Control of Silica Gel Formation in the Acidic Leaching of Calcium Aluminate Slags with Aqueous HCl for Al Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory/Analytical Equipment, Reagents, and Software Used in This Study
2.2. Materials Used
2.3. Experimental Methodology
3. Results
3.1. Metal Extraction Results
3.2. Behavior of Dissolved Si in Relation to the pH
3.3. Mineralogical and Chemical Analysis of Residues
4. Discussion
4.1. Leaching of Aluminium and Behavior of the Impurities
4.2. Silica Gelation Phenomena and Mechanism
5. Conclusions
- The crucial design parameter for successful leaching is the avoidance of SiO2 gelation. In the course of the experimental work, it was shown that efficient leaching of Al was achieved from the slag, even at conditions that favored SiO2 gelation. Leaching with a 15% S/L ratio led to the production of stable, non-gelatinizing PLS containing 34.5 g/L Al;
- Concerning the composition of slags suitable for this process, the phase of gehlenite must be avoided, as its leaching kinetics in aqueous HCl solutions appear to be slower compared to the leaching of pure calcium aluminates;
- SiO2 gelation can be controlled by monitoring the pH of the PLS. Non-gelatinizing residues are produced for pH < 2. This phenomenon was attributed to the behavior of SiO2 in aqueous solutions with pH values below its isoelectric point (IEP);
- When the pH of the PLS at the end of the leaching process is <2, the dissolved CaCl2 and AlCl3 salts accelerate the particle growth of amorphous SiO2 particles, leading to the formation of a filterable leached residue. In this study, during leaching with a 20.2% w/w HCl solution and a 15% S/L ratio at 80 °C, an increase in the D50of the amorphous SiO2 particles was observed from 5.21 μm to 27.51 μm when the duration of leaching increased from 90 min to 120 min.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xing, Z.; Lu, J.; Ji, X. A Brief Review of Metallothermic Reduction Reactions for Materials Preparation. Small Methods 2018, 2, 1800062. [Google Scholar] [CrossRef]
- Güney, H.; Güner, Ö.; Boncuk, F.F.; Kan, S.; Benzeşik, K.; Yücel, O. A Decarbonization Approach for FeCr Production. J. Sustain. Metall. 2023, 9, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Kudyba, A.; Akhtar, S.; Johansen, I.; Safarian, J. Aluminothermic Reduction of Manganese Oxide from Selected MnO-Containing Slags. Materials 2021, 14, 356. [Google Scholar] [CrossRef] [PubMed]
- Safarian, J. A Sustainable Process to Produce Manganese and Its Alloys through Hydrogen and Aluminothermic Reduction. Processes 2022, 10, 27. [Google Scholar] [CrossRef]
- SisAl Pilot—Innovative pilot for Silicon Production with Low Environmental Impact Using Secondary Aluminium and Silicon Raw Materials. Available online: https://www.sisal-pilot.eu/ (accessed on 8 April 2023).
- Brough, D.; Jouhara, H. The aluminium industry: A review on state-of-the-art technologies, environmental impacts and possibilities for waste heat recovery. Int. J. Thermofluids 2020, 1–2, 100007. [Google Scholar] [CrossRef]
- European Commission; Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs; Grohol, M.; Veeh, C. Study on the Critical Raw Materials for the EU 2023—Final Report; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar]
- Nassar, N.T.; Fortier, S.M. Methodology and Technical Input for the 2021 Review and Revision of the U.S. Critical Minerals List; Open-File Report 2021-1045; U.S. Geological Survey: Reston, VA, USA, 2021; 31p. [Google Scholar] [CrossRef]
- The Canadian Critical Minerals Strategy; Cat. No. M34-82/2022E-PDF (Online); Critical Minerals Centre of Excellence (CMCE), 2023. Available online: https://www.canada.ca/content/dam/nrcan-rncan/site/critical-minerals/Critical-minerals-strategyDec09.pdf (accessed on 17 October 2023).
- Sibanda, V.; Ndlovu, S.; Dombo, G.; Shemi, A.; Rampou, M. Towards the Utilization of Fly Ash as a Feedstock for Smelter Grade Alumina Production: A Review of the Developments. J. Sustain. Metall. 2016, 2, 167–184. [Google Scholar] [CrossRef]
- Ding, J.; Ma, S.; Shen, S.; Xie, Z.; Zheng, S.; Zhang, Y. Research and industrialization progress of recovering alumina from fly ash: A concise review. Waste Manag. 2017, 60, 375–387. [Google Scholar] [CrossRef]
- Cao, P.; Li, G.; Jiang, H.; Zhang, X.; Luo, J.; Rao, M.; Jiang, T. Extraction and value-added utilization of alumina from coal fly ash via one-step hydrothermal process followed by carbonation. J. Clean. Prod. 2021, 323, 129174. [Google Scholar] [CrossRef]
- Lin, M.; Liu, Y.-Y.; Lei, S.-M.; Ye, Z.; Pei, Z.-Y.; Li, B. High-efficiency extraction of Al from coal-series kaolinite and its kinetics by calcination and pressure acid leaching. Appl. Clay Sci. 2018, 161, 215–224. [Google Scholar] [CrossRef]
- Pak, V.I.; Kirov, S.S.; Nalivaiko, A.Y.; Ozherelkov, D.Y.; Gromov, A.A. Obtaining Alumina from Kaolin Clay via Aluminum Chloride. Materials 2019, 12, 3938. [Google Scholar] [CrossRef]
- Maria, B.; Efthymios, B.; Dimitrios, P. Exploitation of Kaolin as an Alternative Source in Alumina Production. Mater. Proc. 2021, 5, 24. [Google Scholar] [CrossRef]
- Barry, T.S.; Uysal, T.; Birinci, M.; Erdemoğlu, M. Thermal and Mechanical Activation in Acid Leaching Processes of Non-bauxite Ores Available for Alumina Production—A Review. Min. Metall. Explor. 2019, 36, 557–569. [Google Scholar] [CrossRef]
- Cao, P.; Luo, J.; Jiang, H.; Zhang, X.; Rao, M.; Li, G. Extraction of alumina from low-grade kaolin in the presence of lime and NaOH via multi-stage hydrothermal process. Appl. Clay Sci. 2022, 229, 106675. [Google Scholar] [CrossRef]
- Smirnov, A.; Kibartas, D.; Senyuta, A.; Panov, A. Miniplant Tests of HCl Technology of Alumina Production. In TMS 2018: Light Metals 2018; Springer: Cham, Switzerland, 2018; pp. 57–62. [Google Scholar]
- Azof, F.I.; Safarian, J. Leaching kinetics and mechanism of slag produced from smelting-reduction of bauxite for alumina recovery. Hydrometallurgy 2020, 195, 105388. [Google Scholar] [CrossRef]
- Azof, F.I.; Vafeias, M.; Panias, D.; Safarian, J. The leachability of a ternary CaO-Al2O3-SiO2 slag produced from smelting-reduction of low-grade bauxite for alumina recovery. Hydrometallurgy 2020, 191, 105184. [Google Scholar] [CrossRef]
- ElDeeb, A.B.; Brichkin, V.N.; Bertau, M.; Savinova, Y.A.; Kurtenkov, R.V. Solid state and phase transformation mechanism of kaolin sintered with limestone for alumina extraction. Appl. Clay Sci. 2020, 196, 105771. [Google Scholar] [CrossRef]
- ElDeeb, A.B.; Brichkin, V.N.; Kurtenkov, R.V.; Bormotov, I.S. Extraction of alumina from kaolin by a combination of pyro- and hydro-metallurgical processes. Appl. Clay Sci. 2019, 172, 146–154. [Google Scholar] [CrossRef]
- Vafeias, M.; Bempelou, A.; Georgala, E.; Davris, P.; Balomenos, E.; Panias, D. Leaching of Ca-Rich Slags Produced from Reductive Smelting of Bauxite Residue with Na2CO3 Solutions for Alumina Extraction: Lab and Pilot Scale Experiments. Minerals 2021, 11, 896. [Google Scholar] [CrossRef]
- Queneau, P.B.; Berthold, C.E. Silica in Hydrometallurgy: An Overview. Can. Metall. Q. 1986, 25, 201–209. [Google Scholar] [CrossRef]
- Terry, B. The acid decomposition of silicate minerals part I. Reactivities and modes of dissolution of silicates. Hydrometallurgy 1983, 10, 135–150. [Google Scholar] [CrossRef]
- Terry, B. The acid decomposition of silicate minerals part II. Hydrometallurgical applications. Hydrometallurgy 1983, 10, 151–171. [Google Scholar] [CrossRef]
- Toli, A.; Tsaousi, G.M.; Balomenos, E.; Panias, D.; Heuer, M.; Philipson, H.; Tranell, G. Sustainable Silicon and High Purity Alumina Production from Secondary Silicon and Aluminium Raw Materials through the Innovative SisAl Technology. Mater. Proc. 2021, 5, 85. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- SRM 640f. Line position and Line shape Standard for Powder Diffraction (Silicon Powder). National Institute of Standards and Technology, U.S. Department of Commerce: Gaitherburg, MD, USA, 2019.
- O’Connor, B.H.; Raven, M.D. Application of the Rietveld Refinement Procedure in Assaying Powdered Mixtures. Powder Diffr. 1988, 3, 2–6. [Google Scholar] [CrossRef]
- Gorrepati, E.A.; Wongthahan, P.; Raha, S.; Fogler, H.S. Silica Precipitation in Acidic Solutions: Mechanism, pH Effect, and Salt Effect. Langmuir 2010, 26, 10467–10474. [Google Scholar] [CrossRef] [PubMed]
- Lazaro, A.; Vilanova, N.; Barreto Torres, L.D.; Resoort, G.; Voets, I.K.; Brouwers, H.J.H. Synthesis, Polymerization, and Assembly of Nanosilica Particles below the Isoelectric Point. Langmuir 2017, 33, 14618–14626. [Google Scholar] [CrossRef] [PubMed]
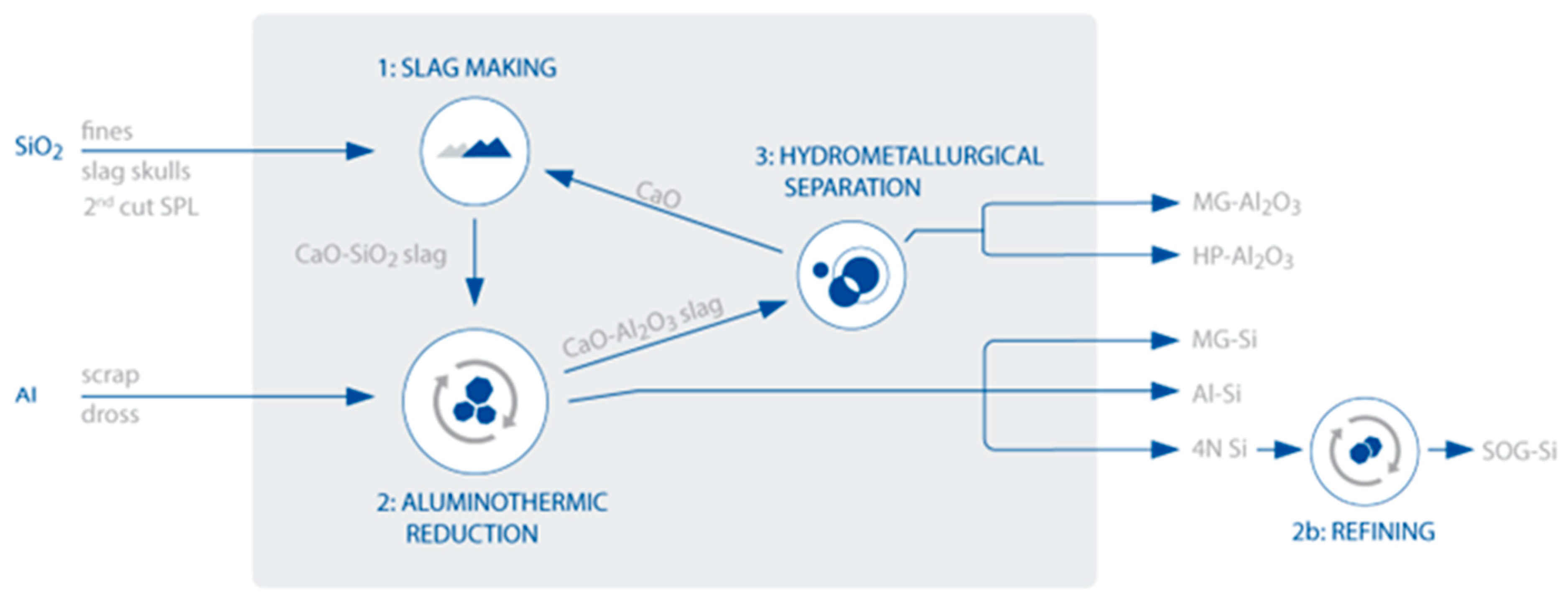




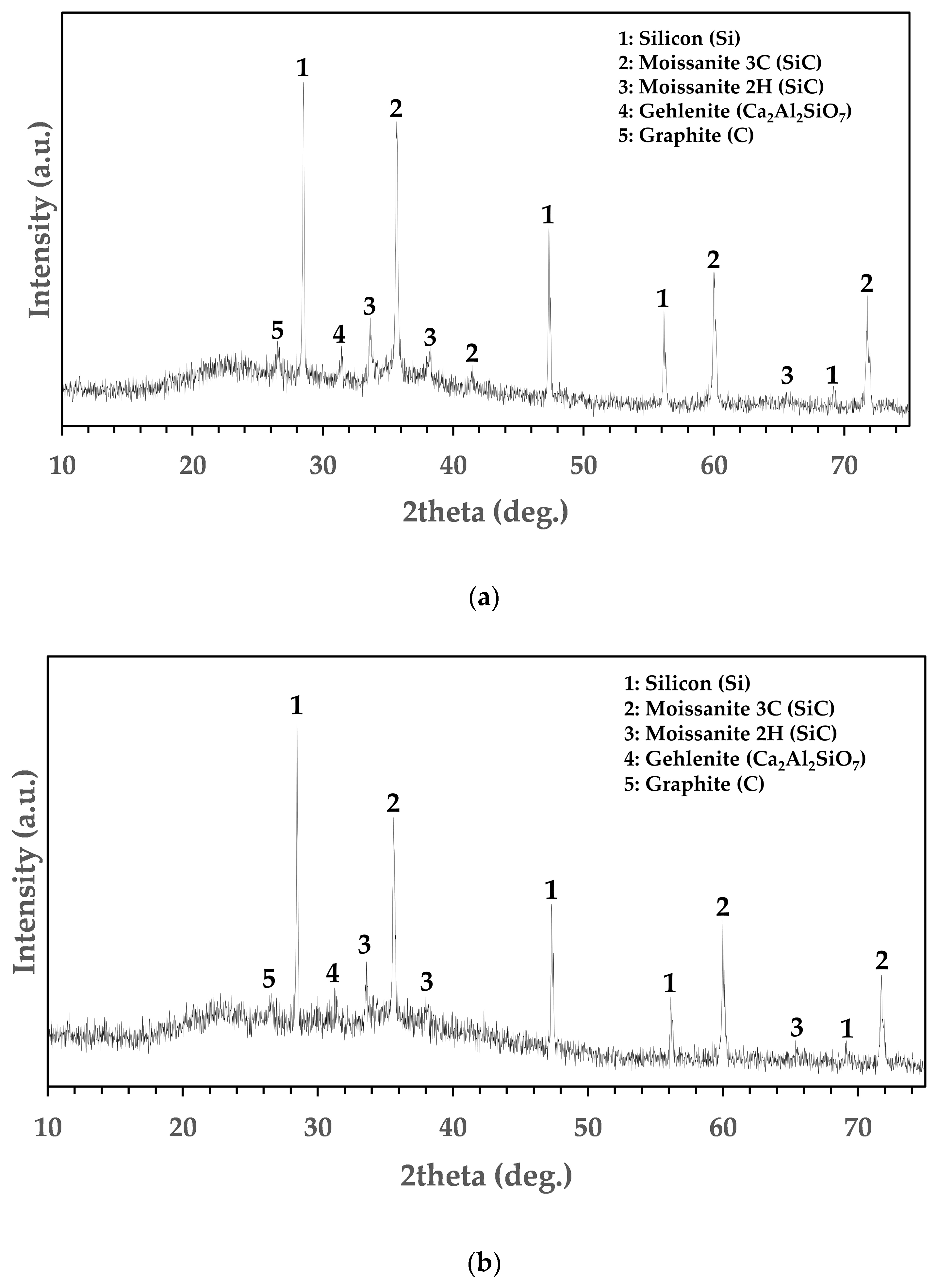
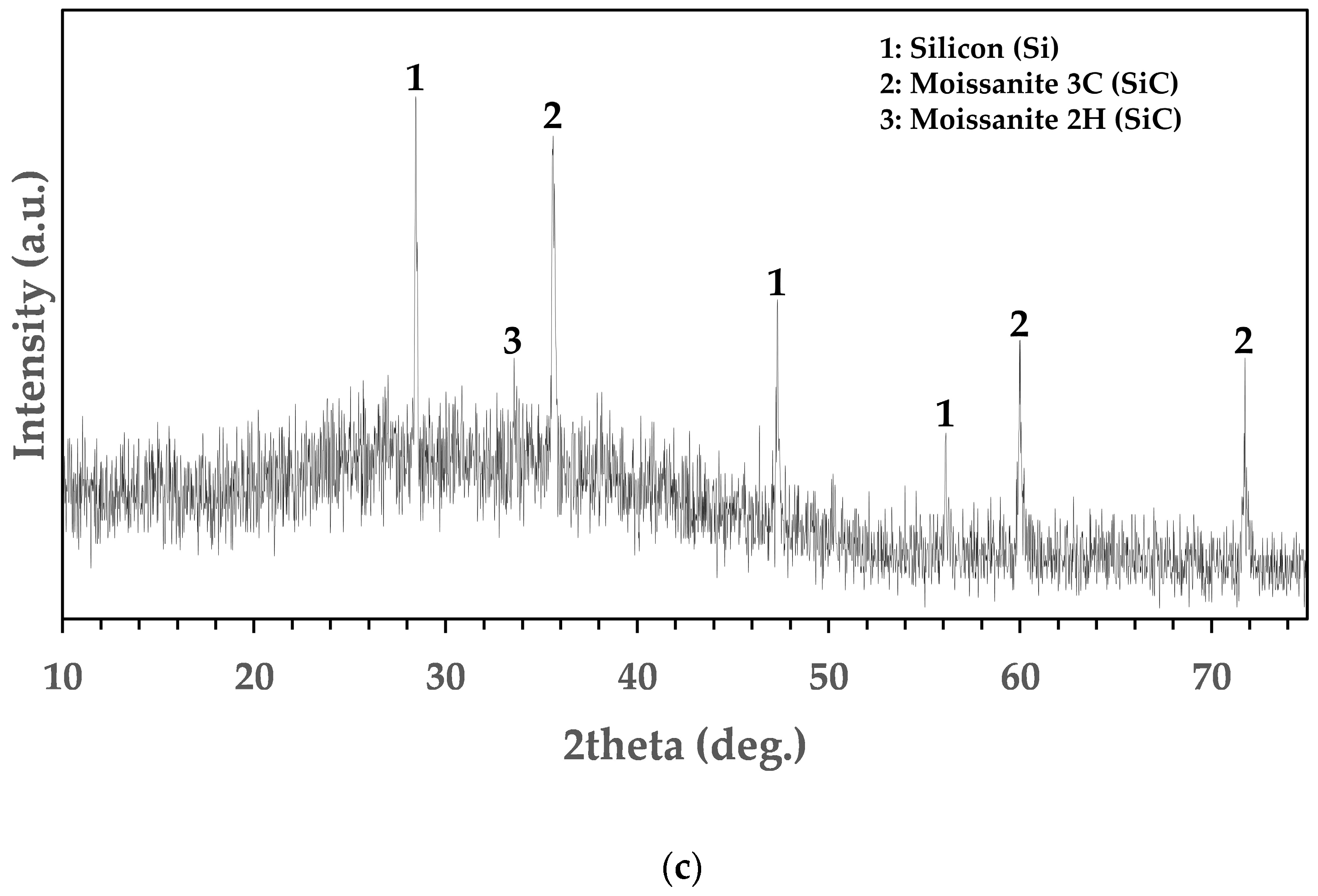

| Al2O3 | CaO | SiO2 | MgO | Fe2O3 | Total |
|---|---|---|---|---|---|
| 49.4% | 37.5% | 15.2% | 0.3% | 0.1% | 102.5% |
| CaAl2O4 (Krotite) | Ca2Al2SiO7 (Gehlenite) | Ca12Al14O33 (Mayenite) | Si (Silicon) | C (Graphite) | SiC (Moissanite 3C) | Amorphous Content | Rwp 1 |
|---|---|---|---|---|---|---|---|
| 32.6% | 13.6% | 28.6% | 1.3% | 0.4% | 3.7 | 21.3% | 6.82% |
| S/L Ratio (%) | Temperature (°C) | Agitation (rpm) | (HCl) (%w/w) | Volume of Solution (mL) | Duration (h) |
|---|---|---|---|---|---|
| 10 | 80 | 300 | 20.2 | 150 | 2 |
| 12.5 | |||||
| 15 | |||||
| 20 |
| Filterability | Average Metal Concentration in PLS | Average % wt. Metal Extraction in PLS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S/L (%) | Silica Gel Observed | Ca (g/l) | Al (g/l) | Si (mg/L) | Fe (mg/L) | Mg (mg/L) | Ca | Al | Si | Fe | Mg |
| 10 | No | 28.9 | 26.4 | 53 | 13 | 272 | 88.9% | 82.7% | 0.6% | 72.5% | 77.2% |
| 12.5 | No | 32.6 | 31.0 | 129 | 18 | 248 | 95.3% | 87.9% | 1.1% | 67.6% | 77.9% |
| 15 | No | 40.1 | 34.5 | 299 | 14 | 275 | 86.4% | 76.5% | 3.2% | 47.8% | 58.5% |
| 20 | Yes | 58.5 | 45.3 | 3400 | 19 | 402 | 97.4% | 80.2% | 21.4% | 45.0% | 63.5% |
| S/L (%) | Si (mg/L) | Si (mmol/L) | Log Si (mmol/L) | pH of PLS |
|---|---|---|---|---|
| 10 | 53 | 1.9 | 0.27 | 0.01 |
| 12.5 | 129 | 4.6 | 0.66 | 0.15 |
| 15 | 299 | 106.5 | 2.03 | 1.35 |
| 20 | 3400 | 121.1 | 2.08 | 1.98 |
| Al2O3 | CaO | SiO2 | MgO | Fe2O3 | LOI | Total |
|---|---|---|---|---|---|---|
| 12.9% | 9.3% | 36.4% | 0.1% | 0.3% | 41.8% | 100.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsaousi, G.M.; Toli, A.; Bempelou, A.; Kotsanis, D.; Vafeias, M.; Balomenos, E.; Panias, D. Control of Silica Gel Formation in the Acidic Leaching of Calcium Aluminate Slags with Aqueous HCl for Al Extraction. Sustainability 2023, 15, 15462. https://doi.org/10.3390/su152115462
Tsaousi GM, Toli A, Bempelou A, Kotsanis D, Vafeias M, Balomenos E, Panias D. Control of Silica Gel Formation in the Acidic Leaching of Calcium Aluminate Slags with Aqueous HCl for Al Extraction. Sustainability. 2023; 15(21):15462. https://doi.org/10.3390/su152115462
Chicago/Turabian StyleTsaousi, Georgia Maria, Aikaterini Toli, Amalia Bempelou, Dimitrios Kotsanis, Michail Vafeias, Efthymios Balomenos, and Dimitrios Panias. 2023. "Control of Silica Gel Formation in the Acidic Leaching of Calcium Aluminate Slags with Aqueous HCl for Al Extraction" Sustainability 15, no. 21: 15462. https://doi.org/10.3390/su152115462
APA StyleTsaousi, G. M., Toli, A., Bempelou, A., Kotsanis, D., Vafeias, M., Balomenos, E., & Panias, D. (2023). Control of Silica Gel Formation in the Acidic Leaching of Calcium Aluminate Slags with Aqueous HCl for Al Extraction. Sustainability, 15(21), 15462. https://doi.org/10.3390/su152115462







