Synthesis and Characterization of Na3SbS4 Solid Electrolytes via Mechanochemical and Sintered Solid-State Reactions: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Synthesis of Na3SbS4 Solid Electrolyte
2.3. Material Characterization
2.4. Electrochemical Characterization
2.5. Cell Assembly for Sodium-Ion Battery Testing
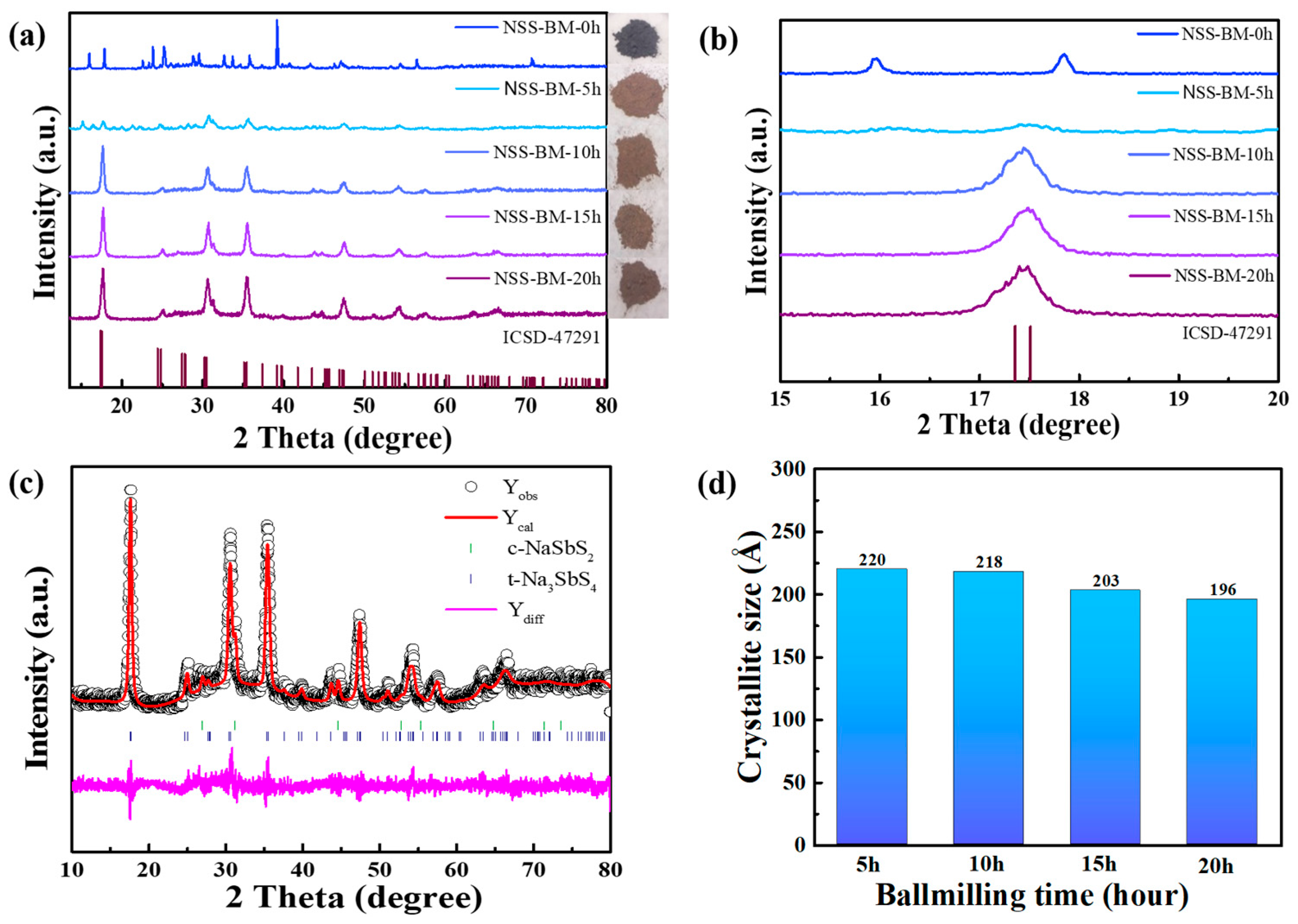
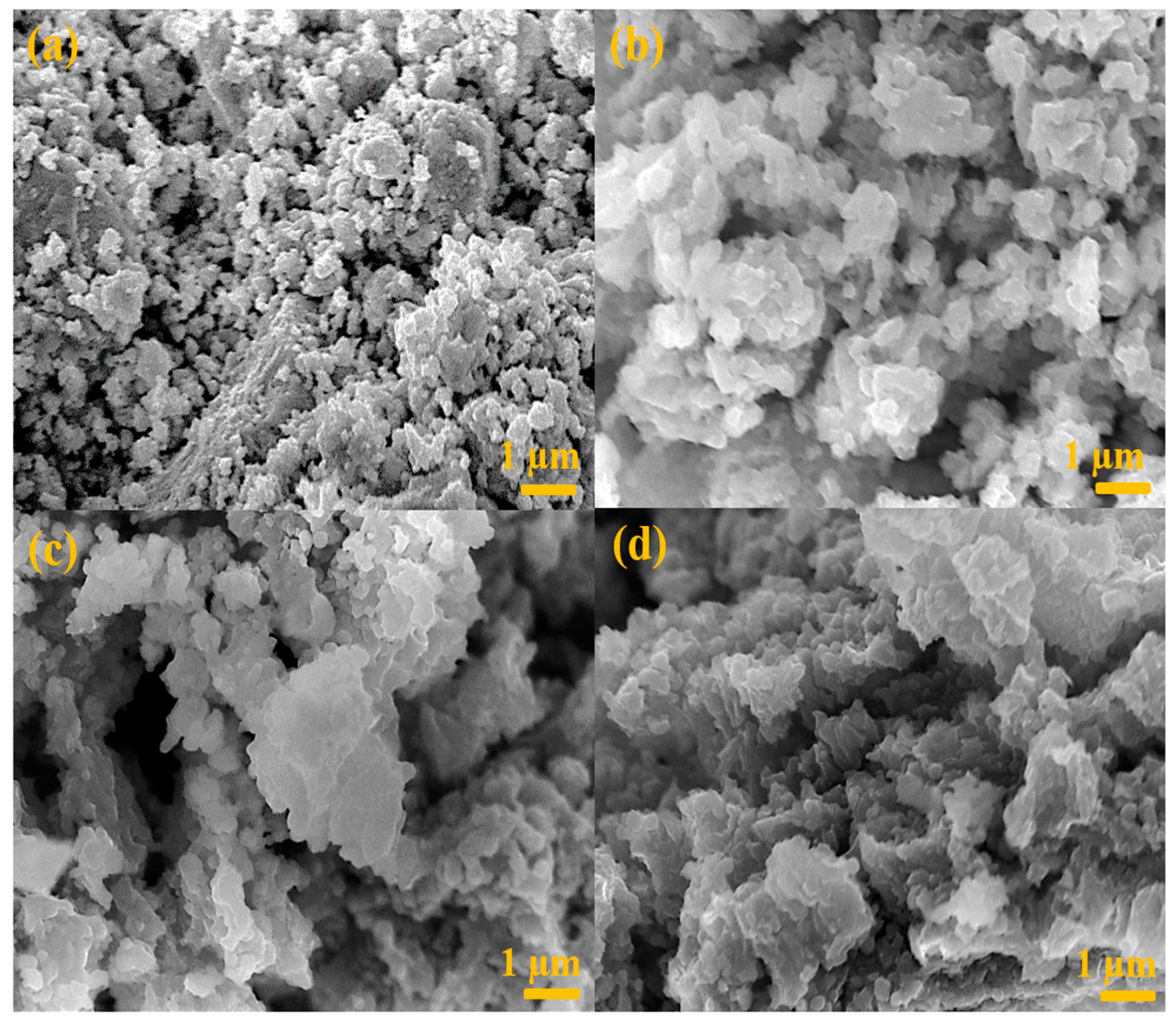
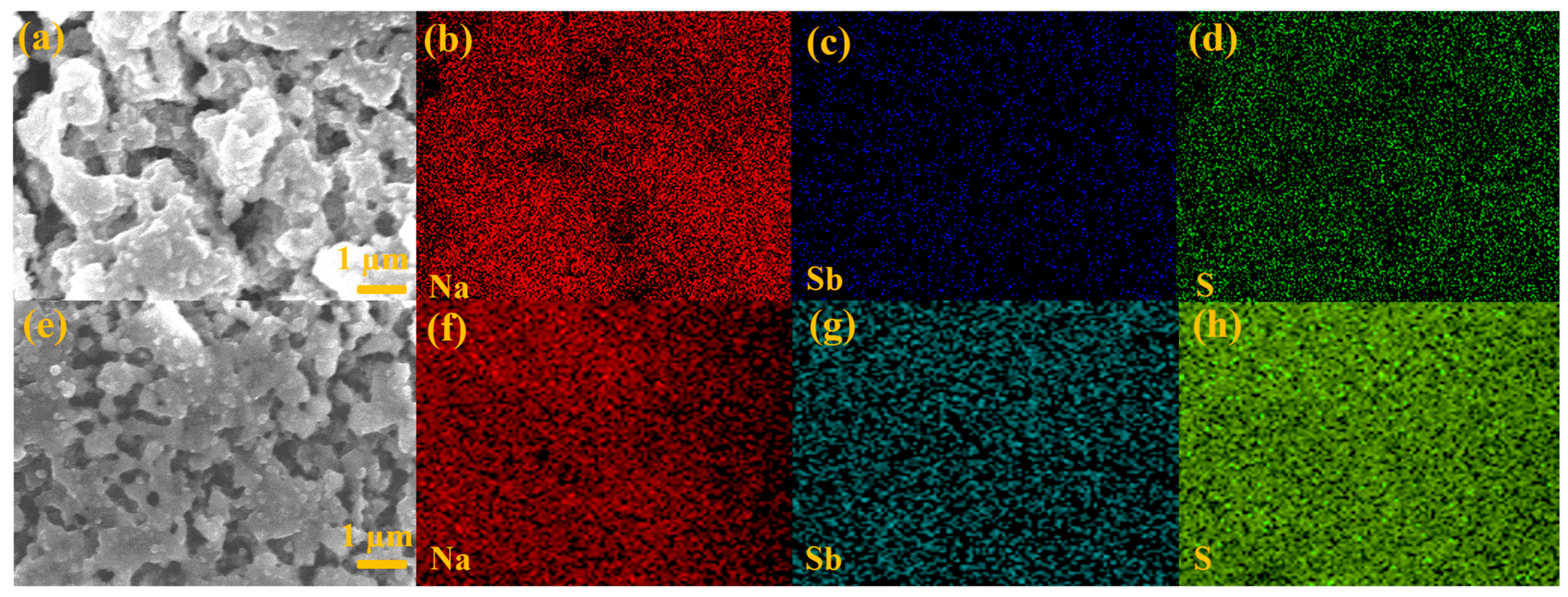
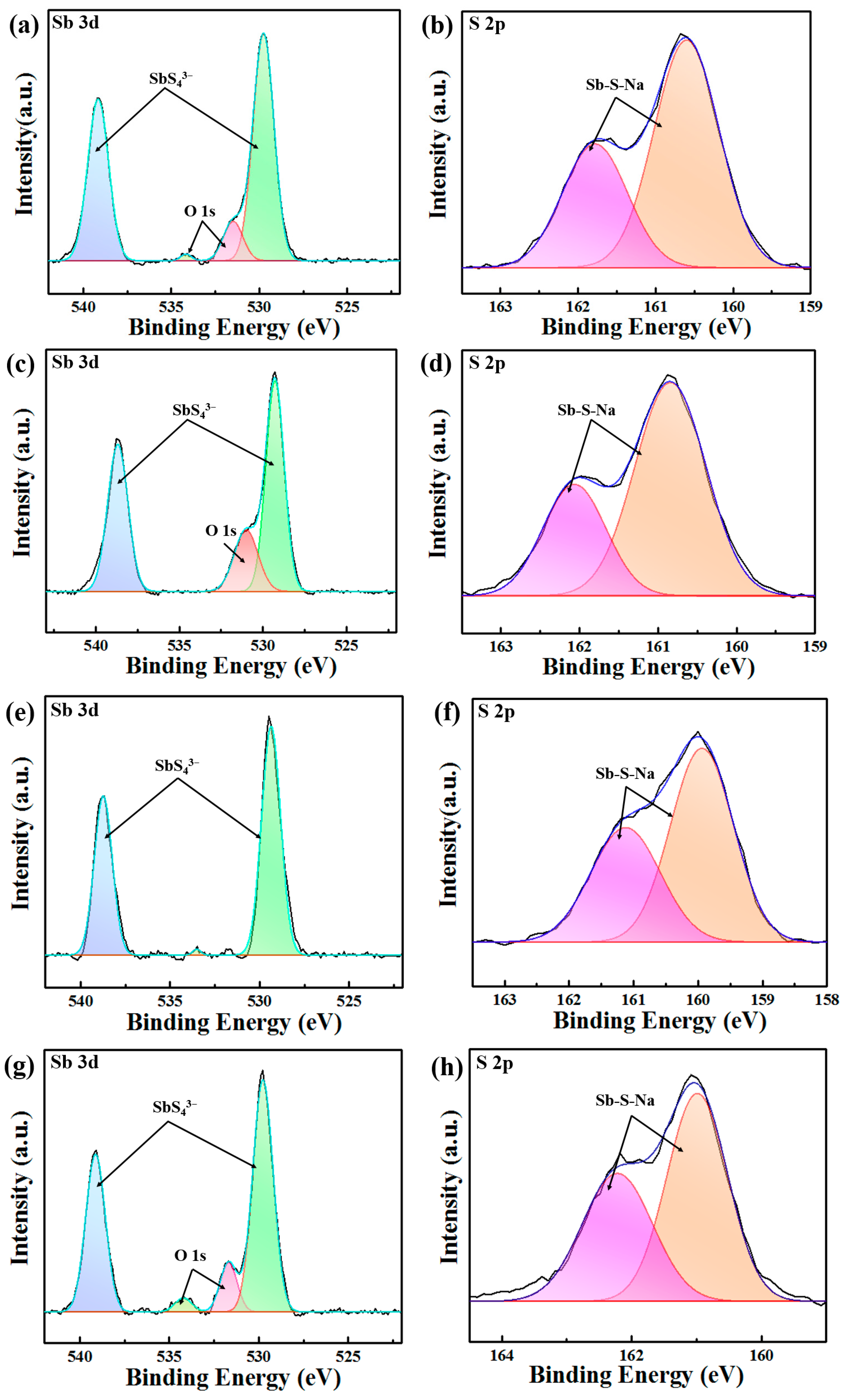
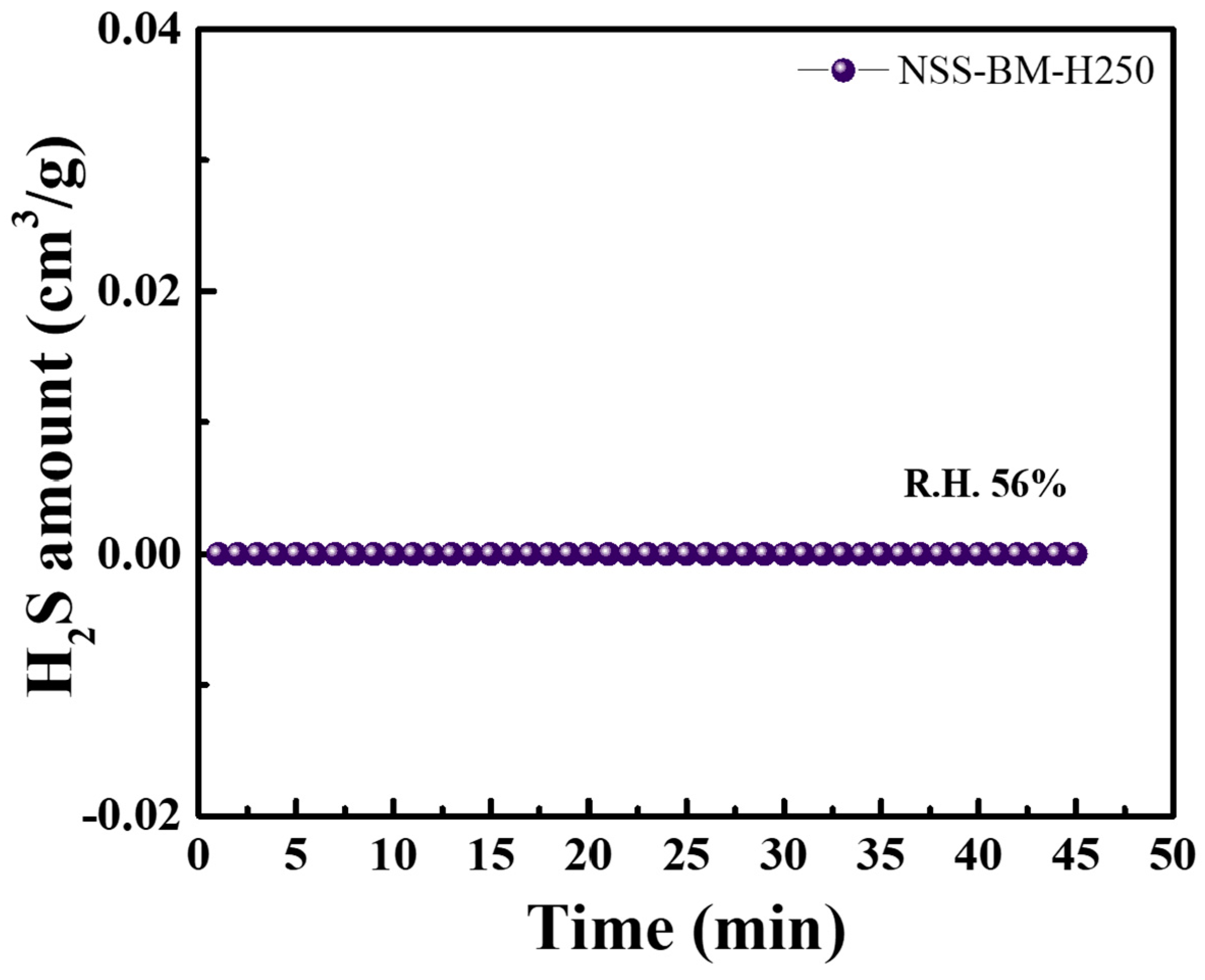
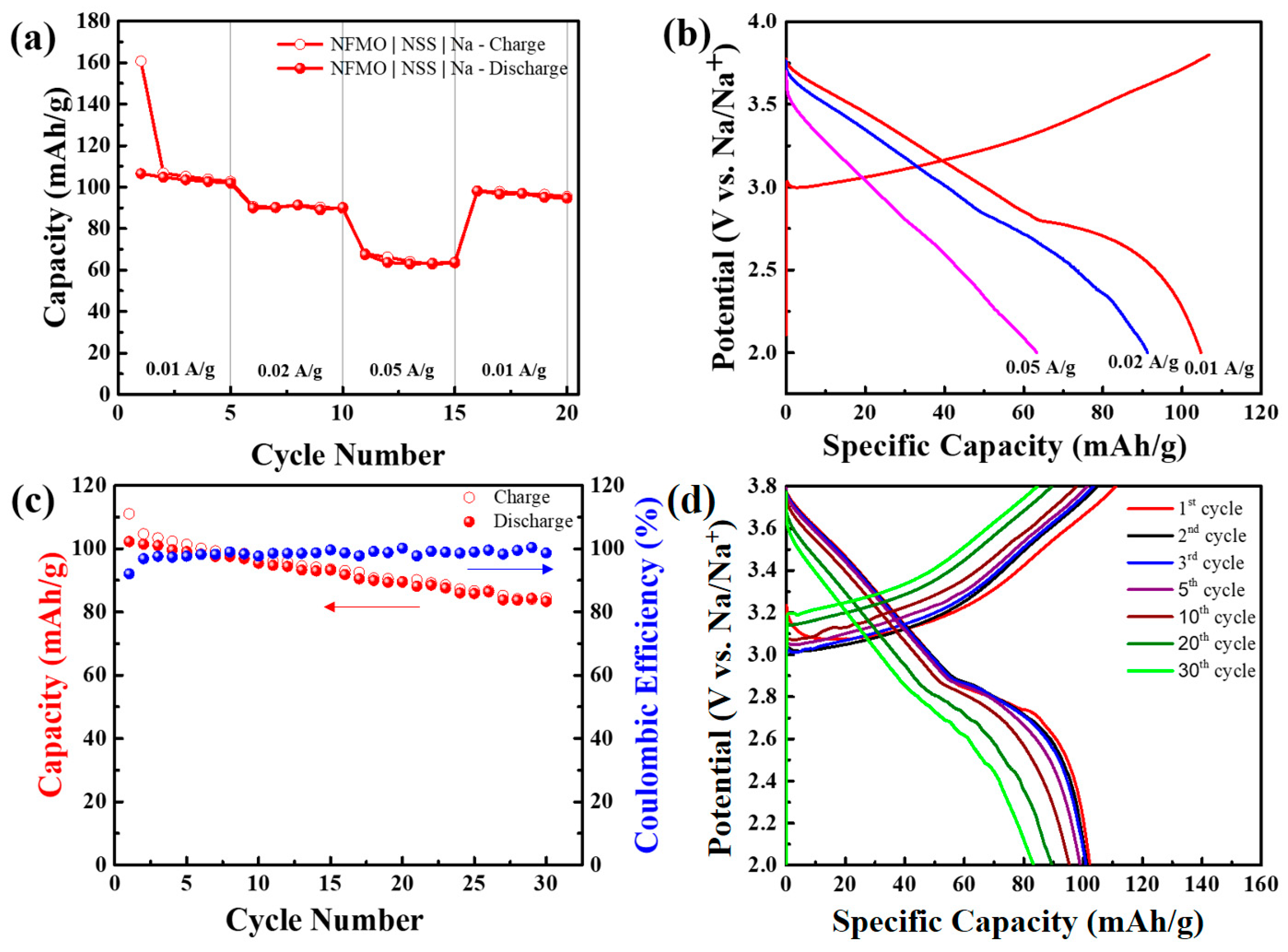
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bron, P.; Johansson, S.; Zick, K.; Schmedt auf der Günne, J.; Dehnen, S.; Roling, B. Li10SnP2S12: An affordable lithium superionic conductor. J. Am. Chem. Soc. 2013, 135, 15694–15697. [Google Scholar] [CrossRef]
- Wang, Y.; Song, S.; Xu, C.; Hu, N.; Molenda, J.; Lu, L. Development of solid-state electrolytes for sodium-ion battery—A short review. Nano Mater. Sci. 2019, 1, 91–100. [Google Scholar] [CrossRef]
- Hayashi, A.; Noi, K.; Sakuda, A.; Tatsumisago, M. Superionic glass-ceramic electrolytes for room-temperature rechargeable sodium batteries. Nat. Commun. 2012, 3, 856. [Google Scholar] [CrossRef]
- Bo, S.H.; Wang, Y.; Kim, J.C.; Richards, W.D.; Ceder, G. Computational and experimental investigations of Na-ion conduction in cubic Na3PSe4. Chem. Mater. 2016, 28, 252–258. [Google Scholar] [CrossRef]
- Banerjee, A.; Park, K.H.; Heo, J.W.; Nam, Y.J.; Moon, C.K.; Oh, S.M.; Jung, Y.S. Na3SbS4: A solution processable sodium superionic conductor for all-solid-state sodium-ion batteries. Angew. Chem. 2016, 128, 9786–9790. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Li, S.; Howard, J.W.; Neuefeind, J.; Ren, Y.; Zhao, Y. Sodium ion transport mechanisms in antiperovskite electrolytes Na3OBr and Na4OI2: An in situ neutron diffraction study. Inorg. Chem. 2016, 55, 5993–5998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Q.; Liu, Z.; Zhou, Z.; Li, S.; Zhu, J.; Zhao, Y. Structural manipulation approaches towards enhanced sodium ionic conductivity in Na-rich antiperovskites. J. Power Source 2015, 293, 735–740. [Google Scholar] [CrossRef]
- Yao, Y.-F.Y.; Kummer, J.T. Ion exchange properties of and rates of ionic diffusion in beta-alumina. J. Inorg. Nucl. Chem. 1967, 29, 2453–2475. [Google Scholar]
- Beevers, C.; Ross, M. The Crystal Structure of “Beta Alumina” Na2O11Al2O3. Zeitschrift für Kristallographie. Cryst. Mater. 1937, 97, 59–66. [Google Scholar]
- Hong, H.Y.-P. Crystal structures and crystal chemistry in the system Na1+xZr2SixP3−xO12. Mater. Res. Bull. 1976, 11, 173–182. [Google Scholar] [CrossRef]
- Von Alpen, U.; Bell, M.F.; Höfer, H.H. Compositional dependence of the electrochemical and structural parameters in the NASICON system (Na1+xSixZr2P3−xO12). Solid State Ion. 1981, 3, 215–218. [Google Scholar] [CrossRef]
- Udovic, T.J.; Matsuo, M.; Unemoto, A.; Verdal, N.; Stavila, V.; Skripov, A.V.; Orimo, S.I. Sodium superionic conduction in Na2B12H12. Chem. Commun. 2014, 50, 3750–3752. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.M.F.; Sequeira, C.A.C. Sodium Borohydride as a fuel for the future. Renew. Sustain. Energy Rev. 2011, 15, 3980–4001. [Google Scholar] [CrossRef]
- Spearing, D.R.; Stebbins, J.F.; Farnan, I. Diffusion and the dynamics of displacive phase transitions in cryolite (Na3AlF6) and chiolite (Na5Al3F14): Multi-nuclear NMR studies. Phys. Chem. Miner. 1994, 21, 373–386. [Google Scholar] [CrossRef]
- Tatsumisago, M.; Hayashi, A. Sulfide glass-ceramic electrolytes for all-solid-state lithium and sodium batteries. Int. J. Appl. Glass Sci. 2014, 5, 226–235. [Google Scholar] [CrossRef]
- Berbano, S.S.; Seo, I.; Bischoff, C.M.; Schuller, K.E.; Martin, S.W. Formation and structure of Na2S + P2S5 amorphous matrials prepared by melt-quenching and mechanical milling. J. Non-Cryst. Solids 2012, 358, 93–98. [Google Scholar] [CrossRef]
- Mizuno, F.; Hayashi, A.; Tadanaga, K.; Tatsumisago, M. New, highly ion-conductive crystals precipitated from Li2S-P2S5 glasses. Adv. Mater. 2005, 17, 918–921. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, D.; Yang, K.; Yan, X.; Wang, L.; Mi, J.; Li, Y. Vacancy-contained tetragonal Na3SbS4 superionic conductor. Adv. Sci. 2016, 3, 1600089. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Hood, Z.D.; Sahu, G.; Pandian, A.S.; Keum, J.K.; Liang, C. An air-stable Na3SbS4 superionic conductor prepared by a rapid and economical synthetic procedure. Angew. Chem. 2016, 128, 8693–8697. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Shang, X.; Wang, W.; Yan, X.; Yu, C.; Wang, L.M. Enhanced electrochemical performance enabled by ionic-liquid-coated Na3SbS4 electrolyte encapsulated in flexible filtration membrane. Chem. Eng. J. 2022, 428, 132094. [Google Scholar] [CrossRef]
- Kim, T.W.; Park, K.H.; Choi, Y.E.; Lee, J.Y.; Jung, Y.S. Aqueous-solution synthesis of Na3SbS 4 solid electrolytes for all-solid-state Na-ion batteries. J. Mater. Chem. A 2018, 6, 840–844. [Google Scholar] [CrossRef]
- Rush, L.E., Jr.; Hood, Z.D.; Holzwarth, N.A.W. Unraveling the electrolyte properties of Na3SbS4 through computation and experiment. Phys. Rev. Mater. 2017, 1, 075405. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Zhao, F.; Zhang, L.; Wang, C.; Li, X.; Sun, X. Gradiently sodiated alucone as an interfacial stabilizing strategy for solid-state Na metal batteries. Adv. Funct. Mater. 2020, 30, 2001118. [Google Scholar] [CrossRef]
- Liang, B.; Yu, L.; Wang, G.; Lin, C.; Gao, C.; Shen, X.; Jiao, Q. Physical and electrochemical behavior of affordable Na3SbS4 solid electrolyte at different heat treatment temperatures. Ceram. Int. 2022, 48, 30144–30150. [Google Scholar] [CrossRef]
- Zhang, D.; Cao, X.; Xu, D.; Wang, N.; Yu, C.; Hu, W.; Zhang, L. Synthesis of cubic Na3SbS4 solid electrolyte with enhanced ion transport for all-solid-state sodium-ion batteries. Electrochim. Acta 2018, 259, 100–109. [Google Scholar] [CrossRef]
- Wu, E.A.; Kompella, C.S.; Zhu, Z.; Lee, J.Z.; Lee, S.C.; Chu, I.H.; Meng, Y.S. New insights into the interphase between the Na metal anode and solid-state sulfide electrolytes: A joint experimental and computational study. ACS Appl. Mater. Interfaces 2018, 10, 10076–10086. [Google Scholar] [CrossRef]
- Li, Y.; Arnold, W.; Halacoglu, S.; Jasinski, J.B.; Druffel, T.; Wang, H. Phase-Transition Interlayer Enables High-Performance Solid-State Sodium Batteries with Sulfide Solid Electrolyte. Adv. Funct. Mater. 2021, 31, 2101636. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, C.; Hood, Z.D.; Chi, M.; Liang, C.; Jalarvo, N.H.; Wang, H. Abnormally low activation energy in cubic Na3SbS4 superionic conductors. Chem. Mater. 2020, 32, 2264–2271. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Hood, Z.D.; Keum, J.K.; Pandian, A.S.; Chi, M.; Sunkara, M.K. Revealing the structural stability and Na-ion mobility of 3D superionic conductor Na3SbS4 at extremely low temperatures. ACS Appl. Energy Mater. 2018, 1, 7028–7034. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Wang, Y.; Feng, Z.; Wang, Y.; Lü, X.; Liang, C. In-situ investigation of pressure effect on structural evolution and conductivity of Na3SbS4 superionic conductor. J. Power Source 2018, 401, 111–116. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Ghosh, A.; Chattopadhyay, S.K. Green synthetic approaches for medium ring–sized heterocycles of biological and pharmaceutical interest. In Green Synthetic Approaches for Biologically Relevant Heterocycles; Elsevier: Amsterdam, The Netherlands, 2021; pp. 617–653. [Google Scholar]
- El-Eskandarany, M.S. The history and necessity of mechanical alloying. Mech. Alloying 2015, 2, 13–47. [Google Scholar]
- Kang, S.J.L. Sintering: Densification, Grain Growth, and Microstructure; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Pasagada, V.K.V.; Yang, N.; Xu, C. Electron beam sintering (EBS) process for Ultra-High Temperature Ceramics (UHTCs) and the comparison with traditional UHTC sintering and metal Electron Beam Melting (EBM) processes. Ceram. Int. 2022, 48, 10174–10186. [Google Scholar] [CrossRef]
- Sottmann, J.; Herrmann, M.; Vajeeston, P.; Hu, Y.; Ruud, A.; Drathen, C.; Wragg, D.S. How Crystallite Size Controls the Reaction Path in Nonaqueous Metal Ion Batteries: The Example of Sodium Bismuth Alloying. Chem. Mater. 2016, 28, 2750–2756. [Google Scholar] [CrossRef]
- Xu, R.C.; Xia, X.H.; Yao, Z.J.; Wang, X.L.; Gu, C.D.; Tu, J.P. Preparation of Li7P3S11 glass-ceramic electrolyte by dissolution- evaporation method for all-solid-state lithium-ion batteries. Electrochim. Acta 2016, 219, 235–240. [Google Scholar] [CrossRef]
- Tsuji, F.; Masuzawa, F.N.; Sakuda, A.; Tatsumisago, M.; Hayashi, A. Preparation and Characterization of Cation-Substituted Na3SbS4 Solid Electrolytes. ACS Appl. Energy Mater. 2020, 3, 11706–11712. [Google Scholar] [CrossRef]
- Nikodimos, Y.; Huang, C.J.; Taklu, B.W.; Su, W.N.; Hwang, B.J. Chemical stability of sulfide solid-state electrolytes: Stability toward humid air and compatibility with solvents and binders. Energy Environ. Sci. 2022, 15, 991–1033. [Google Scholar] [CrossRef]


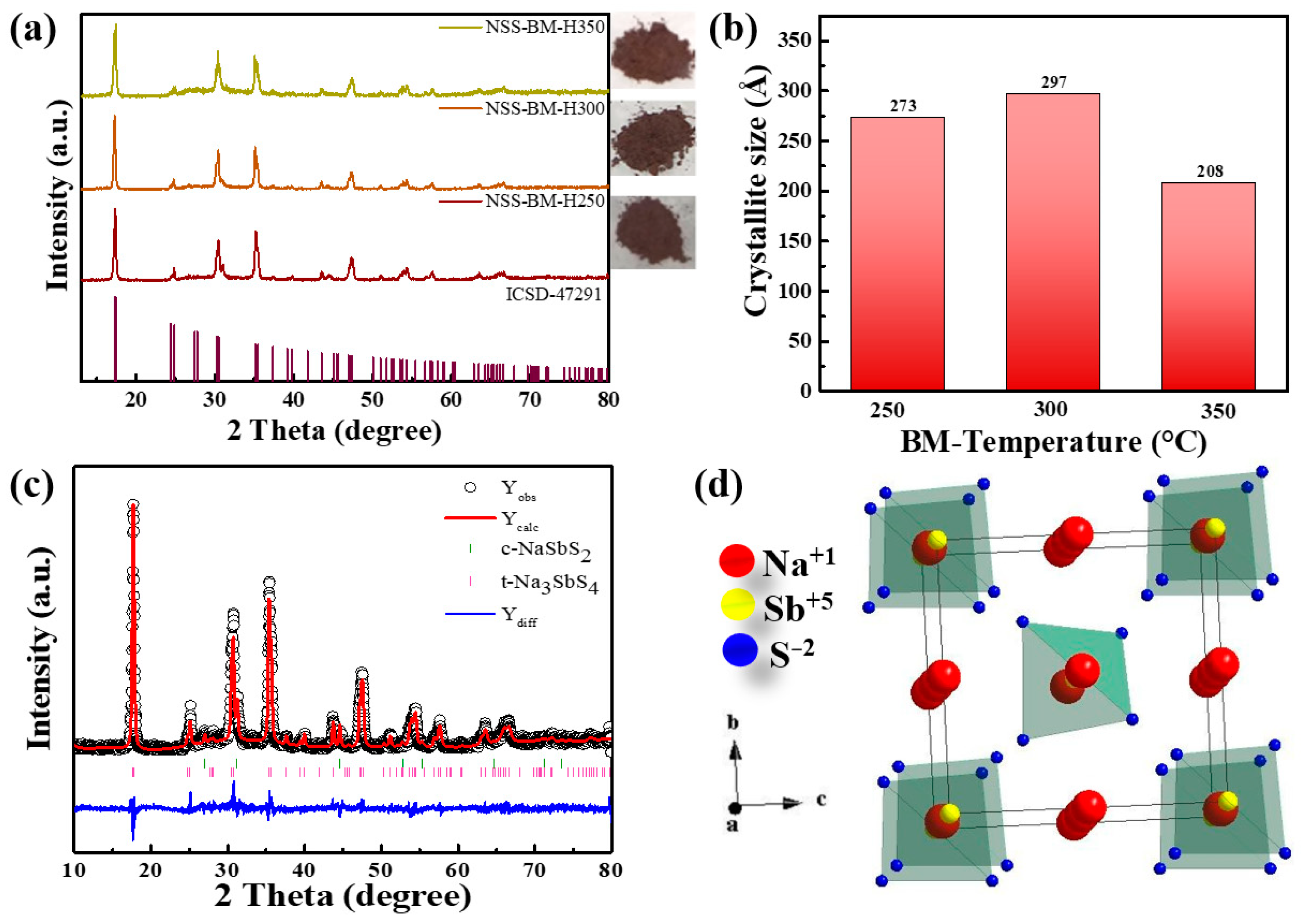

| Atoms | Wyckoff | Occupancy | x/a | y/a | z/c |
|---|---|---|---|---|---|
| Na1 | 4d | 1 | 0 | 1/2 | 1/2 |
| Na2 | 2b | 1 | 0 | 0 | 1/2 |
| Sb | 2a | 1 | 0 | 0 | 0 |
| S | 8e | 1 | 0.29331 | 0.33080 | 0.68745 |
| Samples | L (cm) | S (cm2) | Rb (Ω) | Rgb (Ω) | Rb + Rgb (Ω) | σ25 °C (S cm−1) | Ea (eV) |
|---|---|---|---|---|---|---|---|
| NSS-BM-20 h | 0.044 | 0.7854 | 31 | 171.4 | 202.4 | 2.77 × 10−4 | 0.22 |
| NSS-BM-H250 | 0.043 | 0.7854 | 11.43 | 164.7 | 176.13 | 3.11 × 10−4 | 0.21 |
| NSS-BM-H300 | 0.043 | 0.7854 | 13.38 | 518.9 | 532.28 | 1.03 × 10−4 | 0.23 |
| NSS-BM-H350 | 0.039 | 0.7854 | 26.55 | 439.2 | 465.75 | 1.07 × 10−4 | 0.28 |
| Solid Electrolytes | Ionic Conductivity at RT (mS cm−1) | Activation Energy (eV) | References |
|---|---|---|---|
| Na3SbS4 | 0.31 | 0.21 | This work |
| Na3SbS4 | 0.54 | 0.074 | [24] |
| Na3SbS4 | 0.31 | 0.2 | [27] |
| t-Na3SbS4 | 0.6 | 0.224 | [28] |
| Na3SbS4 | 1.06 | 0.21 | [26] |
| t-Na3SbS4 | 3 | 0.25 | [18] |
| t-Na3SbS4 | 0.8 | 0.3 | [22] |
| Na3SbS4 | 1.05 | 0.22 | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thairiyarayar, C.B.; Huang, C.-H.; Gandomi, Y.A.; Hsieh, C.-T.; Liu, W.-R. Synthesis and Characterization of Na3SbS4 Solid Electrolytes via Mechanochemical and Sintered Solid-State Reactions: A Comparative Study. Sustainability 2023, 15, 15662. https://doi.org/10.3390/su152115662
Thairiyarayar CB, Huang C-H, Gandomi YA, Hsieh C-T, Liu W-R. Synthesis and Characterization of Na3SbS4 Solid Electrolytes via Mechanochemical and Sintered Solid-State Reactions: A Comparative Study. Sustainability. 2023; 15(21):15662. https://doi.org/10.3390/su152115662
Chicago/Turabian StyleThairiyarayar, Celastin Bebina, Chia-Hung Huang, Yasser Ashraf Gandomi, Chien-Te Hsieh, and Wei-Ren Liu. 2023. "Synthesis and Characterization of Na3SbS4 Solid Electrolytes via Mechanochemical and Sintered Solid-State Reactions: A Comparative Study" Sustainability 15, no. 21: 15662. https://doi.org/10.3390/su152115662
APA StyleThairiyarayar, C. B., Huang, C.-H., Gandomi, Y. A., Hsieh, C.-T., & Liu, W.-R. (2023). Synthesis and Characterization of Na3SbS4 Solid Electrolytes via Mechanochemical and Sintered Solid-State Reactions: A Comparative Study. Sustainability, 15(21), 15662. https://doi.org/10.3390/su152115662









