Sustainability Assessment of Araucaria Forest Remnants in Southern Brazil: Insights from Traditional Forest Inventory Surveys
Abstract
1. Introduction
2. Materials and Methods
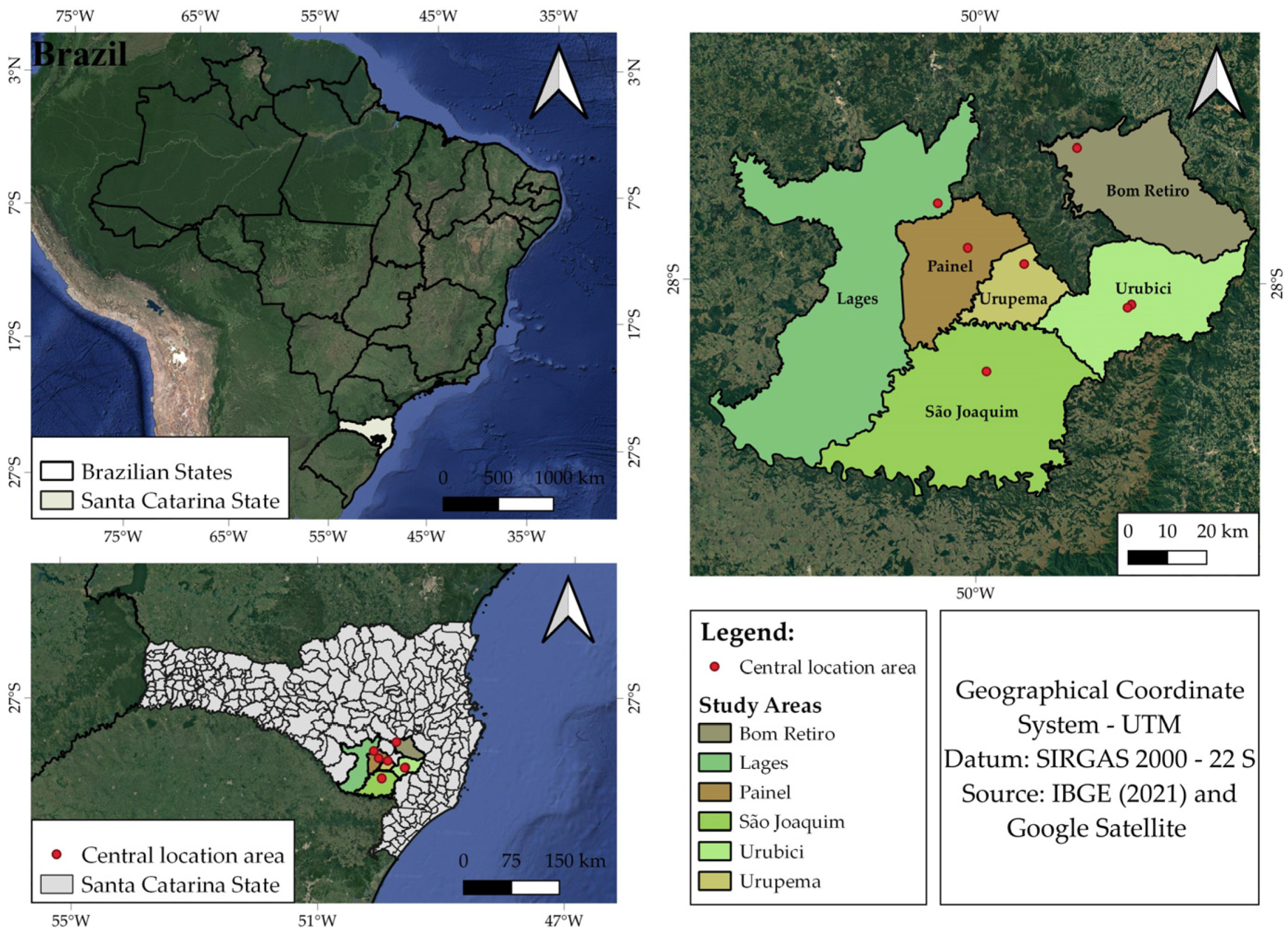
2.1. Description of the Tree Species and the Study Area
2.2. Data Mensuration
3. Results
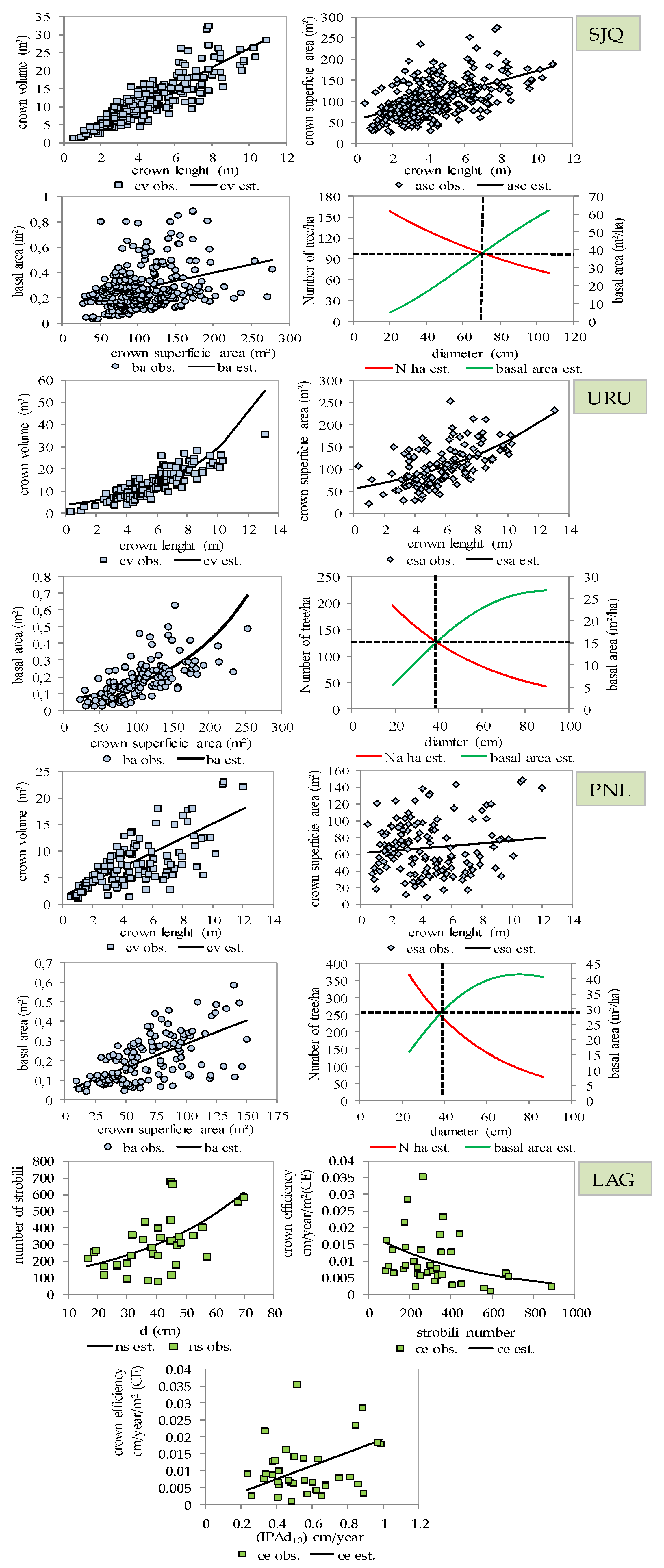
3.1. Characterization of the Forest Component
3.2. Structure, Growth, Form Dimension and Regeneration of the Araucaria Forest
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finger, C.A.G.; Costa, E.A.; Hess, A.F.; Liesenberg, V.; da Bispo, P.C. Simulating sustainable forest management practices using crown attributes: Insights for Araucaria angustifolia trees in southern Brazil. Forests 2023, 14, 1285. [Google Scholar] [CrossRef]
- Péllico, N.; Brena, D.A. Inventário Florestal; Federal University of Paraná Press: Curitiba, Brazil, 1997. [Google Scholar]
- Bergseng, E.; Ask, J.A.; Framstad, E.; Gobakken, T.; Solberg, B.; Hoen, H.F. Biodiversity protection and economics in long term boreal forest management—A detailed case for the valuation of protection measures. For. Pol. Econ. 2012, 15, 12–21. [Google Scholar] [CrossRef]
- Anonymous. Levende Skog-Standard for Baerekraftig Skogforvaltning i Norge (The Living Forest Standards for Sustainable Forest Management in Norway); Levende Skog Norge: Oslo, Norway, 1998; p. 11. [Google Scholar]
- PEFC. Council Information Register. Statistics Figures on PEFC Certification. Available online: https://pefc.org/ (accessed on 3 September 2004).
- Noss, R.F. Indicators for monitoring biodiversity: A hierarchical approach. Conserv. Biol. 1990, 4, 355–364. [Google Scholar] [CrossRef]
- Oliveira, E.K.B.; Rezende, A.V.; Murta Júnior, L.S.; Mazzei, L.; Castro, R.V.O.; d’Oliveira, M.V.N.; Azevedo, G.B. Recruitment models after reduced impact logging in the Amazon rainforest. For. Ecol. Manag. 2023, 549, 121471. [Google Scholar] [CrossRef]
- Pukkala, T.; Kangas, J.; Kniivilä, M.; Tiainen, A.-M. Integrating forest-level and compartment-level indices of species diversity with numerical forest planning. Silva Fenn. 1997, 31, 417–429. [Google Scholar] [CrossRef]
- Beckert, S.M.; Rosot, M.A.D.; Rosot, N.C. Crescimento e dinâmica de Araucaria angustifólia (Bert.) O. Ktze. Fragm. Floresta Ombrófila Mista. Sci. For. 2014, 42, 209–218. [Google Scholar]
- Hess, A.F.; Loiola, T.; Souza, I.A.; Nascimento, B. Morphometry of the crown of Araucaria angustifolia in natural sites in southern Brazil. Bosque 2016, 37, 603–611. [Google Scholar] [CrossRef][Green Version]
- Roman, M.; Bressan, D.A.; Durlo, M.A. Morphometric variables and interdimensional relations for Cordia trichotoma (Vell.) Arráb. ex Steud. Ciência Florest. 2009, 19, 473–480. [Google Scholar] [CrossRef]
- Durlo, M.A.; Denardi, L. Morphometry for Cabralea canjerana in native secondary forests in Rio Grande do Sul. Ciência Florest. 1998, 8, 55–66. [Google Scholar] [CrossRef]
- de Maria, T.R.B.C.; Bomm, B.F.H.; Nesi, J.; Ho, T.L.; Bobrowski, R. Canopy architecture and morphometry of tree species used in the urban forest. Floresta 2020, 50, 1892–1901. [Google Scholar]
- Da Silva Jardim, F.C. Natural regeneration in tropical forest. Rev. Ciências Agrárias Amaz. J. Agric. Environ. Sci. 2015, 58, 105–113. [Google Scholar] [CrossRef]
- Zhu, J.J.; Liu, Z.G. A review on disturbance ecology forest. Chin. J. Appl. Ecol. 2004, 15, 1703–1710. (In Chinese) [Google Scholar]
- Salles, J.C.; Schiavini, I. Estrutura e composição do estrato de regeneração em um fragmento florestal urbano: Implicações para a dinâmica e a conservação da comunidade arbórea. Acta Bot. Bras. 2007, 31, 223–233. [Google Scholar] [CrossRef][Green Version]
- Pukkala, T. Measuring the social performance of forest management. J. For. Res. 2021, 32, 1803–1818. [Google Scholar] [CrossRef]
- Andrae, F.H.; Schneider, P.R.; Durlo, M.A. Importância do manejo de florestas nativas para a renda da propriedade e abastecimento do mercado madeireiro. Ciência Florest. 2018, 28, 1293–1302. [Google Scholar] [CrossRef]
- Elliott, K.J.; Hewitt, D. Forest species diversity in upper elevation hardwood forest in the southern Appalachian moutains. Castanea 1997, 62, 32–42. [Google Scholar]
- Peltzer, D.A.; Bast, M.L.; Wilson, S.D.; Gerry, A.K. Plant diversity and tree responses following contrasting disturbances in boreal forest. For. Ecol. Manag. 2000, 127, 97–203. [Google Scholar] [CrossRef]
- Meng, S.M.; Lieffers, V.J.; Huang, S. Modeling crown volume of lodgepole pine based upon the uniform stress theory. For. Ecol. Manag. 2007, 25, 174–181. [Google Scholar] [CrossRef]
- Barbosa, L.O.; Finger, C.A.G.; Barbosa, L.O.; Finger, C.A.G.; Costa, E.A.; Campoe, O.C.; Schons, C.T. Using crown characterisation variables as indicator of the vigor, competition and growth of Brazilian pine. South. For. J. For. Sci. 2021, 83, 240–253. [Google Scholar] [CrossRef]
- Bezerra, T.G.; Ruschel, A.R.; Emmert, F.; Nascimento, R.G.M. Changes caused by forest logging in structure and floristic diversity of natural regeneration: Relationship between climate variables and forest dynamics in the easthern Amazon. For. Ecol. Manag. 2021, 482, 1–11. [Google Scholar] [CrossRef]
- Montigny, L. Establishments Report for STEMS 1, Snowden Demonstration Forest; Technical Report, 017; Silviculture Treatments for Ecosystem Management in the Sayward (STEMS): Vancouver Island, BC, Canada, 2004. [Google Scholar]
- Li, Y.; Kröber, W.; Bruelheide, H.; Härdtle, W.; von Oheimb, G. Crown and leaf traits as predictors of subtropical tree sapling growth rates. J. Plant Ecol. 2017, 10, 136–145. [Google Scholar] [CrossRef]
- Wang, B.; Bu, Y.; Tao, G.; Yan, C.; Zhou, X.; Li, W.; Zhao, P.; Yang, Y.; Gou, R. Quantifying the effect of crow vertical position on individual tree competition: Total overlap index and its application in sustainable forest management. Sustainability 2020, 12, 7498. [Google Scholar] [CrossRef]
- Waring, R.H.; Theis, W.G.; Muscato, D. Stem growth per unit of leaf area-a measure of tree vigor. For. Sci. 1980, 26, 112–117. [Google Scholar]
- Reid, D.E.B.; Lieffers, V.J.; Silins, U. Growth and crown efficiency of height repressed lodgepole pine; are suppressed trees more efficient? Trees 2004, 18, 390–398. [Google Scholar] [CrossRef]
- Gough, C.M.; Seiler, J.R.; Maier, C.A. Short-term effects of fertilization on lodlloly pine (Pinus taeda L.) physiology. Plant Cell Environ. 2004, 27, 876–886. [Google Scholar] [CrossRef]
- Vose, J.M. Patterns of leaf area distribution within crowns of nitrogen and phosphorus fertilized loblolly pine trees. For. Sci. 1988, 34, 564–573. [Google Scholar] [CrossRef]
- Gillespie, A.R.; Allen, H.L.; Vose, J.M. Amount and vertical distribution of foliage of young loblolly pine trees as affected by canopy position and silvicultural tratament. Can. J. For. Res. 1994, 24, 1337–1344. [Google Scholar] [CrossRef]
- Wendling, I.; Zanette, F. Araucária: Particularidades, Propagação e Manejo de Plantios; Embrapa: Brasília, Brasil, 2017. [Google Scholar]
- Zanon, M.L.B.; Finger, C.A.G.; Schneider, P.R. Proporção da dioicia e distribuição diamétrica de árvores masculinas e femininas de Araucaria angustifolia (Bertol.) Kuntze, em povoamentos implantados. Ciência Florest. 2009, 19, 425–431. [Google Scholar] [CrossRef][Green Version]
- Anselmini, J.I.; Zanette, F. Polinização controlada em Araucaria angustifolia. Cerne 2012, 18, 247–255. [Google Scholar] [CrossRef][Green Version]
- Mantovani, A.; Morellato, P.C.; Reis, M.S. Fenologia reprodutiva e produção de sementes em Araucaria angustifolia (Bert.) O. Kuntze. Rev. Bras. Botânica 2004, 27, 787–796. [Google Scholar] [CrossRef]
- Ferri, G.K. Araucaria Angustifolia: Milhões de anos de História. Ed. 86. Available online: https://www.academia.edu/34876510/Araucaria_angustifolia_milh%C3%B5es_de_anos_de_hist%C3%B3ria (accessed on 28 April 2023).
- Schorr, L.P.B. Dinâmica e Relações Alométricas para Espécies Arbóreas em Floresta Ombrófila Mista Sob Regime de Não Manejo No Sul Do Brasil. Master’s Thesis, Santa Catarina State University, Lages, Brazil, 2019. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparoveck, G. Köppen climate classification map for Brazil. Meteor. Zeitsc. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Burkhart, H.E.; Tomé, M. Modeling Forest Trees and Stands; Springer: Berlin, Germany, 2012. [Google Scholar]
- Turkman, M.A.A.; Silva, G.L. Modelos Lineares Generalizados—Da Teoria à Prática. Universidade de Lisboa, 2000. Available online: https://www.spestatistica.pt/publicacoes/publicacao/modelos-lineares-generalizados-da-teoria-pratica (accessed on 12 September 2023).
- SAS. The SAS System for Windows; SAS Institute: Cary, NC, USA, 2004. [Google Scholar]
- Demétrio, L.; Hess, A.F.; de Sousa, A.N.; Costa, E.A.; Liesenberg, V.; Freisleben, M.J.; Schimalski, M.B.; Finger, C.A.G.; dos Hofiço, N.S.A.; da Bispo, P.C. Can we predict male strobili production in Araucaria angustifolia trees with dendrometric and morphometric attributes? Forests 2022, 13, 2074. [Google Scholar] [CrossRef]
- Kaps, M.; Lamberson, W.R. Biostatitistics for Animal Science; CABI Publishing: London, UK, 2004. [Google Scholar]
- Hess, A.F.; Minatti, M.; Liesenberg, V.; de Mattos, P.P.; Braz, E.M.; Costa, E.A. Brazilian pine diameter at breast height and growth in mixed Ombrophilous forest in southern Brazil. Austral. J. Crop Sci. 2018, 12, 770–777. [Google Scholar] [CrossRef]
- Hess, A.F.; Ricken, P.; Ciarnoschi, L.D. Dendrochronology, increment and forest management in araucaria forest, Santa Catarina State. Ciência Florest. 2018, 28, 1568–1582. [Google Scholar] [CrossRef]
- Hess, A.F.; Schiitter, S.; dos Santos, D.V.; Costa, E.A.; Minatti, M.; Ricken, P.; Klein, D.R.; da Silveira, A.C.; Liesenberg, V.; de Sousa, A.N.; et al. Form of distribution of dendro/morphometric variables for Brazilian Pine in Southern Brazil. J. Agric. Sci. 2021, 13, 69–82. [Google Scholar] [CrossRef]
- Hess, A.F.; Loiola, T.; Souza, I.A.; Minatti, M.; Ricken, P.; Borsoi, G.A. Forest management for the conservation of Araucaria angustifolia in southern Brazil. Floresta 2018, 48, 373–382. [Google Scholar] [CrossRef]
- Hess, A.F.; Atanazio, K.A.; Borsoi, G.A.; Schorr, L.P.B.; Souza, I.A.; Costa, E.A.; Klein, D.R.; Krefta, S.M.; Stepka, T.F.; Abatti, R. Crown efficiency and pine cones production for Brazilian pine (Araucaria angustifolia (Bertol.) Kuntze) in south Brazil. J. Agric. Sci. 2019, 11, 247–259. [Google Scholar] [CrossRef]
- Atanazio, K.A.; Hess, A.F.; Krefta, S.M.; Schorr, L.P.B.; Sousa, I.A.; Domiciano, C.A.R.; Cuchi, T.; Moraes, G.C. Modelagem das relações morfométricas com a produção de pinhas de Araucaria angustifolia (Bertol.) Kuntze no sul do Brasil. Ciência Florest. 2022, 32, 1247–1267. [Google Scholar] [CrossRef]
- Costa, E.A.; Finger, C.A.G.; Schneider, P.R.; Hess, A.F.; Liesenberg, V.; Schons, C.T. Modeling competition indices for Araucaria angustifolia at two sites in southern Brazil. Bosque 2020, 41, 65–75. [Google Scholar]
- Pretzsch, H. Forest Dynamics, Growth and Yield; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Thomson, J.D.; Barret, S.C.H. Selection of autocrossing sexual selection and devolution of dioecy in plants. Amer. Nat. 1981, 18, 443–449. [Google Scholar] [CrossRef]
- Mendes, F.S.; Jardim, F.C.S.; Carvalho, J.O.P.; Lima, T.T.S.; Souza, D.V. Dinâmica da composição florística do sub-bosque em floresta tropical manejada, no município de Moju, estado do Pará, Brasil. Rev. Ciências Agrárias Amaz. J. Agric. Environ. Sci. 2012, 55, 117–123. [Google Scholar] [CrossRef]
- Krůček, M.; Trochta, J.; Cibulka, M.; Král, K. Beyond the cones: How crown shape plasticity alters aboveground competition for space and light—Evidence from terrestrial laser scanning. Agric. For. Meteor. 2019, 264, 188–189. [Google Scholar] [CrossRef]
- Binkley, D.; Campoe, O.C.; Gspalti, M.; Forrester, D.I. Light absorption and use efficiency in forests: Why patterns differ for trees and stands. For. Ecol. Manag. 2013, 310, 577–588. [Google Scholar] [CrossRef]
- Pretzsch, H. Canopy space filling and tree crown morphology in mixed-species stands compared with monocultures. For. Ecol. Manag. 2014, 327, 251–264. [Google Scholar] [CrossRef]
- Iida, Y.; Kohyama, T.S.; Kubo, T.; Kassim, A.R.; Poorter, L.; Sterck, F.; Potts, M.D. The architecture and life-history strategies across 200 co-occurring tropical tree species. Funct. Ecol. 2011, 25, 1260–1268. [Google Scholar] [CrossRef]
- Seidel, D.; Leuschner, C.; Muller, A.; Krause, B. Crown plasticity in mixed forests—Quantifying asymmetry as a measure of competition using terrestrial laser scanning. For. Ecol. Manag. 2011, 261, 2123–2132. [Google Scholar] [CrossRef]
- Rickli-Horst, H.C.; Bona, C.; Sant’Anna Santos, B.F.; Koehler, H.S.; Wendling, I.; Zuffellato-Ribas, K.C. Visual and anatomical analisys of welding quality x scion survival in Araucaria angustifolia. Acta Sci. 2021, 43, e45509. [Google Scholar]
- Binkley, D.; Stape, J.L.; Ryan, M.J.; Barnard, H.R.; Fownes, J. Age-related decline in forest ecosystem growth: An individual-tree, stand-structure hypothesis. Ecosystems 2002, 5, 58–67. [Google Scholar] [CrossRef]
- Luyssaert, S.; Schulze, E.D.; Borner, A.; Knohl, A.; Hessenmoller, D.; Law, B.E.; Ciais, P.; Grace, J. Old-growth forests as global carbon sinks. Nature 2008, 445, 213–215. [Google Scholar] [CrossRef]
- Meinzer, F.C.; Lachenbruch, B.; Dawson, T.E. (Eds.) Size and Age-Related Changes in Tree Structure and Function; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Messier, C.; Puettmann, K.J.; Coates, K.D. Managing Forests as Complex Adaptative Systems: Building Resilience to the Challenge of Global Change; Routledge: London, UK, 2013. [Google Scholar]
- Cattaneo, N.; Schneider, R.; Bravo, F.; Bravo-Oviedo, A. Inter-specific competition of tree congeners induces changes in crown architecture in Mediterranean pine mistures. For. Ecol. Manag. 2020, 476, 1–13. [Google Scholar] [CrossRef]
- Hardiman, B.S.; Bohrer, G.; Gough, C.M.; Vogel, C.S.; Curtis, P.S.; Vogel, S.; Curtis, S.; Hardiman, S. The role of canopy structural complexity in wood net primary production of a maturing northern deciduous forest. Ecology 2012, 92, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.E. Phenotipic plasticity for plant development, function and life history. Trends Plant Sci. 2000, 5, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.A.; Finger, C.A.G.; Hess, A.F. Modelo de incremento em área basal para árvores de araucária de uma floresta inequiânea. Pesq. Flor. Bras. 2015, 35, 239–245. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J. A review of processes behind diversity-productivity relationships in forests. Curr. For. Rep. 2016, 2, 45–61. [Google Scholar] [CrossRef]
- Beltrán, H.A.; Pastur, G.M.; Ivancich, H.; Lencinas, M.V.; Chauchard, L.M. Tree health influences diameter growth along site quality, crown classes and age gradients in Nothogaus forests of southern Patagonia. J. For. Sci. 2013, 59, 328–336. [Google Scholar] [CrossRef]
- Baurele, P.; Rutherford, P.; Lafranco, D. Defoliadores de roble (Nothofagus obliqua) raulí (N. alpina), coigữe (N. dombeyi) y lenga (N. pumilio). [Oak (Nothofagus obliqua), raulí (N. alpina), coigữe (N. dombeyi) y lenga (N. pumilio) defoliators]. Bosque 1997, 18, 97–107. [Google Scholar] [CrossRef]
- Diamantopoulou, M.J. Simulation of over-bark tree bole diameters, through the RFr (Random Forest Regression) algorithm. Folia Oecologica 2022, 49, 93–101. [Google Scholar] [CrossRef]
- Reich, K.F.; Kuns, M.; Bitter, A.W.; Oheimb, G.V. Do different indices of forest structural heterogeneity yield consistent results? iForest 2022, 15, 424–432. [Google Scholar] [CrossRef]
- Gustafsson, L.; Baker, S.C.; Bauhus, J.; Beese, W.J.; Brodie, A.; Kouki, J.; Lindermayer, D.B.; Lõhmus, A.; Pastur, G.M.; Messier, C.; et al. Retention forestry to maintanin multifunctional forests: A world perspective. BioScience 2012, 62, 633–645. [Google Scholar] [CrossRef]
- Bauhus, J.; Püttmann, K.J.; Kühne, C. Close-to-nature forest management in Europe: Does it support complexity and adaptability of forest ecosystems? In Managing Forests as Complex Adaptive System: Building Resilience to the Challenge of Global Change; The Earthscan Forest Library, Routledge: London, UK, 2013. [Google Scholar]
- Brang, P.; Spathelf, P.; Larsen, J.B.; Bauhus, J.; Boncina, A.; Chauvin, C.; Drossler, L.; Garcia-Guemes, C.; Heiri, C.; Kerr, G.; et al. Suitability of close-to-nature silvivulture for adapting temprerate European forests to climate change. Forestryni 2014, 87, 492–503. [Google Scholar] [CrossRef]
- Felipe-Lucia, M.R.; Soliveres, S.; Penone, C.; Manning, P.; Van Der Plas, F.; Boch, S.; Prati, D.; Ammer, C.; Schall, P.; Gossner, M.M.; et al. Multiple forest attributes underpin the supply of multiple ecosystem services. Nat. Commun. 2018, 9, 4839. [Google Scholar] [CrossRef] [PubMed]
- Schall, P.; Gossner, M.M.; Heinrichs, S.; Fischer, M.; Boch, S.; Prati, D.; Jung, K.; Baumgartner, V.; Blaser, S.; Bohm, S.; et al. The impactof even-aged and uneven-aged forest management on regional biodiversity of multiple taxa in European beech forests. J. Appl. Ecol. 2018, 55, 267–278. [Google Scholar] [CrossRef]
- Schuldt, A.; Ebeling, A.; Kunz, M.; Staab, M.; Guimaraes- Steinicke, C.; Bachmann, D.; Buchmann, N.; Durka, W.; Fichtner, A.; Fornoff, F.; et al. Multiple plant diversity components drive consumer communities across ecosytems. Nat. Commun. 2019, 10, 1460. [Google Scholar] [CrossRef] [PubMed]
- Forrester, D.I. Ecological and physiological processes in mixed versus monospecific stands. In Mixed-Species Forests: Ecology and Management; Pretzsch, H., Forrester, D.I., Bauhus, J., Eds.; Springer: Berlin, Germany, 2017. [Google Scholar] [CrossRef]
- Bauhus, J.; Pyttel, P. Managed forests. In Routledge Handbook of Forest Ecology; Peh, K.S.H., Corlett, T.T., Bergeron, Y., Eds.; Routledge: London, UK, 2015. [Google Scholar]
- Pretzsch, H. Individual tree structure and growth in mixed compared with monospecific stands. In Mixed-Species Forests: Ecology and Management; Pretzsch, H., Forrester, D.I., Bauhus, J., Eds.; Springer: Berlin, Germany, 2017. [Google Scholar] [CrossRef]
- Smith, T.M.; Smith, R.L. Elements of Ecology, 7th ed.; Pearson International Edition; Benjamin Cummings: San Francisco, CA, USA, 2009. [Google Scholar]


| Site | d | cd | cl | cv | csa | g | N | |
|---|---|---|---|---|---|---|---|---|
| SJQ | max | 106.6 | 16.6 | 10.9 | 32.4 | 276.8 | 0.89 | 368.4 |
| min | 20.1 | 4.2 | 0.5 | 1.4 | 27.1 | 0.03 | 36.1 | |
| mean | 57.4 | 9.8 | 4.4 | 11.2 | 106.8 | 0.28 | 112.6 | |
| sd | 16.7 | 2.2 | 2.1 | 5.9 | 44.3 | 0.17 | 54.7 | |
| URU | max | 89.4 | 15.9 | 13.1 | 36.0 | 253.3 | 0.62 | 447.5 |
| min | 18.7 | 4.1 | 0.3 | 0.9 | 22.3 | 0.02 | 39.5 | |
| mean | 46.3 | 8.9 | 5.8 | 13.6 | 107.0 | 0.18 | 113.9 | |
| sd | 14.3 | 2.2 | 2.1 | 6.3 | 44.2 | 0.11 | 61.2 | |
| PNL | max | 86.6 | 12.3 | 12.0 | 23.3 | 149.6 | 0.56 | 1145.9 |
| min | 23.6 | 1.25 | 0.5 | 1.2 | 8.7 | 0.04 | 66.8 | |
| mean | 49.5 | 7.1 | 4.5 | 7.5 | 67.7 | 0.21 | 197.6 | |
| sd | 13.4 | 2.7 | 2.5 | 4.4 | 31.9 | 0.11 | 143.9 | |
| URUB | max | 91.9 | 24.9 | 14.3 | 93.0 | 739.5 | 0.66 | 513.9 |
| min | 12.9 | 4.6 | 1.4 | 1.7 | 14.5 | 0.01 | 13.5 | |
| mean | 37.4 | 10.0 | 6.3 | 19.1 | 147.3 | 0.13 | 111.3 | |
| sd | 14.4 | 3.5 | 3.5 | 15.4 | 111.5 | 0.10 | 90.7 | |
| BRT | max | 75.4 | 18.9 | 7.4 | 33.9 | 324.6 | 0.45 | 227.4 |
| min | 25.5 | 7.4 | 1.0 | 1.9 | 43.9 | 0.05 | 30.8 | |
| mean | 47.2 | 10.8 | 4.0 | 11.9 | 122.9 | 0.18 | 100.4 | |
| sd | 1.9 | 2.7 | 1.9 | 7.7 | 65.8 | 0.08 | 45.2 | |
| LAG | max | 69.7 | 19.6 | 12.2 | 45.2 | 301.7 | 0.16 | 576.4 |
| min | 16.4 | 4.7 | 2.7 | 4.4 | 17.3 | 0.001 | 33.1 | |
| mean | 40.1 | 9.6 | 6.9 | 17.6 | 80.2 | 0.03 | 188.3 | |
| sd | 9.0 | 3.3 | 2.2 | 9.3 | 59.7 | 0.03 | 124.3 |
| Site | Eq. | b0 * | b1 * | D | AIC | BIC | LF |
|---|---|---|---|---|---|---|---|
| SJQ | 7 | −0.2805 | 2.6439 | 14.4 | 1216.6 | 1227.5 | G-µ |
| 8 | 56.4195 | 11.5695 | 35.5 | 2731.9 | 2742.7 | G-µ | |
| 9 | 0.1377 | 0.0013 | 80.7 | −323.4 | −312.6 | G-µ | |
| 10 | 5.2547 | −0.0095 | 46.1 | 2828.7 | 2839.5 | G-ln(µ) | |
| URU | 7 | 1.3476 | 0.2034 | 10.9 | 705 | 713.6 | G-ln(µ) |
| 8 | 4.0251 | 0.1072 | 16.6 | 1310.5 | 1319.1 | G-ln(µ) | |
| 9 | −2.786 | 0.0095 | 29.9 | −304.4 | −295.8 | G-ln(µ) | |
| 10 | 5.6796 | −0.0215 | 14.9 | 1308.6 | 1317.2 | G-ln(µ) | |
| PNL | 7 | 1.4752 | 1.3733 | 20.8 | 628.2 | 636.8 | G-µ |
| 8 | 4.1156 | 0.0218 | 32.9 | 1282.1 | 1290.7 | G-ln(µ) | |
| 9 | 0.0446 | 0.0024 | 26.1 | −286.1 | −277.4 | G-µ | |
| 10 | 6.5288 | −0.0265 | 24.5 | 1514.7 | 1523.3 | G-ln(µ) | |
| LAG | 12 | 4.7573 | 0.0237 | 7.5 | 456.7 | 461.4 | G-ln(µ) |
| 13 | 5.9724 | −22.0764 | 9.9 | 466.9 | 471.7 | G-ln(µ) | |
| 14 | −0.0004 | 0.0197 | 14.3 | −269.8 | −265.1 | G-µ | |
| SITES SJQ-URU-PNL | 15 | 4.6481 | −0.0784 | 33.5 | 708.6 | 717.3 | G-ln(µ) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hess, A.F.; Demétrio, L.; de Sousa, A.N.; Costa, E.A.; Liesenberg, V.; Biffi, L.J.; Finger, C.A.G.; Borsoi, G.A.; Stepka, T.F.; Ransoni, J.G.R.d.L.; et al. Sustainability Assessment of Araucaria Forest Remnants in Southern Brazil: Insights from Traditional Forest Inventory Surveys. Sustainability 2024, 16, 3361. https://doi.org/10.3390/su16083361
Hess AF, Demétrio L, de Sousa AN, Costa EA, Liesenberg V, Biffi LJ, Finger CAG, Borsoi GA, Stepka TF, Ransoni JGRdL, et al. Sustainability Assessment of Araucaria Forest Remnants in Southern Brazil: Insights from Traditional Forest Inventory Surveys. Sustainability. 2024; 16(8):3361. https://doi.org/10.3390/su16083361
Chicago/Turabian StyleHess, André Felipe, Laryssa Demétrio, Alex Nascimento de Sousa, Emanuel Arnoni Costa, Veraldo Liesenberg, Leonardo Josoé Biffi, César Augusto Guimarães Finger, Geedre Adriano Borsoi, Thiago Floriani Stepka, José Guilherme Raitz de Lima Ransoni, and et al. 2024. "Sustainability Assessment of Araucaria Forest Remnants in Southern Brazil: Insights from Traditional Forest Inventory Surveys" Sustainability 16, no. 8: 3361. https://doi.org/10.3390/su16083361
APA StyleHess, A. F., Demétrio, L., de Sousa, A. N., Costa, E. A., Liesenberg, V., Biffi, L. J., Finger, C. A. G., Borsoi, G. A., Stepka, T. F., Ransoni, J. G. R. d. L., Silva, E. I. M. d., Ferreira, M. B., & Bispo, P. d. C. (2024). Sustainability Assessment of Araucaria Forest Remnants in Southern Brazil: Insights from Traditional Forest Inventory Surveys. Sustainability, 16(8), 3361. https://doi.org/10.3390/su16083361













