Abstract
Perlite waste materials with different particle sizes were evaluated as potential candidates for removing the malachite green (MG) and Congo red (CR) dyes from contaminated water. Two types of waste, referred to as coarse (CP) and fine (FP), with particle sizes of 0.075 mm, 0.045 mm, and 0.037 mm, were used. The samples were characterized using X-ray diffraction, X-ray fluorescence, Fourier transform infrared spectroscopy, and N2 adsorption/desorption. The adsorption efficiency of MG and CR was investigated by varying the parameters of pH, contact time, and initial concentration. The reduction in particle size significantly influenced the removal of the CR dye, leading to an increase in the adsorption rate of 23.9% and 45.5% for CP and FP, respectively. Conversely, the adsorption of the MG dye on the residues was not affected by different particle sizes. CP and FP exhibited a removal rate exceeding 70% for both dyes. The adsorption of MG and CR on the wastes was well-described by the Sips isotherm model. The results of adsorption kinetics were best fit by the Elovich model. Perlite waste materials have demonstrated significant potential for the adsorptive remove of cationic and anionic dyes from aqueous solutions.
1. Introduction
The contamination of water, resulting from natural and anthropogenic actions, poses significant risks to both public health and the global economy [1]. Rapid population growth, industrialization, and urbanization have significantly increased the consumption of drinking water and the discharge of wastewater, exacerbating contamination and/or scarcity of water resources on a global scale [2,3]. Industrial activities are the primary contributors to water pollution, especially in the textile, agricultural, pharmaceutical, cosmetic, and plastic sectors [4,5]. Effluents from these industries often contain a significant amount of dyes. Dye molecules have a negative impact on the biological cycle of aquatic life, as they block sunlight, reduce oxygen levels, and hinder the process of photosynthesis [6,7]. Furthermore, organic dyes, especially those classified as cationic and anionic, possess mutagenic, allergenic, and carcinogenic properties. This presents a significant concern for human well-being, as these dyes have the potential to rapidly accumulate within living cells, so impacting the entirety of the food chain [8,9].
Dyes such as malachite green (MG) and Congo red (CR) are often used in the paper and textile industries [10]. MG is a highly water-soluble cationic dye that is biopersistent and can cause harm to health even at extremely low concentrations [11]. It exhibits high toxicity, carcinogenicity, mutagenicity, and teratogenicity [12]. MG can cause damage to multiple organs such as the kidneys and liver, impair the immune and reproductive systems, and lead to developmental abnormalities in some mammals [13]. CR is a highly stable anionic azo dye that is light-resistant and easily soluble in water. Exposure to CR causes severe irritation to the eyes, skin, and digestive tract [14]. Moreover, this dye has the potential to induce the development of neoplastic growths in the human body, along with the manifestation of renal and cardiovascular disorders, as well as the occurrence of amyloidosis. Its process of degradation results in the formation of benzidine, a compound known for its mutagenic and carcinogenic characteristics, which has led to its prohibition in European countries, the United States, and Canada [15]. Considering the adverse impacts of dyes, it is essential to search for sustainable and effective technologies to remove or reduce the environmental pollution caused. Dye removal has become an very important, urgent and imperative environmental issue to protect human health and save aquatic ecosystems [16,17].
Numerous techniques have been established for the purpose of eliminating dyes, encompassing ion exchange [18], nanofiltration [19], membrane filtration [20], oxidation [21], and adsorption [22]. Among them, adsorption is considered the most promising solution due to its high efficiency, ease of use, low operational cost, and wide applicability to various types of organic and inorganic pollutants [23,24]. Activated carbon is the most employed adsorbent for the purpose of dye removal [25]. However, it is relatively expensive, experiences rapid saturation, and is challenging to regenerate [8]. This factor has continuously driven the search and development of efficient, environmentally sustainable, and cost-effective adsorbents for the treatment of contaminated water.
Perlite is an amorphous aluminum-silica volcanic rock that typically contains a high silica content (>72%) [26]. When exposed to heat, it expands and becomes a lightweight material widely used in the construction industry [27]. In recent times, perlite has garnered significant attention as a viable substitute adsorbent that is natural, low-cost, and non-toxic, with high adsorption properties [28,29,30,31,32,33,34]. On the other hand, both during the extraction of raw perlite and the production and processing of expanded perlite, a significant amount of waste is generated, which is often discarded in landfill or inappropriately in construction dumps. These wastes are difficult to handle and use, and create dust due to their extremely low density (50–150 kg/m3) [35]. As a result, their applicability is limited, mostly as a partial substitute for cement in concrete composition [36]. Hence, it is imperative to identify ways for promoting the utilization of perlite waste to reduce negative impacts on the environment, which mainly includes air and water pollution. It is extremely important to value the reuse and recycling of this waste, seeking new applications thus, promoting sustainable development [37].
In a recent study conducted by Selengil and Yildiz [38], expanded perlite waste was employed for the purpose of adsorbing methylene blue dye from aqueous solutions. The researchers successfully achieved a maximum adsorption capacity of 9.91 mg/g. However, the adsorptive potential of perlite waste has not yet been fully explored, and there is a significant knowledge gap in this area. Therefore, the objective of this work was to investigate the potential utilization of perlite waste with varying particle sizes as potential adsorbents for the remediation of water contaminated with MG and CR dyes. The parameters of pH, contact time, and initial concentrations of MG and CR were investigated. The adsorption mechanism was evaluated through the conduction of isothermal and kinetic studies.
2. Materials and Methods
2.1. Materials
Perlite wastes were generated as a residual by-product from a company located in northeastern Brazil. Two types of perlite waste were used: coarse waste (CP) and a fine waste (FP). CP waste was generated during the mining of perlite, while FP waste was generated after expansion perlite (heated to >900 °C). Table 1 presents the chemical composition of CP and FP wastes.

Table 1.
Chemical composition (wt%) of CP and FP wastes utilized in this study.
The waste was crushed using a hammer mill (Servirtec, CT-12061, Tubarão, Brazil) operating at a rotational speed of 3400 rpm for 5 min. After processing, the waste went through the dry sieving process to obtain perlite waste in different particle sizes. Three sizes of sieves (Bertel Indústria Metalúrgica, Caieiras, Brazil) were used to provide particle sizes of 0.075 mm, 0.045 mm, and 0.037 mm. Table 2 displays the nomenclatures of the samples.

Table 2.
Nomenclature of the samples of the perlite wastes in distinct granulometries.
2.2. Chemicals
The malachite green dye, with a chemical formula of [C23H25ClN2] and a molecular weight of 364.90 g/mol, was purchased from Synth (Diadema, Brazil). The Congo Red dye, with a chemical formula of [C32H22N6Na2O6S2] and a molecular weight of 696.68 g/mol, was acquired from Dinâmica Química (Indaiatuba, Brazil). Ammonium hydroxide (NH4OH, 25% NH3 basis) and hydrochloric acid (HCl, 37% analytical grade) were provided from VETEC (Duque de Caxias, Brazil).
2.3. Waste Characterizations
X-ray diffraction (Shimadzu, XRD-6000, Kyoto, Japan) is the main technique employed for obtaining information into the crystalline structure of a material [39], and was performed using CuKα (λ = 1.54 Å) radiation, operated at 40 kV and 30 mA, in the 2θ angular range of 10–40° with a step size of 0.02°. Chemical composition was analyzed by X-ray fluorescence spectrometry (Shimadzu, EDX-720, Kyoto, Japan). Fourier-transform infrared spectra (FTIR) were recorded in the scan range from 4000 cm−1 to 400 cm−1, with a resolution of 4 cm−1 and 32 scans (Bruker, Vertex-70, Billerica, MA, USA). The surface area and average pore diameter were determined by nitrogen adsorption/desorption measurements at 77 K (Quantachrome, Autosorb iQ, Anton Paar, Graz, Austria). The surface area was determined using the Brunner–Emmett–Teller (BET) method [40]. The results obtained were processed using the OriginLab 2018 software.
2.4. Batch Adsorption Studies
Adsorption experiments were performed to examine the adsorptive capacity of CP and FP wastes under the following parameters: pH variation, dye concentration variation, and contact time. During the adsorption experiments, a single parameter was manipulated while keeping all other variables constant. The adsorbent–solution systems subjected to agitation at 150 rpm in a refrigerated shaker incubator (Novatecnica, NT 735, Piracicaba, Brazil) at a temperature of 25 °C for up to 360 min. After this period, the samples were subjected to centrifugation (Fanem, 206, Guarulhos, Brazil) for 10 min at 3600 rpm. The residual concentration of MG and CR in the supernatants was determined using a UV-Vis spectrophotometer (Shimadzu, UV–1800, Kyoto, Japan) at wavelengths of 618 nm and 501 nm for MG and CR, respectively. The experiments were conducted in triplicate.
To analyze the effect of the initial concentrations of MG and CR dyes, 20 mg of CP and FP wastes were added to amber glass bottles, each containing 20 mL of a dye solution with concentrations ranging from 5 to 200 mg/L. The experiment was conducted at a pH of 7 for a duration of 360 min. The contact time (15 min to 360 min) and pH (3 to 11) parameters were analyzed using 20 mg of wastes in 20 mL of solution (MG or CR) with a concentration of 50 mg/L. The adsorption data obtained were plotted in the OriginLab 2018 software. Isothermal and kinetic constants were calculated using nonlinear regression with the help of OriginLab 2018 software.
The adsorbed amount at equilibrium (qe) and the percentage of dye removal (%R) were determined according to Equations (1) and (2), respectively:
where qe (mg/g) is the amount of dye adsorbed by the waste in the equilibrium, Co (mg/L) is the initial concentration of dye, Ce (mg/L) is the concentration of dye when equilibrium is reached, V (L) is volume of dye solution, and m (g) is the mass of the waste.
qe = [(Co − Ce) V]/m
%R = [(Co − Ce)/Co] × 100
3. Results and Discussion
3.1. Characterizations
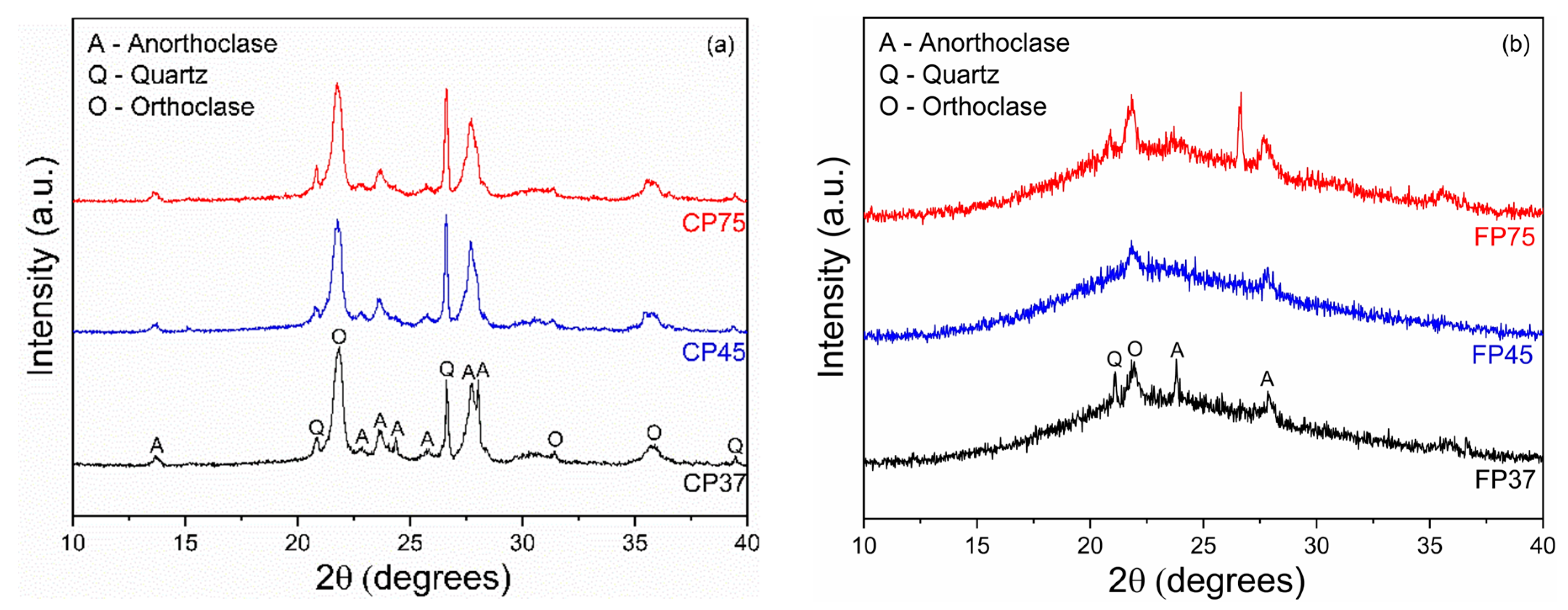
Figure 1a,b present the XRD patterns of CP and FP wastes in different particle sizes. The wastes exhibited crystalline phases of quartz (JCPDS 46-1045), anorthoclase (alkali feldspar, JCPDS 09-0478), and orthoclase (potassium feldspar, JCPDS 31-0966). These phases are related to the early formation of microcrystals during the ascent of magma toward the Earth’s surface [41,42]. Additionally, the presence of an amorphous halo is observed in the 2θ range between 20 to 30° (CP waste) and between 15 to 30° (FP waste), characteristic of perlite [43]. Similar findings have been documented in the existing literature [27,44,45].
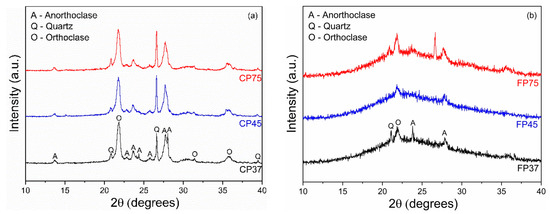
Figure 1.
XRD patterns of (a) coarse and (b) fine perlite waste samples at different particle sizes.
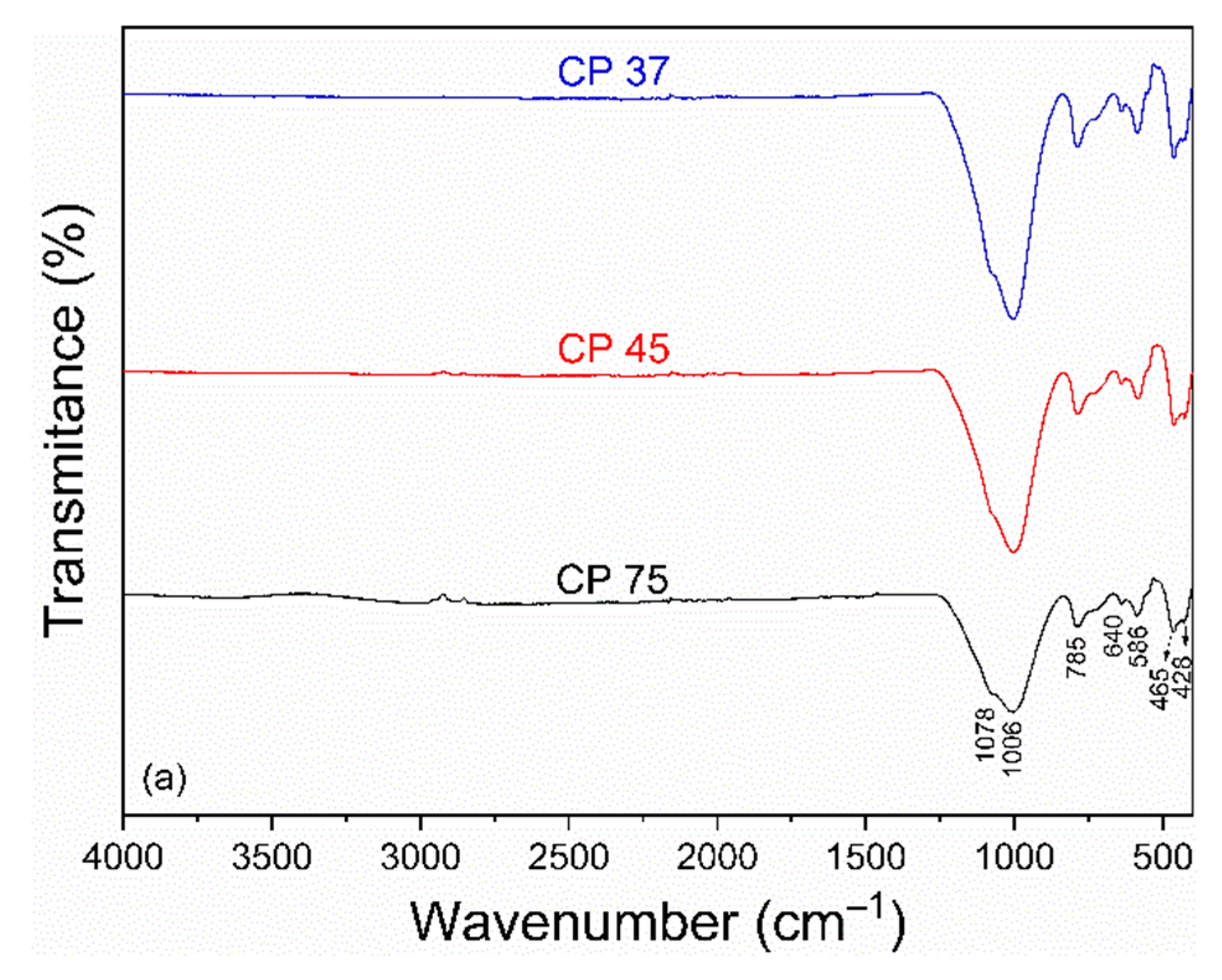
The FTIR spectra of the samples CP and FP wastes in different particle sizes are shown in Figure 2a,b. For CP waste (Figure 2a), bands at 1078 and 640 cm–1 were observed, related to the asymmetric stretching vibration of the Si-O-Si group [46]. The band at 785 cm–1 was attributed to the symmetric stretching vibration of the Si-O-Si group, characteristic of amorphous silicates [47]. Bands at 1006 and 586 cm–1 were associated with the stretching vibration of the Si-O-Al group [46]. Bands located at 465 and 428 cm–1 corresponded to the stretching of the O-Si-O and Si-O groups, respectively [48].
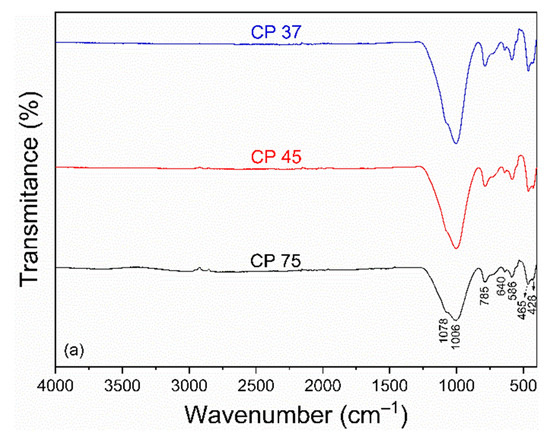
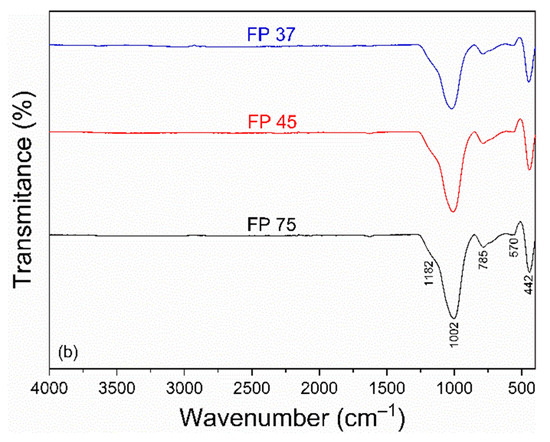
Figure 2.
FTIR spectra of (a) coarse and (b) fine perlite waste samples at different particle sizes.
The spectrum of FP waste (Figure 2b) reveals the disappearance of bands at 640, 586, and 465 cm–1. The remaining bands were slightly shifted, appearing at 1182, 1002, 570, and 442 cm–1. The observed modifications are linked to the condensation process of silanol groups during to the thermal treatment to produce expanded perlite [49]. It is worth noting that the different particle sizes resulted in slight changes in the FTIR spectra, which were reflected in the intensities of the bands. The spectra of FP showed bands with lower intensities compared to the bands in the CP spectra. The researchers Udvardi et al. [50] noticed this trend during their analysis of the impact of particle size on monomineralic powders utilizing FTIR.
The specific surface area (SSA) and the average pore diameter (Dp) CP and FP wastes in different particle sizes are listed in Table 3. The reduction in particle size is accompanied by an increase in the surface area. FP waste exhibits a higher SSA compared to CP waste. These values may be related to a more porous structure obtained after the thermal treatment of natural perlite [46]. On the other hand, FP waste showed lower Dp values compared to CP waste, potentially attributable to the generation and distribution of novel pores during the process of perlite calcination.

Table 3.
Values of the specific surface area (SSA) and the average pore diameter (Dp) of the samples of coarse and fine perlite wastes in different particle sizes.
3.2. Adsorption Study
3.2.1. Effect of pH Variation
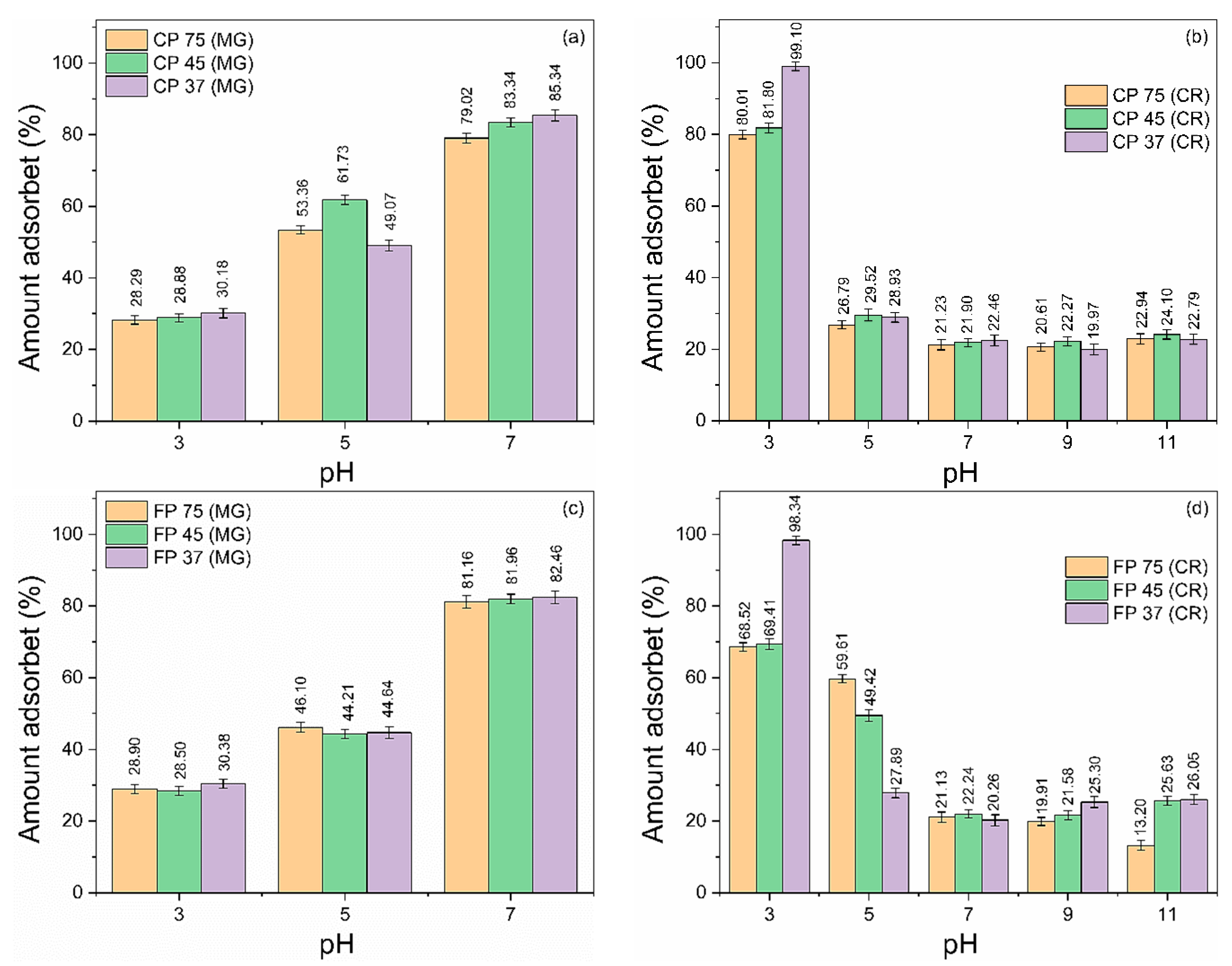
pH is one of the most critical and essential parameters that significantly impact the adsorption phenomena. The pH has a dual impact on the adsorbent, influencing both its surface charge and the ionization and speciation of the adsorbate [51,52]. Figure 3a–d shows the effect of pH on the adsorption of MG and CR on CP and FP waste samples. The experiments were carried out using initial concentrations of MG and CR dyes of 50 mg/L and a waste quantity of 20 mg, in the pH ranges of 3 to 7 (for MG) and 3 to 11 (for CR) for 360 min. pH adjustments of the solutions were made using 1 M HCl or NH4OH. Above pH 7, the MG dye solution gradually loses color intensity, becoming completely colorless. This phenomenon is commonly referred to as alkali fading [53,54]. Thus, in this study, the effects of pH at higher levels (pH > 7) could not be estimated. The adsorbed amount of MG and CR dyes on the wastes was highly influenced by the initial pH levels. The amount of adsorbed MG increased proportionally to the increase in pH, while the adsorbed amount of CR was inversely proportional.
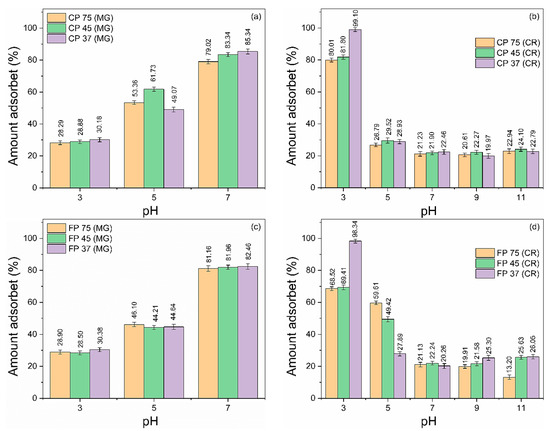
Figure 3.
Influence of pH variation on the adsorption of (a,c) CV and (b,d) CR dyes in coarse and fine perlite wastes with different particle sizes.
In an acidic environment, the H+ ions existing in the dye solution promote the protonation of the surface functional groups of the perlite, increasing its positive surface charge [29]. The lower adsorption rate observed can be attributed to the competitive interaction between the MG dye molecules and the H+ ions by adsorption sites, together with the repulsion generated between by the positively charged surface of the wastes and positive MG molecules [55]. On the other hand, the CR molecules with a negative charge demonstrated a significant electrostatic attraction to the waste’s positively charged surface, resulting in an increased effectiveness of adsorption (>96%) [56].
Raising pH increases the concentration of OH– ions, promoting the deprotonation of the surface of CP and FP waste, progressively increasing the negative surface charge [57], which favors the adsorption of positively charged MG molecules at higher pH levels, reaching the highest removal percentages (>79%). In contrast, negatively charged CR molecules compete with the OH– ions in the solution, in addition to the repulsion that occurs between such molecules and the negative surface of the wastes, significantly reducing the removal efficiency [58].
At pH 3, for the CR dye, samples with smaller particle sizes (CP 37 and FP 37) showed removal exceeding 98%. At pH 7, the CP 37 and FP 37 samples provided removal efficiency exceeding 80% for MG. Based on these results, pH values of 3 (for CR) and 7 (for MG) were considered ideal for conducting further adsorption experiments as they provided the highest removal efficiency.
Furthermore, in general, it was noted that the percentage of dye removal increased with a decrease in the particle size of the waste. Such a trend was expected since the adsorption process is a surface phenomenon, and reducing particle sizes lead to an increase in surface area (see Table 3), resulting in a higher removal rate [59]. However, very small particles acquire high reactivity, which facilitates aggregation and reduces the adsorption capacity of the adsorbent [60], which would explain, in some cases, why the CP 45 and FP 45 samples adsorbed more than the CP 37 and FP 37 samples.
3.2.2. Effect of Initial Dye Concentration and Isothermal Study
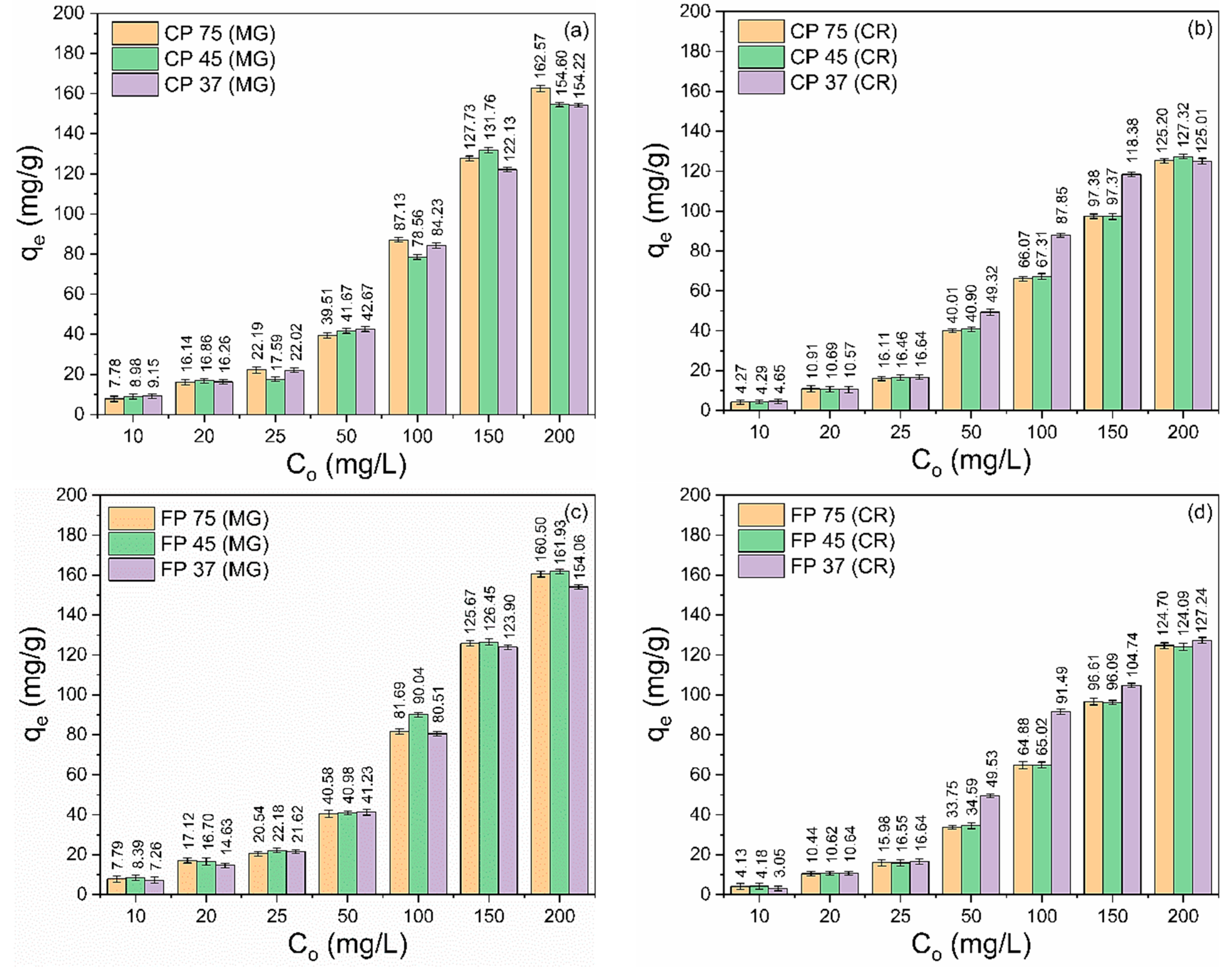
The variation in the initial concentration of MG and CR dyes on the adsorption capacity of CP and FP wastes is illustrated in Figure 4a–d. Different concentrations of MG and CR dyes (10–200 mg/L) were analyzed at pH 7 (for MG) and pH 3 (for CR), with a contact time of 360 min and a waste quantity of 20 mg. The data clearly demonstrate that there is a notable enhancement in the adsorption capability of the wastes as the starting concentration increases (Co). The observed phenomenon can be attributed to the increase in the driving force at the solid–liquid interface caused by the rising concentration gradient, which facilitates mass transfer and increases the likelihood of collision between the waste surface and dye molecules, providing higher adsorption capacity [61,62,63].
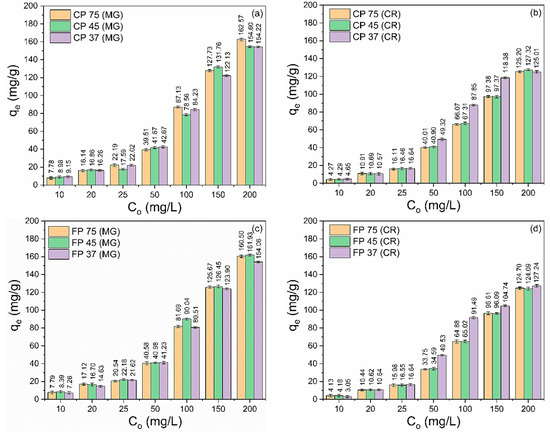
Figure 4.
Effect of variation initial concentration of (a,c) MG and CR (b,d) dyes on the adsorption capacity of (a,b) coarse and (c,d) fine perlite wastes with different particle sizes.
For MG adsorption (Figure 4a,c), the values of the maximum adsorption capacity followed the order: CP 75 (162.57 mg/g) > FP 45 (161.93 mg/g) > FP 75 (160.50 mg/g) > CP 45 (154.60 mg/g) > CP 37 (154.22 mg/g) > FP 37 (154.06 mg/g). On the other hand, for CR adsorption (Figure 4b,d), the values of the maximum adsorption capacity were CP 45 (127.32 mg/g) > FP 75 (127.24 mg/g) > CP 75 (125.20 mg/g) > CP 37 (125.01 mg/g) > FP 45 (124.69 mg/g) > FP 37 (124.09 mg/g).
Isothermal models are essential to qualitatively explain the adsorbent–adsorbate interaction, the mechanism of the adsorption process, and the adsorptive capacity of the adsorbent [64]. Experimental data for MG and CR adsorption were fitted to the nonlinear Langmuir, Freundlich, Redlich–Peterson (R–P), Temkin, and Sips models, corresponding to Equations (3)–(7), respectively [22,65]:
where qe (mg/g) is the amount of dye adsorbed by the waste in the equilibrium; qmax (mg/g) is the maximum adsorption capacity of the waste; Ce (mg/L) is the concentration of dye adsorbed at equilibrium; KL (L/mg), KF ((mg/g) (L/mg)1/n), KRP (L/g), and KS (L/mg) are the constants for the Langmuir, Freundlich, Redlich–Peterson (R–P), and Sips models, respectively; n and ns correspond to the heterogeneity factor; β and αRP (1/mg) are the constants for the R–P model; AT (L/mg) and bT (J/mol) are the constants for the Temkin model.
qe = (qmax KL Ce)/(1 + KL Ce)
qe = KF Cen–1
qe = (KRP Ce)/(1 + αRP Ceβ)
qe = (RT/bT) lnAT + (RT/bT) lnCe
qe = (qmax KS Cens)/(1 + KS Cens)
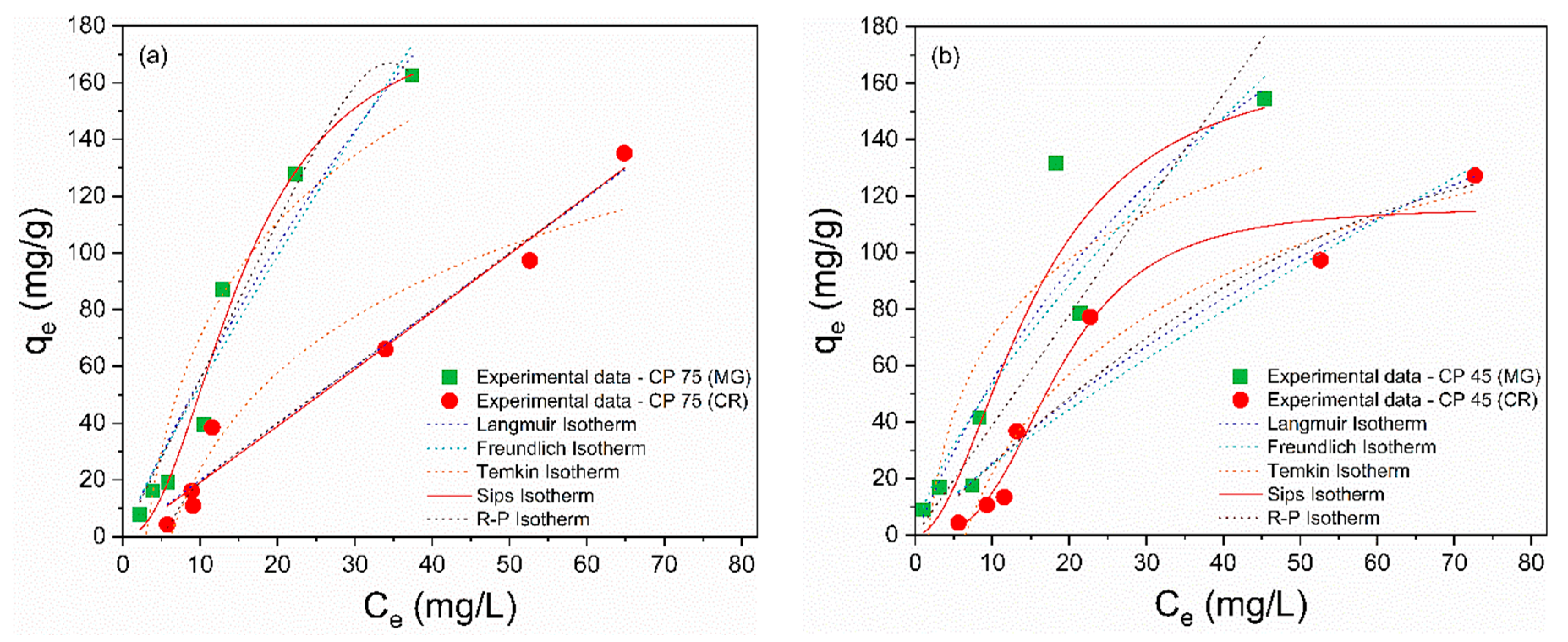
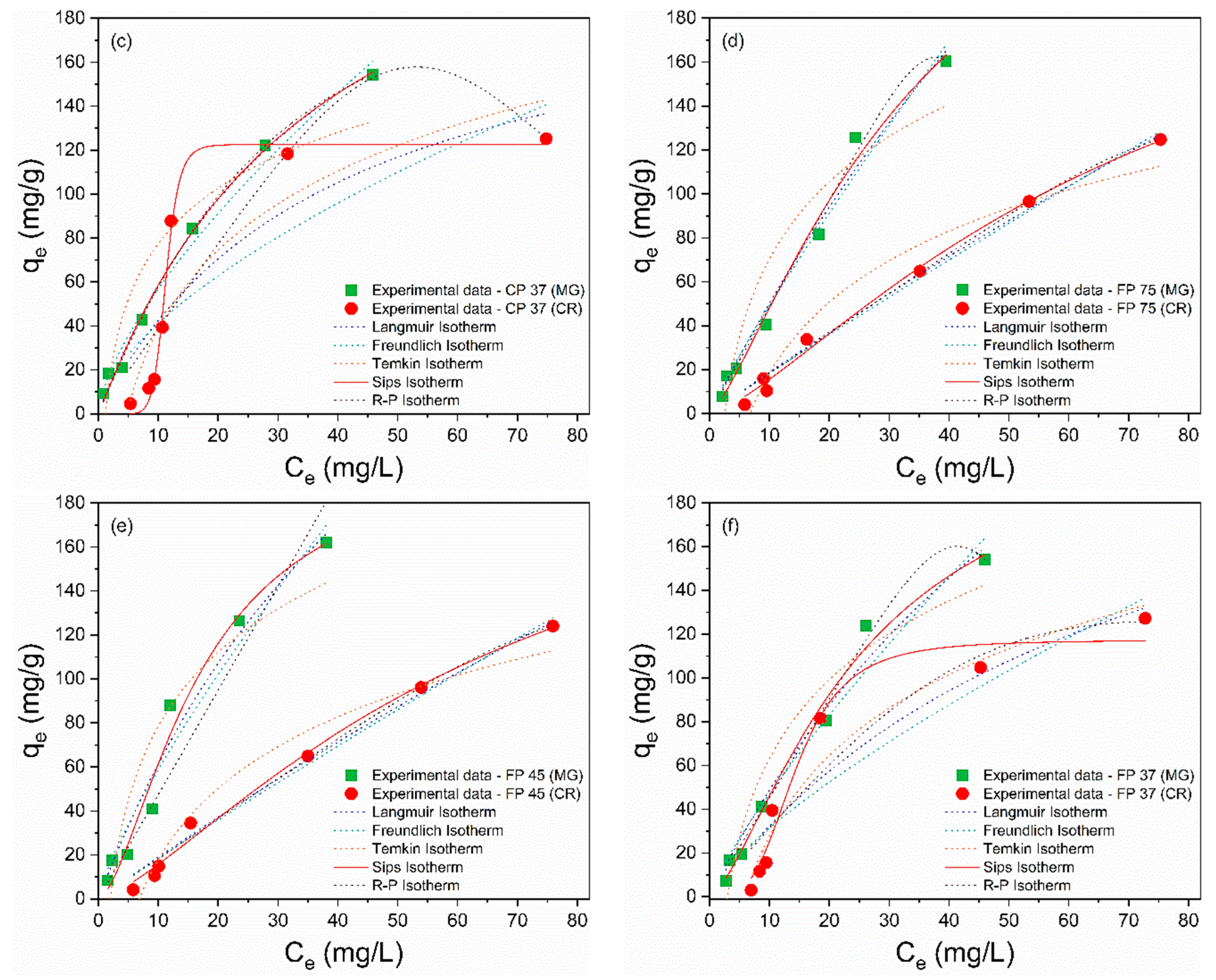
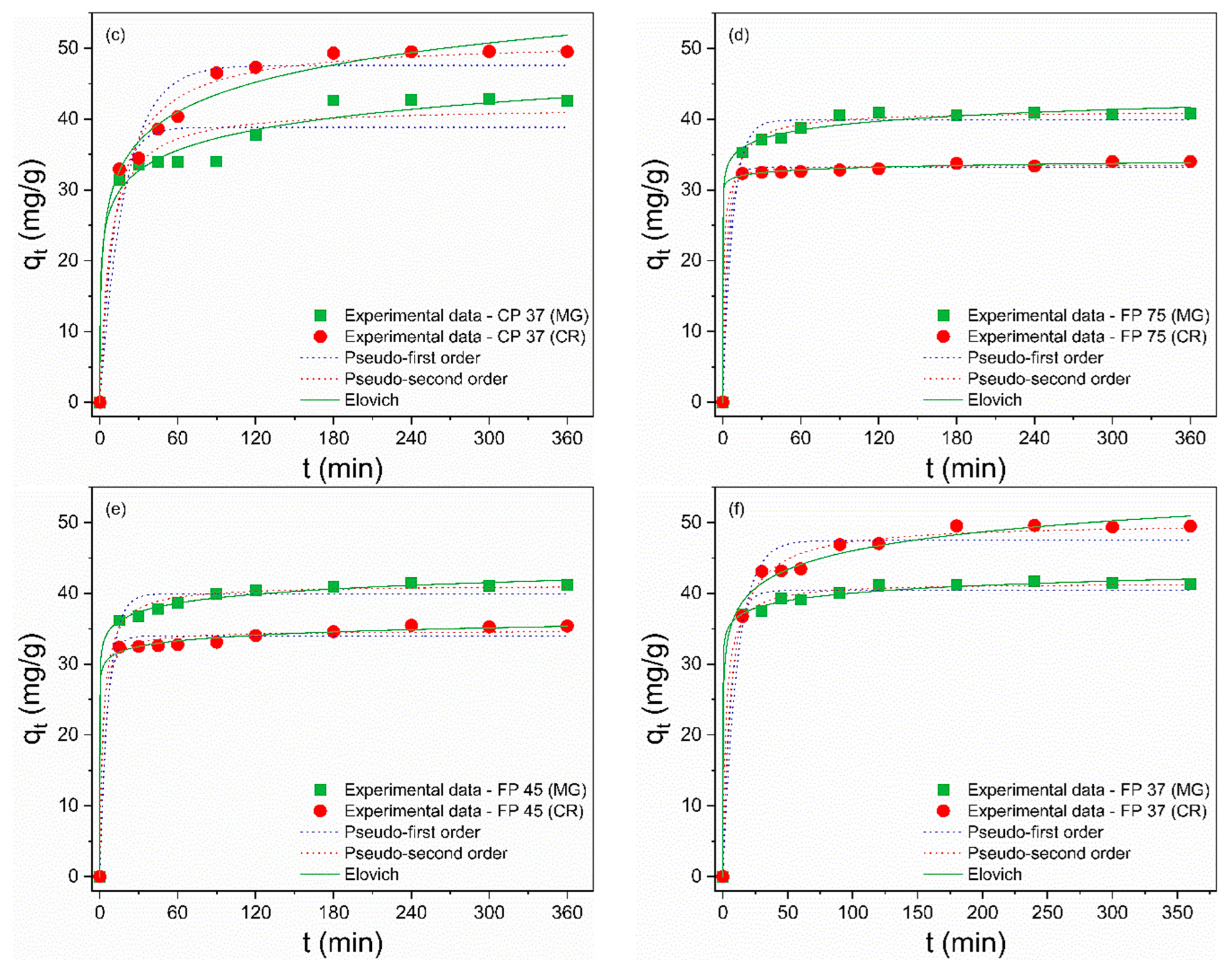
The adsorption isotherms are depicted in Figure 5a–f, and the calculated isothermal parameters are presented in Table 4 and Table 5. According to the obtained data, the Sips model provided a better description of the adsorption process of MG and CR on perlite waste samples, with correlation coefficients (R²) closer to unity and the smallest error values. Furthermore, the qmax values from the Sips model were more consistent with the experimental data. The Sips model is a combination of the Langmuir and Freundlich models and is used to describe adsorption on heterogeneous surfaces [66]. When the heterogeneity constant of Sips (ns) is greater than or equal to 1, adsorption approaches the Langmuir isotherm with monolayer adsorption, while ns < 1 indicates adsorption approaching the Freundlich isotherm with multilayer adsorption [67]. Therefore, the ideal fit of the experimental data to the Sips isothermal model proposes that the adsorption of MG and CR molecules is a heterogeneous phenomenon occurring in a monolayer on the surface of CP and FP wastes [68], as the ns values were greater than 1.
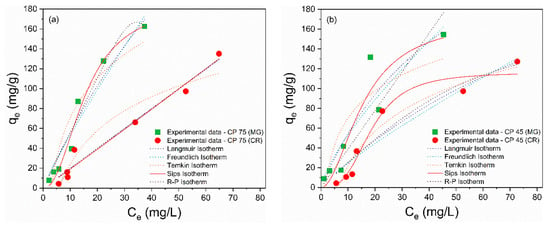
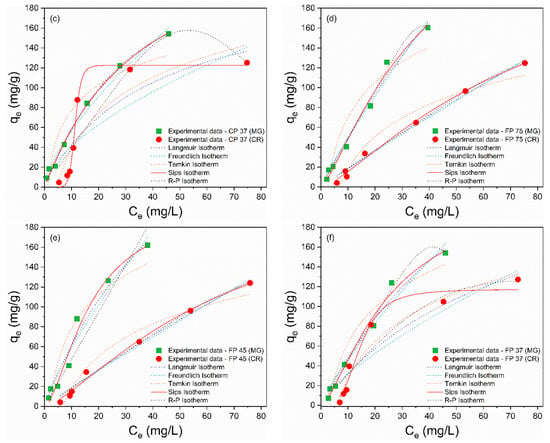
Figure 5.
Nonlinear isothermal models (Langmuir, Freundlich, Temkin, Sips, and R–P) of MG and CR for (a) CP 75, (b) CP 45, (c) CP 37, (d) FP 75, (e) FP 45, and (f) FP 37.

Table 4.
Langmuir, Freundlich, Temkin, Sips, and Redlich–Peterson (R–P) isothermal parameters for MG adsorption.

Table 5.
Langmuir, Freundlich, Temkin, Sips, and Redlich–Peterson (R–P) isothermal parameters for CR adsorption.
3.2.3. Effect of Contact Time and Kinetic Study
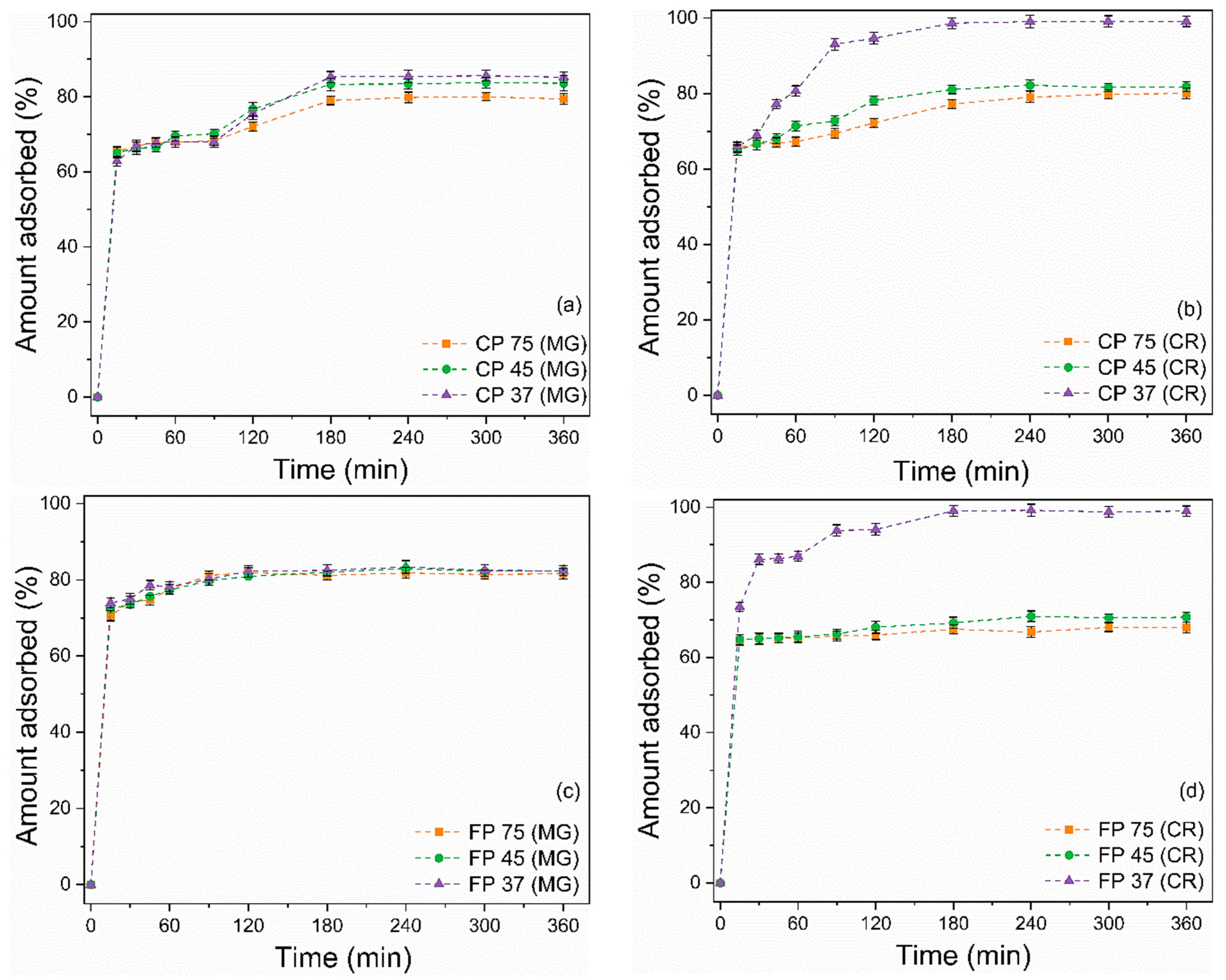
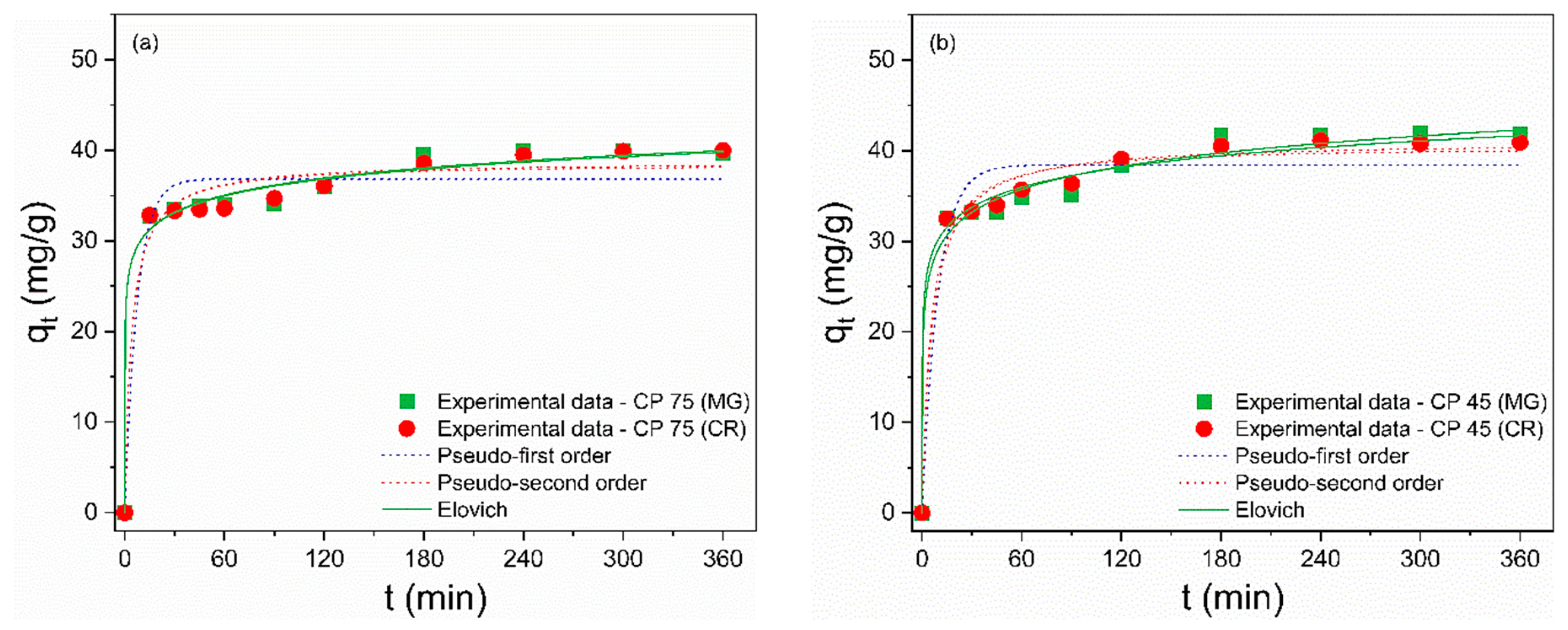
Figure 6a–d illustrates the effect of contact time on the adsorption of the dyes MG and CR by CP and FP wastes. Solutions of MG and CR presenting concentrations of 50 mg/L, a quantity of 20 mg of waste, pH 7 (for MG), and pH 3 (for CR) were used to assess adsorption kinetics. At first, the adsorption process for MG and CR was relatively fast, as the wastes were able to adsorb more than 60% of both dyes within the first 15 min. Over time, there was a decrease in the increasing trend of the adsorption rate until equilibrium was reached at 180 min. The rapid adsorption in the initial stages is attributed to the presence of many active sites available on the surface of the adsorbent [69]. The following decline in the adsorption rate can be explained by the saturation of active sites and a reduction in available active sites [70].
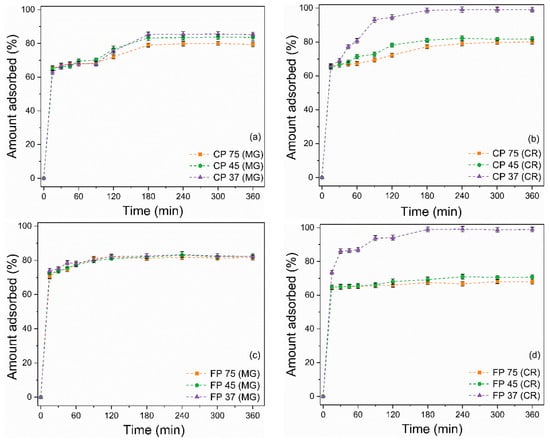
Figure 6.
Effect of contact time on the adsorption of (a,c) MG and (b,d) CR on (a,b) coarse and (c,d) fine perlite wastes with different particle sizes.
When equilibrium was reached, the removal values for MG were 70.4%, 83.6%, 85.2%, 81.6%, 82.4%, and 82.3% for CP 75, CP 45, CP 37, FP 75, FP 45, and FP 37, respectively. For CR, the removal values were 80%, 81.8%, 99.1%, 68.1%, 70.7%, and 98.9% for CP 75, CP 45, CP 37, FP 75, FP 45, and FP 37, respectively. It is worth highlighting that for the CR dye, reducing the particle size from CP 75 to CP 37 resulted in a 23.9% increase in removal efficiency. Similarly, by reducing the particle size from FP 75 to FP 37, there was a 45.5% increase in the adsorption rate. Conversely, for the adsorption of MG dye, reducing the particle size did not prove effective in increasing the adsorptive capacity of the wastes.
The investigation of adsorption kinetics is an essential factor that yields information about the mass transfer mechanism and the rate of adsorption of the adsorbate by the adsorbent [71]. To describe the kinetic process, the nonlinear pseudo-first-order (PFO), pseudo-second-order (PSO), and Elovich models were used to fit the experimental data and are described by Equations (8)–(10), respectively [72,73]:
where k1 (min–1) and k2 (g/(mg·min)) are the rate constants for the pseudo-first-order (PFO) and pseudo-second-order (PSO) adsorption, respectively; qt (mg/g) is the adsorption capacity of the waste at time t (min), and qe (mg/g) is the adsorption capacity of the waste in the equilibrium; α (mg·g–1/min) and β (g/mg) are the constants for the Elovich model. The kinetic constants were calculated from the nonlinear curves (Figure 7a–f) and are presented in Table 6 and Table 7. The Elovich model provided the best fits to the experimental data for the MG and CR dyes, as it showed R² values closer to unity and the smallest error values. This model describes that the dominant adsorption mechanism is chemisorption and assumes that the surfaces of the residues are energetically heterogeneous [74,75]. Furthermore, the constants α and β refer to adsorption and desorption rates, respectively. The adsorption process is considered viable when the values of α are much greater than the values of β, implying that the adsorption rate is higher than the desorption rate [76], suggesting the feasibility of MG and CR adsorption on CP and FP wastes. This kinetic model was also supported by the fact that the Sips model demonstrated the highest level of agreement with the isothermal data.
qt = qe [1 − exp(−k1 t)]
qt = (qe2 k2 t)/(1 + k2 qe t)
qt = α + βlnt
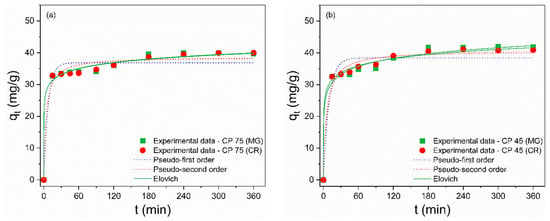
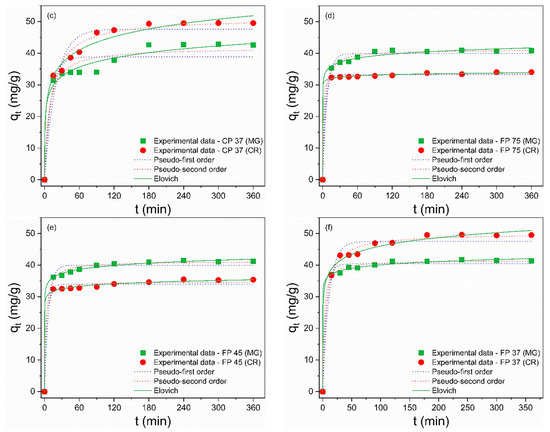
Figure 7.
Nonlinear curves of the pseudo-first order, pseudo-second-order, and Elovich adsorption kinetic models of MG and CR dyes for (a) CP 75, (b) CP 45, (c) CP 37, (d) FP 75, (e) FP 45 and (f) FP 37.

Table 6.
Constants of the pseudo-first-order, pseudo-second-order, and Elovich kinetic models for MG dye adsorption.

Table 7.
Constants of the pseudo-first-order, pseudo-second-order, and Elovich kinetic models for CR dye adsorption.
3.2.4. Comparison with Other Sustainable Adsorbents
Table 8 compares the maximum adsorption capacity of MG and CR adsorbed on the wastes with previously published work elsewhere that worked with other sustainable adsorbents. Therefore, it is evident that perlite wastes exhibit superior adsorptive properties for both cationic and anionic dyes compared to alternative low-cost materials. This demonstrates the potential of CP and FP samples to treat wastewater contaminated with dyes, thus presenting alternative solutions to address the ecological challenges associated with the accumulation of waste.

Table 8.
Comparison of the maximum adsorption capacity (Qmax) of different adsorbents for MG and CR dyes.
4. Conclusions
The adsorptive potentials of coarse and fine perlite waste for the removal of the MG and CR dyes were investigated. Smaller particle sizes significantly influenced the adsorption of the anionic dye CR. Samples CP 75, FP 75, CP 45, and FP 45 exhibited an adsorption rate of approximately 70% to 80%, while samples CP 37 and FP 37 achieved a removal rate exceeding 98%. On the other hand, the reduction in particle size had no substantial impact on the adsorption of the cationic dye MG. Based on the kinetic study, the experimental data for the adsorption of MG and CR fit well with the Elovich model. The utilization of isotherm models demonstrated that the adsorption data exhibited a better fit to the Sips model. The results revealed that CP and FP wastes demonstrated satisfactory potential for the adsorption of cationic and anionic dyes, with an adsorption rate exceeding 70% for MG and CR. Therefore, wastes derived from perlite mining and processing have shown promise as alternative and sustainable options for the elimination of anionic and cationic dyes from contaminated water, providing a simple, environmentally friendly, and economically efficient solution for transforming mining waste into highly effective adsorbent.
Ultimately, future research could investigate the use of perlite waste to remove other types of dyes, as well as mixing between them, since several types of dyes are found in the same effluent. We seek to optimize the conditions of the adsorption process to work under optimal conditions and carry out studies on the recyclability of perlite waste, seeking sustainable and functional regeneration to make the most of the use of this waste.
Author Contributions
Experimental data, data curation, and formal analysis, J.I.S.F., V.C.S. and P.F.S.; conceptualization, funding acquisition, and project administration, G.A.N., R.R.M. and J.M.C.; formal analysis and writing—review and editing, V.C.S., R.R.M. and A.M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Coordenação de Aperfeiçoamento de Pessoal e Nível Superior–Brasil (CAPES)–Finance Code 001 (scholarships granted to Josenildo Isidro Santos Filho, to Vanderlane Cavalcanti da Silva and to Paulysendra Felipe Silva), grants number, 88887.838730/2023-00, 88887.814270/2023–00 and 88887.814267/2023-00; Brazilian research funding agency CNPq, grant Nos. 420004/2018-1, 309771/2021-8, and 309234/2020-4, to the FAPESQ, grant No. Notice 006/2018 PRONEX, and for the financial support.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Babuji, P.; Thirumalaisamy, S.; Duraisamy, K.; Periyasamy, G. Human Health Risks Due to Exposure to Water Pollution: A Review. Water 2023, 15, 2532. [Google Scholar] [CrossRef]
- Yu, Q.; Sun, Z.; Shen, J.; Xu, X.; Han, Q.; Zhu, M. The Nonlinear Effect of New Urbanization on Water Pollutant Emissions: Empirical Analysis Based on the Panel Threshold Model. J. Environ. Manage. 2023, 345, 118564. [Google Scholar] [CrossRef]
- Islam, T.; Repon, M.R.; Islam, T.; Sarwar, Z.; Rahman, M.M. Impact of Textile Dyes on Health and Ecosystem: A Review of Structure, Causes, and Potential Solutions. Environ. Sci. Pollut. Res. 2023, 30, 9207–9242. [Google Scholar] [CrossRef] [PubMed]
- Reghioua, A.; Barkat, D.; Jawad, A.H.; Abdulhameed, A.S.; Khan, M.R. Synthesis of Schiff’s Base Magnetic Crosslinked Chitosan-Glyoxal/ZnO/Fe3O4 Nanoparticles for Enhanced Adsorption of Organic Dye: Modeling and Mechanism Study. Sustain. Chem. Pharm. 2021, 20, 100379. [Google Scholar] [CrossRef]
- Farrokhi, Z.; Sadjadi, S.; Raouf, F.; Bahri-Laleh, N. Novel Bio-Based Pd/Chitosan-Perlite Composite Bead as an Efficient Catalyst for Rapid Decolorization of Azo Dye. Inorg. Chem. Commun. 2022, 143, 109734. [Google Scholar] [CrossRef]
- Ahmad, A.; Tariq, S.; Zaman, J.U.; Martin Perales, A.I.; Mubashir, M.; Luque, R. Recent Trends and Challenges with the Synthesis of Membranes: Industrial Opportunities towards Environmental Remediation. Chemosphere 2022, 306, 135634. [Google Scholar] [CrossRef]
- Singh, A.; Kaushik, V.; Kumari, V.; Goswami, A.; Nain, S. Impact of Variable Metal Oxide Loadings on Photocatalytic and Antibacterial Behaviour of CNT-NiO Nanocomposites. Bionanoscience 2023. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, S.; Tian, Z.; Duan, G.; Pan, H.; Yue, Y.; Li, S.; Jian, S.; Yang, W.; Liu, K. MOFs Meet Wood: Reusable Magnetic Hydrophilic Composites toward Efficient Water Treatment with Super-High Dye Adsorption Capacity at High Dye Concentration. Chem. Eng. J. 2022, 446, 136851. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, L.; Su, Y.; Yu, H.; Liu, H.; Qian, S.; Zheng, W.; Zhao, Y. Zr-MOFs Loaded on Polyurethane Foam by Polydopamine for Enhanced Dye Adsorption. J. Environ. Sci. 2021, 101, 177–188. [Google Scholar] [CrossRef]
- Sreelekshmi, P.B.; Pillai, R.R.; Meera, A.P. Controlled Synthesis of Novel Graphene Oxide Nanoparticles for the Photodegradation of Organic Dyes. Top. Catal. 2022, 65, 1659–1668. [Google Scholar] [CrossRef]
- You, X.; Zhou, R.; Zhu, Y.; Bu, D.; Cheng, D. Adsorption of Dyes Methyl Violet and Malachite Green from Aqueous Solution on Multi-Step Modified Rice Husk Powder in Single and Binary Systems: Characterization, Adsorption Behavior and Physical Interpretations. J. Hazard. Mater. 2022, 430, 128445. [Google Scholar] [CrossRef]
- Qiao, Q.; Zhou, H.; Guo, F.; Shu, R.; Liu, S.; Xu, L.; Dong, K.; Bai, Y. Facile and Scalable Synthesis of Mesoporous Composite Materials from Coal Gasification Fine Slag for Enhanced Adsorption of Malachite Green. J. Clean. Prod. 2022, 379, 134739. [Google Scholar] [CrossRef]
- Gharavi-nakhjavani, M.S.; Niazi, A.; Hosseini, H.; Aminzare, M.; Dizaji, R.; Tajdar-oranj, B.; Mirza Alizadeh, A. Malachite Green and Leucomalachite Green in Fish: A Global Systematic Review and Meta-Analysis. Environ. Sci. Pollut. Res. 2023, 30, 48911–48927. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-Y.; Li, W.; Du, N.; Lu, H.-Q.; Meng, L.-D.; Huang, K.-Y.; Li, K. Preparation of Quaternary Ammonium Magnetic Chitosan Microspheres and Their Application for Congo Red Adsorption. Carbohydr. Polym. 2022, 297, 119995. [Google Scholar] [CrossRef] [PubMed]
- Extross, A.; Waknis, A.; Tagad, C.; Gedam, V.V.; Pathak, P.D. Adsorption of Congo Red Using Carbon from Leaves and Stem of Water Hyacinth: Equilibrium, Kinetics, Thermodynamic Studies. Int. J. Environ. Sci. Technol. 2023, 20, 1607–1644. [Google Scholar] [CrossRef]
- Ahmadian, M.; Jaymand, M. Interpenetrating Polymer Network Hydrogels for Removal of Synthetic Dyes: A Comprehensive Review. Coord. Chem. Rev. 2023, 486, 215152. [Google Scholar] [CrossRef]
- Shi, Y.; Chang, Q.; Zhang, T.; Song, G.; Sun, Y.; Ding, G. A Review on Selective Dye Adsorption by Different Mechanisms. J. Environ. Chem. Eng. 2022, 10, 108639. [Google Scholar] [CrossRef]
- Li, D.-P.; Zhang, Y.-R.; Zhao, X.-X.; Zhao, B.-X. Magnetic Nanoparticles Coated by Aminoguanidine for Selective Adsorption of Acid Dyes from Aqueous Solution. Chem. Eng. J. 2013, 232, 425–433. [Google Scholar] [CrossRef]
- Cao, X.-L.; Yan, Y.-N.; Zhou, F.-Y.; Sun, S.-P. Tailoring Nanofiltration Membranes for Effective Removing Dye Intermediates in Complex Dye-Wastewater. J. Memb. Sci. 2020, 595, 117476. [Google Scholar] [CrossRef]
- Ravadelli, M.; da Costa, R.E.; Lobo-Recio, M.A.; Akaboci, T.R.V.; Bassin, J.P.; Lapolli, F.R.; Belli, T.J. Anoxic/Oxic Membrane Bioreactor Assisted by Electrocoagulation for the Treatment of Azo-Dye Containing Wastewater. J. Environ. Chem. Eng. 2021, 9, 105286. [Google Scholar] [CrossRef]
- Hao, N.; Nie, Y.; Xu, Z.; Jin, C.; Fyda, T.J.; Zhang, J.X.J. Microfluidics-Enabled Acceleration of Fenton Oxidation for Degradation of Organic Dyes with Rod-like Zero-Valent Iron Nanoassemblies. J. Colloid Interface Sci. 2020, 559, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.C.; Araújo, M.E.B.; Rodrigues, A.M.; Cartaxo, J.M.; Menezes, R.R.; Neves, G.A. Adsorption Behavior of Acid-Treated Brazilian Palygorskite for Cationic and Anionic Dyes Removal from the Water. Sustainability 2021, 13, 3954. [Google Scholar] [CrossRef]
- Wan, X.; Rong, Z.; Zhu, K.; Wu, Y. Chitosan-Based Dual Network Composite Hydrogel for Efficient Adsorption of Methylene Blue Dye. Int. J. Biol. Macromol. 2022, 222, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Jadoun, S.; Fuentes, J.P.; Urbano, B.F.; Yáñez, J. A Review on Adsorption of Heavy Metals from Wastewater Using Conducting Polymer-Based Materials. J. Environ. Chem. Eng. 2023, 11, 109226. [Google Scholar] [CrossRef]
- Sultana, M.; Rownok, M.H.; Sabrin, M.; Rahaman, M.H.; Alam, S.M.N. A Review on Experimental Chemically Modified Activated Carbon to Enhance Dye and Heavy Metals Adsorption. Clean. Eng. Technol. 2022, 6, 100382. [Google Scholar] [CrossRef]
- Babas, H.; Kaichouh, G.; Khachani, M.; Karbane, M.E.; Chakir, A.; Guenbour, A.; Bellaouchou, A.; Warad, I.; Zarrouk, A. Equilibrium and Kinetic Studies for Removal of Antiviral Sofosbuvir from Aqueous Solution by Adsorption on Expanded Perlite: Experimental, Modelling and Optimization. Surf. Interfaces 2021, 23, 100962. [Google Scholar] [CrossRef]
- Różycka, A.; Pichór, W. Effect of Perlite Waste Addition on the Properties of Autoclaved Aerated Concrete. Constr. Build. Mater. 2016, 120, 65–71. [Google Scholar] [CrossRef]
- Sassi, W.; Msaadi, R.; Ardhaoui, N.; Ammar, S.; Nafady, A. Selective/Simultaneous Batch Adsorption of Binary Textile Dyes Using Amorphous Perlite Powder: Aspects of Central Composite Design Optimization and Mechanisms. J. Environ. Health Sci. Eng. 2023, 21, 441–454. [Google Scholar] [CrossRef]
- Khoshraftar, Z.; Masoumi, H.; Ghaemi, A. On the Performance of Perlite as a Mineral Adsorbent for Heavy Metals Ions and Dye Removal from Industrial Wastewater: A Review of the State of the Art. Case Stud. Chem. Environ. Eng. 2023, 8, 100385. [Google Scholar] [CrossRef]
- Msaadi, R.; Yahia, A.; Sassi, W.; Ammar, S. Adsorption of Methyl Orange onto Perlite: Optimization, Adsorption Kinetics, and Thermodynamic Studies. Chem. Africa 2023, 6, 933–944. [Google Scholar] [CrossRef]
- Saufi, H.; El Alouani, M.; Alehyen, S.; El Achouri, M.; Aride, J.; Taibi, M. Photocatalytic Degradation of Methylene Blue from Aqueous Medium onto Perlite-Based Geopolymer. Int. J. Chem. Eng. 2020, 2020, 9498349. [Google Scholar] [CrossRef]
- Damiyine, B.; Guenbour, A.; Boussen, R. Adsorption of Rhodamine B Dye onto Expanded Perlite from Aqueous Solution: Kinetics, Equilibrium and Thermodynamics. J. Mater. Environ. Sci. 2017, 8, 345–355. [Google Scholar]
- Loqman, A.; El Bali, B.; El Gaidoumi, A.; Boularbah, A.; Kherbeche, A.; Lützenkirchen, J. The First Application of Moroccan Perlite as Industrial Dyes Removal. Silicon 2021, 14, 2813–2838. [Google Scholar] [CrossRef]
- Kumar, M.D.K.M.; Padmanaban, R.; Kumar, M. Adsorption Modelling Studies for the Removal of Rose Bengal Dye from Aqueous Solutions Using a Natural Adsorbent Perlite. Indian J. Chem. Technol. 2022, 28, 604–611. [Google Scholar]
- Kotwica, Ł.; Pichór, W.; Kapeluszna, E.; Różycka, A. Utilization of Waste Expanded Perlite as New Effective Supplementary Cementitious Material. J. Clean. Prod. 2017, 140, 1344–1352. [Google Scholar] [CrossRef]
- Osacký, M.; Pálková, H.; Hudec, P.; Czímerová, A.; Galusková, D.; Vítková, M. Effect of Alkaline Synthesis Conditions on Mineralogy, Chemistry and Surface Properties of Phillipsite, P and X Zeolitic Materials Prepared from Fine Powdered Perlite by-Product. Microporous Mesoporous Mater. 2020, 294, 109852. [Google Scholar] [CrossRef]
- Golik, V.I.; Klyuev, R.V.; Martyushev, N.V.; Zyukin, D.A.; Karlina, A.I. Technology for Nonwaste Recovery of Tailings of the Mizur Mining and Processing Plant. Metallurgist 2023, 66, 1476–1480. [Google Scholar] [CrossRef]
- Selengil, U.; Yildiz, D. Investigation of the Methylene Blue Adsorption onto Waste Perlite. Desalin. Water Treat. 2022, 262, 235–247. [Google Scholar] [CrossRef]
- Bosikov, I.I.; Martyushev, N.V.; Klyuev, R.V.; Tynchenko, V.S.; Kukartsev, V.A.; Eremeeva, S.V.; Karlina, A.I. Complex Assessment of X-Ray Diffraction in Crystals with Face-Centered Silicon Carbide Lattice. Crystals 2023, 13, 528. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Dişçi, E.; Polat, R. The Influence of Nano-CaO and Nano-Al2O3 and Curing Conditions on Perlite Based Geopolymer Concrete Produced by the One-Part Mixing Method. Constr. Build. Mater. 2022, 346, 128484. [Google Scholar] [CrossRef]
- Lexa, J.; Varga, P.; Uhlik, P.; Koděra, P.; Biroň, A.; Rajnoha, M. Perlite Deposits of the Central Slovakia Volcanic Field (Western Carpathians): Geology and Properties. Geol. Carpathica 2021, 72, 253–281. [Google Scholar] [CrossRef]
- Aziz, A.; Bellil, A.; El Amrani El Hassani, I.-E.; Fekhaoui, M.; Achab, M.; Dahrouch, A.; Benzaouak, A. Geopolymers Based on Natural Perlite and Kaolinic Clay from Morocco: Synthesis, Characterization, Properties, and Applications. Ceram. Int. 2021, 47, 24683–24692. [Google Scholar] [CrossRef]
- de Oliveira Neto, R.E.; Cartaxo, J.D.M.; Rodrigues, A.M.; Barros, S.V.A.; da Costa, F.P.; Neves, G.D.A.; Menezes, R.R. New Sustainable Mortar Compositions Containing Perlite Waste. Clean Technol. Environ. Policy 2022, 24, 1403–1415. [Google Scholar] [CrossRef]
- Chakir, A.; Bessiere, J.; Kacemi, K.E.L.; Marouf, B. A Comparative Study of the Removal of Trivalent Chromium from Aqueous Solutions by Bentonite and Expanded Perlite. J. Hazard. Mater. 2002, 95, 29–46. [Google Scholar] [CrossRef]
- Raji, M.; Nekhlaoui, S.; El Hassani, I.-E.E.A.; Essassi, E.M.; Essabir, H.; Rodrigue, D.; Bouhfid, R.; el kacem Qaiss, A. Utilization of Volcanic Amorphous Aluminosilicate Rocks (Perlite) as Alternative Materials in Lightweight Composites. Compos. Part B Eng. 2019, 165, 47–54. [Google Scholar] [CrossRef]
- Wheelwright, W.; Cooney, R.P.; Ray, S.; Zujovic, Z.; de Silva, K. Ultra-High Surface Area Nano-Porous Silica from Expanded Perlite: Formation and Characterization. Ceram. Int. 2017, 43, 11495–11504. [Google Scholar] [CrossRef]
- Sarı, A.; Sahinoglu, G.; Tuzen, M. Antimony (III) Adsorption from Aqueous Solution Using Raw Perlite and Mn-Modified Perlite: Equilibrium, Thermodynamic, and Kinetic Studies. Ind. Eng. Chem. Res. 2012, 51, 6877–6886. [Google Scholar] [CrossRef]
- Roulia, M.; Chassapis, K.; Kapoutsis, J.A.; Kamitsos, E.I.; Savvidis, T. Influence of Thermal Treatment on the Water Release and the Glassy Structure of Perlite. J. Mater. Sci. 2006, 41, 5870–5881. [Google Scholar] [CrossRef]
- Udvardi, B.; Kovács, I.J.; Fancsik, T.; Kónya, P.; Bátori, M.; Stercel, F.; Falus, G.; Szalai, Z. Effects of Particle Size on the Attenuated Total Reflection Spectrum of Minerals. Appl. Spectrosc. 2016, 71, 1157–1168. [Google Scholar] [CrossRef]
- Ali, F.; Ali, N.; Bibi, I.; Said, A.; Nawaz, S.; Ali, Z.; Salman, S.M.; Iqbal, H.M.N.; Bilal, M. Adsorption Isotherm, Kinetics and Thermodynamic of Acid Blue and Basic Blue Dyes onto Activated Charcoal. Case Stud. Chem. Environ. Eng. 2020, 2, 100040. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Ukhurebor, K.E.; Onyancha, R.B.; Osibote, O.A.; Darmokoesoemo, H.; Kusuma, H.S. Fly Ash-Based Adsorbent for Adsorption of Heavy Metals and Dyes from Aqueous Solution: A Review. J. Mater. Res. Technol. 2021, 14, 2751–2774. [Google Scholar] [CrossRef]
- Kooravand, M.; Haddadi, H.; Asadpour, S.; Farhadian, S.; Sarmast, N.; Asfaram, A. Simply Synthesized Ca-Al-LDH-Thiosulfate as Adsorbent for Removal of Malachite Green from Aqueous Solution. Polyhedron 2023, 246, 116653. [Google Scholar] [CrossRef]
- Widiartyasari Prihatdini, R.; Suratman, A.; Siswanta, D. Linear and Nonlinear Modeling of Kinetics and Isotherm of Malachite Green Dye Adsorption to Trimellitic-Modified Pineapple Peel. Mater. Today Proc. 2023, 88, 33–40. [Google Scholar] [CrossRef]
- Haladu, S.A. Highly Efficient Adsorption of Malachite Green Dye onto a Cross-Linked PH-Responsive Cycloterpolymer Resin: Kinetic, Equilibrium and Thermodynamic Studies. J. Mol. Liq. 2022, 357, 119115. [Google Scholar] [CrossRef]
- Silva, V.C.; Araújo, M.E.B.; Rodrigues, A.M.; Vitorino, M.D.B.C.; Cartaxo, J.M.; Menezes, R.R.; Neves, G.A. Adsorption Behavior of Crystal Violet and Congo Red Dyes on Heat-Treated Brazilian Palygorskite: Kinetic, Isothermal and Thermodynamic Studies. Materials 2021, 14, 5688. [Google Scholar] [CrossRef]
- Mohammed, I.; Yaseri, A.; Al Shehri, D.; Mahmoud, M. Basalt Mineral Surface Charge and the Effect of Mineralization on Its Colloidal Stability: Implications of Subsurface CO2 Storage. Fuel 2024, 356, 129569. [Google Scholar] [CrossRef]
- Madan, S.; Shaw, R.; Tiwari, S.; Tiwari, S.K. Adsorption Dynamics of Congo Red Dye Removal Using ZnO Functionalized High Silica Zeolitic Particles. Appl. Surf. Sci. 2019, 487, 907–917. [Google Scholar] [CrossRef]
- Alam, M.Z.; Bari, M.N.; Kawsari, S. Statistical Optimization of Methylene Blue Dye Removal from a Synthetic Textile Wastewater Using Indigenous Adsorbents. Environ. Sustain. Indic. 2022, 14, 100176. [Google Scholar] [CrossRef]
- Yang, D.-L.; Liu, R.-K.; Wei, Y.; Sun, Q.; Wang, J.-X. Micro-Sized Nanoaggregates: Spray-Drying-Assisted Fabrication and Applications. Particuology 2024, 85, 22–48. [Google Scholar] [CrossRef]
- Ramirez Arenas, L.; Le Coustumer, P.; Ramseier Gentile, S.; Zimmermann, S.; Stoll, S. Removal Efficiency and Adsorption Mechanisms of CeO2 Nanoparticles onto Granular Activated Carbon Used in Drinking Water Treatment Plants. Sci. Total Environ. 2023, 856, 159261. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jia, X.; Jin, M.; Guo, R.; Niu, B.; Yan, H.; Wang, H. A Magnetically Recyclable Carboxyl-Functionalized Chitosan Composite for Efficiently Removing Methyl Orange from Wastewater: Isotherm, Kinetics, Thermodynamic, and Adsorption Mechanism. Int. J. Biol. Macromol. 2023, 253, 126631. [Google Scholar] [CrossRef] [PubMed]
- Abbou, B.; Lebkiri, I.; Ouaddari, H.; El Amri, A.; Achibat, F.E.; Kadiri, L.; Ouass, A.; Lebkiri, A.; Rifi, E.H. Improved Removal of Methyl Orange Dye by Adsorption Using Modified Clay: Combined Experimental Study Using Surface Response Methodology. Inorg. Chem. Commun. 2023, 155, 111127. [Google Scholar] [CrossRef]
- Dana, M.; Jamshidi, P.; Shemirani, F. Effect of Ionic Liquid to Highly Selective Removal of Cd2+ onto Carbon Nanotube: Optimized by Plackett–Burman Design Method. Chem. Pap. 2023. [Google Scholar] [CrossRef]
- Araújo, M.E.B.; Silva, V.C.; Fernandes, J.V.; Cartaxo, J.M.; Rodrigues, A.M.; Menezes, R.R.; de Araújo Neves, G. Innovative Adsorbents Based on Bentonite Mining Waste for Removal of Cationic Dyes from Wastewater. Environ. Sci. Pollut. Res. 2022, 29, 90446–90462. [Google Scholar] [CrossRef]
- Petrović, J.; Ercegović, M.; Simić, M.; Kalderis, D.; Koprivica, M.; Milojković, J.; Radulović, D. Novel Mg-Doped Pyro-Hydrochars as Methylene Blue Adsorbents: Adsorption Behavior and Mechanism. J. Mol. Liq. 2023, 376, 121424. [Google Scholar] [CrossRef]
- Watwe, V.; Kulkarni, S.; Kulkarni, P. Development of Dried Uncharred Leaves of Ficus Benjamina as a Novel Adsorbent for Cationic Dyes: Kinetics, Isotherm, and Batch Optimization. Ind. Crops Prod. 2023, 195, 116449. [Google Scholar] [CrossRef]
- Nascimento, V.X.; Pinto, D.; Lütke, S.F.; da Silva, M.C.F.; Machado, F.M.; Lima, É.C.; Silva, L.F.O.; Dotto, G.L. Brilliant Blue FCF Dye Adsorption Using Magnetic Activated Carbon from Sapelli Wood Sawdust. Environ. Sci. Pollut. Res. 2023, 30, 58684–58696. [Google Scholar] [CrossRef]
- Gohr, M.S.; Abd-Elhamid, A.I.; El-Shanshory, A.A.; Soliman, H.M.A. Adsorption of Cationic Dyes onto Chemically Modified Activated Carbon: Kinetics and Thermodynamic Study. J. Mol. Liq. 2022, 346, 118227. [Google Scholar] [CrossRef]
- Vakili, A.; Zinatizadeh, A.A.; Rahimi, Z.; Zinadini, S.; Mohammadi, P.; Azizi, S.; Karami, A.; Abdulgader, M. The Impact of Activation Temperature and Time on the Characteristics and Performance of Agricultural Waste-Based Activated Carbons for Removing Dye and Residual COD from Wastewater. J. Clean. Prod. 2023, 382, 134899. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Kinetic Models: Physical Meanings, Applications, and Solving Methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zang, Z.; Zhang, S.; Ouyang, G.; Han, R. Enhanced Fluoride Adsorption from Aqueous Solution by Zirconium (IV)-Impregnated Magnetic Chitosan Graphene Oxide. Int. J. Biol. Macromol. 2021, 182, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Piri, F.; Mollahosseini, A.; Hosseini, M.M. Enhanced Adsorption of Dyes on Microwave-Assisted Synthesized Magnetic Zeolite-Hydroxyapatite Nanocomposite. J. Environ. Chem. Eng. 2019, 7, 103338. [Google Scholar] [CrossRef]
- Khnifira, M.; Boumya, W.; Attarki, J.; Mahsoune, A.; Abdennouri, M.; Sadiq, M.; Kaya, S.; Barka, N. Adsorption Characteristics of Dopamine by Activated Carbon: Experimental and Theoretical Approach. J. Mol. Struct. 2023, 1278, 134964. [Google Scholar] [CrossRef]
- Wei, W.; Jiao, L.; Li, W.; Tang, X.; Xie, W.; Yu, H.; Li, W.; Lei, F. Removal of High-Molecular-Weight Hexose Alkaline Degradation Products by Rosin-Based Anion Adsorbent: Kinetics, Thermodynamics, and Mechanisms. Food Control 2023, 145, 109410. [Google Scholar] [CrossRef]
- de la Luz-Asunción, M.; Pérez-Ramírez, E.E.; Martínez-Hernández, A.L.; García-Casillas, P.E.; Luna-Bárcenas, J.G.; Velasco-Santos, C. Adsorption and Kinetic Study of Reactive Red 2 Dye onto Graphene Oxides and Graphene Quantum Dots. Diam. Relat. Mater. 2020, 109, 108002. [Google Scholar] [CrossRef]
- Gupta, N.; Kushwaha, A.K.; Chattopadhyaya, M.C. Application of Potato (Solanum tuberosum) Plant Wastes for the Removal of Methylene Blue and Malachite Green Dye from Aqueous Solution. Arab. J. Chem. 2016, 9, S707–S716. [Google Scholar] [CrossRef]
- Sharma, N.; Nandi, B.K. Utilization of Sugarcane Baggase, an Agricultural Waste to Remove Malachite Green Dye from Aqueous Solutions. J. Mater. Environ. Sci 2013, 4, 1052–1065. [Google Scholar]
- Sonawane, G.H.; Shrivastava, V.S. Kinetics of Decolourization of Malachite Green from Aqueous Medium by Maize Cob (Zea maize): An Agricultural Solid Waste. Desalination 2009, 247, 430–441. [Google Scholar] [CrossRef]
- Khan, T.A.; Rahman, R.; Khan, E.A. Adsorption of Malachite Green and Methyl Orange onto Waste Tyre Activated Carbon Using Batch and Fixed-Bed Techniques: Isotherm and Kinetics Modeling. Model. Earth Syst. Environ. 2017, 3, 38. [Google Scholar] [CrossRef]
- Mishra, S.P.; Patra, A.R.; Das, S. Methylene Blue and Malachite Green Removal from Aqueous Solution Using Waste Activated Carbon. Biointerface Res. Appl. Chem 2020, 11, 7410–7421. [Google Scholar]
- Porkodi, K.; Vasanth Kumar, K. Equilibrium, Kinetics and Mechanism Modeling and Simulation of Basic and Acid Dyes Sorption onto Jute Fiber Carbon: Eosin Yellow, Malachite Green and Crystal Violet Single Component Systems. J. Hazard. Mater. 2007, 143, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, C.; Arasi, D.J.S.E. Removal of Congo Red from Wastewater by Adsorption onto Waste Red Mud. Chemosphere 1997, 34, 401–417. [Google Scholar] [CrossRef]
- Somasekhara Reddy, M.C.; Sivaramakrishna, L.; Varada Reddy, A. The Use of an Agricultural Waste Material, Jujuba Seeds for the Removal of Anionic Dye (Congo Red) from Aqueous Medium. J. Hazard. Mater. 2012, 203–204, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Stjepanović, M.; Velić, N.; Galić, A.; Kosović, I.; Jakovljević, T.; Habuda-Stanić, M. From Waste to Biosorbent: Removal of Congo Red from Water by Waste Wood Biomass. Water 2021, 13, 279. [Google Scholar] [CrossRef]
- Abbas, M.; Trari, M. Kinetic, Equilibrium and Thermodynamic Study on the Removal of Congo Red from Aqueous Solutions by Adsorption onto Apricot Stone. Process Saf. Environ. Prot. 2015, 98, 424–436. [Google Scholar] [CrossRef]
- Tor, A.; Cengeloglu, Y. Removal of Congo Red from Aqueous Solution by Adsorption onto Acid Activated Red Mud. J. Hazard. Mater. 2006, 138, 409–415. [Google Scholar] [CrossRef]
- Namasivayam, C.; Jeyakumar, R.; Yamuna, R.T. Dye Removal from Wastewater by Adsorption on ‘Waste’ Fe(III)/Cr(III) Hydroxide. Waste Manag. 1994, 14, 643–648. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).