Removal of Base Metals from Mine Tailings in Chloride- and Seawater-Based Media Followed by Solvent Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mine Tailings Sample
2.2. Chemicals
2.3. Leaching Experiments
2.4. Design of Experiments
2.5. Leaching Time and Seawater Tests
2.6. Metals Recovery
3. Results and Discussion
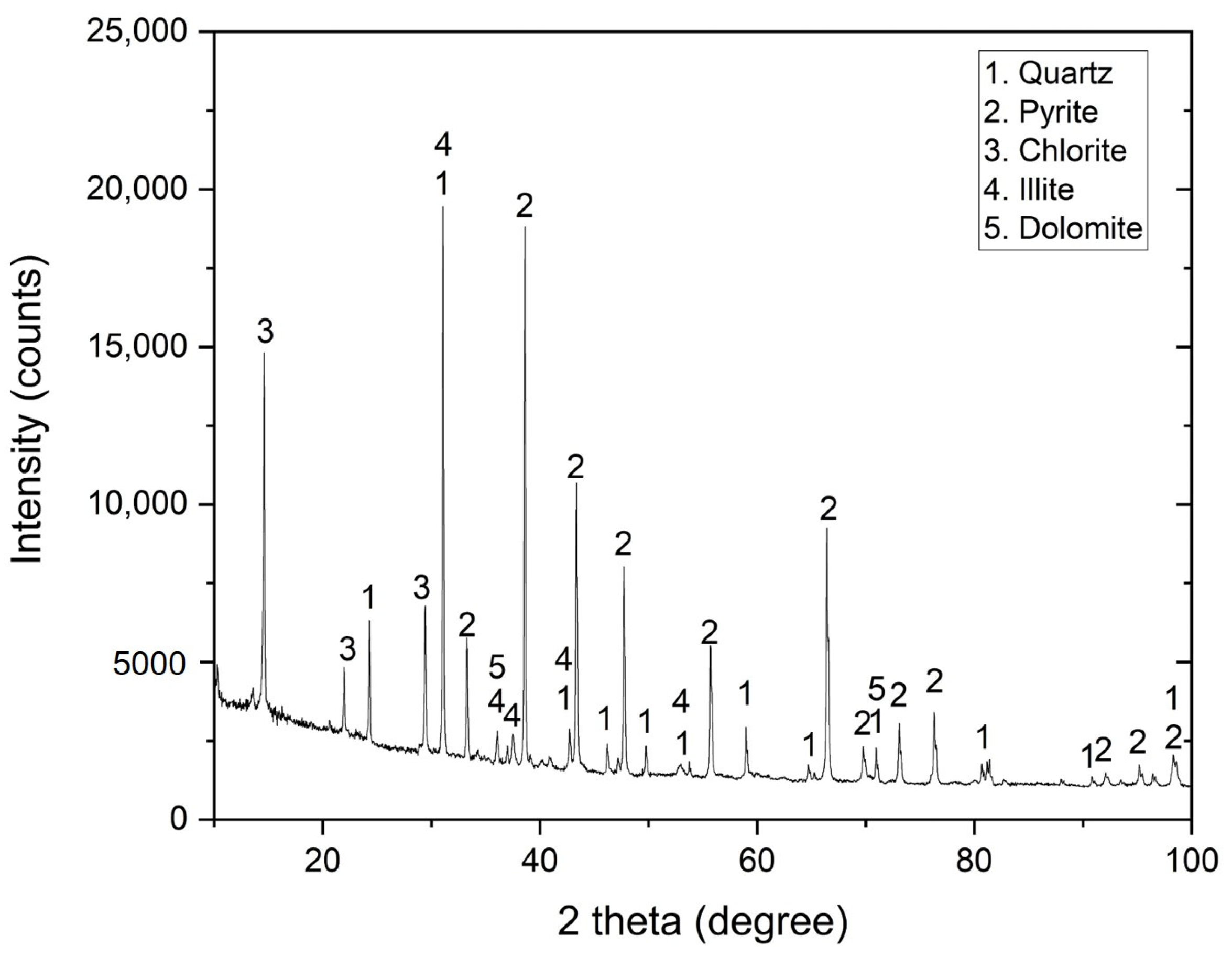
3.1. Tailings Sample Characterization
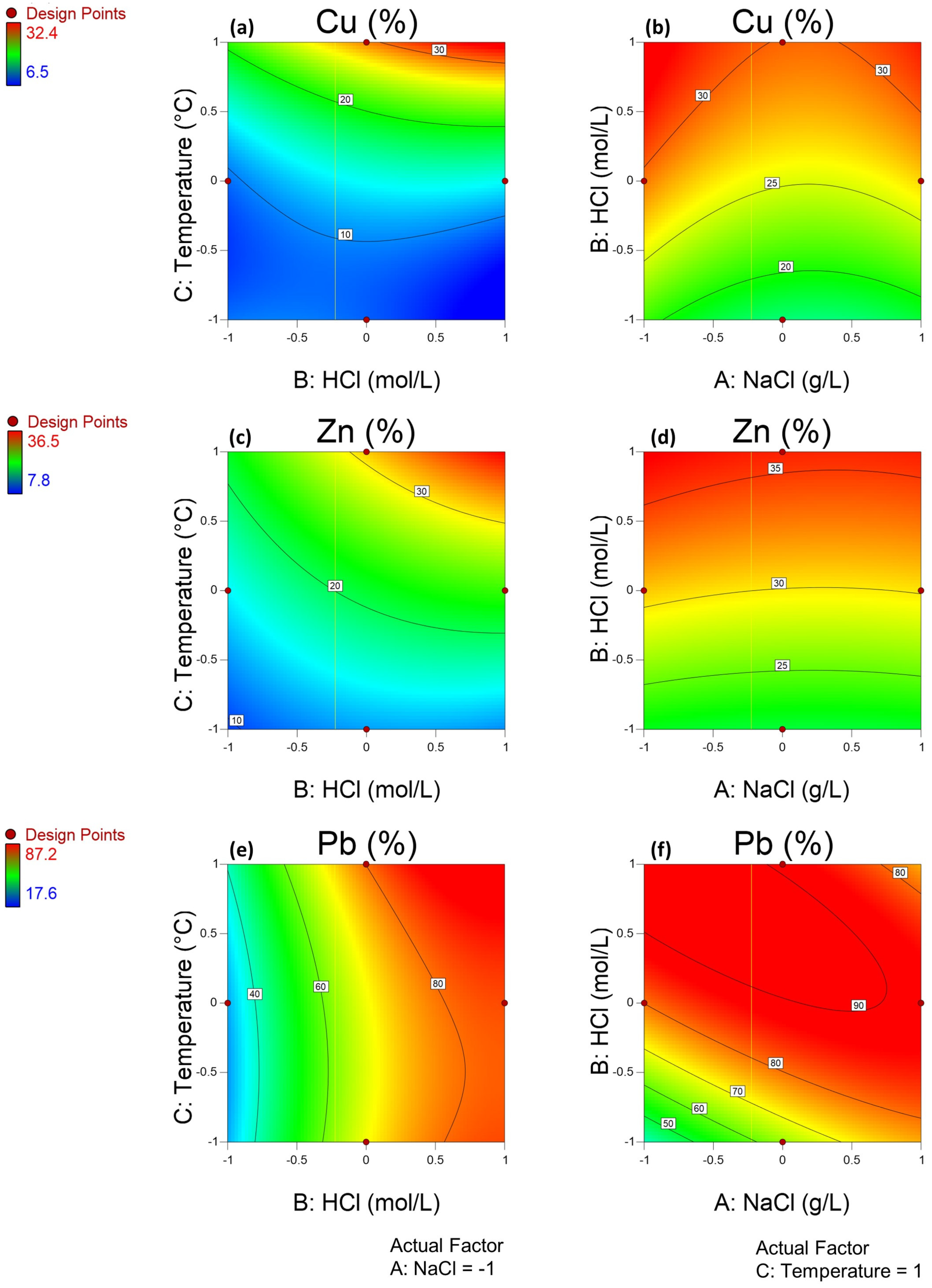
3.2. Leaching of Base Metals from the Mine Tailings Sample
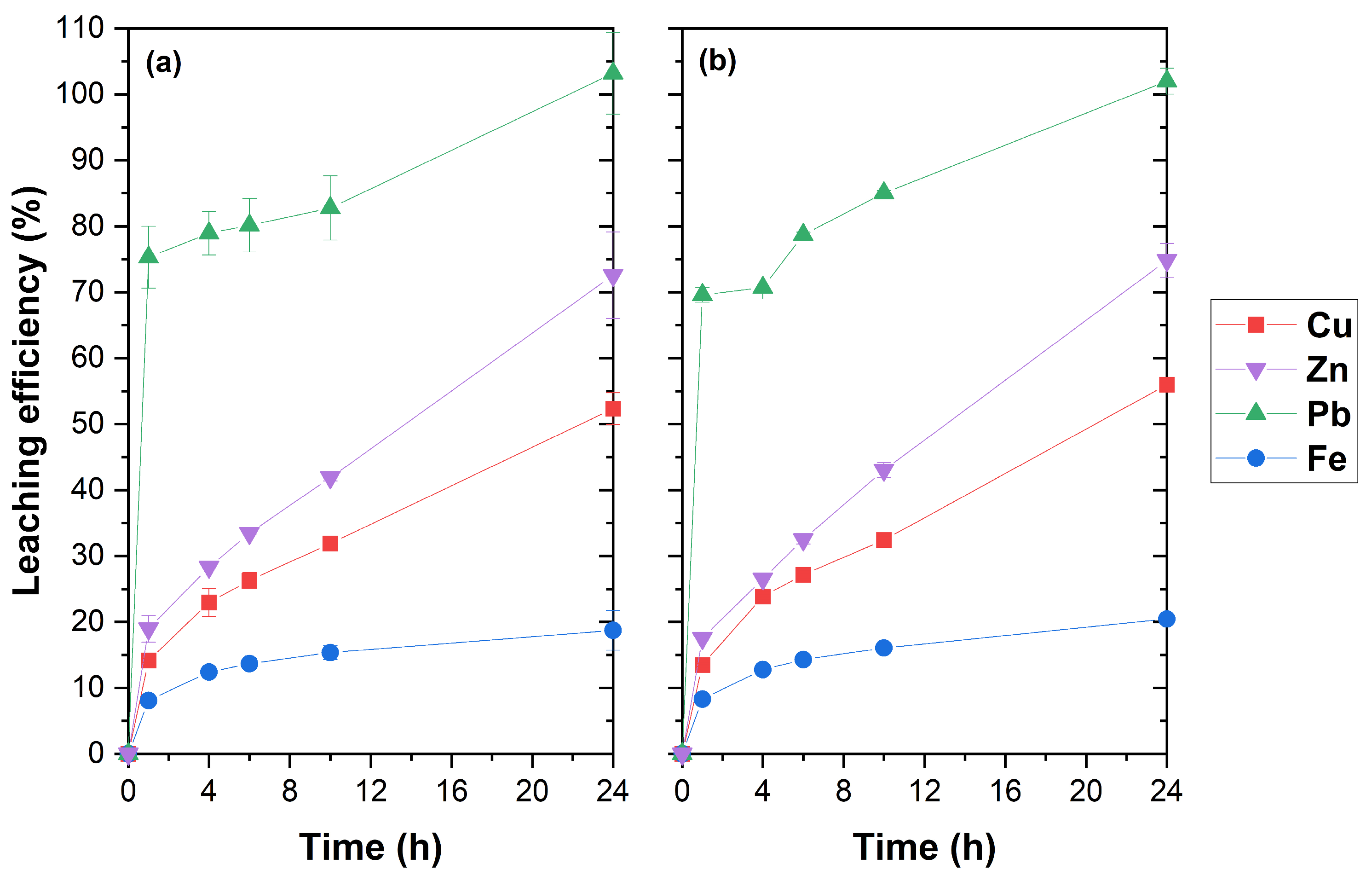
3.3. Effect of Time and Artificial Seawater
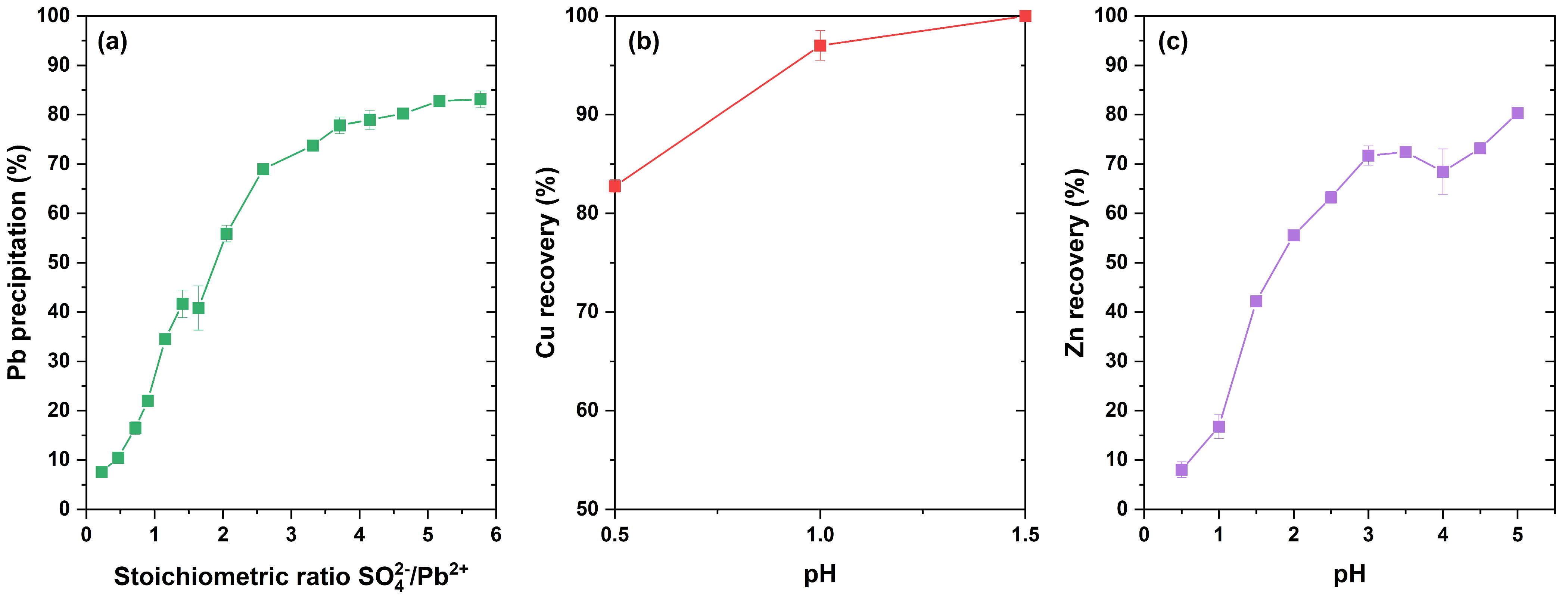
3.4. Metals Recovery
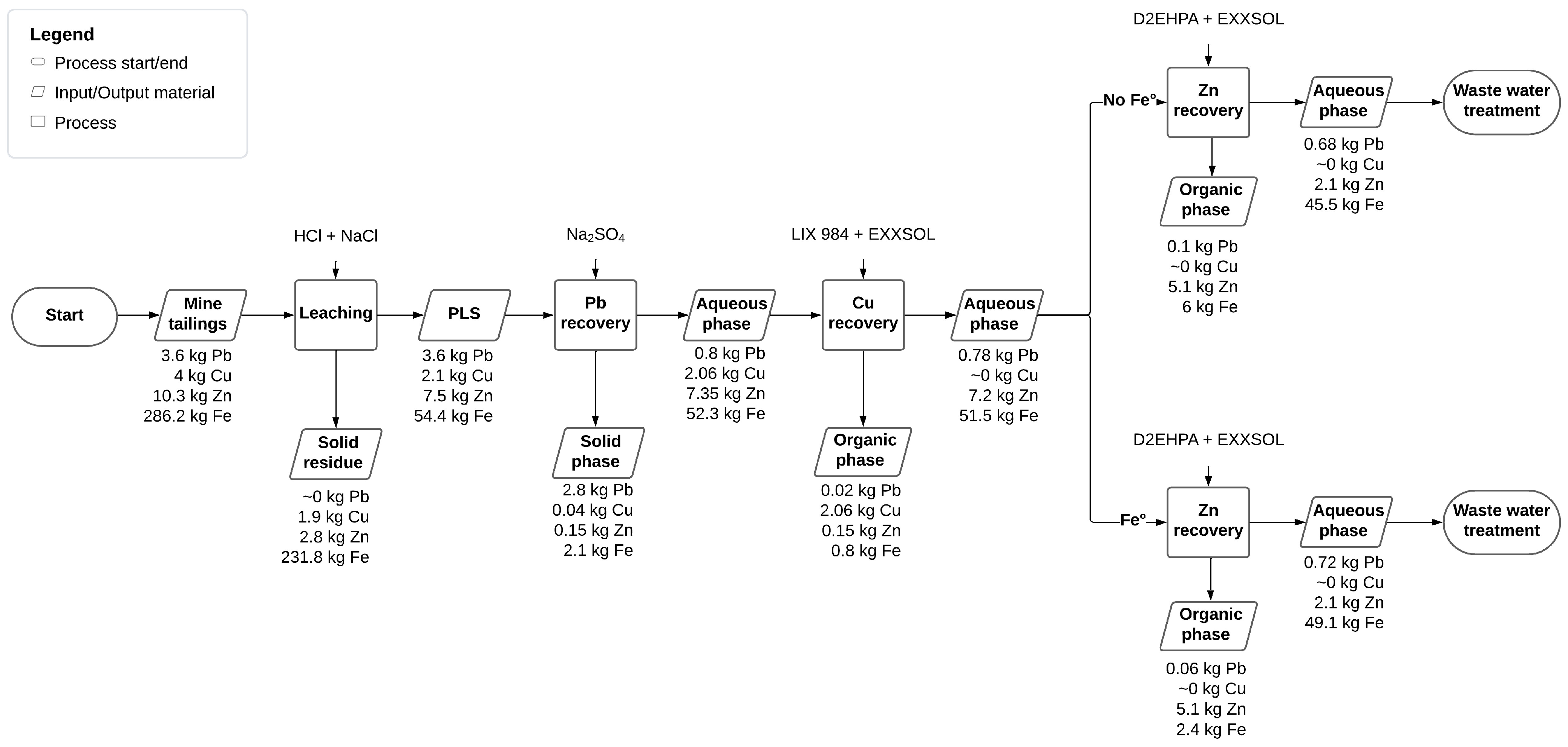
3.5. Proposed Multi-Stage Recovery Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kossoff, D.; Dubbin, W.E.; Alfredsson, M.; Edwards, S.J.; Macklin, M.G.; Hudson-Edwards, K.A. Mine tailings dams: Characteristics, failure, environmental impacts, and remediation. Appl. Geochem. 2014, 51, 229–245. [Google Scholar] [CrossRef]
- Kinnunen, P.; Karhu, M.; Yli-Rantala, E.; Kivikytö-Reponen, P.; Mäkinen, J. A review of circular economy strategies for mine tailings. Clean. Eng. Technol. 2022, 8, 100499. [Google Scholar] [CrossRef]
- Marín, O.A.; Kraslawski, A.; Cisternas, L.A. Estimating processing cost for the recovery of valuable elements from mine tailings using dimensional analysis. Miner. Eng. 2022, 184, 107629. [Google Scholar] [CrossRef]
- Simate, G.S.; Ndlovu, S. Acid mine drainage: Challenges and opportunities. J. Environ. Chem. Eng. 2014, 2, 1785–1803. [Google Scholar] [CrossRef]
- dos Santos Vergilio, C.; Lacerda, D.; Da Silva Souza, T.; de Oliveira, B.C.V.; Fioresi, V.S.; de Souza, V.V.; Da Rocha Rodrigues, G.; de Araujo Moreira Barbosa, M.K.; Sartori, E.; Rangel, T.P.; et al. Immediate and long-term impacts of one of the worst mining tailing dam failure worldwide (Bento Rodrigues, Minas Gerais, Brazil). Sci. Total Environ. 2021, 756, 143697. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.R.; Kemp, D.; Lèbre, É.; Svobodova, K.; Pérez Murillo, G. Catastrophic tailings dam failures and disaster risk disclosure. Int. J. Disaster Risk Reduct. 2020, 42, 101361. [Google Scholar] [CrossRef]
- Cisternas, L.A.; Gálvez, E.D. The use of seawater in mining. Miner. Process. Extr. Metall. Rev. 2018, 39, 18–33. [Google Scholar] [CrossRef]
- Xanthopoulos, P.; Bevandić, S.; Spooren, J.; Binnemans, K.; Kukurugya, F. Recovery of copper, zinc and lead from photovoltaic panel residue. RSC Adv. 2022, 12, 2351–2360. [Google Scholar] [CrossRef]
- Winand, R. Chloride hydrometallurgy. Hydrometallurgy 1991, 27, 285–316. [Google Scholar] [CrossRef]
- Texeira, L.; Calisaya-Azpilcueta, D.; Cruz, C.; Botero, Y.L.; Cisternas, L.A. Impact of the use of seawater on acid mine drainage from mining wastes. J. Clean. Prod. 2023, 383, 135516. [Google Scholar] [CrossRef]
- Kinnunen, P.H.-M.; Kaksonen, A.H. Towards circular economy in mining: Opportunities and bottlenecks for tailings valorization. J. Clean. Prod. 2019, 228, 153–160. [Google Scholar] [CrossRef]
- Falagán, C.; Grail, B.M.; Johnson, D.B. New approaches for extracting and recovering metals from mine tailings. Miner. Eng. 2017, 106, 71–78. [Google Scholar] [CrossRef]
- Free, M. Hydrometallurgy: Fundamentals and Applications/Michael L. Free; TMS-Wiley: Hoboken, NJ, USA, 2013; ISBN 9781118230770. [Google Scholar]
- Torres, C.M.; Taboada, M.E.; Graber, T.A.; Herreros, O.O.; Ghorbani, Y.; Watling, H.R. The effect of seawater based media on copper dissolution from low-grade copper ore. Miner. Eng. 2015, 71, 139–145. [Google Scholar] [CrossRef]
- Watling, H.R. Chalcopyrite hydrometallurgy at atmospheric pressure: 2. Review of acidic chloride process options. Hydrometallurgy 2014, 146, 96–110. [Google Scholar] [CrossRef]
- Dutrizac, J.E. The leaching of sulphide minerals in chloride media. Hydrometallurgy 1992, 29, 1–45. [Google Scholar] [CrossRef]
- Schueler, T.A.; de Aguiar, P.F.; Vera, Y.M.; Goldmann, D. Leaching of Cu, Zn, and Pb from Sulfidic Tailings Under the Use of Sulfuric Acid and Chloride Solutions. J. Sustain. Metall. 2021, 7, 1523–1536. [Google Scholar] [CrossRef]
- Senanayake, G.; Muir, D.M. Chloride processing of metal sulphides: Review of fundamentals and applications. In Proceedings of the Hydrometallurgy 2003: 5th International Symposium Honoring Professor Ian M. Ritchie; Volume 1: Leaching and Solution Purification, Vancouver, BC, Canada, 24–27 August 2003; TMS (The Minerals, Metals & Materials Society). pp. 517–531. [Google Scholar]
- Abo Atia, T.; Spooren, J. Microwave assisted chloride leaching of zinc plant residues. J. Hazard. Mater. 2020, 398, 122814. [Google Scholar] [CrossRef]
- Miki, H.; Nicol, M. The kinetics of the copper-catalysed oxidation of iron(II) in chloride solutions. In Proceedings of the Hydrometallurgy 2008: Proceedings of the 6th International Symposium, Phoenix, AZ, USA, 17–21 August 2008; pp. 971–979. [Google Scholar]
- Hernández, P.; Dorador, A.; Martínez, M.; Toro, N.; Castillo, J.; Ghorbani, Y. Use of Seawater/Brine and Caliche’s Salts as Clean and Environmentally Friendly Sources of Chloride and Nitrate Ions for Chalcopyrite Concentrate Leaching. Minerals 2020, 10, 477. [Google Scholar] [CrossRef]
- Lawson, F.; Cheng, C.-Y.; Lee, L.S.Y. Leaching of Copper Sulphides and Copper Mattes in Oxygenated Chloride/Sulphate Leachants. Miner. Process. Extr. Metall. Rev. 1992, 8, 183–203. [Google Scholar] [CrossRef]
- Miki, H.; Nicol, M. The dissolution of chalcopyrite in chloride solutions. IV. The kinetics of the auto-oxidation of copper(I). Hydrometallurgy 2011, 105, 246–250. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Montes, K.S.; Padilla, R. Chalcopyrite leaching in sulfate–chloride media at ambient pressure. Hydrometallurgy 2011, 109, 37–42. [Google Scholar] [CrossRef]
- Opara, C.B.; Kamariah, N.; Spooren, J.; Pollmann, K.; Kutschke, S. Interesting Halophilic Sulphur-Oxidising Bacteria with Bioleaching Potential: Implications for Pollutant Mobilisation from Mine Waste. Microorganisms 2023, 11, 222. [Google Scholar] [CrossRef]
- Noguchi, H.; Okibe, N. The role of bioleaching microorganisms in saline water leaching of chalcopyrite concentrate. Hydrometallurgy 2020, 195, 105397. [Google Scholar] [CrossRef]
- Escobar, A.G.; Relvas, J.M.R.S.; Pinto, Á.M.M.; Oliveira, M. Physical–Chemical Characterization of the Neves Corvo Extractive Mine Residues: A Perspective Towards Future Mining and Reprocessing of Sulfidic Tailings. J. Sustain. Metall. 2021, 7, 1483–1505. [Google Scholar] [CrossRef]
- De Carvalho, A.L.C.B.; de Carvalho, V.A.; Blannin, R.; Escobar, A.G.; Frenzel, M.; Rudolph, M.; Silva, A.C.; Goldmann, D. A study on the desulfurization of sulfidic mine tailings for the production of a sulfur-poor residue. Miner. Eng. 2023, 202, 108285. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; Da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Box, G.E.P.; Behnken, D.W. Some New Three Level Designs for the Study of Quantitative Variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- D 1141-98 (Reapproved 2003); Standard Practice for the Preparation of Substitute Ocean Water. ASTM International: West Conshohocken, PA, USA, 2003.
- Habashi, F. (Ed.) Handbook of Extractive Metallurgy; Wiley-VCH: Weinheim, Germany, 1997; ISBN 3527287922. [Google Scholar]
- Habashi, F. Textbook of Hydrometallurgy, 2nd ed.; Métallurgie Extractive Québec: Québec, QC, Canada, 1999; ISBN 2-980-3247-7-9. [Google Scholar]
- Johnson, D. The Evolution, Current Status, and Future Prospects of Using Biotechnologies in the Mineral Extraction and Metal Recovery Sectors. Minerals 2018, 8, 343. [Google Scholar] [CrossRef]
- Calvo, G.; Mudd, G.; Valero, A.; Valero, A. Decreasing Ore Grades in Global Metallic Mining: A Theoretical Issue or a Global Reality? Resources 2016, 5, 36. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 9th ed.; John Wiley & Sons Inc: Hoboken, NJ, USA, 2017. [Google Scholar]
- Zhong, S.; Li, Y. An improved understanding of chalcopyrite leaching kinetics and mechanisms in the presence of NaCl. J. Mater. Res. Technol. 2019, 8, 3487–3494. [Google Scholar] [CrossRef]
- Aydogan, S.; Aras, A.; Canbazoglu, M. Dissolution kinetics of sphalerite in acidic ferric chloride leaching. Chem. Eng. J. 2005, 114, 67–72. [Google Scholar] [CrossRef]
- Lei, C.; Yan, B.; Chen, T.; Wang, X.-L.; Xiao, X.-M. Silver leaching and recovery of valuable metals from magnetic tailings using chloride leaching. J. Clean. Prod. 2018, 181, 408–415. [Google Scholar] [CrossRef]
- Carneiro, M.; Leão, V.A. The role of sodium chloride on surface properties of chalcopyrite leached with ferric sulphate. Hydrometallurgy 2007, 87, 73–82. [Google Scholar] [CrossRef]
- Byrne, R.H.; Yao, W.; Luo, Y.; Millero, F.J. Complexation of Pb(II) by Chloride Ions in Aqueous Solutions. Aquat. Geochem. 2010, 16, 325–335. [Google Scholar] [CrossRef]
- Altinkaya, P.; Liipo, J.; Kolehmainen, E.; Haapalainen, M.; Leikola, M.; Lundström, M. Leaching of Trace Amounts of Metals from Flotation Tailings in Cupric Chloride Solutions. Min. Metall. Explor. 2019, 36, 335–342. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Jeffrey, M.I.; Lawson, F. The effect of chloride ions on the dissolution of chalcopyrite in acidic solutions. Hydrometallurgy 2000, 56, 189–202. [Google Scholar] [CrossRef]
- Farahmand, F.; Moradkhani, D.; Safarzadeh, M.S.; Rashchi, F. Brine leaching of lead-bearing zinc plant residues: Process optimization using orthogonal array design methodology. Hydrometallurgy 2009, 95, 316–324. [Google Scholar] [CrossRef]
- Behnajady, B.; Moghaddam, J.; Behnajady, M.A.; Rashchi, F. Determination of the Optimum Conditions for the Leaching of Lead from Zinc Plant Residues in NaCl–H 2 SO 4 –Ca(OH) 2 Media by the Taguchi Method. Ind. Eng. Chem. Res. 2012, 51, 3887–3894. [Google Scholar] [CrossRef]
- Hernández, P.C.; Taboada, M.E.; Herreros, O.O.; Torres, C.M.; Ghorbani, Y. Chalcopyrite dissolution using seawater-based acidic media in the presence of oxidants. Hydrometallurgy 2015, 157, 325–332. [Google Scholar] [CrossRef]
- Copper. Available online: https://energy-resources.basf.com/global/en/mining_solutions/solvent_extraction/copper.html#accordion_v2-0abbbc400b-item-0e2e7ad963 (accessed on 19 June 2023).
- Jafari, H.; Abdollahi, H.; Gharabaghi, M.; Balesini, A.A. Solvent extraction of zinc from synthetic Zn-Cd-Mn chloride solution using D2EHPA: Optimization and thermodynamic studies. Sep. Purif. Technol. 2018, 197, 210–219. [Google Scholar] [CrossRef]
- Soltani, F.; Darabi, H.; Aram, R.; Ghadiri, M. Leaching and solvent extraction purification of zinc from Mehdiabad complex oxide ore. Sci. Rep. 2021, 11, 1566. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.K.; Begum, D.A. Solvent extraction of Fe3+ from chloride solution by D2EHPA in kerosene. Hydrometallurgy 1998, 50, 153–168. [Google Scholar] [CrossRef]
- Escobar, A.; Schimmel, K.A.; de Gyves, J.; de San Miguel, E.R. Hollow-fiber dispersion-free extraction and stripping of Pb(II) in the presence of Cd(II) using D2EHPA under recirculating operation mode. J. Chem. Technol. Biotechnol. 2004, 79, 961–973. [Google Scholar] [CrossRef]
- Martins, N.P.; Srivastava, S.; Simão, F.V.; Niu, H.; Perumal, P.; Snellings, R.; Illikainen, M.; Chambart, H.; Habert, G. Exploring the Potential for Utilization of Medium and Highly Sulfidic Mine Tailings in Construction Materials: A Review. Sustainability 2021, 13, 12150. [Google Scholar] [CrossRef]
- Veiga Simão, F.; Chambart, H.; Vandemeulebroeke, L.; Cappuyns, V. Incorporation of sulphidic mining waste material in ceramic roof tiles and blocks. J. Geochem. Explor. 2021, 225, 106741. [Google Scholar] [CrossRef]
- Wilson, A.M.; Bailey, P.J.; Tasker, P.A.; Turkington, J.R.; Grant, R.A.; Love, J.B. Solvent extraction: The coordination chemistry behind extractive metallurgy. Chem. Soc. Rev. 2014, 43, 123–134. [Google Scholar] [CrossRef]


| Levels | ||||
|---|---|---|---|---|
| Factors | Unit | −1 | 0 | +1 |
| A: NaCl | g/L | 10 | 35 | 60 |
| B: HCl | mol/L | 0.1 | 0.5 | 1.0 |
| C: Temperature | °C | 20 | 45 | 70 |
| Element | wt% | Element | wt% |
|---|---|---|---|
| Fe | 28.6 | Na | 0.2 |
| S | 24.9 | Ti | 0.2 |
| Si | 12.1 | Mn | 0.06 |
| Al | 3.0 | Ni | 0.03 |
| Zn | 1.0 | Sb | 0.03 |
| Mg | 0.9 | Co | 0.02 |
| Ca | 0.5 | P | 0.01 |
| As | 0.5 | Ba | 0.01 |
| K | 0.4 | Cr | 0.01 |
| Cu | 0.4 | Sr | 0.003 |
| Pb | 0.4 | Ag | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo Schueler, T.; Fernandes de Aguiar, P.; Yagmurlu, B.; Goldmann, D. Removal of Base Metals from Mine Tailings in Chloride- and Seawater-Based Media Followed by Solvent Extraction. Sustainability 2023, 15, 15515. https://doi.org/10.3390/su152115515
Azevedo Schueler T, Fernandes de Aguiar P, Yagmurlu B, Goldmann D. Removal of Base Metals from Mine Tailings in Chloride- and Seawater-Based Media Followed by Solvent Extraction. Sustainability. 2023; 15(21):15515. https://doi.org/10.3390/su152115515
Chicago/Turabian StyleAzevedo Schueler, Tamara, Paula Fernandes de Aguiar, Bengi Yagmurlu, and Daniel Goldmann. 2023. "Removal of Base Metals from Mine Tailings in Chloride- and Seawater-Based Media Followed by Solvent Extraction" Sustainability 15, no. 21: 15515. https://doi.org/10.3390/su152115515
APA StyleAzevedo Schueler, T., Fernandes de Aguiar, P., Yagmurlu, B., & Goldmann, D. (2023). Removal of Base Metals from Mine Tailings in Chloride- and Seawater-Based Media Followed by Solvent Extraction. Sustainability, 15(21), 15515. https://doi.org/10.3390/su152115515






