the Mekong Delta. Wetl. Ecol. Manag. 2016, 24, 231–244. [Google Scholar] [CrossRef]
- Amelia, R.; Basyuni, M.; Alfinsyahri, A.; Sulistiyono, N.; Slamet, B.; Bimantara, Y.; Harahap, S.S.H.; Harahap, M.; Harahap, I.M.; Al Mustaniroh, S.S.; et al. Evaluation of Plant Growth and Potential of Carbon Storage in the Restored Mangrove of an Abandoned Pond in Lubuk Kertang, North Sumatra, Indonesia. Forests 2023, 14, 158. [Google Scholar] [CrossRef]
- Bournazel, J.; Kumara, M.P.; Jayatissa, L.P.; Viergever, K.; Morel, V.; Huxham, M. The impacts of shrimp farming on land-use and carbon storage around Puttalam lagoon, Sri Lanka. Ocean Coast. Manag. 2015, 113, 18–28. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta analysis. Glob. Chang. Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Updated Nationality Determined Contribution Republic of Indonesia. Indonesia Submission of Updated Nationality Deter-mined Contribution (NDC) and of Long-term Strategy on Low Carbon and Climate Resilience 2050; Minister for the Environment and Forestry: Jakarta, Indonesia, 2021. Available online: https://unfccc.int/sites/default/files/NDC/2022-06/Updated%20NDC%20Indonesia%202021%20-%20corrected%20version.pdf (accessed on 17 October 2022).
- Bouillon, S.; Borges, A.V.; Castaneda, E.; Diele, K.; Dittmar, T.; Duke, N.C.; Kristensen, E.; Lee, S.Y.; Marchand, C.; Middelburg, J.; et al. Mangrove production and carbon sinks: A revision of global budget estimates. Glob. Biogeochem. Cycles 2008, 22, GB2013. [Google Scholar] [CrossRef]
- Chen, G.; Tam, N.; Ye, Y. Summer fluxes of atmospheric greenhouse gases N2O, CH4 and CO2 from mangrove soil in South China. Sci. Total. Environ. 2010, 408, 2761–2767. [Google Scholar] [CrossRef] [PubMed]
- Tomotsune, M.; Yoshitake, S.; Iimura, Y.; Kida, M.; Fujitake, N.; Koizumi, H.; Ohtsuka, T. Effects of soil temperature and tidal condition on variation in carbon dioxide flux from soil sediment in a subtropical mangrove forest. J. Trop. Ecol. 2018, 34, 268–275. [Google Scholar] [CrossRef]
- Chanda, A.; Akhand, A.; Manna, S.; Dutta, S.; Hazra, S.; Das, I.; Dadhwal, V.K. Characterizing spatial and sea-sonal variability of carbon dioxide and water vapour fluxes above a tropical mixed mangrove forest canopy, India. J. Earth Syst. Sci. 2013, 122, 503–513. [Google Scholar] [CrossRef]
- Nozarpour, R.; Shojaei, M.G.; Naderloo, R.; Nasi, F. Crustaceans functional diversity in mangroves and adjacent mudflats of the Persian Gulf and Gulf of Oman. Mar. Environ. Res. 2023, 186, 105919. [Google Scholar] [CrossRef] [PubMed]
- Costa, Y.; Martins, I.; Carvalho, G.; Barros, F. Sea-level rise effects on macrozoobenthos distribution within an estuarine gradient using Species Distribution Modeling. Ecol. Inform. 2022, 71, 101816. [Google Scholar] [CrossRef]
- Pradisty, N.A.; Sidik, F.; Bimantara, Y.; Susetya, I.E.; Basyuni, M. Litterfall and Associated Macrozoobenthic of Restored Mangrove Forests in Abandoned Aquaculture Ponds. Sustainability 2022, 14, 8082. [Google Scholar] [CrossRef]


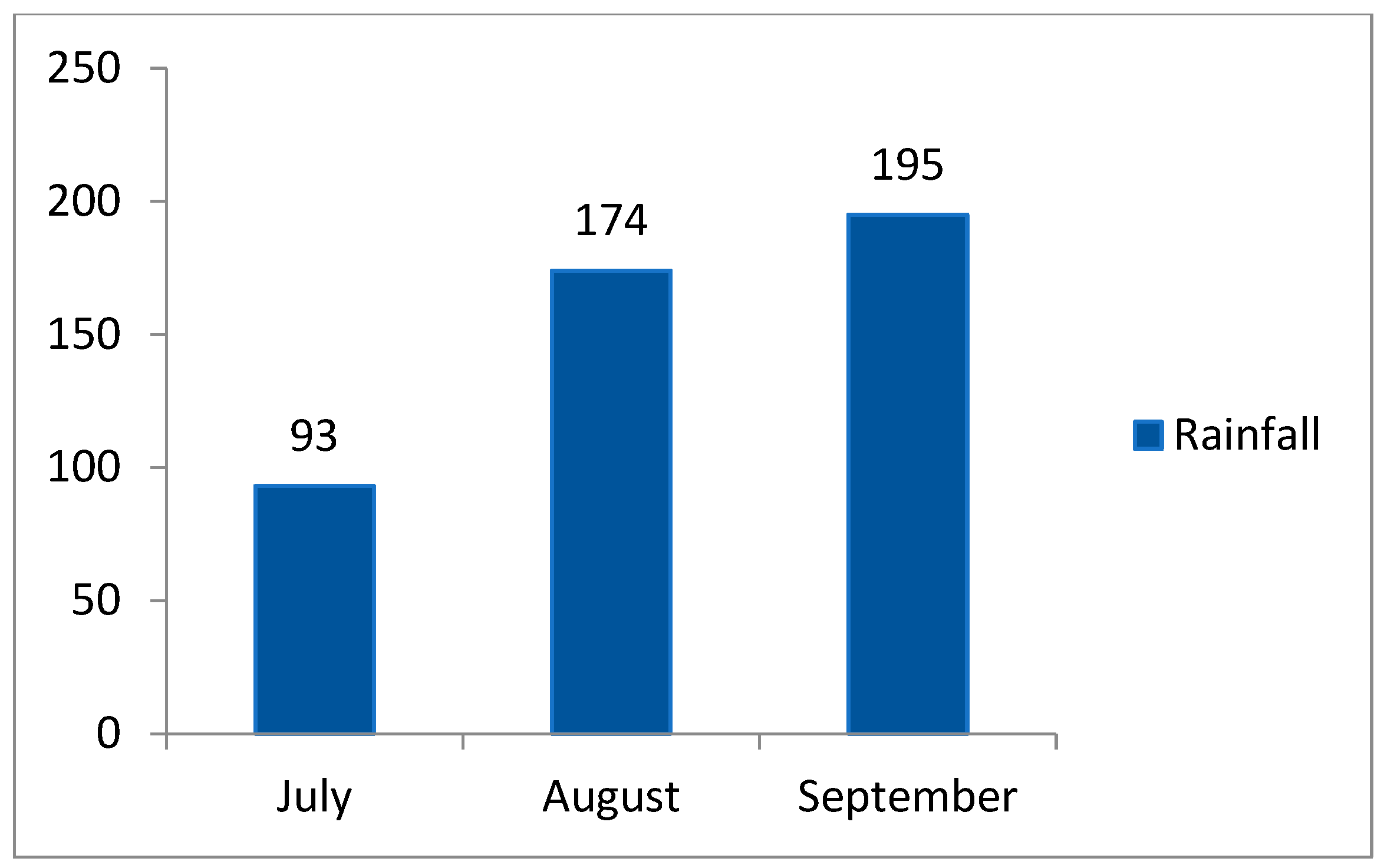

| Species | Natural Forest | Restoration | Oil Palm | Mixed Agriculture |
|---|---|---|---|---|
| Avicennia officinalis | 76 | 11 | Na | Na |
| Bruguiera gymnorrhiza | 101 | 137 | Na | Na |
| Bruguiera parviflora | 379 | 195 | Na | Na |
| Ceriops tagal | 11 | 33 | Na | Na |
| Excoecaria agallocha | 274 | 119 | Na | Na |
| Rhizophora apiculata | 755 | 1181 | Na | Na |
| Scyphiphora hydrophyllacea | 47 | Na | Na | Na |
| Xylocarpus granatum | 144 | 340 | Na | Na |
| Elaeis guineensis | Na | Na | 155 | Na |
| Persea americana | Na | Na | Na | 7 |
| Artocarpus communis | Na | Na | Na | 18 |
| Artocarpus heterophyllus | Na | Na | Na | 7 |
| Cocus nucifera | Na | Na | Na | 11 |
| Durio zibethinus | Na | Na | Na | 7 |
| Psidium guajava | Na | Na | Na | 11 |
| Citrus sinensis | Na | Na | Na | 15 |
| Calophyllum inophyllum | Na | Na | Na | 25 |
| Areca catechu L. | Na | Na | Na | 4 |
| Annona muricata | Na | Na | Na | 25 |
| Artocarpus altilis | Na | Na | Na | 4 |
| Total | 1787 | 1762 | 155 | 134 |
| No. | Land Cover Type | Diameter (cm) | Height (m) | Basal Area (m2) |
|---|---|---|---|---|
| 1 | Natural forest | 11.83 ± 5.07 | 11.97 ± 1.78 | 0.84 ± 0.76 |
| 2 | Restoration | 10.03 ± 4.30 | 9.83 ± 1.53 | 0.64 ± 0.94 |
| 3 | Oil palm | 0.80 ± 0.12 | 4.51 ± 0.93 | 0.00 ± 0.00 |
| 4 | Mixed agriculture | 9.43 ± 5.45 | 4.91 ± 1.99 | 0.66 ± 0.49 |
| No. | Land Covers | Above-Ground Carbon (MgC ha−1) | Below-Ground Carbon (MgC ha−1) | Woody Debris (MgC ha−1) | Soil Carbon (MgC ha−1) | Total Carbon (MgC ha−1) |
|---|---|---|---|---|---|---|
| 1 | Natural forest | 242.93 | 32.51 | 17.06 | 423.59 | 716.09 |
| 2 | Restoration | 145.99 | 17.69 | 13.93 | 356.53 | 534.14 |
| 3 | Oil palm | 7.29 | Na | 6.73 | 65.05 | 79.07 |
| 4 | Mixed agriculture | 6.95 | Na | 0.18 | 50.44 | 57.45 |
| 5 | Rice fields | Na | Na | Na | 215.79 | 215.79 |
| 6 | Pond | Na | Na | Na | 303.07 | 308.07 |
| Paired Differences | T | Df | Sig. (2-Tailed) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Std. Deviation | Std. Error Mean | 95% Confidence Interval of the Difference | ||||||
| Lower | Upper | ||||||||
| Pair 1 | Natural forest | −360.547 | 696.503 | 284.346 | −1091.482 | 370.88 | −1.268 | 5 | 0.261 |
| - | - | - | - | - | - | - | |||
| Pair 2 | Restoration | 261.410 | 2617.274 | 1068.498 | −2485.250 | 3008.071 | 0.245 | 5 | 0.816 |
| - | - | - | - | - | - | - | |||
| Pair 3 | Mixed agriculture | 264.099 | 1077.378 | 439.838 | −866.540 | 1394.738 | 0.600 | 5 | 0.574 |
| - | - | - | - | - | - | - | - | ||
| Pair 4 | Rice fields | −1391.762 | 1679.508 | 531.107 | −2593.210 | −190.315 | −2.620 | 9 | 0.028 |
| - | - | - | - | - | - | ||||
| Pair 5 | Pond | −65.137 | 998.099 | 315.627 | −779.134 | 648.860 | −0.206 | 9 | 0.841 |
| - | - | - | - | - | - | - | - | ||
| Pair 6 | Oil palm plantation | −1302.605 | 1417.934 | 578.869 | −2790.636 | 185.425 | −2.250 | 5 | 0.074 |
| - | - | - | - | - | - | ||||
| CO2 Flux | Number of Macrozoobenthos Holes | ||
|---|---|---|---|
| CO2 Flux | Pearson correlation | 1 | 0.069 |
| Sig. (2-tailed) | 0.815 | ||
| N | 14 | 14 | |
| Number of macrozoobenthos holes | Pearson correlation | 0.069 | 1 |
| Sig. (2-tailed) | 0.815 | ||
| N | 14 | 14 | |
| CO2 Flux | Hole Area | ||
|---|---|---|---|
| CO2 flux | Pearson Correlation | 1 | −0.185 |
| Sig. (2-tailed) | 0.527 | ||
| N | 14 | 14 | |
| Hole area | Pearson Correlation | −0.185 | 1 |
| Sig. (2-tailed) | 0527 | ||
| N | 14 | 14 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harahap, M.; Basyuni, M.; Sulistiyono, N.; Sasmito, S.D.; Latifah, S.; Delvian; Amelia, R.; Bimantara, Y.; Harahap, S.S.H.; Larekeng, S.H.; et al. Carbon Stock and CO2 Fluxes in Various Land Covers in Karang Gading and Langkat Timur Laut Wildlife Reserve, North Sumatra, Indonesia. Sustainability 2023, 15, 15196. https://doi.org/10.3390/su152115196
Harahap M, Basyuni M, Sulistiyono N, Sasmito SD, Latifah S, Delvian, Amelia R, Bimantara Y, Harahap SSH, Larekeng SH, et al. Carbon Stock and CO2 Fluxes in Various Land Covers in Karang Gading and Langkat Timur Laut Wildlife Reserve, North Sumatra, Indonesia. Sustainability. 2023; 15(21):15196. https://doi.org/10.3390/su152115196
Chicago/Turabian StyleHarahap, Mikrajni, Mohammad Basyuni, Nurdin Sulistiyono, Sigit D. Sasmito, Siti Latifah, Delvian, Rizka Amelia, Yuntha Bimantara, Salma Safrina Hashilah Harahap, Siti Halimah Larekeng, and et al. 2023. "Carbon Stock and CO2 Fluxes in Various Land Covers in Karang Gading and Langkat Timur Laut Wildlife Reserve, North Sumatra, Indonesia" Sustainability 15, no. 21: 15196. https://doi.org/10.3390/su152115196
APA StyleHarahap, M., Basyuni, M., Sulistiyono, N., Sasmito, S. D., Latifah, S., Delvian, Amelia, R., Bimantara, Y., Harahap, S. S. H., Larekeng, S. H., Sumarga, E., Al Mustaniroh, S. S., Slamet, B., Arifanti, V. B., & Ali, H. M. (2023). Carbon Stock and CO2 Fluxes in Various Land Covers in Karang Gading and Langkat Timur Laut Wildlife Reserve, North Sumatra, Indonesia. Sustainability, 15(21), 15196. https://doi.org/10.3390/su152115196









