Exploring the Impact of Potassium on Growth, Photosynthetic Performance, and Nutritional Status of Lemon Trees (cv. Adamopoulou) Grafted onto Sour Orange and Volkamer Lemon Rootstocks
Abstract
:1. Introduction
- To assess the impact of K fertilization on plant growth parameters, such as dry matter production and its allocation among various plant parts;
- To investigate how K influences leaf gas exchange parameters like net photosynthesis, stomatal conductance, and transpiration rate;
- To investigate how K affects nutrient uptake and distribution within plant tissues.
2. Materials and Methods
2.1. Plant Material and Experimental Setup
2.2. Experimental Procedure
2.3. Plant Growth
2.4. Leaf Photosynthesis, Chlorophylls, and Carotenoids
2.5. Determination of Plant Nutrient Concentrations
2.6. Statistical Data Analysis
3. Results and Discussion
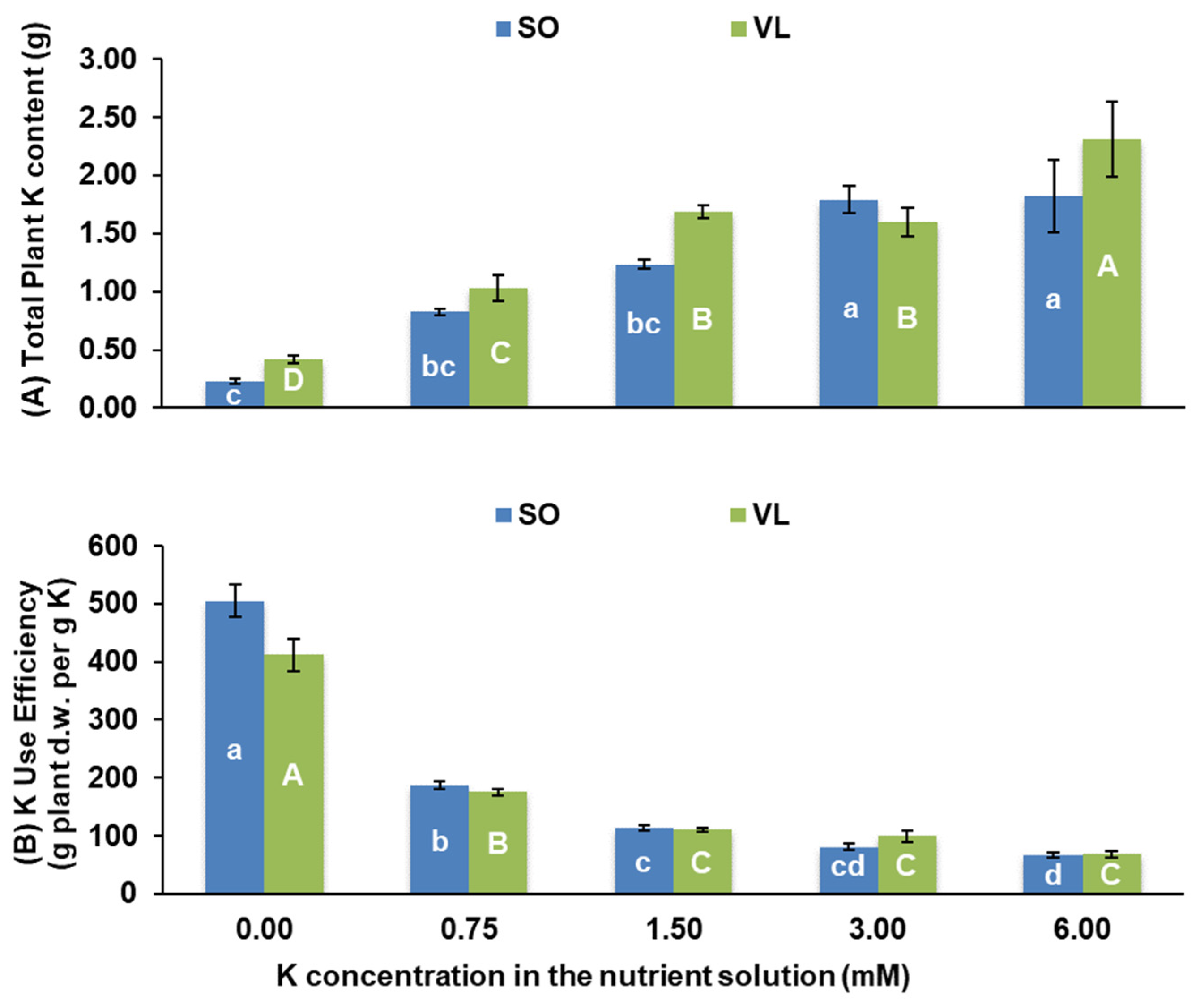
3.1. Plant Growth
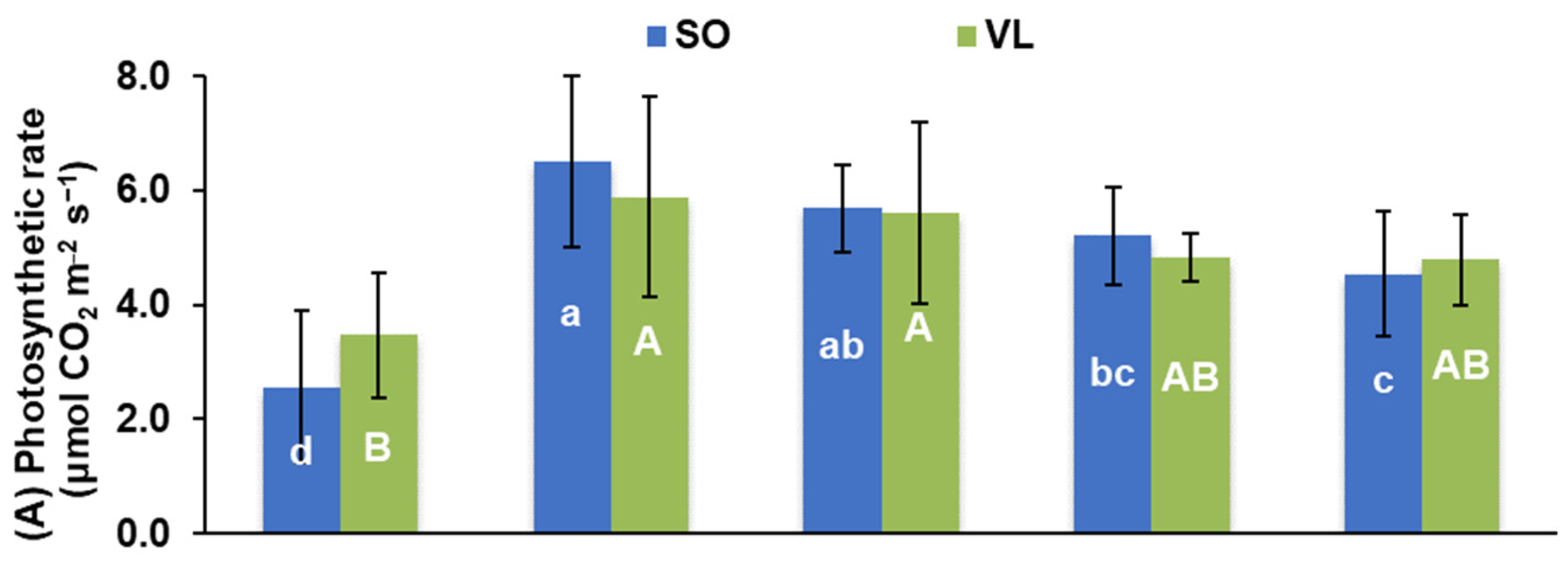
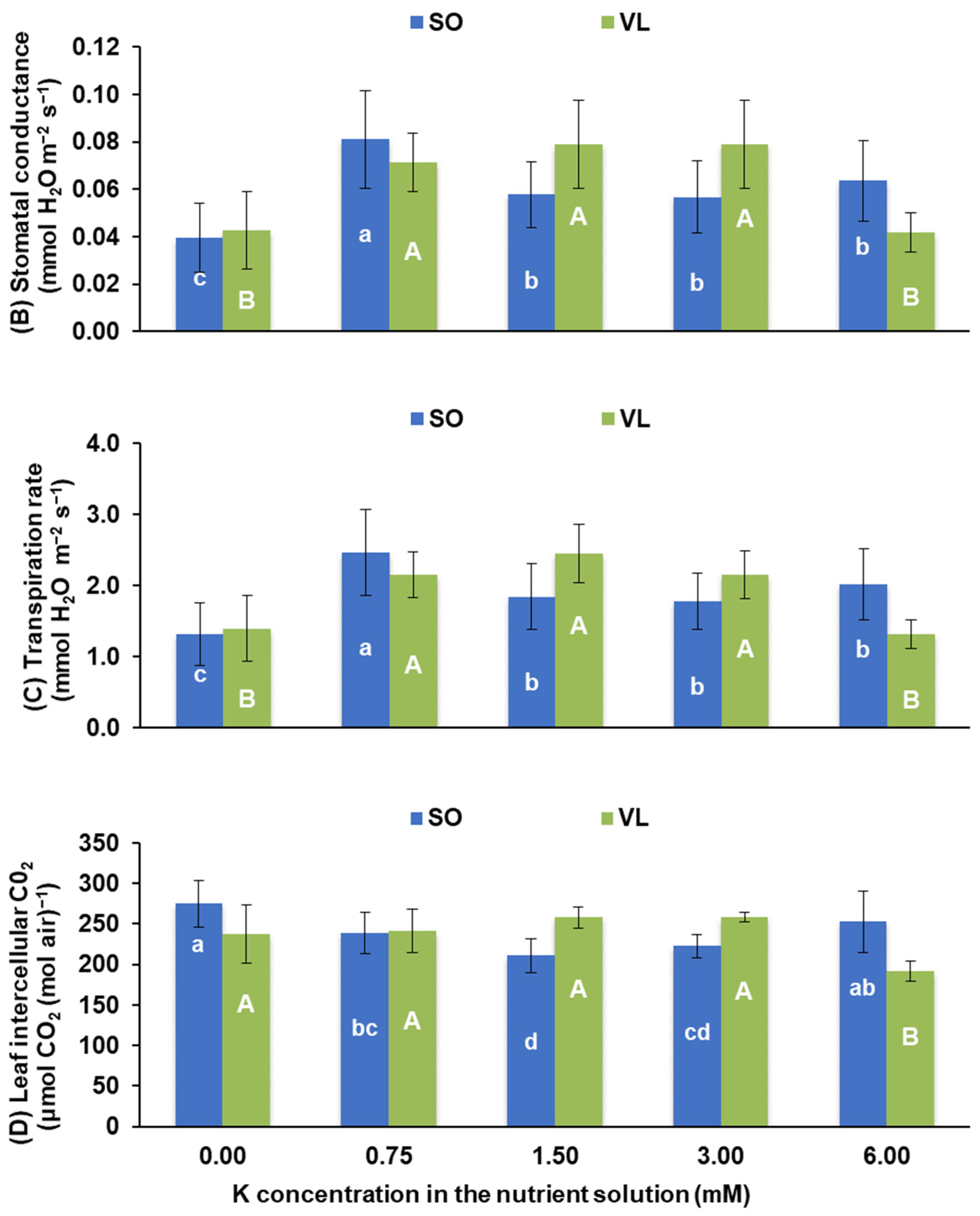
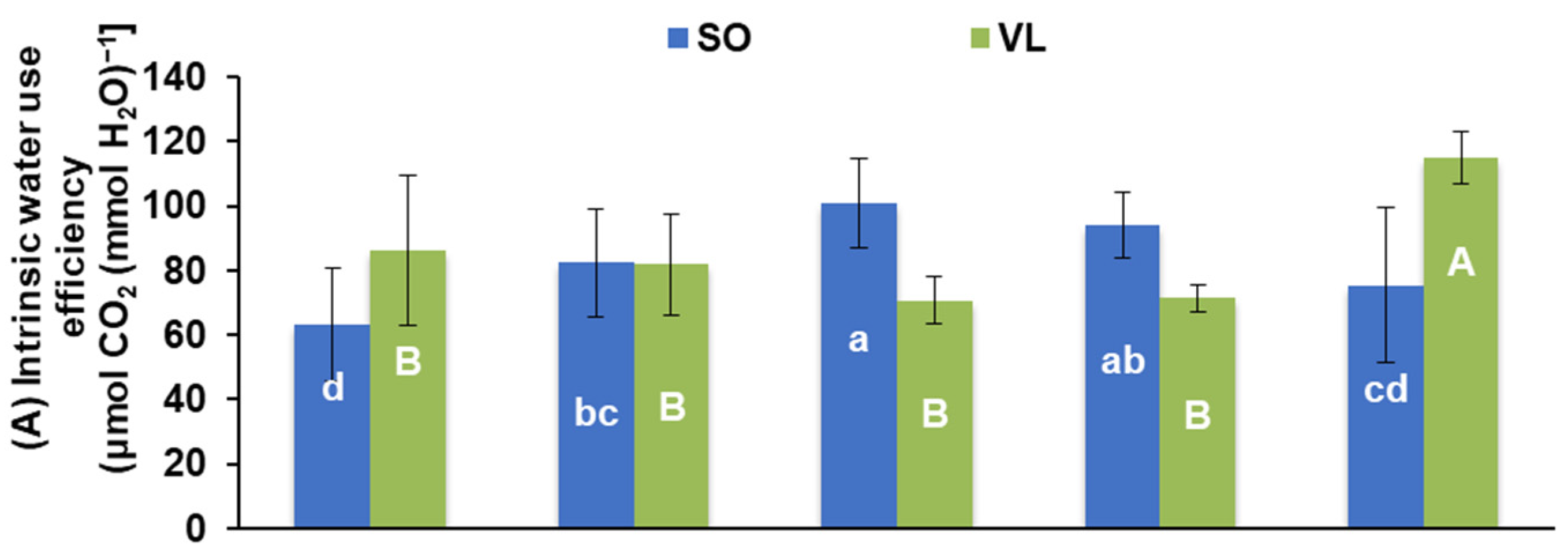
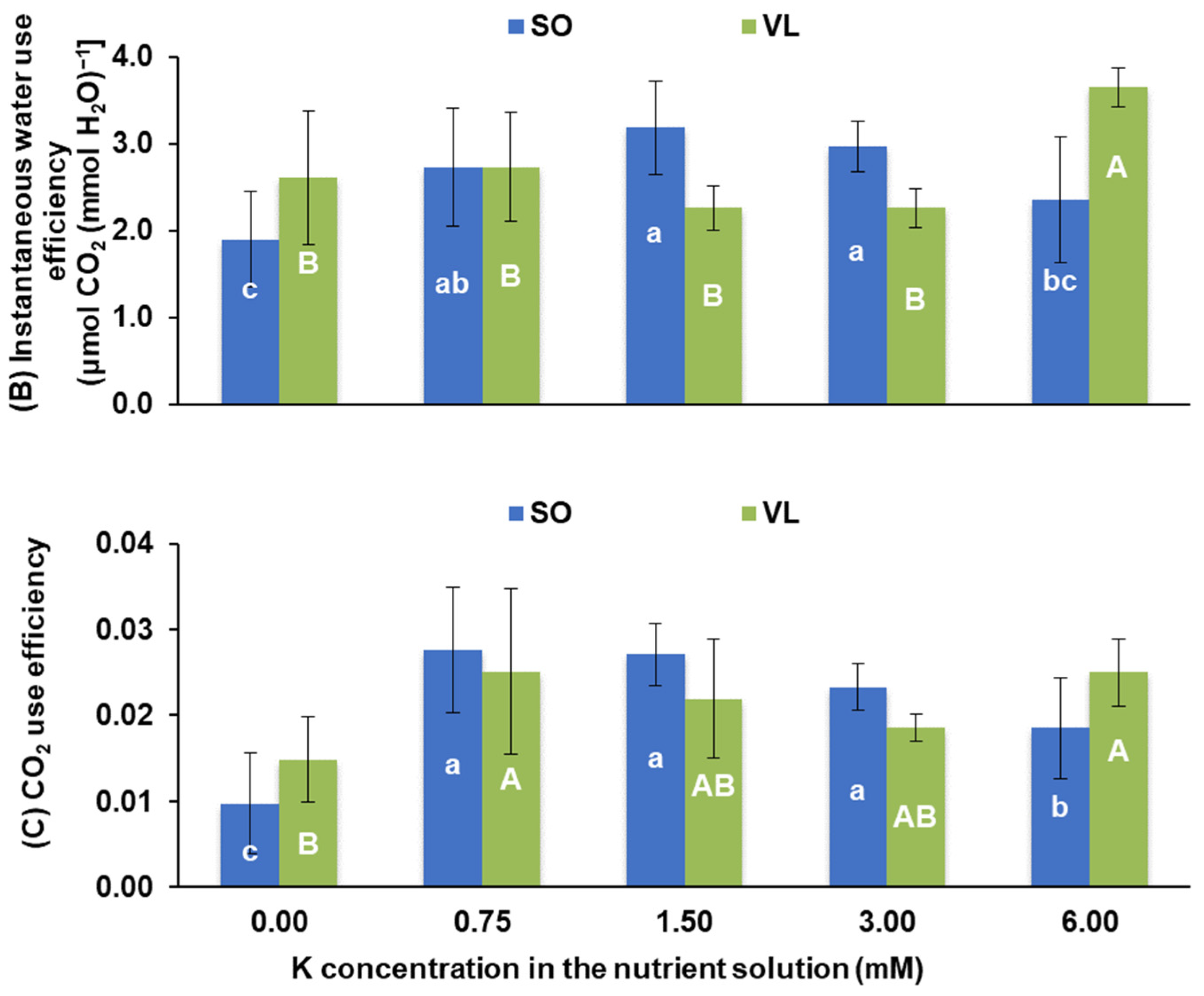
3.2. Photosynthesis and Pigments
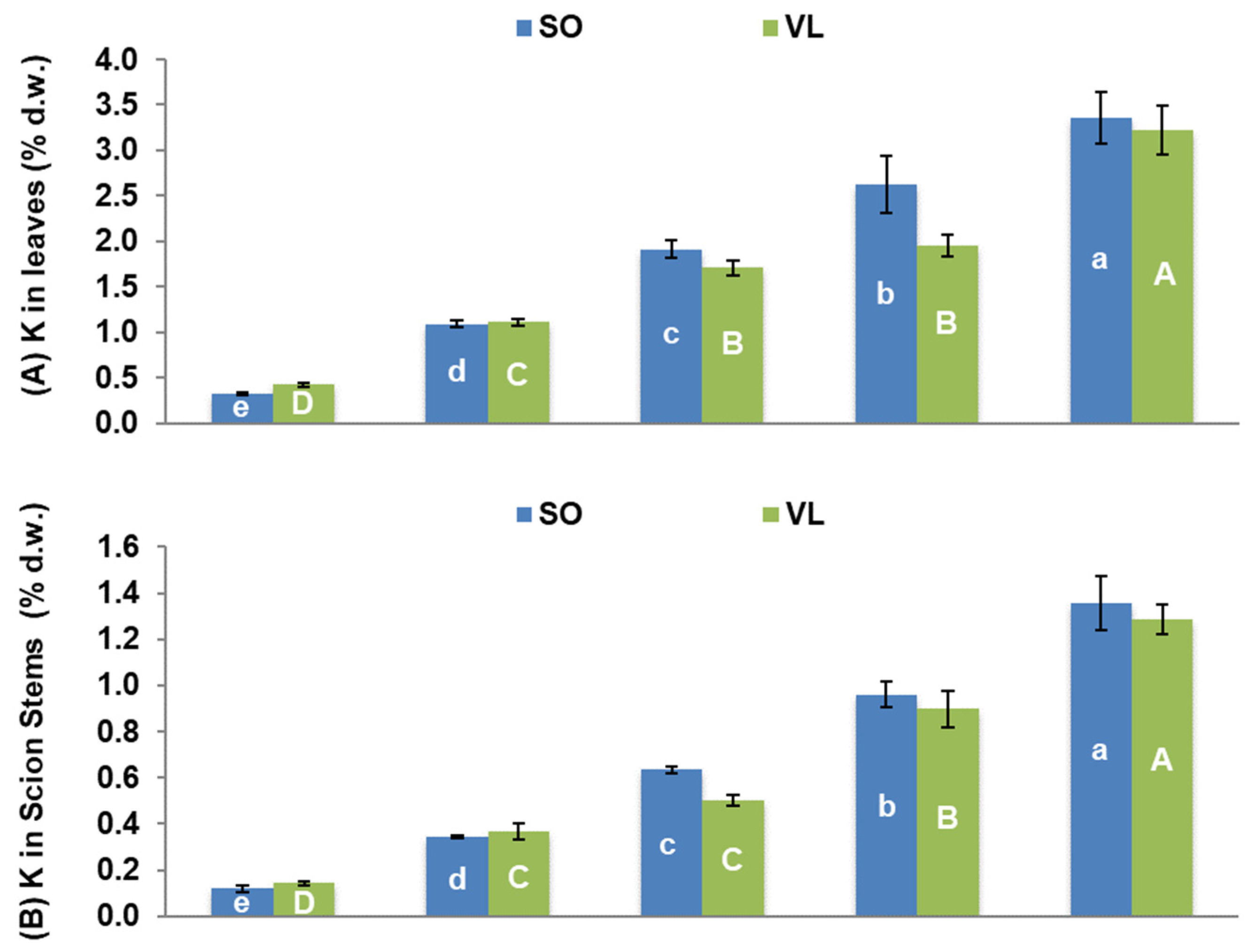
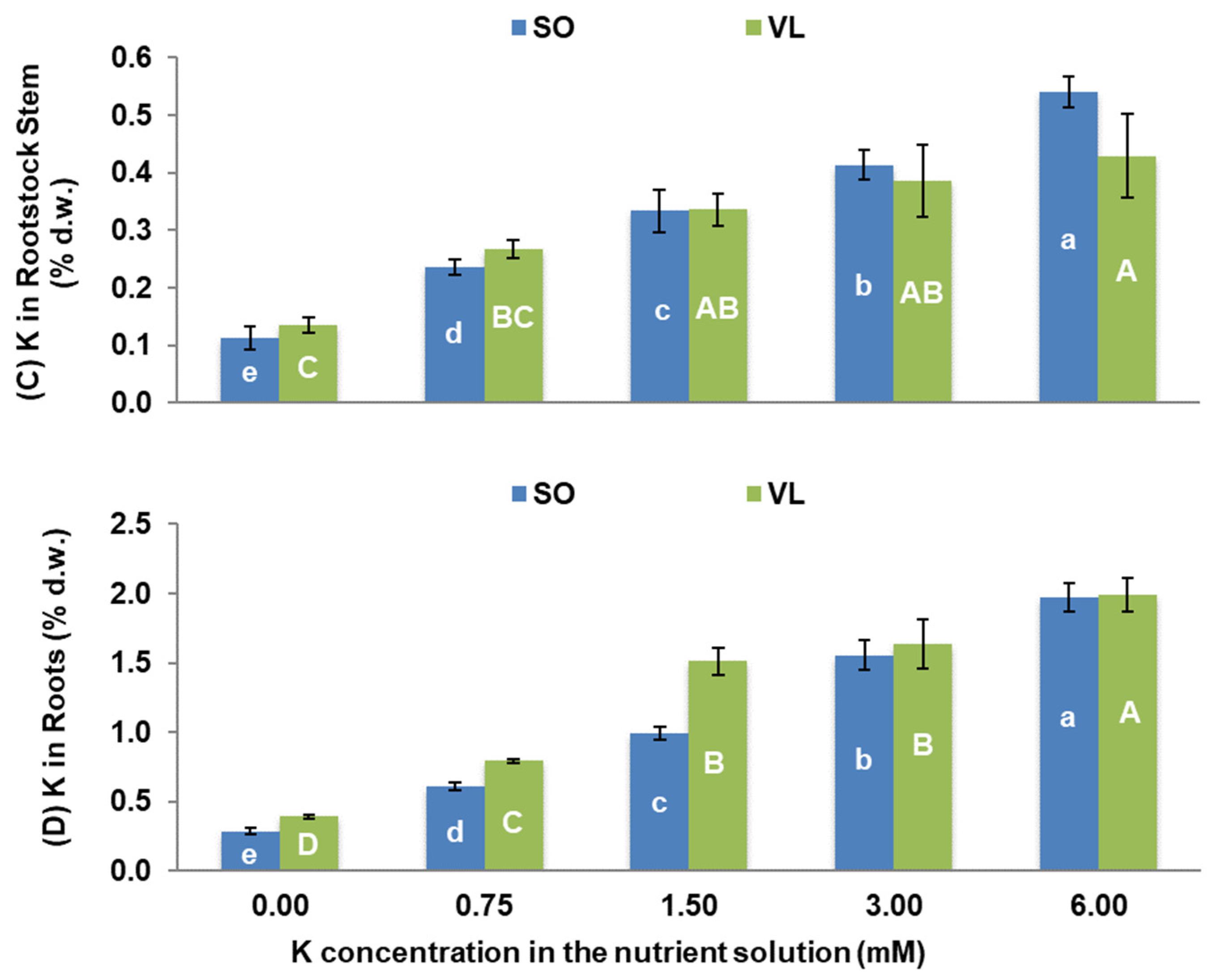
3.3. Potassium Nutrition of Plants and Interactions with Other Elements
4. Conclusions
- Potassium deficiency symptoms: when experiencing K deficiency, lemon plants displayed characteristic chlorotic and necrotic symptoms, which were more pronounced in older leaves and somewhat more severe in SO-grafted plants;
- Rootstock-dependent K concentrations: lemon trees grafted onto SO rootstock had higher K concentrations in all plant parts, except for the roots, compared to those grafted onto VL rootstock, when they were treated with moderate to high K. The opposite was found for the 0 mM K treatment;
- Efficient K management: SO-grafted plants exhibited superior K utilization and management during its deficiency, surpassing VL-grafted plants;
- Reduced nutrient uptake: increasing K concentration in the nutrient solution consistently and linearly reduced the total uptake of essential nutrients, including P, Ca, Mg, B, Mn, and Zn, especially at the highest K treatment;
- Impact on nutrient concentrations: elevated K levels negatively affected the concentrations of P, Ca, Mg, and Zn in scion tissues, including both leaves and scion stems, leading to mineral nutrient imbalances;
- Physiological responses: potassium deficiency adversely affected various physiological processes in lemon leaves, including a decrease in photosynthesis rate, stomatal conductance, transpiration rate, intercellular CO2 concentration, water use efficiency, CO2 utilization efficiency, chlorophyll a/b ratio, and carotenoid concentrations. Low rates of leaf photosynthesis under K deficiency were due to both stomatal and non-stomatal limitations;
- Rootstock-dependent effects: rootstock-dependent responses were observed in scion leaf and stem growth as well as mineral nutrient concentration, uptake, and distribution across plant parts. Additionally, variations were noted in leaf intercellular CO2 concentration, water use efficiency, carboxylation efficiency, Chl a/b ratio, and total carotenoid concentration under varying K conditions, highlighting the complexity of rootstock interactions.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations Statistics Division, FAOSTAT DATABASE. Available online: https://www.fao.org/faostat/en/ (accessed on 29 September 2023).
- Matheyambath, A.C.; Padmanabhan, P.; Paliyath, G. Citrus Fruits. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 136–140. [Google Scholar] [CrossRef]
- Lemon (Citrus limon). Available online: https://www.hort.purdue.edu/newcrop/morton/lemon.html (accessed on 29 September 2023).
- Klimek-Szczykutowicz, M.; Szopa, A.; Ekiert, H. Citrus limon (Lemon) Phenomenon—A Review of the Chemistry, Pharmacological Properties, Applications in the Modern Pharmaceutical, Food, and Cosmetics Industries, and Biotechnological Studies. Plants 2020, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Mattos, D.; Kadyampakeni, D.M.; Oliver, A.Q.; Boaretto, R.M.; Morgan, K.T.; Quaggio, J.A. Soil and Nutrition Interactions. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 311–331. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2011; 672p. [Google Scholar]
- Oosterhuis, D.M.; Loka, D.A.; Kawakami, E.M.; Pettigrew, W.T. The Physiology of Potassium in Crop Production. Adv. Agron. 2014, 126, 203–233. [Google Scholar] [CrossRef]
- Rawat, J.; Sanwal, P.; Saxena, J. Potassium and Its Role in Sustainable Agriculture. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: New Delhi, India, 2016; pp. 235–253. [Google Scholar] [CrossRef]
- Cakmak, I. The Role of Potassium in Alleviating Detrimental Effects of Abiotic Stresses in Plants. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in Plants: Growth Regulation, Signaling, and Environmental Stress Tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Morris, J.R.; Sims, C.A.; Cawthon, D.L. Effects of Excessive Potassium Levels On PH, Acidity and Color of Fresh and Stored Grape Juice. Am. J. Enol. Vitic. 1983, 34, 35–39. [Google Scholar] [CrossRef]
- Dibb, D.W.; Thompson, W.R. Interaction of Potassium with Other Nutrients. In Potassium in Agriculture; Wiley Online Library: Hoboken, NJ, USA, 2015; pp. 515–533. [Google Scholar] [CrossRef]
- Daliparthy, J.; Barker, A.V.; Mondal, S.S. Potassium Fractions with Other Nutrients in Crops: A Review Focusing on the Tropics. J. Plant Nutr. 1994, 17, 1859–1886. [Google Scholar] [CrossRef]
- Fageria, V.D. Nutrient Interactions in Crop Plants. J. Plant Nutr. 2001, 24, 1269–1290. [Google Scholar] [CrossRef]
- Xu, X.; Du, X.; Wang, F.; Sha, J.; Chen, Q.; Tian, G.; Zhu, Z.; Ge, S.; Jiang, Y. Effects of Potassium Levels on Plant Growth, Accumulation and Distribution of Carbon, and Nitrate Metabolism in Apple Dwarf Rootstock Seedlings. Front. Plant Sci. 2020, 11, 534048. [Google Scholar] [CrossRef] [PubMed]
- Quaggio, J.A.; Mattos, D.; Cantarella, H.; Almeida, E.L.E.; Cardoso, S.A.B. Lemon Yield and Fruit Quality Affected by NPK Fertilization. Sci. Hortic. 2002, 96, 151–162. [Google Scholar] [CrossRef]
- Alva, A.K.; Mattos, D.; Paramasivam, S.; Patil, B.; Dou, H.; Sajwan, K.S. Potassium Management for Optimizing Citrus Production and Quality. Int. J. Fruit Sci. 2006, 6, 3–43. [Google Scholar] [CrossRef]
- Vijay, V.; Dalal, R.P.S.; Beniwal, B.S.; Saini, H. Impact of Foliar Application of Potassium and Its Spray Schedule on Yield and Quality of Sweet Orange (Citrus sinensis) Cv. Jaffa. J. Appl. Nat. Sci. 2016, 8, 1893–1898. [Google Scholar] [CrossRef]
- Ben Mimoun, M.; Dbara, S.; Lahmar, K.; Marchand, M. Effects of Potassium Nutrition on Fruit Yield and Quality of ‘Maltaise’ Citrus (Citrus sinensis L.). Acta Hortic. 2018, 1217, 225–230. [Google Scholar] [CrossRef]
- Davies, F.S.; Albrigo, L.G. Citrus; CAB International: Wallingford, UK, 1994; 254p. [Google Scholar]
- Al-Jaleel, A.; Zekri, M.; Hammam, Y. Yield, Fruit Quality, and Tree Health of ‘Allen Eureka’ Lemon on Seven Rootstocks in Saudi Arabia. Sci. Hortic. 2005, 105, 457–465. [Google Scholar] [CrossRef]
- Papadakis, I.E.; Dimassi, K.N.; Bosabalidis, A.M.; Therios, I.N.; Patakas, A.; Giannakoula, A. Boron Toxicity in ‘Clementine’ Mandarin Plants Grafted on Two Rootstocks. Plant Sci. 2004, 166, 539–547. [Google Scholar] [CrossRef]
- Papadakis, I.E.; Dimassi, K.N.; Bosabalidis, A.M.; Therios, I.N.; Patakas, A.; Giannakoula, A. Effects of B Excess on Some Physiological and Anatomical Parameters of ‘Navelina’ Orange Plants Grafted on Two Rootstocks. Environ. Exp. Bot. 2004, 51, 247–257. [Google Scholar] [CrossRef]
- Martínez-Nicolas, J.J.; Núñez-Gómez, D.; Lidón, V.; Martínez-Font, R.; Melgarejo, P.; Hernández, F.; Legua, P. Physico-Chemical Attributes of Lemon Fruits as Affected by Growing Substrate and Rootstock. Foods 2022, 11, 2487. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 23–32. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Fageria, N.K.; Oliveira, J.P. Nitrogen, Phosphorus and Potassium Interactions in Upland Rice. J. Plant Nutr. 2014, 37, 1586–1600. [Google Scholar] [CrossRef]
- Hamouda, H.A.; Eldahshouri, M.F.; Manal, F.M.; Thalooth, A.T. Growth, Yield and Nutrient Status of Wheat Plants as Affected by Potassium and Iron Foliar Application in Sandy Soil. Int. J. Chemtech Res. 2015, 8, 1473–1481. [Google Scholar]
- Gerardeaux, E.; Jordan-Meille, L.; Constantin, J.; Pellerin, S.; Dingkuhn, M. Changes in Plant Morphology and Dry Matter Partitioning Caused by Potassium Deficiency in Gossypium hirsutum (L.). Environ. Exp. Bot. 2010, 67, 451–459. [Google Scholar] [CrossRef]
- Çelik, H.; Aşik, B.; Gürel, S.; Katkat, A.V. Effects of Potassium and Iron on Macro Element Uptake of Maize. Zemdirb. Agric. 2010, 97, 11–22. [Google Scholar]
- Sun, T.; Zhang, J.; Zhang, Q.; Li, X.; Li, M.; Yang, Y.; Zhou, J.; Wei, Q.; Zhou, B. Transcriptional and Metabolic Responses of Apple to Different Potassium Environments. Front. Plant Sci. 2023, 14, 1131708. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, F.; Xing, Y.; Liu, J.; Lv, M.; Meng, H.; Du, X.; Zhu, Z.; Ge, S.; Jiang, Y. Appropriate and Constant Potassium Supply Promotes the Growth of M9T337 Apple Rootstocks by Regulating Endogenous Hormones and Carbon and Nitrogen Metabolism. Front. Plant Sci. 2022, 13, 827478. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, V.S.; Prado, R.; Almeida, H.J.; Cruz, F.; Santos, D.M.M. Gaseous Exchanges, Growth and Foliar Anatomy of Sugarcane Plants Grown in Potassium (K) Deprived Nutrient Solution. Aust. J. Crop Sci. 2015, 9, 577–584. [Google Scholar]
- Römheld, V.; Kirkby, E.A. Research on Potassium in Agriculture: Needs and Prospects. Plant Soil 2010, 335, 155–180. [Google Scholar] [CrossRef]
- Jákli, B.; Tavakol, E.; Tränkner, M.; Senbayram, M.; Dittert, K. Quantitative Limitations to Photosynthesis in K Deficient Sunflower and Their Implications on Water-Use Efficiency. J. Plant Physiol. 2017, 209, 20–30. [Google Scholar] [CrossRef]
- Çalişkan, B.; Çalişkan, A.C. Potassium Nutrition in Plants and Its Interactions with Other Nutrients in Hydroponic Culture. In Potassium—Improvement of Quality in Fruits and Vegetables Through Hydroponic Nutrient Management; InTechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Pettigrew, W.T.; Gerik, T.J. Cotton Leaf Photosynthesis and Carbon Metabolism. Adv. Agron. 2007, 94, 209–236. [Google Scholar] [CrossRef]
- Jin, S.H.; Huang, J.Q.; Li, X.Q.; Zheng, B.S.; Wu, J.S.; Wang, Z.J.; Liu, G.H.; Chen, M. Effects of Potassium Supply on Limitations of Photosynthesis by Mesophyll Diffusion Conductance in Carya cathayensis. Tree Physiol. 2011, 31, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Bednarz, C.W.; Oosterhuis, D.M.; Evans, R.D. Leaf Photosynthesis and Carbon Isotope Discrimination of Cotton in Response to Potassium Deficiency. Environ. Exp. Bot. 1998, 39, 131–139. [Google Scholar] [CrossRef]
- Hu, W.; Lu, Z.; Meng, F.; Li, X.; Cong, R.; Ren, T.; Sharkey, T.D.; Lu, J. The Reduction in Leaf Area Precedes That in Photosynthesis under Potassium Deficiency: The Importance of Leaf Anatomy. New Phytol. 2020, 227, 1749–1763. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, R.R.; de Oliveira Freire, A.L. Gas Exchange of Oiticica Seedlings in Response to Potassium Fertilization and Water Turning. J. Exp. Agric. Int. 2022, 44, 35–46. [Google Scholar] [CrossRef]
- Pervez, H.; Ashraf, M.; Makhdum, M.I. Influence of Potassium Nutrition on Gas Exchange Characteristics and Water Relations in Cotton (Gossypium hirsutum L.). Photosynthetica 2004, 42, 251–255. [Google Scholar] [CrossRef]
- Mohd Zain, N.A.; Ismail, M.R. Effects of Potassium Rates and Types on Growth, Leaf Gas Exchange and Biochemical Changes in Rice (Oryza sativa) Planted under Cyclic Water Stress. Agric. Water Manag. 2016, 164, 83–90. [Google Scholar] [CrossRef]
- Du, Q.; Zhao, X.H.; Xia, L.; Jiang, C.J.; Wang, X.G.; Han, Y.; Wang, J.; Yu, H.-Q. Effects of Potassium Deficiency on Photosynthesis, Chloroplast Ultrastructure, ROS, and Antioxidant Activities in Maize (Zea mays L.). J. Integr. Agric. 2019, 18, 395–406. [Google Scholar] [CrossRef]
- Quaggio, J.A.; Mattos Junior, D.; Boaretto, R.M. Sources and Rates of Potassium for Sweet Orange Production. Sci. Agric. 2011, 68, 369–375. [Google Scholar] [CrossRef]
- Ashraf, M.Y.; Gul, A.; Ashraf, M.; Hussain, F.; Ebert, G. Improvement in Yield and Quality of Kinnow (Citrus deliciosa × Citrus nobilis) by Potassium Fertilization. J. Plant Nutr. 2010, 33, 1625–1637. [Google Scholar] [CrossRef]
- Khoshbakht, D.; Ghorbani, A.; Baninasab, B.; Naseri, L.A.; Mirzaei, M. Effects of Supplementary Potassium Nitrate on Growth and Gas-Exchange Characteristics of Salt-Stressed Citrus Seedlings. Photosynthetica 2014, 52, 589–596. [Google Scholar] [CrossRef]
- Singh, G.; Rattanpal, H.S.; Chahal, T.S.; Singh, K.; Gupta, M. Effects of Potassium Application on Vegetative Growth, Fruiting, and Nutrient Status of Kinnow Mandarin under Indian Sub-Tropical Conditions. J. Plant Nutr. 2023, 46, 1197–1206. [Google Scholar] [CrossRef]
- Papadakis, I.E.; Antonopoulou, C.; Sotiropoulos, T.; Chatzissavvidis, C.; Therios, I. Effect of Magnesium on Mineral Nutrition, Chlorophyll, Proline and Carbohydrate Concentrations of Sweet Orange (Citrus sinensis Cv. Newhall) Plants. Appl. Sci. 2023, 13, 7995. [Google Scholar] [CrossRef]
- Papadakis, I.E.; Antonopoulou, C.; Chatzissavvidis, C.; Protopapadakis, E.; Therios, I. Rootstock Effects on Nutritional Status, Yield and Fruit Quality of Washington Navel Sweet Orange [Citrus sinensis (L.) Osbeck] Trees. Agrochimica 2018, 62, 367–378. [Google Scholar] [CrossRef]
- Dubey, A.K.; Sharma, R.M. Effect of Rootstocks on Tree Growth, Yield, Quality and Leaf Mineral Composition of Lemon (Citrus limon (L.) Burm.). Sci. Hortic. 2016, 200, 131–136. [Google Scholar] [CrossRef]
- Taylor, B.K.; Dimsey, R.T. Rootstock and Scion Effects on the Leaf Nutrient Composition of Citrus Trees. Aust. J. Exp. Agric. 1993, 33, 363. [Google Scholar] [CrossRef]
- Toplu, C.; Uygur, V.; Kaplankiran, M.; Demirkeser, T.H.; Yildiz, E. Leaf Mineral Composition of “Nova”, “robinson” and “Fremont” Mandarin Cultivars on Different Rootstocks. J. Plant Nutr. 2010, 33, 602–612. [Google Scholar] [CrossRef]
- El-Sayed, O.M. Mutual Effect Between Three Orange Cvs. And Sour Orange and Volkameriana Rootstocks in Newly Reclaimed Lands. Arab Univ. J. Agric. Sci. 2013, 21, 219–233. [Google Scholar]
- White, P.J.; Bell, M.J.; Djalovic, I.; Hinsinger, P.; Rengel, Z. Potassium Use Efficiency of Plants. In Improving Potassium Recommendations for Agricultural Crops; Murrell, T.S., Mikkelsen, R.L., Sulewski, G., Norton, R., Thompson, M.L., Eds.; Springer: Cham, Switzerland, 2020; pp. 119–145. [Google Scholar] [CrossRef]
- Robson, A.D.; Pitman, M.G. Interactions Between Nutrients in Higher Plants. In Inorganic Plant Nutrition; Springer: Berlin/Heidelberg, Germany, 1983; pp. 147–180. [Google Scholar]
- Xie, K.; Cakmak, I.; Wang, S.; Zhang, F.; Guo, S. Synergistic and Antagonistic Interactions between Potassium and Magnesium in Higher Plants. Crop J. 2021, 9, 249–256. [Google Scholar] [CrossRef]
- Mengel, K. Potassium. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 91–120. [Google Scholar]

| Rootstock | Dry Weights (g) | K Concentration in the Nutrient Solution (mM) | ||||
|---|---|---|---|---|---|---|
| 0.00 | 0.75 | 1.50 | 3.00 | 6.00 | ||
| SO | Leaves | 28.31 ± 3.69 b | 37.7 ± 1.75 a | 32.81 ± 1.99 ab | 34.13 ± 2.75 a | 23.45 ± 4.26 b |
| Scion Stems | 19.31 ± 2.04 c | 31.01 ± 1.51 a | 25.38 ± 0.93 abc | 29.4 ± 4.28 a | 21.87 ± 3.66 bc | |
| Rootstock Stem | 43.48 ± 2.37 a | 56.22 ± 2.52 a | 54.87 ± 2.46 a | 53.05 ± 4.53 a | 48.09 ± 3.42 a | |
| Root | 22.09 ± 3.95 a | 28.92 ± 1.86 a | 26.88 ± 1.11 a | 26.82 ± 1.57 a | 22.22 ± 2.56 a | |
| Total Plant | 113.19 ± 11.19 b | 153.84 ± 7.11 a | 139.93 ± 4.95 ab | 143.4 ± 12.32 ab | 115.62 ± 12.27 b | |
| VL | Leaves | 37.96 ± 3.69 a | 43.39 ± 8.02 a | 44.76 ± 3.43 a | 32.39 ± 1.64 a | 32.98 ± 3.19 a |
| Scion Stems | 35.43 ± 2.13 a | 44.14 ± 8.67 a | 49.61 ± 3.38 a | 42.76 ± 3.74 a | 38.72 ± 3.78 a | |
| Rootstock Stem | 64.49 ± 2.6 a | 62.45 ± 1.54 a | 60.07 ± 3.69 a | 58.06 ± 2.64 a | 54.74 ± 2.77 a | |
| Root | 29.48 ± 0.77 ab | 29.4 ± 3.59 a | 32.15 ± 2.39 a | 21.97 ± 1.64 c | 24.46 ± 1.66 bc | |
| Total Plant | 167.37 ± 5.3 a | 179.38 ± 20.84 a | 186.59 ± 8.73 a | 155.17 ± 5.99 a | 150.89 ± 8.45 a | |
| Rootstock | Parameter | K Concentration in the Nutrient Solution (mM) | ||||
|---|---|---|---|---|---|---|
| 0.00 | 0.75 | 1.50 | 3.00 | 6.00 | ||
| SO | Chla | 1.07 ± 0.31 a | 1.55 ± 0.16 a | 1.65 ± 0.19 a | 1.44 ± 0.44 a | 1.61 ± 0.22 a |
| Chlb | 0.44 ± 0.1 a | 0.56 ± 0.08 a | 0.6 ± 0.08 a | 0.53 ± 0.16 a | 0.6 ± 0.08 a | |
| Chla + Chlb | 1.51 ± 0.41 a | 2.11 ± 0.24 a | 2.25 ± 0.27 a | 1.97 ± 0.59 a | 2.21 ± 0.3 a | |
| Chla/Chlb | 2.41 ± 0.2 b | 2.78 ± 0.13 a | 2.75 ± 0.07 a | 2.72 ± 0.09 a | 2.69 ± 0.06 a | |
| Carotenoids | 0.36 ± 0.08 b | 0.48 ± 0.04 a | 0.51 ± 0.06 a | 0.47 ± 0.1 ab | 0.52 ± 0.07 a | |
| VL | Chla | 1.39 ± 0.32 a | 1.53 ± 0.15 a | 1.75 ± 0.26 a | 1.69 ± 0.46 a | 1.49 ± 0.11 a |
| Chlb | 0.56 ± 0.13 a | 0.57 ± 0.04 a | 0.73 ± 0.17 a | 0.7 ± 0.23 a | 0.62 ± 0.1 a | |
| Chla + Chlb | 1.95 ± 0.44 a | 2.1 ± 0.19 a | 2.48 ± 0.42 a | 2.39 ± 0.68 a | 2.11 ± 0.21 a | |
| Chla/Chlb | 2.46 ± 0.05 a | 2.69 ± 0.1 a | 2.42 ± 0.25 a | 2.44 ± 0.24 a | 2.43 ± 0.23 a | |
| Carotenoids | 0.43 ± 0.09 a | 0.49 ± 0.05 a | 0.54 ± 0.03 a | 0.51 ± 0.09 a | 0.45 ± 0.02 a | |
| Rootstock | Plant Part | K Concentration in the Nutrient Solution (mM) | ||||
|---|---|---|---|---|---|---|
| 0.00 | 0.75 | 1.50 | 3.00 | 6.00 | ||
| SO | Leaves | 40.96 ± 3.19 a | 49.79 ± 1.06 a | 50.59 ± 2.18 a | 48.95 ± 2.48 a | 43.03 ± 4.03 a |
| Scion Stems | 10.19 ± 0.68 b | 12.97 ± 0.62 ab | 13.1 ± 0.83 ab | 15.45 ± 1.12 a | 16.39 ± 1.81 a | |
| Rootstock Stem | 21.8 ± 3.4 a | 15.99 ± 0.41 b | 14.67 ± 1.22 b | 12.34 ± 1.46 b | 15.04 ± 1.76 b | |
| Roots | 27.05 ± 1.48 a | 21.25 ± 0.85 a | 21.64 ± 1.32 a | 23.26 ± 1.54 a | 25.53 ± 3.74 a | |
| Scion (Total) | 51.16 ± 3.1 b | 62.76 ± 0.94 a | 63.69 ± 2.08 a | 64.4 ± 2.81 a | 59.42 ± 4.93 ab | |
| Rootstock (Total) | 48.84 ± 3.1 a | 37.24 ± 0.94 b | 36.31 ± 2.08 b | 35.6 ± 2.81 b | 40.58 ± 4.93 ab | |
| VL | Leaves | 38.59 ± 2.4 a | 45.34 ± 2.49 a | 44.76 ± 1.04 a | 39.73 ± 1.86 a | 46.47 ± 2.66 a |
| Scion Stems | 12.37 ± 1.05 b | 15.23 ± 1.38 b | 14.7 ± 0.8 b | 23.75 ± 1.38 a | 22.04 ± 1.89 a | |
| Rootstock Stem | 20.82 ± 0.86 a | 16.9 ± 2.34 ab | 12.06 ± 1.56 bc | 14.12 ± 2.28 bc | 9.98 ± 0.32 c | |
| Roots | 28.22 ± 1.59 a | 22.54 ± 0.68 b | 28.47 ± 1.06 a | 22.41 ± 2.32 b | 21.5 ± 0.82 b | |
| Scion (Total) | 50.96 ± 1.76 c | 60.57 ± 1.9 b | 59.47 ± 1.11 b | 63.47 ± 2.45 ab | 68.51 ± 0.78 a | |
| Rootstock (Total) | 49.04 ± 1.76 a | 39.43 ± 1.9 b | 40.53 ± 1.11 b | 36.53 ± 2.45 bc | 31.49 ± 0.78 c | |
| Element | Plant Part | K Concentration in the Nutrient Solution (mM) | ||||
|---|---|---|---|---|---|---|
| 0.00 | 0.75 | 1.50 | 3.00 | 6.00 | ||
| N (% d.w.) | Leaves | 2.98 ± 0.19 a | 3.01 ± 0.06 a | 2.99 ± 0.12 a | 2.93 ± 0.05 a | 2.75 ± 0.13 a |
| Scion Stems | 1.45 ± 0.05 a | 1.29 ± 0.11 ab | 1.25 ± 0.04 ab | 1.13 ± 0.05 bc | 0.95 ± 0.1 c | |
| Rootstock Stem | 0.88 ± 0.01 a | 0.79 ± 0.03 a | 0.88 ± 0.02 a | 0.94 ± 0.08 a | 0.93 ± 0.05 a | |
| Roots | 2.55 ± 0.12 a | 2.06 ± 0.06 b | 2.16 ± 0.08 b | 2.18 ± 0.05 b | 2.04 ± 0.06 b | |
| Ca (% d.w.) | Leaves | 4.44 ± 0.37 a | 3.56 ± 0.05 b | 2.73 ± 0.19 c | 2.07 ± 0.09 d | 1.49 ± 0.08 d |
| Scion Stems | 1.54 ± 0.08 ab | 1.4 ± 0.02 ab | 1.56 ± 0.06 a | 1.3 ± 0.13 b | 0.97 ± 0.05 c | |
| Rootstock Stem | 0.99 ± 0.05 a | 1.13 ± 0.03 a | 1.14 ± 0.05 a | 1.12 ± 0.1 a | 0.98 ± 0.07 a | |
| Roots | 0.98 ± 0.12 b | 1.72 ± 0.08 a | 1.7 ± 0.08 a | 1.74 ± 0.02 a | 1.92 ± 0.17 a | |
| Mg (% d.w.) | Leaves | 0.4 ± 0.03 a | 0.35 ± 0.01 ab | 0.32 ± 0.01 bc | 0.27 ± 0.01 c | 0.19 ± 0.02 d |
| Scion Stems | 0.18 ± 0.02 a | 0.16 ± 0 ab | 0.19 ± 0.01 a | 0.13 ± 0.01 bc | 0.1 ± 0.01 c | |
| Rootstock Stem | 0.06 ± 0.01 a | 0.05 ± 0 a | 0.05 ± 0 a | 0.04 ± 0 a | 0.04 ± 0 a | |
| Roots | 0.16 ± 0.01 a | 0.16 ± 0.01 a | 0.16 ± 0.01 a | 0.12 ± 0.01 b | 0.09 ± 0.01 c | |
| P (% d.w.) | Leaves | 0.68 ± 0.05 a | 0.67 ± 0.01 a | 0.63 ± 0.02 a | 0.63 ± 0.03 a | 0.5 ± 0.03 b |
| Scion Stems | 0.91 ± 0.06 a | 0.8 ± 0.04 ab | 0.85 ± 0.03 ab | 0.73 ± 0.03 b | 0.57 ± 0.05 c | |
| Rootstock Stem | 0.47 ± 0.07 a | 0.43 ± 0.03 ab | 0.51 ± 0.03 a | 0.46 ± 0.03 ab | 0.35 ± 0.02 b | |
| Roots | 1.02 ± 0.06 a | 0.98 ± 0.02 a | 0.89 ± 0.03 a | 0.96 ± 0.02 a | 0.9 ± 0.07 a | |
| Fe (mg kg−1 d.w.) | Leaves | 35.68 ± 1.21 c | 49.53 ± 1.83 bc | 51.19 ± 4.43 bc | 75.02 ± 13.43 a | 63.48 ± 5.1 ab |
| Scion Stems | 15.08 ± 1.29 a | 22.26 ± 3.18 a | 23.82 ± 1.66 a | 21.08 ± 1.75 a | 22.89 ± 3.81 a | |
| Rootstock Stem | 18.74 ± 1.81 a | 19.34 ± 2.65 a | 16.61 ± 0.57 a | 15.89 ± 1.2 a | 16.04 ± 1.58 a | |
| Roots | 361.28 ± 27.35 bc | 504.65 ± 33.93 a | 477.6 ± 41.48 a | 433.98 ± 37.37 ab | 308.68 ± 26.25 c | |
| Mn (mg kg−1 d.w.) | Leaves | 53.58 ± 2.38 a | 49.09 ± 1 a | 45.96 ± 1.16 ab | 40.16 ± 3.65 b | 22.61 ± 3.32 c |
| Scion Stems | 6.07 ± 0.28 b | 6.1 ± 0.2 b | 8.09 ± 0.35 a | 8.31 ± 0.8 a | 8.89 ± 0.9 a | |
| Rootstock Stem | 5.87 ± 0.73 a | 5.61 ± 0.34 a | 6.22 ± 0.25 a | 6.93 ± 0.62 a | 6.93 ± 0.24 a | |
| Roots | 585.08 ± 49 a | 593.35 ± 23.2 a | 539.95 ± 42.38 a | 498.15 ± 12.68 ab | 401.6 ± 25.19 b | |
| Zn (mg kg−1 d.w.) | Leaves | 29.58 ± 2.85 b | 36.91 ± 1.13 a | 29.35 ± 2.09 b | 28.92 ± 2.34 b | 16.93 ± 2.97 c |
| Scion Stems | 37.69 ± 3.99 a | 25.28 ± 0.87 b | 26.02 ± 0.67 b | 22.6 ± 1.56 bc | 17 ± 1.54 c | |
| Rootstock Stem | 13.15 ± 2.29 a | 9.36 ± 0.23 a | 9.71 ± 0.58 a | 10.82 ± 1.39 a | 9.19 ± 0.54 a | |
| Roots | 324.1 ± 26.91 a | 303.45 ± 17.05 ab | 257.25 ± 10.15 bc | 257.23 ± 18.19 bc | 209.75 ± 11.09 c | |
| Cu (mg kg−1 d.w.) | Leaves | 7.71 ± 0.99 b | 10.04 ± 0.17 a | 10 ± 0.22 a | 9.89 ± 0.74 a | 6.18 ± 0.13 b |
| Scion Stems | 7.67 ± 0.66 b | 8.48 ± 0.37 ab | 9.41 ± 0.14 a | 8.68 ± 0.5 ab | 5.94 ± 0.62 c | |
| Rootstock Stem | 5.41 ± 0.23 a | 6.21 ± 0.2 a | 6.61 ± 0.44 a | 6.82 ± 0.62 a | 5.52 ± 0.5 a | |
| Roots | 13.37 ± 0.4 b | 15.92 ± 0.51 a | 13.57 ± 0.52 b | 12.85 ± 0.4 b | 12.09 ± 0.46 b | |
| B (mg kg−1 d.w.) | Leaves | 103.72 ± 10.57 a | 88.52 ± 6.96 ab | 74.75 ± 7.91 bc | 60.8 ± 2.73 c | 67.49 ± 2.1 bc |
| Scion Stems | 19.02 ± 1.04 a | 15.64 ± 1.68 ab | 13.97 ± 0.6 b | 14.93 ± 1.61 b | 14.48 ± 1 b | |
| Rootstock Stem | 16.1 ± 1 a | 14.05 ± 0.56 a | 15.78 ± 1.71 a | 15.2 ± 1.57 a | 13.71 ± 0.18 a | |
| Roots | 14.51 ± 0.36 a | 15.84 ± 0.35 a | 16.6 ± 1.08 a | 16.04 ± 0.82 a | 15.45 ± 0.43 a | |
| Element | Plant Part | K Concentration in the Nutrient Solution (mM) | ||||
|---|---|---|---|---|---|---|
| 0.00 | 0.75 | 1.50 | 3.00 | 6.00 | ||
| N (% d.w.) | Leaves | 2.93 ± 0.15 a | 2.89 ± 0.1 a | 3.02 ± 0.11 a | 3.02 ± 0.11 a | 2.86 ± 0.17 a |
| Scion Stems | 1.1 ± 0.06 b | 1.32 ± 0.07 a | 0.91 ± 0.05 c | 1.03 ± 0.05 bc | 0.85 ± 0.07 c | |
| Rootstock Stem | 0.75 ± 0.05 a | 0.83 ± 0.07 a | 0.65 ± 0.03 a | 0.76 ± 0.03 a | 0.65 ± 0.03 a | |
| Roots | 2.35 ± 0.11 ab | 2.23 ± 0.07 ab | 2.07 ± 0.12 b | 2.49 ± 0.04 a | 2.08 ± 0.09 b | |
| Ca (% d.w.) | Leaves | 4.6 ± 0.3 a | 3.6 ± 0.17 b | 3.06 ± 0.12 c | 2.18 ± 0.14 d | 1.27 ± 0.06 e |
| Scion Stems | 1.19 ± 0.07 ab | 1.37 ± 0.12 a | 1.21 ± 0.08 ab | 0.97 ± 0.1 b | 0.65 ± 0.03 c | |
| Rootstock Stem | 0.7 ± 0.04 a | 0.68 ± 0.04 a | 0.65 ± 0.03 a | 0.63 ± 0.02 a | 0.51 ± 0.02 b | |
| Roots | 0.96 ± 0.04 b | 1.02 ± 0.16 b | 0.82 ± 0.05 b | 1.52 ± 0.13 a | 1.16 ± 0.12 b | |
| Mg (% d.w.) | Leaves | 0.33 ± 0.01 a | 0.3 ± 0.02 a | 0.26 ± 0.01 b | 0.27 ± 0.01 b | 0.2 ± 0 c |
| Scion Stems | 0.14 ± 0.01 a | 0.15 ± 0.01 a | 0.13 ± 0.01 ab | 0.12 ± 0.01 b | 0.07 ± 0 c | |
| Rootstock Stem | 0.07 ± 0 a | 0.07 ± 0 ab | 0.06 ± 0 bc | 0.06 ± 0 bc | 0.05 ± 0 c | |
| Roots | 0.26 ± 0.03 a | 0.27 ± 0.01 b | 0.22 ± 0.01 b | 0.16 ± 0 c | 0.11 ± 0 c | |
| P (% d.w.) | Leaves | 0.71 ± 0.02 a | 0.7 ± 0.03 a | 0.67 ± 0.02 ab | 0.61 ± 0.01 bc | 0.58 ± 0.01 c |
| Scion Stems | 0.72 ± 0.04 a | 0.72 ± 0.03 a | 0.72 ± 0.05 a | 0.63 ± 0.01 ab | 0.56 ± 0.02 b | |
| Rootstock Stem | 0.78 ± 0.03 a | 0.76 ± 0.04 a | 0.71 ± 0.04 ab | 0.63 ± 0.03 b | 0.47 ± 0.05 c | |
| Roots | 1.43 ± 0.08 b | 1.54 ± 0.15 b | 1.38 ± 0.02 b | 2.02 ± 0.07 a | 1.41 ± 0.1 b | |
| Fe (mg kg−1 d.w.) | Leaves | 71.01 ± 14.24 a | 64.9 ± 3.13 a | 73.45 ± 4.71 a | 82.63 ± 3.8 a | 85.39 ± 4.6 a |
| Scion Stems | 22.12 ± 2.27 a | 24.97 ± 0.84 a | 22.94 ± 2.44 a | 24.44 ± 1.97 a | 20.1 ± 0.42 a | |
| Rootstock Stem | 18.41 ± 1.49 a | 18.65 ± 1.23 a | 16.4 ± 2.65 a | 16.81 ± 0.85 a | 14.98 ± 0.25 a | |
| Roots | 431.88 ± 23.1 ab | 524.25 ± 46.53 a | 489.88 ± 27.42 a | 475 ± 36.05 a | 339.4 ± 15.43 b | |
| Mn (mg kg−1 d.w.) | Leaves | 68.7 ± 6.02 a | 75.42 ± 5.31 a | 68.71 ± 5.35 a | 64.49 ± 2.63 a | 43.07 ± 2.5 b |
| Scion Stems | 5.88 ± 0.37 c | 7.62 ± 0.44 b | 8.2 ± 0.86 b | 10.18 ± 0.33 a | 10.59 ± 0.57 a | |
| Rootstock Stem | 4.74 ± 0.1 a | 5.25 ± 0.27 a | 6.35 ± 1.13 a | 6.7 ± 0.27 a | 7.06 ± 0.42 a | |
| Roots | 480.1 ± 36.33 a | 525.9 ± 57.71 a | 490.13 ± 58.66 a | 551.23 ± 35.95 a | 419.33 ± 31.24 a | |
| Zn (mg kg−1 d.w.) | Leaves | 31.98 ± 1.86 ab | 34.17 ± 2.46 a | 34.41 ± 0.23 a | 26.83 ± 1.95 b | 19.92 ± 1.47 c |
| Scion Stems | 27.38 ± 3.07 a | 31.01 ± 4.04 a | 24.71 ± 3.04 a | 22.17 ± 1.11 a | 19.58 ± 1.11 a | |
| Rootstock Stem | 18.89 ± 1.18 a | 17.74 ± 1.12 a | 15.35 ± 2.65 a | 16.04 ± 0.84 a | 19.37 ± 2.01 a | |
| Roots | 247.1 ± 11.46 a | 258.83 ± 31.12 a | 191.1 ± 19.58 a | 223.28 ± 20.3 a | 186.13 ± 13.11 a | |
| Cu (mg kg−1 d.w.) | Leaves | 4.69 ± 0.3 c | 6.19 ± 0.58 b | 7.86 ± 0.21 a | 7.13 ± 0.36 ab | 6.64 ± 0.17 b |
| Scion Stems | 5.78 ± 0.28 ab | 6.11 ± 0.35 ab | 6.57 ± 0.5 a | 5.41 ± 0.28 b | 4.36 ± 0.05 c | |
| Rootstock Stem | 4.14 ± 0.16 ab | 4.72 ± 0.32 a | 3.91 ± 0.25 bc | 3.8 ± 0.09 bc | 3.3 ± 0.11 c | |
| Roots | 15.09 ± 0.73 ab | 16.27 ± 1.33 a | 11.86 ± 1.35 bc | 12.57 ± 1.08 bc | 10.39 ± 0.39 c | |
| B (mg kg−1 d.w.) | Leaves | 107.39 ± 6.75 a | 101.07 ± 6.3 ab | 84.13 ± 5.34 bc | 74.62 ± 4.15 c | 70.52 ± 7.59 c |
| Scion Stems | 9.88 ± 0.67 a | 9.58 ± 1.39 a | 7.67 ± 0.29 a | 8.47 ± 0.8 a | 8.29 ± 0.67 a | |
| Rootstock Stem | 14.33 ± 0.47 a | 14.15 ± 1.18 a | 11.42 ± 0.39 a | 12.62 ± 0.66 a | 12.42 ± 0.92 a | |
| Roots | 12.33 ± 0.12 b | 13.68 ± 0.91 b | 13.32 ± 0.48 b | 16.3 ± 1.04 a | 14.02 ± 0.97 ab | |
| Rootstock | VL (Volkamer Lemon) | SO (Sour Orange) | ||||||
|---|---|---|---|---|---|---|---|---|
| Plant Part | Roots | Rootstock Stems | Scion Stems | Leaves | Roots | Rootstock Stems | Scion Stems | Leaves |
| Ν (% d.w.) | −0.213 n.s. | −0.357 n.s. | −0.567 ** | −0.082 n.s. | −0.489 ** | 0.382 n.s | −0.764 *** | −0.385 n.s |
| P (% d.w.) | 0.083 n.s. | −0.871 *** | −0.712 *** | −0.786 *** | −0.336 n.s | −0.483 * | −0.810 *** | −0.733 *** |
| Κ (% d.w.) | 0.846 *** | 0.685 ** | 0.965 *** | 0.944 *** | 0.943 *** | 0.910 *** | 0.951 *** | 0.901 *** |
| Ca (% d.w.) | 0.368 n.s. | −0.754 *** | −0.805 *** | −0.925 *** | 0.622 ** | −0.187 n.s | −0.795 *** | −0.874 *** |
| Mg (% d.w.) | −0.891 *** | −0.727 *** | −0.867 *** | −0.885 *** | −0.877 *** | −0.502 * | −0.776 *** | −0.893 *** |
| B (mg kg−1 d.w.) | 0.326 n.s. | −0.324 n.s. | −0.275 n.s. | −0.714 *** | 0.078 n.s | −0.268 n.s | −0.376 n.s | −0.599 ** |
| Mn (mg kg−1 d.w.) | −0.261 n.s. | 0.586 ** | 0.791 *** | −0.755 *** | −0.762 *** | 0.465 * | 0.651 ** | −0.922 *** |
| Zn (mg kg−1 d.w.) | −0.459 * | 0.115 n.s. | −0.557 ** | −0.813 *** | −0.744 *** | −0.299 n.s | −0.755 *** | −0.726 *** |
| Fe (mg kg−1 d.w.) | −0.558 * | −0.411 n.s. | −0.285 n.s. | 0.446 * | −0.449 * | −0.332 n.s | 0.289 n.s | 0.537 * |
| Cu (mg kg−1 d.w.) | −0.634 ** | −0.665 ** | −0.675 ** | 0.347 n.s. | −0.57 ** | −0.064 n.s | −0.531 ** | −0.475 * |
| Rootstock | VL (Volkamer Lemon) | SO (Sour Orange) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Plant Part | Total Plant Content | Roots | Rootstock Stems | Scion Stems | Leaves | Total Plant Content | Roots | Rootstock Stems | Scion Stems | Leaves |
| Ν | −0.609 ** | −0.091 n.s. | −0.021 n.s. | −0.076 n.s. | 0.118 n.s. | −0.439 n.s. | 0.101 n.s. | 0.680 ** | −0.454 * | −0.544 * |
| P | −0.764 *** | 0.506 * | −0.606 ** | 0.273 n.s. | −0.088 n.s. | −0.526 * | 0.483 * | 0.171 n.s. | −0.318 n.s. | −0.614 ** |
| Κ | 0.820 *** | −0.509 * | −0.657 ** | 0.727 *** | 0.300 n.s. | 0.788 *** | 0.095 n.s. | −0.378 n.s. | 0.681 ** | −0.102 n.s. |
| Ca | −0.903 *** | 0.875 *** | 0.744 *** | 0.311 n.s. | −0.882 *** | −0.692 ** | 0.879 *** | 0.814 *** | 0.012 n.s. | −0.905 *** |
| Mg | −0.829 *** | −0.763 *** | 0.581 ** | 0.207 n.s. | 0.656 ** | −0.800 *** | −0.344 n.s. | 0.692 ** | 0.422 n.s. | −0.502 * |
| B | −0.709 *** | 0.736 *** | 0.450 n.s. | 0.520 ** | −0.708 *** | −0.713 *** | 0.683 ** | −0.646 ** | 0.520 * | −0.803 *** |
| Mn | −0.653 ** | 0.155 n.s. | 0.805 *** | 0.808 *** | −0.595 ** | −0.576 ** | 0.331 n.s. | 0.696 ** | 0.877 *** | −0.684 ** |
| Zn | −0.711 *** | −0.171 n.s. | 0.613 ** | 0.120 n.s. | −0.391 n.s. | −0.650 ** | 0.253 n.s. | 0.277 n.s. | −0.328 n.s. | −0.451 * |
| Fe | −0.582 ** | −0.585 ** | 0.110 n.s. | 0.415 n.s. | 0.561 * | −0.297 n.s. | −0.498 ** | 0.083 n.s. | 0.546 ** | 0.510 * |
| Cu | −0.603 * | −0.510 * | −0.082 n.s. | 0.092 n.s. | 0.571 ** | −0.369 n.s. | 0.097 n.s. | 0.598 ** | −0.138 n.s. | −0.633 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadakis, I.E.; Ladikou, E.-V.; Oikonomou, A.; Chatzistathis, T.; Chatziperou, G. Exploring the Impact of Potassium on Growth, Photosynthetic Performance, and Nutritional Status of Lemon Trees (cv. Adamopoulou) Grafted onto Sour Orange and Volkamer Lemon Rootstocks. Sustainability 2023, 15, 15858. https://doi.org/10.3390/su152215858
Papadakis IE, Ladikou E-V, Oikonomou A, Chatzistathis T, Chatziperou G. Exploring the Impact of Potassium on Growth, Photosynthetic Performance, and Nutritional Status of Lemon Trees (cv. Adamopoulou) Grafted onto Sour Orange and Volkamer Lemon Rootstocks. Sustainability. 2023; 15(22):15858. https://doi.org/10.3390/su152215858
Chicago/Turabian StylePapadakis, Ioannis E., Evangelia-Vasiliki Ladikou, Alexia Oikonomou, Theocharis Chatzistathis, and Georgia Chatziperou. 2023. "Exploring the Impact of Potassium on Growth, Photosynthetic Performance, and Nutritional Status of Lemon Trees (cv. Adamopoulou) Grafted onto Sour Orange and Volkamer Lemon Rootstocks" Sustainability 15, no. 22: 15858. https://doi.org/10.3390/su152215858
APA StylePapadakis, I. E., Ladikou, E.-V., Oikonomou, A., Chatzistathis, T., & Chatziperou, G. (2023). Exploring the Impact of Potassium on Growth, Photosynthetic Performance, and Nutritional Status of Lemon Trees (cv. Adamopoulou) Grafted onto Sour Orange and Volkamer Lemon Rootstocks. Sustainability, 15(22), 15858. https://doi.org/10.3390/su152215858








