Life Cycle Assessment of Polyol Production from Lignin via Organosolv and Liquefaction Treatments
Abstract
:1. Introduction
1.1. Relevance of the Study
1.2. Objectives
2. Materials and Methods
2.1. Life Cycle Assessment Methodology
2.2. Goal and Scope Definition
2.3. System Boundaries and Functional Unity
2.4. Life Cycle Inventory
2.4.1. Data Quality
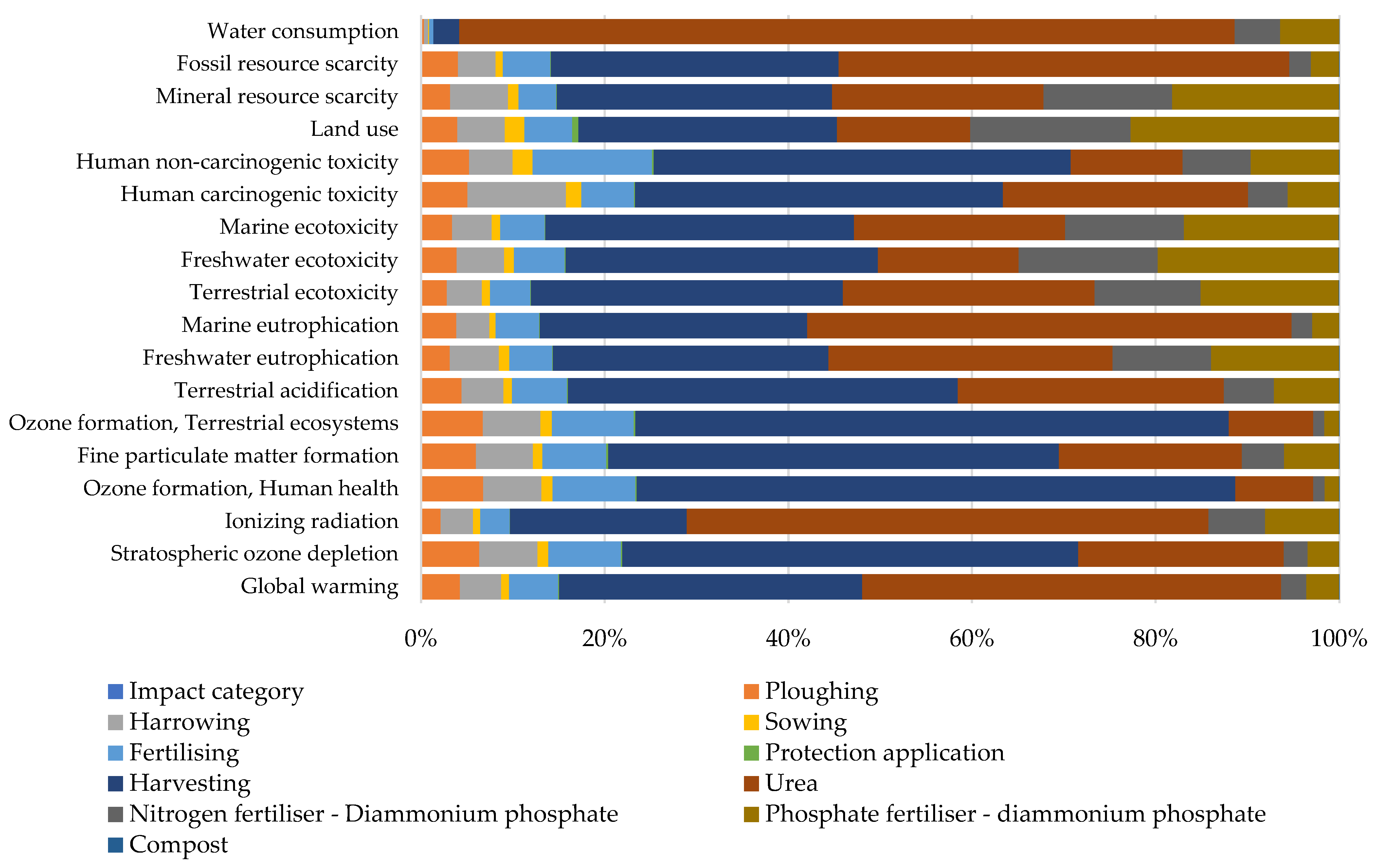
2.4.2. Crop Production
2.5. Organosolv and Liquefaction Processes
2.5.1. Laboratory Processes and Optimization
2.5.2. Organosolv-Mediated Lignin Extraction
2.5.3. OL Liquefaction
2.5.4. Hydroxyl Number and Acid Number
2.6. Scale Up
2.7. Life Cycle Impact Assessment and Interpretation
3. Results
3.1. Laboratory Results: Compositional Analysis and Liquefaction Process
3.2. Life Cycle Inventory
3.3. Life Cycle Impact Assessment
3.3.1. Midpoint Level—Characterization
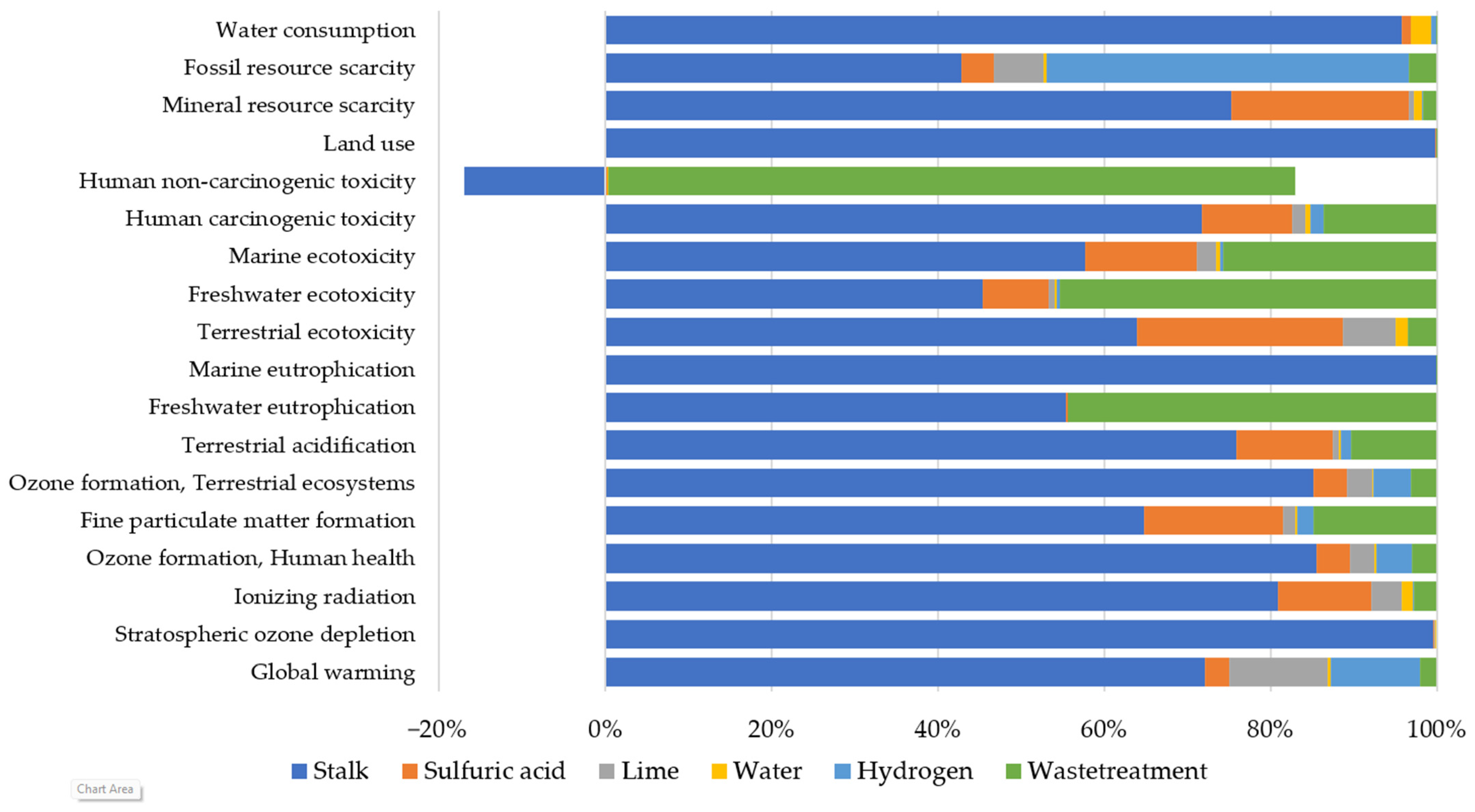
Cardoon Production
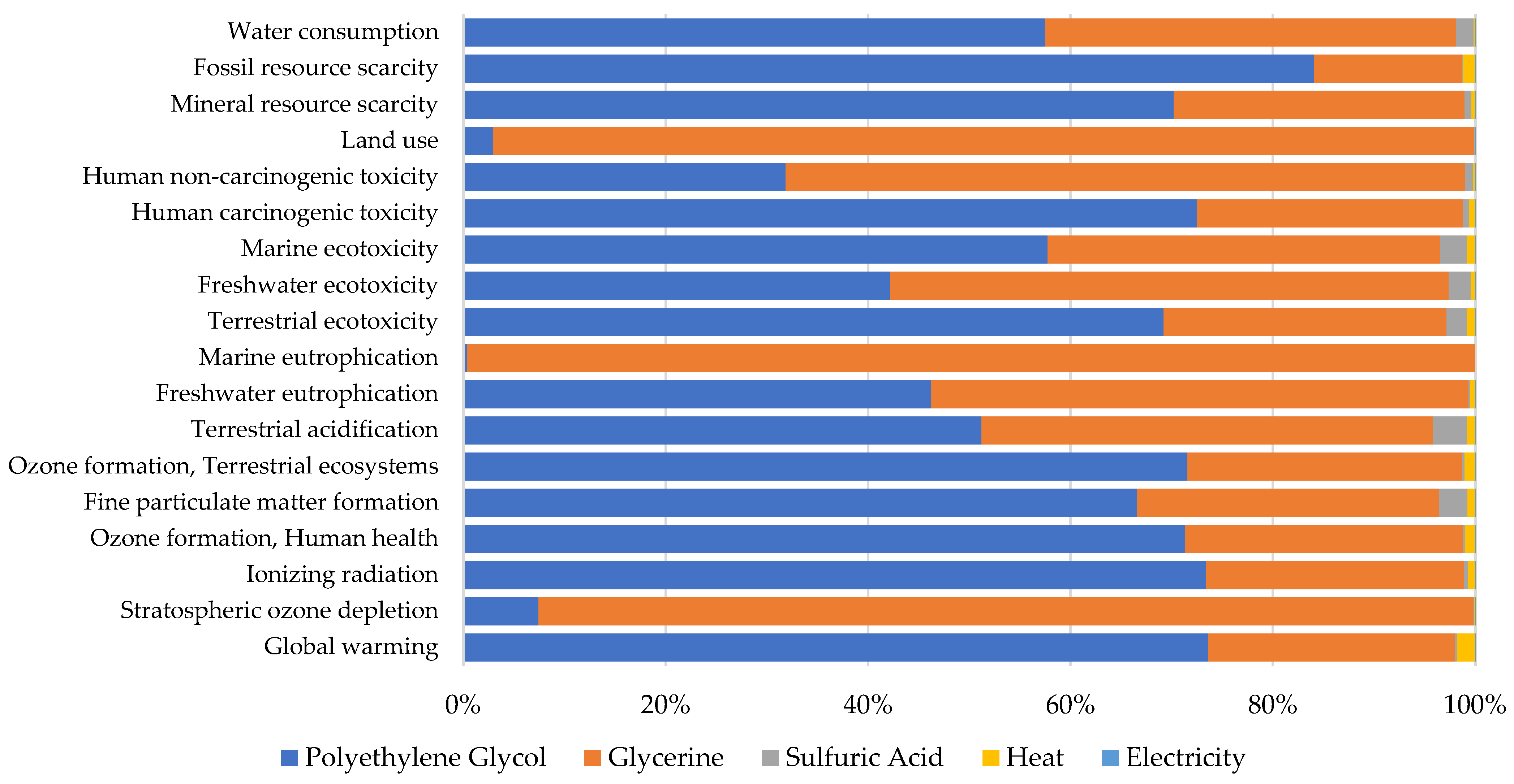
Organosolv
Liquefaction
3.3.2. ReCiPe Endpoint
4. Discussion
4.1. LCIA Midpoint Level
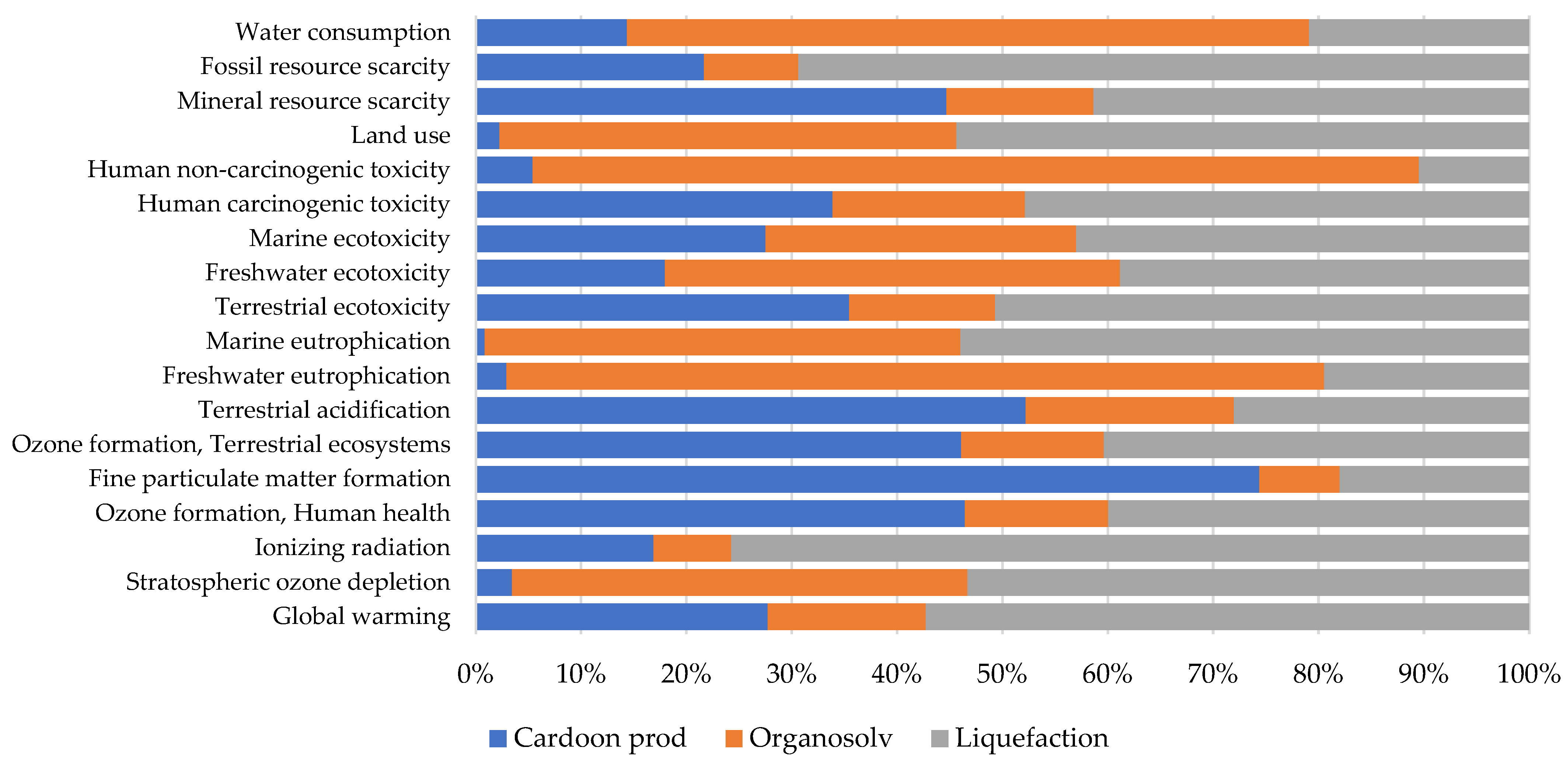
4.1.1. Crop Production
4.1.2. Organosolv
4.1.3. Liquefaction
4.1.4. Comparison GWP
4.2. LCIA Endpoint Level
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Cardoon Production | ||
|---|---|---|
| Urea | 57.5 | kg/ha |
| Diamonium phosphate—N | 4.5 | kg/ha |
| Diamonium phosphate—P | 11.5 | kg/ha |
| Compost | 20 | kg/ha |
| Pendimetalin | 151.7 | g/ha |
| Ploughing | 0.17 | ha |
| Harrowing | 0.33 | ha |
| Sowing | 0.17 | ha |
| Fertilizing | 1 | ha |
| Plant protection application | 0.17 | ha |
| Harvesting | 1 | ha |
| Emission—N2O | 0.8358 | kg/ha |
| Emission—NH3 | 9.3 | kg/ha |
| Emission—CO2 | 11.5 | kg/ha |
| GVL Production | ||
|---|---|---|
| Biomass | 4.601 | kg |
| H2SO4 | 0.046 | kg |
| Lime | 0.035 | kg |
| H2O | 1.872 | kg |
| H2 | 0.016 | kg |
| Waste gypsum | 0.081 | kg |
| Wood as mixture | 0.286 | Kg |
| Values in kg per kg of GVL | ||
| Impact Category | Unit | Crop Production | Organosolv | Liquefaction | Total |
|---|---|---|---|---|---|
| Global warming | kg CO2 eq | 0.911 | 0.492 | 1.880 | 3.283 |
| Stratospheric ozone depletion | kg CFC11 eq | 2.09 × 10−7 | 2.62 × 10−6 | 3.23 × 10−6 | 6.05 × 10−6 |
| Ionizing radiation | kBq Co-60 eq | 1.64 × 10−3 | 7.15 × 10−4 | 7.36 × 10−3 | 9.71 × 10−3 |
| Ozone formation, Human health | kg NOx eq | 4.91 × 10−3 | 1.44 × 10−3 | 4.23 × 10−3 | 1.06 × 10−2 |
| Fine particulate matter formation | kg PM2.5 eq | 1.16 × 10−2 | 1.19 × 10−3 | 2.80 × 10−3 | 1.56 × 10−2 |
| Ozone formation, Terrestrial ecosystems | kg NOx eq | 5.08 × 10−3 | 1.49 × 10−3 | 4.45 × 10−3 | 1.10 × 10−2 |
| Terrestrial acidification | kg SO2 eq | 1.45 × 10−2 | 5.49 × 10−3 | 7.77 × 10−3 | 2.78 × 10−2 |
| Freshwater eutrophication | kg P eq | 1.42 × 10−5 | 3.79 × 10−4 | 9.50 × 10−5 | 4.89 × 10−4 |
| Marine eutrophication | kg N eq | 1.70 × 10−5 | 9.03 × 10−4 | 1.08 × 10−3 | 2.00 × 10−3 |
| Terrestrial ecotoxicity | kg 1,4-DCB | 4.170 | 1.632 | 5.965 | 11.767 |
| Freshwater ecotoxicity | kg 1,4-DCB | 9.44 × 10−4 | 2.27 × 10−3 | 2.04 × 10−3 | 5.26 × 10−3 |
| Marine ecotoxicity | kg 1,4-DCB | 3.48 × 10−3 | 3.73 × 10−3 | 5.43 × 10−3 | 1.26 × 10−2 |
| Human carcinogenic toxicity | kg 1,4-DCB | 1.40 × 10−2 | 7.57 × 10−3 | 1.98 × 10−2 | 4.14 × 10−2 |
| Human non-carcinogenic toxicity | kg 1,4-DCB | 0.518 | 8.068 | 1.004 | 9.590 |
| Land use | m2a crop eq | 0.026 | 0.495 | 0.621 | 1.142 |
| Mineral resource scarcity | kg Cu eq | 5.82 × 10−3 | 1.82 × 10−3 | 5.39 × 10−3 | 1.30 × 10−2 |
| Fossil resource scarcity | kg oil eq | 0.268 | 0.111 | 0.860 | 1.239 |
| Water consumption | m3 | 2.08 × 10−2 | 9.38 × 10−2 | 3.03 × 10−2 | 1.45 × 10−1 |
References
- Vieira, F.R.; Magina, S.; Evtuguin, D.V.; Barros-Timmons, A. Lignin as a Renewable Building Block for Sustainable Polyurethanes. Materials 2022, 15, 6182. [Google Scholar] [CrossRef]
- Polyurathane Market Size & COVID-19 Impact Analysis. Available online: https://www.fortunebusinessinsights.com/industry-reports/polyurethane-pu-market-101801 (accessed on 5 September 2023).
- Cateto, C.A.; Barreiro, M.F.; Rodrigues, A.E.; Belgacem, M.N. Optimization study of lignin oxypropylation in view of the preparation of polyurethane rigid foams. Ind. Eng. Chem. Res. 2009, 48, 2583–2589. [Google Scholar] [CrossRef]
- Kamm, B.; Gruber, P.R.; Kamm, M. Biorefineries-Industrial Processes and Products; Wiley-VCH Weinheim: Weinheim, Germany, 2006; Volume 2. [Google Scholar]
- Tribot, A.; Amer, G.; Alio, M.A.; de Baynast, H.; Delattre, C.; Pons, A.; Mathias, J.-D.; Callois, J.-M.; Vial, C.; Michaud, P. Wood-lignin: Supply, extraction processes and use as bio-based material. Eur. Polym. J. 2019, 112, 228–240. [Google Scholar] [CrossRef]
- Luan, P.; Zhao, X.; Copenhaver, K.; Ozcan, S.; Zhu, H. Turning Natural Herbaceous Fibers into Advanced Materials for Sustainability. Adv. Fiber Mater. 2022, 4, 736–757. [Google Scholar] [CrossRef]
- Borand, M.N.; Karaosmanoğlu, F. Effects of organosolv pretreatment conditions for lignocellulosic biomass in biorefinery applications: A review. J. Renew. Sustain. Energy 2018, 10, 033104. [Google Scholar] [CrossRef]
- Kautto, J.; Realff, M.J.; Ragauskas, A.J. Design and simulation of an organosolv process for bioethanol production. Biomass Convers. Biorefinery 2013, 3, 199–212. [Google Scholar] [CrossRef]
- Sannigrahi, P.; Ragauskas, A.J.; Miller, S.J. Lignin structural modifications resulting from ethanol organosolv treatment of loblolly pine. Energy Fuels 2010, 24, 683–689. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Jin, Y.; Ruan, X.; Cheng, X.; Lü, Q. Liquefaction of lignin by polyethyleneglycol and glycerol. Bioresour. Technol. 2011, 102, 3581–3583. [Google Scholar] [CrossRef]
- da Silva, S.H.F.; Egüés, I.; Labidi, J. Liquefaction of Kraft lignin using polyhydric alcohols and organic acids as catalysts for sustainable polyols production. Ind. Crops Prod. 2019, 137, 687–693. [Google Scholar] [CrossRef]
- Zhang, X.; Kim, Y.; Elsayed, I.; Taylor, M.; Eberhardt, T.L.; Hassan, E.B.; Shmulsky, R. Rigid polyurethane foams containing lignin oxyalkylated with ethylene carbonate and polyethylene glycol. Ind. Crops Prod. 2019, 141, 111797. [Google Scholar] [CrossRef]
- Hu, S.; Luo, X.; Li, Y. Polyols and polyurethanes from the liquefaction of lignocellulosic biomass. ChemSusChem 2014, 7, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Zhao, G.-J.; Alma, M.H. Polycondensation reaction and its mechanism during lignocellulosic liquefaction by an acid catalyst: A review. For. Stud. China 2011, 13, 71–79. [Google Scholar] [CrossRef]
- Soares, B.; Gama, N.; Freire, C.; Barros-Timmons, A.; Brandao, I.; Silva, R.; Pascoal Neto, C.; Ferreira, A. Ecopolyol production from industrial cork powder via acid liquefaction using polyhydric alcohols. ACS Sustain. Chem. Eng. 2014, 2, 846–854. [Google Scholar] [CrossRef]
- Jasiukaitytė-Grojzdek, E.; Kunaver, M.; Crestini, C. Lignin structural changes during liquefaction in acidified ethylene glycol. J. Wood Chem. Technol. 2012, 32, 342–360. [Google Scholar] [CrossRef]
- Jasiukaitytė, E.; Kunaver, M.; Crestini, C. Lignin behaviour during wood liquefaction—Characterization by quantitative 31P, 13C NMR and size-exclusion chromatography. Catal. Today 2010, 156, 23–30. [Google Scholar] [CrossRef]
- Yip, J.; Chen, M.; Szeto, Y.; Yan, S. Comparative study of liquefaction process and liquefied products from bamboo using different organic solvents. Bioresour. Technol. 2009, 100, 6674–6678. [Google Scholar] [CrossRef]
- Faris, A.H.; Ibrahim, M.N.M.; Rahim, A.A.; Hussin, M.H.; Brosse, N. Preparation and characterization of lignin polyols from the residues of oil palm empty fruit bunch. BioResources 2015, 10, 7339–7352. [Google Scholar] [CrossRef]
- Cinelli, P.; Anguillesi, I.; Lazzeri, A. Green synthesis of flexible polyurethane foams from liquefied lignin. Eur. Polym. J. 2013, 49, 1174–1184. [Google Scholar] [CrossRef]
- Mohammadpour, R.; Mir Mohamad Sadeghi, G. Effects of some material parameters on lignin biopolymer liquefaction by microwave heating. Iran. Polym. J. 2020, 29, 147–159. [Google Scholar] [CrossRef]
- Xue, B.-L.; Wen, J.-L.; Sun, R.-C. Producing lignin-based polyols through microwave-assisted liquefaction for rigid polyurethane foam production. Materials 2015, 8, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, M. Chemistry and Technology Of Polyols for Polyurethanes; Smithers Rapra Publishing: Shawbury, UK, 2005. [Google Scholar]
- ISO 14040a; Environmental Management—Life Cycle Assessment—Principles and Framework. ISO: Geneva, Switzerland, 2006.
- ISO 14040b; Environmental Management—Life Cycle Assessment—Principles and Framework. ISO: Geneva, Switzerland, 2006.
- SimaPro. Sustainability SimaPro. Available online: https://simapro.com/ (accessed on 5 September 2023).
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The ecoinvent database version 3 (part I): Overview and methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- Tortoioli, S.; Paolotti, L.; Romagnoli, F.; Boggia, A.; Rocchi, L. Environmental Assessment of Bio-Oil Transformation from Thistle in the Italian Context: An LCA Study. Environ. Clim. Technol. 2020, 24, 430–446. [Google Scholar] [CrossRef]
- Barros Lovate Temporim, R.; Cavalaglio, G.; Petrozzi, A.; Coccia, V.; Cotana, F.; Nicolini, A. Life Cycle Assessment of Cynara cardunculus L. -Based Polygeneration and Biodiesel Chains. Sustainability 2022, 14, 13868. [Google Scholar] [CrossRef]
- Giannoni, T.; Gelosia, M.; Bertini, A.; Fabbrizi, G.; Nicolini, A.; Coccia, V.; Iodice, P.; Cavalaglio, G. Fractionation of Cynara cardunculus L. by Acidified Organosolv Treatment for the Extraction of Highly Digestible Cellulose and Technical Lignin. Sustainability 2021, 13, 8714. [Google Scholar] [CrossRef]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. From laboratory to industrial scale: A scale-up framework for chemical processes in life cycle assessment studies. J. Clean. Prod. 2016, 135, 1085–1097. [Google Scholar] [CrossRef]
- IPCC. Guidelines for National Greenhouse Gas Inventories; IPCC: Geneva, Switzerland, 2006. [Google Scholar]
- Sluiter, J.B.; Ruiz, R.O.; Scarlata, C.J.; Sluiter, A.D.; Templeton, D.W. Compositional analysis of lignocellulosic feedstocks. 1. Review and description of methods. J. Agric. Food Chem. 2010, 58, 9043–9053. [Google Scholar] [CrossRef]
- Sequeiros, A.; Serrano, L.; Briones, R.; Labidi, J. Lignin liquefaction under microwave heating. J. Appl. Polym. Sci. 2013, 130, 3292–3298. [Google Scholar] [CrossRef]
- ASTM D4274-21; Standard Test Method for Testing Polyurethane Raw Materials: Determination of Hydroxyl Numbers of Polyols. ASTM: West Conshohocken, PA, USA, 2005.
- Bello, S.; Ríos, C.; Feijoo, G.; Moreira, M.T. Comparative evaluation of lignocellulosic biorefinery scenarios under a life-cycle assessment approach. Biofuels Bioprod. Biorefin. 2018, 12, 1047–1064. [Google Scholar] [CrossRef]
- Uihlein, A.; Schebek, L. Environmental impacts of a lignocellulose feedstock biorefinery system: An assessment. Biomass Bioenergy 2009, 33, 793–802. [Google Scholar] [CrossRef]
- Jeswani, H.K.; Falano, T.; Azapagic, A. Life cycle environmental sustainability of lignocellulosic ethanol produced in integrated thermo-chemical biorefineries. Biofuels Bioprod. Biorefin. 2015, 9, 661–676. [Google Scholar] [CrossRef]
- Naimi, L.J.; Sokhansanj, S. Data-based equation to predict power and energy input for grinding wheat straw, corn stover, switchgrass, miscanthus, and canola straw. Fuel Process. Technol. 2018, 173, 81–88. [Google Scholar] [CrossRef]
- Han, J.; Son, M.; Kang, D. Process design and environmental analysis for catalytic production of gamma-valerolactone from Kenaf. J. Ind. Eng. Chem. 2023, 120, 254–260. [Google Scholar] [CrossRef]
- Green, D.W.; Perry, R.H. Perry’s Chemical Engineers’ Handbook; McGraw-Hill Education: New York, NY, USA, 2008. [Google Scholar]
- Anlauf, H. Mechanical Solid–Liquid Separation, Introduction. In Ullmann’s Encyclopedia of Industrial Chemistry; Verlag Chemie: Hoboken, NJ, USA, 2000; pp. 1–13. [Google Scholar]
- Barbanera, M.; Castellini, M.; Tasselli, G.; Turchetti, B.; Cotana, F.; Buzzini, P. Prediction of the environmental impacts of yeast biodiesel production from cardoon stalks at industrial scale. Fuel 2021, 283, 118967. [Google Scholar] [CrossRef]
- Koch, D.; Paul, M.; Beisl, S.; Friedl, A.; Mihalyi, B. Life cycle assessment of a lignin nanoparticle biorefinery: Decision support for its process development. J. Clean. Prod. 2020, 245, 118760. [Google Scholar] [CrossRef]
- Huijbregts, M.A.; Steinmann, Z.J.; Elshout, P.M.; Stam, G.; Verones, F.; Vieira, M.; Zijp, M.; Hollander, A.; Van Zelm, R. ReCiPe2016: A harmonised life cycle impact assessment method at midpoint and endpoint level. Int. J. Life Cycle Assess. 2017, 22, 138–147. [Google Scholar] [CrossRef]
- Antunes, A.; Amaral, E.; Belgacem, M. Cynara cardunculus L.: Chemical composition and soda-anthraquinone cooking. Ind. Crops Prod. 2000, 12, 85–91. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod. Biorefin. 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Fabbrizi, G.; Giannoni, T.; Lorenzi, L.; Nicolini, A.; Iodice, P.; Coccia, V.; Cavalaglio, G.; Gelosia, M. High Solid and Low Cellulase Enzymatic Hydrolysis of Cardoon Stems Pretreated by Acidified γ-Valerolactone/Water Solution. Energies 2022, 15, 2600. [Google Scholar] [CrossRef]
- Barros Lovate Temporim, R.; Cavalaglio, G.; Petrozzi, A.; Coccia, V.; Iodice, P.; Nicolini, A.; Cotana, F. Life Cycle Assessment and Energy Balance of a Polygeneration Plant Fed with Lignocellulosic Biomass of Cynara cardunculus L. Energies 2022, 15, 2397. [Google Scholar] [CrossRef]
- Deligios, P.A.; Sulas, L.; Spissu, E.; Re, G.A.; Farci, R.; Ledda, L. Effect of input management on yield and energy balance of cardoon crop systems in Mediterranean environment. Eur. J. Agron. 2017, 82, 173–181. [Google Scholar] [CrossRef]
- Caloiero, T.; Coscarelli, R.; Gaudio, R.; Leonardo, G.P. Precipitation trend and concentration in the Sardinia region. Theor. Appl. Climatol. 2019, 137, 297–307. [Google Scholar] [CrossRef]
- Helling, R.K.; Russell, D.A. Use of life cycle assessment to characterize the environmental impacts of polyol production options. Green Chem. 2009, 11, 380–389. [Google Scholar] [CrossRef]
- von der Assen, N.; Bardow, A. Life cycle assessment of polyols for polyurethane production using CO2 as feedstock: Insights from an industrial case study. Green Chem. 2014, 16, 3272–3280. [Google Scholar] [CrossRef]
- Manzardo, A.; Marson, A.; Roso, M.; Boaretti, C.; Modesti, M.; Scipioni, A.; Lorenzetti, A. Life Cycle Assessment Framework To Support the Design of Biobased Rigid Polyurethane Foams. ACS Omega 2019, 4, 14114–14123. [Google Scholar] [CrossRef] [PubMed]





| Process/Phase | Data | Source | Reference |
|---|---|---|---|
| Crop Production | Yields and chemical inputs (fertilizers) | Primary data from Ramoon et al. and Tortoioli et al. | [29,30] |
| Machinery | Secondary data from Ramoon el al. | [30] | |
| Emissions (N2O, NH3, and CO2 from soil maintenance) | Estimation based on Tortoioli et al. | [29] | |
| Organosolv and Liquefaction | Condition (temperatures, cooling), efficiency conversion, yields, inputs (solvents and reactants) and outputs (products, by-products, and residues) at laboratory scale | Primary data from Giannoni et al. | [31] |
| Processes, mass, and energy flows at large scale | Estimation based on the Piccino et al. methodology | [32] |
| Cellulose w/w | Hemicellulose w/w | Acetyl Group w/w | Pectin w/w | Lignin a w/w | Extractives w/w | Ash w/w |
|---|---|---|---|---|---|---|
| 30.5% ± 0.15 | 17.2% ± 0.07 | 5.0% ± 0.12 | 4.7% ± 0.33 | 14.2% ± 0.18 | 7.6% ± 0.16 | 8.8% ± 0.19 |
| Organosolv Treatment | Liquefaction | ||||
|---|---|---|---|---|---|
| Milling | PEG 400 | 0.66 | kg | ||
| Lignocellulosic Biomass | 1.15 | kg | Glycerol | 0.16 | kg |
| Electricity | 4.70 × 10−2 | MJ | H2SO4 | 2.55 × 10−2 | kg |
| Organosolv Reaction | Heat | 0.30 | MJ | ||
| H2O | 1.08 | kg | Electricity-Pump | 1.02 × 10−4 | MJ |
| GVL | 5.38 | kg | |||
| H2SO4 | 0.20 | kg | |||
| Heat-Organosolv | 3.02 | MJ | |||
| Electricity-Pumps | 1.07 × 10−3 | MJ | |||
| Filtration Organosolv Products | |||||
| Electricity-Filtration | 0.37 | MJ | |||
| Electricity-Pump | 5.50 × 10−4 | MJ | |||
| Pulp Washing | |||||
| H2O | 0.72 | kg | |||
| GVL | 5.38 | kg | |||
| Electricity-Pump | 1.42 × 10−3 | MJ | |||
| Pulp Filtration | |||||
| Electricity-Filtration | 0.48 | MJ | |||
| Electricity-Pump | 6.91 × 10−4 | MJ | |||
| Lignin Washing | |||||
| H2O | 21.06 | kg | |||
| Electricity-Pump | 8.96 × 10−3 | MJ | |||
| Lignin Filtration | |||||
| Electricity-Filtration | 0.32 | MJ | |||
| Electricity-Pump | 4.70 × 10−4 | MJ | |||
| Wastewater Treatment | |||||
| Water Treatment | 92.27 | kg | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalaglio, G.; Mecca, I.; Iodice, P.; Giannoni, T.; Gelosia, M.; Nicolini, A.; Barros Lovate Temporim, R. Life Cycle Assessment of Polyol Production from Lignin via Organosolv and Liquefaction Treatments. Sustainability 2023, 15, 15905. https://doi.org/10.3390/su152215905
Cavalaglio G, Mecca I, Iodice P, Giannoni T, Gelosia M, Nicolini A, Barros Lovate Temporim R. Life Cycle Assessment of Polyol Production from Lignin via Organosolv and Liquefaction Treatments. Sustainability. 2023; 15(22):15905. https://doi.org/10.3390/su152215905
Chicago/Turabian StyleCavalaglio, Gianluca, Ippolita Mecca, Paola Iodice, Tommaso Giannoni, Mattia Gelosia, Andrea Nicolini, and Ramoon Barros Lovate Temporim. 2023. "Life Cycle Assessment of Polyol Production from Lignin via Organosolv and Liquefaction Treatments" Sustainability 15, no. 22: 15905. https://doi.org/10.3390/su152215905






