Abstract
The formation of soil seed banks could be an important survival strategy for desert plant species that determine their persistence under harsh conditions, where temperature is extremely high, and chances of rainfall are low and unpredictable. Therefore, the assessment of the seed viability and germination potential of in-situ stored seeds could be important for understanding their reproductive strategies. Seeds of the studied species were collected in 2017 and divided into two batches. The first batch (fresh seeds) of each species was tested for seed germination within one week after collection. However, the second batch (in-situ stored seeds) was tested for seed germination in the first week of October 2022. In the current study, the germination potential of in-situ stored seeds was investigated in order to determine their ability to remain viable under natural conditions. Stored seeds of studied species showed higher germination percentages (53–89%) than fresh seeds (3–34%), except for Peganum harmala, indicating the presence of seed dormancy at the time of seed maturation. Seed germination percentages of all the species were significantly enhanced by storage, indicating their ability to form persistent soil seed banks, although the extent was species-specific. Fresh seeds of all the species attained higher germination in light as compared to complete darkness, suggesting that the germination of seeds can be restricted if they are buried deep in the soil under natural conditions. However, depending on species, in-situ seed storage changed the light requirement for germination. Additionally, the absence of an increase in the ratio of dead seeds between fresh and stored seeds indicates their ability to remain persistent in a soil seed bank and thus may offer great potential for maintaining and restoring desert ecosystems.
1. Introduction
Integration of both ex-situ and in-situ conservation methods are essential for conserving plant biodiversity. Ex-situ storage of seeds in a seed bank is considered to be one of the most cost-effective methods for conserving plant diversity and also offers a vital source of material for restoration [1]. In-situ stored seeds in a soil seed bank is considered to be a key factor for ecosystem restoration because these seeds have the potential to replace the adult plants [2,3]. Therefore, the soil seed bank is considered as a biodiversity reservoir and plays an important role in (i) species recruitment and establishment, (ii) long term conservation of genetic variation, (iii) determining the spatio-temporal distribution of species, and (iv) having direct implications for restoration and effective ecosystem management [1,4,5,6,7,8]. Moreover, seed persistence in a soil seed bank also contributes to determining the fate of plant populations and thus plays an important role in determining species survival and plant diversity [9,10,11]. Therefore, understanding the seed longevity in a soil seed bank could be crucial for predicting species distribution under changing climatic conditions as well as understanding the community dynamics in both short- and long-term perspectives [12,13]. In-situ storage of seeds can provide more accurate information on the viability loss and germinability over time because these seeds remain exposed to natural environmental conditions.
In arid deserts, plants are subjected to extremely high temperatures, high evaporation rates, low water availability, high salinity, and acute nutrient deficiencies [14,15]. All these factors contribute to creating this region as one of the most hostile places for plant growth and development. Maintaining soil seed banks in desert environmental conditions could be the adaptation strategy that enhances their chances of establishment under such unpredictable extreme weather conditions [16,17]. Usually, both dormant and non-dormant seeds are present in soil seed banks and consequently increases the chances of species persistence by synchronizing the germination timing with favorable seasons or in the absence of enough seed production during a particular year [17,18,19]. Since seed production in the desert is usually inconsistent and low due to scanty and irregular rainfall events [20,21], understanding the ability of desert species to form a soil seed bank could be important for designing restoration and conservation plans. Here the natural vegetation has been already degraded as a result of several factors such as overgrazing, off-road driving, and camping [22].
In a soil seed bank, seeds may either remain on the soil surface, partially buried, or completely buried, depending on the size and amount of litter cover [23]. Moreover, different species may exhibit differences in seed longevity in a soil seed bank. Seed banks have been categorized into three categories: (i) transient (<1 year), (ii) short-term persistent (1–5 years), and (iii) persistent (>5 years), based on their ability to remain viable in the soil [24]. Various factors such as temperature, light, moisture, soil properties, predation, and burial depth affect the seed persistence ability [25,26]. Therefore, understanding the seed longevity during storage is of prime concern and could be important for implementing suitable conservation and management strategies.
Understanding the factors influencing the viability of seeds in a soil seed bank could be useful for developing restoration strategies for desert habitats. However, the precise documentation of time duration for seed longevity in soil seed banks could be important for determining the success of restoration efforts. Seed longevity determines the period in which seeds can germinate and produce new individuals and consequently regulates the spatio-temporal dynamics of plant populations [27]. However, the ability to remain viable during desiccation is one of the most important aspects that affects seed longevity [28]. Most of the species that occur in arid hot regions produce orthodox seeds (i.e., desiccation tolerant) and have the ability to form soil seed banks [29,30]. Until now, few seed longevity studies have been conducted for ex-situ [31,32] and in-situ stored seeds [17,33]. Therefore, attention needs to be given to understanding how seed longevity is affected under natural conditions. Identifying the seed bank longevity by detecting the seed viability (i.e., germinability) could be crucial for implementing natural restoration measures. However, despite their importance for restoration, little is known about the potential of soil seed bank formation as well as seed longevity under natural conditions for Arabian desert species. We hypothesize that the seeds of desert species may have sufficient longevity to form persistent soil seed banks and therefore they can increase restoration potential by serving as a direct source of natural restoration. Hence, we compared the seed germination potential of fresh and in-situ stored seeds to address the following questions: (i) Do fresh and stored seeds have the same ability to germinate, (ii) whether seeds can maintain their viability and germination capacity under in-situ storage, and (iii) if yes, are there any differences in seed longevity among species?
2. Materials and Methods
2.1. Seed Collection
We collected seeds of 6 species [3 annuals (Centaurea pseudosinaica, Gypsophila capillaris, Lotus halophilus) and 3 perennials (Astragalus spinosus, Peganum harmala, Panicum turgidum)] from naturally occurring populations at the time of their dispersal (during May–June 2017). These species are relatively common and have both ecological and economic importance (Table 1, Figure 1 and Figure 2). Approximately 30–35 plants of each species were randomly sampled to represent the genetic diversity of respective populations. Fresh seed mass for each species was determined at the time of collection from 50 seeds, replicated four times, using an electronic balance (Sartorius Co., Goettingen, Germany). Seed color and seed shape were recorded using a Stereo Microscope (Nikon SMZ800N; Nikon Instruments Inc., Melville, NY, USA). The seeds were cleaned immediately after collection using a hand-made rubber thresher and care was taken not to scarify the seed coating while cleaning the seeds. After cleaning, seeds were divided into two batches: batch 1 as fresh seeds and batch 2 as stored seeds.

Table 1.
Seed collection location and uses of the studied species.
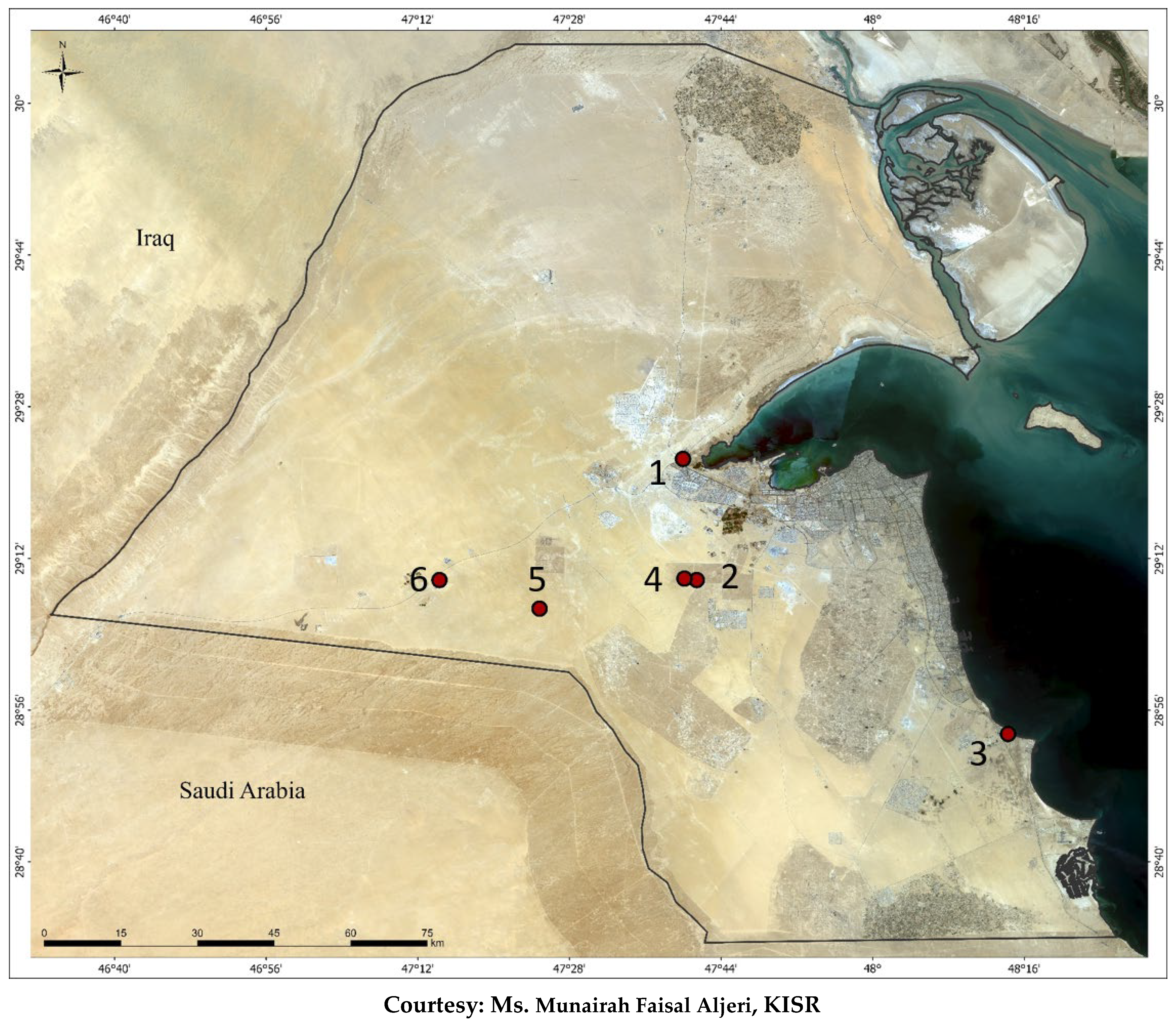
Figure 1.
Seed collection location in Kuwait of (1) Centaurea pseudosinaica; (2) Gypsophila capillaris; (3) Astagalus spinosus; (4) Peganum harmala; (5) Panicum turgidum; and (6) Lotus halophilus.

Figure 2.
Seeds of (A) Centaurea pseudosinaica; (B) Gypsophila capillaris; (C) Astagalus spinosus; (D) Peganum harmala; (E) Panicum turgidum; and (F) Lotus halophilus.
The first batch (fresh seeds) of each species was tested for seed germination within one week after collection. The second batch was stored (in situ) in a nylon bag (mesh size 0.2 mm) and placed outside in the soil at a depth of 2–4 cm from May–June 2017 to the last week of September 2022 (stored seeds). Whenever rain was forecasted, seeds of the natural storage (batch 2) were taken from the soil and were returned back shortly after improvement of the weather. The stored seeds of each species were retrieved by the first week of October 2022 and tested for germinability.
The Kuwait climate is characterized by a long, dry, and hot summer with daytime temperatures occasionally exceeding 50 °C, and cooler winters with extreme lows below 4 °C. Precipitation is scarce, with less than 114 mm annually and mostly occurring during winter between October and March [40].
2.2. Germination Trials
Seeds (both fresh and stored) of the selected species were germinated in a growth chamber (Caron 7306, Caron Products and Services, Inc., Marietta, OH, USA) set at 12/12 h daily thermoperiods and two light regimes (12 h light at 30 °C and 12 h dark at 20 °C per day) that were termed as light and dark treatments. Light was provided by a 50-W white fluorescent lamp with the light period coinciding with the high temperature. The temperature used to incubate the seeds was chosen because it was found to be optimal for the germination of these species during preliminary study. Moreover, this temperature regime represents the field conditions between October to early April when there is a higher chance of rain [40].
Light and dark treatments were used to mimic exposed and buried seeds, respectively. The higher temperature regime coincided with the light cycle in the 12 h light photoperiod. Seeds were placed in 9 cm Petri dishes lined with filter paper (Whatman No. 1) moistened with 5 mL of distilled water. Four replications of 25 seeds per Petri-dish were used in each treatment for each species. Constant darkness was maintained by wrapping Petri dishes in two layers of aluminum foil. Germinated seeds were counted and removed daily from the 12 h light treatments, while seeds under the dark treatments were evaluated only at the end of the experiment (after 28 days). Germination was defined as the presence of a radicle (≥2 mm). At the end of the germination trials, all the non-germinated seeds from the 12 h light treatments were dissected to evaluate the embryo status and viability of the non-germinated seeds (living and therefore white; turgid and brown and therefore dead) under a stereo microscope (LEICA).
2.3. Data Analysis
The effect of photoperiod and storage on germination was evaluated separately for each species using a Generalized Linear Model (GLM) with binomial distribution with logit function. The data were analyzed using ®Genstat Software 22nd Edition (VSN International Ltd., Oxford, UK). The photoperiod, storage, and their interaction were explanatory variables in the model. Post-hoc Bonferroni testing was used to determine the differences among the treatment means. Mean germination time (MGT) was calculated using the formula (∑TiNi)/G, where Ti = the day of germination (from planting); Ni = number of seeds germinated at Ti; and G = total number of germinated seeds [41]. Mean germination time (MGT) was calculated separately for each species and analyzed using one-way ANOVA for light treatment only with storage as the factor. Ratio of dead seeds to viable seeds (germinated + dormant) was arcsine transformed and analyzed using one-way ANOVA considering storage as the factor. Non-transformed data is shown in the graph. Relative light germination (RLG) Index [42] was derived by dividing the germination percentage in light by the sum of germination percentage in light and dark. Data on RLG was analyzed using one-way ANOVA for each species separately having storage as the explanatory variable.
3. Results
3.1. Seed Morphological Traits
Seed morphological traits showed a variation among species (Table 2). For example, seed shape was reniform in two species (G. capillaris and A. spinosus) but oblong-inflated, triangular, ovate, and suborbicular in other species. Similarly, the seed coat color and mode of seed dispersal also varied among the species. The seed mass varied from 100.9 ± 0.51 in L. halophilus to 527.4 ± 62.02g in C. pseudosinaica.

Table 2.
Seed traits of the studied species.
3.2. Seed Germination
Seed germination percentages of all the species were affected by both storage and photoperiod, although the effects were species specific (Figure 3). Stored seeds of all the species showed significantly higher germination percentages as compared to the fresh seeds, although the germination percentages of both fresh and stored seeds varied considerably among species. Fresh seeds of four species (i.e., C. pseudosinaica, G. capillaris, A. spinosus, and P. turgidum) had a very low germination percentage (<20%), while P. harmala and L. halophilus seeds showed better germination percentages. Fresh seeds of A. spinosus achieved the lowest (7.0%) and P. harmala achieved the highest germination percentages (64%) in light.
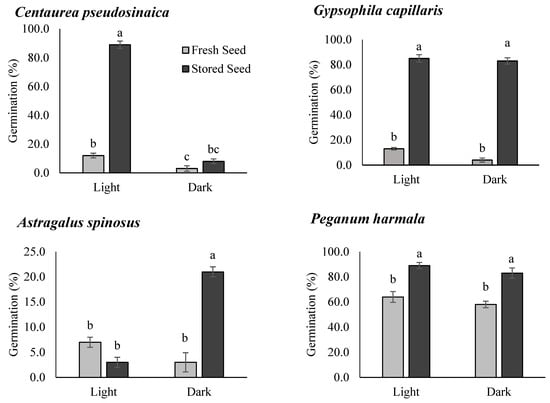
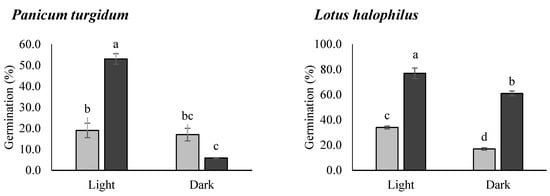
Figure 3.
Germination percentage (means ± SE) of the fresh and stored seeds under light and dark conditions. Different letters in the bars indicate significant differences among treatments (each species analyzed separately).
Stored seeds of all the species achieved more than 50.0% final germination percentages, except for A. spinosus. Three species, namely C. pseudosinaica, P. turgidum, and L. halophilus, showed significantly higher germination percentages in the presence of light after storage. Fresh seeds of A. spinosus showed poor germination, irrespective of the presence or absence of light. Stored seeds of A. spinosus exhibited significantly higher germination in dark conditions than the fresh seeds. However, stored seeds of G. capillaris showed significantly higher germination irrespective of the presence or absence of light. A similar trend was detected in P. harmala but the reduction in germination percentage was less than for G. capillaris, especially when the seeds were exposed to light conditions. Additionally, there was no significant increase in the ratio of dead seeds to viable seeds after the storage period in all the species (Figure 4).
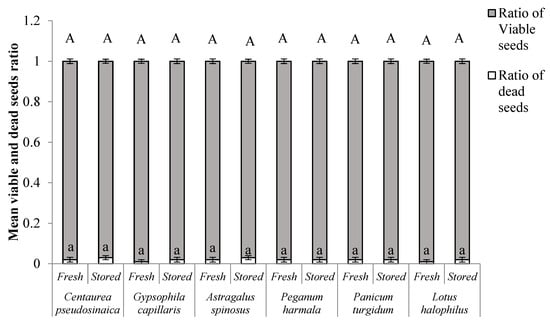
Figure 4.
Ratio of dead and viable (germinated + dormant) seeds (means ± SE) of the fresh and stored seeds of the selected species. Different letters in the bars indicate significant differences among treatments (each species analyzed separately). ‘A’ is the comparison between viable seeds of each species and ‘a’ is the comparison between dead seeds of each species.
The Generalized Linear Model (GLM) analysis indicated that photoperiod (p ≤ 0.025), storage (p ≤ 0.001), and their interaction (p ≤ 0.013) had significant effects on the seed germination of C. pseudosinaica. However, only the main factors (i.e., photoperiod and storage) had significant effects on the seed germination of G. capillaris (photoperiod—p ≤ 0.031; storage—p ≤ 0.001) and L. halophilus (photoperiod—p ≤ 0.007; storage—p ≤ 0.001). Conversely, only storage had significant effects on the seed germination of A. spinosus, P. harmala, and P. turgidum (Table 3).

Table 3.
Generalized linear models with binomial distribution of seed germination. Significant values (p < 0.05) are highlighted in bold.
The RLG Index (Table 4) of fresh seeds was significantly higher than the stored seeds in G. capillaris (p ≤ 0.018) and L. halophilus (p ≤ 0.001). Overall, stored seeds showed lower MGT than the fresh seeds in all the species, except in P. turgidum. However, a significant reduction in MGT has been shown by the stored seeds of C. pseudosinaica, G. capillaris, and L. halophilus (Table 5).

Table 4.
RLG for fresh and stored seeds. Letters indicate significant differences among treatments within each species.

Table 5.
Mean germination times (days) for fresh and stored seeds under 12 h light per day. Letters indicate significant differences among treatments within each species.
4. Discussion
Seeds play a vital role in plant restoration because most of the restoration projects are generally initiated from seed. Therefore, understanding the longevity of long-term in-situ stored seeds through germination assessment is crucial for (i) recognizing their ability to form persistent soil seed banks and (ii) identifying the potential of in-situ stored seeds for ecosystem restoration. The ability to remain persistent in soil seed banks for long periods of time could be an important strategy that may assist in avoiding sporadic events above ground by contributing to post-disturbance regeneration. The current study revealed that all the test species have the ability to remain persistent in soil seed banks without losing their viability. However, interspecific variation was observed in seed traits (i.e., seed mass, shape, and color) among the studied species. This interspecific variation could be the reflection of genetic differences among the species [50]. Nevertheless, seed traits (i.e., color, shape, and mode of dispersal) did not show any relation with germination, indicating that these traits are not sufficient to predict the germination performance. Therefore, further investigation incorporating other closely related species is required in order to understand the impact of these traits on germination.
Freshly matured seeds of P. harmala and L. halophilus were able to germinate up to 64.0% and 34.0%, respectively. However, fresh seeds of other species exhibited lower germination percentages (<20%) as compared to the stored seeds. These results indicate that the seeds of these species may have different types and levels of dormancy at the time of maturity [51] that prevents the germination of freshly harvested seeds. For example, previous studies reported that C. pseudosinaica, G. capillaris, P. harmala, and P. turgidum seeds have different levels of physiological dormancy [17,52,53], while A. spinosus and L. halophilus seeds are reported to have physical dormancy due to the presence of a hard seed coating that restricts water permeability [18].
The presence of different levels and types of dormancies at the time of seed maturity could be the survival mechanism in desert species that prevents the germination of freshly matured seeds until the occurrence of favorable conditions for germination and seedling establishment. Seeds of all these species mature and disperse during summer (May to June) when the temperature is extremely high and the chances of rainfall are minimum in their natural habitats. Therefore, possessing dormancy at the time of maturation could be the adaptation strategy that may allow them to escape from extreme environmental conditions during summer. Additionally, retaining dormancy could promote species persistence under desert conditions by assisting in (i) synchronizing the germination timing with favorable seasons, (ii) the formation of seedbanks, and (iii) reducing the competition for resources (i.e., nutrients and water) at the seedling proliferation stage [51,54]. Generally, seed production in deserts is erratic and low due to scanty and irregular rainfall events [21]; therefore, forming seed banks could be the adaptation strategy to survive under such conditions, especially when the production of seeds may be reduced for long periods. Moreover, the lower germination of fresh seeds has been suggested as a survival strategy that ensures species persistence by the formation of soil seed banks, even with the repeated droughts that usually occur in desert environments [55,56].
Our results showed that the stored seeds not only maintained their viability (i.e., germinability) but also enhanced their germination percentage significantly, indicating that seeds of these species had greater ecological longevity. Moreover, in-situ seed storage did not affect seed quality that can cause seed death (Figure 4), demonstrating their ability to remain persistent in the soil seed bank for longer durations and thus could serve as a potential source for species reinforcements by contributing to the restoration of declining plant populations [57,58]. Producing orthodox seeds by desert plants could be the survival strategy that allows these species to survive under such extreme environmental conditions [59,60]. Moreover, retaining high viability under in-situ storage conditions demonstrates that these species produce orthodox seeds (i.e., desiccation tolerant).
Orthodox seeds can remain viable for long periods at high temperatures and in dry soils [61,62]. However, seed moisture content plays an important role in determining seed longevity during the storage period [63]. Therefore, quantifying the relations between temperature and seed moisture content and longevity can assist in the better prediction of the storage life of seeds. Therefore, further experiments are required to be conducted considering all the parameters for better understanding seed longevity under in-situ storage conditions.
An increase in the germination percentages of C. pseudosinaica, G. capillaris, P. harmala, and P. turgidum after storage indicates that in-situ storage might be effective for breaking physiological dormancy in these species. Generally, the interaction of temperature and moisture over time has been reported to be responsible for breaking the physiological dormancy and enhancing the germination in physiologically dormant seeds [17,64,65]. In the present case, stored seeds were exposed to daily (day and night) and seasonal (summer and winter) temperature fluctuations, which might be responsible for alleviating the physiological dormancy by accelerating the after-ripening process [17,66]. Similarly, extreme soil temperatures during summer as well as seasonal temperature fluctuations have been found effective in breaking physical dormancy in various species [18,67,68]. In the present case, stored seeds of A. spinosus showed higher germination but the overall germination percentage was very low (<22%), indicating that factors other than temperature and humidity fluctuations under natural conditions might be responsible for breaking their seed dormancy. Inherent seed physical and physiological properties might be accountable for retaining dormancy in this species [69]. The existence of different types and levels of seed dormancy in the studied species might be advantageous by allowing seeds to germinate at different times (i.e., from few days to years) and consequently would ensure the chances of their population persistence under such extreme environmental conditions. Moreover, the percentages of dead seeds were very low after in-situ storage (<4.0%), indicating that these species have higher longevity and thus have higher chances to remain persistent in soil seed banks for longer durations.
Fresh seeds germinated significantly better in light when compared to dark, indicating that the seeds are light sensitive and hence will germinate better if they remain on or near the soil surface. Previous studies suggested that seeds that require light for germination have higher potential to form persistent seed banks [51,70], which was clearly corroborated by our results. However, storage decreased the light-sensitivity in G. capillaris and P. harmala for germination indicating that the light sensitivity of seeds for germination is affected due to the alteration of phytochrome response during storage [71,72]. Other species showed higher germination in light as compared to dark, indicating the species-specific differences in light sensitivity during storage. Alterations in seasonal temperature and moisture are found to be responsible for changing the seed dormancy status [69]. Changes in light requirements or RLG during storage could provide a better understanding about the changes in light sensitivity, although capturing the light response only based on RLG is difficult because various other factors such as the level of seed dormancy, photon fluence, and temperature also play an important role in determining light sensitivity during germination [42].
Temperature and relative humidity are known to be important factors which play an important role in regulating seed dormancy and viability status during storage [51]. In the present study, mean germination times were lower in the stored seeds, indicating that temperature and relative humidity might have played a role in affecting MGT by altering the dormancy status under storage and consequently reducing the MGT, depending on species as reported previously in various desert species [17,18]. Usually, germination timing is correlated with dormancy alleviation [73] and reduction in MGT by stored seeds may be the adaptation strategy in desert conditions where germination occurs rapidly after a rainfall event [74].
5. Conclusions
Restoration is a costly and challenging task in deserts due to extreme climatic conditions. However, it may be possible to reduce the cost if information about seed persistency and viability in soil seed banks is available. Understanding the ability to maintain seed viability under in-situ storage conditions could be important for developing conservation and restoration programs in arid environmental conditions. Seeds of all the tested species were dormant at maturity; however, in-situ seed storage enhanced the germination percentage and reduced the MGT. The results of the present study suggested that all the studied species have the ability to form persistent seed banks and they can be classified as orthodox seeds in terms of storage behavior. Moreover, the lower percentage of dead seeds after in-situ storage (<4.0%) indicates that these species have higher longevity and thus have higher chances to remain persistent in soil seed banks for longer durations. The ability to remain viable in soil seed banks will possibly give support for direct implications for restoration efforts by serving as a reservoir of regeneration potential.
Author Contributions
M.K.S.: conception and design, manuscript writing, final approval of manuscript; A.B.: conception and design, manuscript writing, visualization, manuscript reviewing; S.J.: manuscript reviewing, editing, and statistical analysis; R.R.T.: manuscript reviewing and editing; M.T.S.: editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Kuwait Institute for Scientific Research (KISR).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Munairah Faisal Aljeri, KISR for her help in mapping the seed collection locations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chapman, T.; Miles, S.; Trivedi, C. Capturing, protecting and restoring plant diversity in the UK: RBG Kew and the Millennium Seed Bank. Plant Divers. 2019, 41, 124–131. [Google Scholar] [CrossRef]
- González-Alday, J.; Marrs, R.H.; Martínez-Ruiz, C. Soil seed bank formation during early revegetation after hydroseeding in reclaimed coal wastes. Ecol. Eng. 2009, 35, 1062–1069. [Google Scholar] [CrossRef]
- Mohammed, S.A.; Denboba, M.A. Study of soil seed banks in ex-closures for restoration of degraded lands in the central Rift Valley of Ethiopia. Sci. Rep. 2020, 10, 956. [Google Scholar] [CrossRef]
- Bossuyt, B.; Honnay, O. Can the seed bank be used for ecological restoration? An overview of seed bank characteristics in European communities. J. Veg. Sci. 2008, 19, 875–884. [Google Scholar] [CrossRef]
- Münzbergová, Z.; Šurinová, M.; Husáková, I.; Brabec, J. Strong fluctuations in aboveground population size do not limit genetic diversity in populations of an endangered biennial species. Oecologia 2018, 187, 863–872. [Google Scholar] [CrossRef]
- Basto, S.; Thompson, K.; Grime, J.P.; Fridley, J.D.; Calhim, S.; Askew, A.P. Severe effects of long-term drought on calcareous grassland seed banks. NPJ Clim. Atmospher. Sci. 2018, 1, 1. [Google Scholar] [CrossRef]
- Yang, X.; Baskin, C.C.; Baskin, J.M.; Pakeman, R.J.; Huang, Z.; Gao, R.; Cornelissen, J.H. Global patterns of potential future plant diversity hidden in soil seed banks. Nat. Commun. 2021, 12, 7023. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L. Revising the trait-based filtering framework to include interacting filters: Lessons from grassland restoration. J. Ecol. 2021, 109, 3466–3472. [Google Scholar] [CrossRef]
- Pake, C.E.; Venable, D.L. Seed banks in desert annuals: Implications for persistence and coexistence in variable environments. Ecology 1996, 77, 1427–1436. [Google Scholar] [CrossRef]
- Cabin, R.J.; Mitchell, R.J.; Marshall, D.L. Do surface plant and soil seed bank populations differ genetically? A multipopulation study of the desert mustard Lesquerella fendleri (Brassicaceae). Am. J. Bot. 1998, 85, 1098–1109. [Google Scholar] [CrossRef]
- Silvertown, J.; McConway, K.; Gowing, D.; Dodd, M.; Fay, M.F.; Joseph, J.A.; Dolphin, K. Absence of phylogenetic signal in the niche structure of meadow plant communities. Proc. R. Soc. B Biol. 2006, 273, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Ooi, M.K.J. Seed bank persistence and climate change. Seed Sci. Res. 2012, 22, S53–S60. [Google Scholar] [CrossRef]
- Martens, B.; Waegeman, W.; Dorigo, W.A.; Verhoest, N.E.; Miralles, D.G. Terrestrial evaporation response to modes of climate variability. NPJ Clim. Atmos. Sci. 2018, 1, 43. [Google Scholar] [CrossRef]
- Abu Sukar, H.K.; Almerri, F.H.; Almurekki, A.A. Agro-Hydro-Meteorological Data Book for the State of Qatar, Doha. DAWR 2007. [Google Scholar]
- Rewald, B.; Leuschner, C.; Wiesman, Z.; Ephrath, J.E. Influence of salinity on root hydraulicproperties of three olive varieties. Plant Biosyst. 2011, 145, 12–22. [Google Scholar] [CrossRef]
- Wijayratne, U.C.; Pyke, D.A. Burial increases seed longevity of two Artemisia tridentata (Asteraceae) subspecies. Am. J. Bot. 2012, 99, 438–447. [Google Scholar] [CrossRef]
- Bhatt, A.; Gallacher, D.J.; Souza-Filho, P.R.M. Germination strategies of annual and short-lived perennial species in the Arabian Desert. J. Arid Land 2020, 12, 1071–1082. [Google Scholar] [CrossRef]
- Bhatt, A.; Bhat, N.R.; Phartyal, S.S.; Gallacher, D. Dry-storage and light exposure reduce dormancy of Arabian desert legumes more than temperature. Seed Sci. Technol. 2020, 48, 247–255. [Google Scholar] [CrossRef]
- Qiuyan, L.; Haiyan, F.; Qiangguo, C. Persistent soil seed banks along altitudinal gradients in the Qilian Mountains in China and their significance for conservation management. Afr. J. Agric. Res. 2011, 6, 2329–2340. [Google Scholar]
- Ernest, S.M.; Brown, J.H.; Parmenter, R.R. Rodents, plants, and precipitation: Spatial and temporal dynamics of consumers and resources. Oikos 2000, 88, 470–482. [Google Scholar] [CrossRef]
- Pol, R.G.; Pirk, G.I.; Marone, L. Grass seed production in the central Monte desert during successive wet and dry years. Plant Ecol. 2010, 208, 65–75. [Google Scholar] [CrossRef]
- Omar, S.; Roy, W.Y. Biodiversity and climate change in Kuwait. Int. J. Clim. Chang. Strateg. Manag. 2010, 2, 68–83. [Google Scholar] [CrossRef]
- Zhang, R.; Hu, X.; Baskin, J.M.; Baskin, C.C.; Wang, Y. Effects of litter on seedling emergence and seed persistence of three common species on the Loess Plateau in Northwestern China. Front. Plant Sci. 2017, 8, 103. [Google Scholar] [CrossRef]
- Thompson, K.; Band, S.R. Survival of a lowland heathland seed bank after a 33-year burial. Seed Sci. Res. 1997, 7, 409–411. [Google Scholar] [CrossRef]
- Long, R.L.; Gorecki, M.J.; Renton, M.; Scott, J.K.; Colville, L.; Goggin, D.E.; Commander, L.E.; Westcott, D.A.; Cherry, H.; Finch-Savage, W.E. The ecophysiology of seed persistence: A mechanistic view of the journey to germination or demise. Biol. Rev. 2015, 90, 31–59. [Google Scholar] [CrossRef]
- Ma, M.J.; Dalling, J.W.; Ma, Z.; Zhou, X.H. Soil environmental factors drive seed density across vegetation types on the Tibetan Plateau. Plant Soil. 2017, 419, 349–361. [Google Scholar] [CrossRef]
- Joet, T.; Ourcival, J.M.; Dussert, S. Ecological significance of seed desiccation sensitivity in Quercus ilex. Ann. Bot. 2013, 111, 693–701. [Google Scholar] [CrossRef]
- Tweddle, J.C.; Dickie, J.B.; Baskin, C.C.; Baskin, J.M. Ecological aspects of seed desiccation sensitivity. J. Ecol. 2003, 91, 294–304. [Google Scholar] [CrossRef]
- Murdoch, A.J.; Ellis, R.H. Dormancy, viability and longevity. In Seeds: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CAB International: Wallingford, UK, 2000; pp. 183–214. [Google Scholar]
- Berjak, P.; Pammenter, N.W. From Avicennia to Zizania: Seed recalcitrance in perspective. Ann. Bot. 2008, 101, 213–228. [Google Scholar] [CrossRef]
- Bhatt, A.; Bhat, N.R.; Murru, V.; Santo, A. Eco-physiological studies on desert plants: Germination of Halothamnus iraqensis Botsch. Seeds under different conditions. J. Arid Land 2019, 11, 75–85. [Google Scholar]
- Bhatt, A.; Bhat, N.R.; Suleiman, M.K.; Santo, A. Effects of storage, mucilage presence, photoperiod, thermoperiod and salinity on germination of Farsetia aegyptia Turra (Brassicaceae) seeds: Implications for restoration and seed banks in Arabian Desert. Plant Biosyst. 2019, 153, 280–287. [Google Scholar] [CrossRef]
- Bhatt, A.; Carón, M.M.; Gallacher, D.; Souza-Filho, P.R.M. Storage duration, light, temperature and salinity exposure influence germination of the glycophyte Rhanterium epapposum. Botany 2021, 99, 261–267. [Google Scholar] [CrossRef]
- Al-Jaloud, A.A.; Chaudhary, S.A.; Bashour, I.I.; Qureshit, S.; Al-Shanghitti, A. Nutrient evaluation of some arid range plants in Saudi Arabia. J. Arid Environ. 1994, 28, 299–311. [Google Scholar] [CrossRef]
- Elgamal, M.H.A.; Soliman, H.S.; Karawya, M.S.; Mikhova, B.; Duddeck, H. Isolation of triterpene saponins from Gypsophila capillaris. Phytochemistry 1995, 38, 1481–1485. [Google Scholar] [CrossRef]
- Omar, S.; Zaman, S. Kuwait rangelands status, development and research priorities. Sustain. Dev. Arid Zones 1998, 2, 403–421. [Google Scholar]
- Bidak, L.M.; Kamal, S.A.; Halmy, M.W.A.; Heneidy, S.Z. Goods and services provided by native plants in desert ecosystems: Examples from the northwestern coastal desert of Egypt. Glob. Ecol. Conserv. 2015, 3, 433–447. [Google Scholar] [CrossRef]
- Akhter, R.; Arshad, M. Arid rangelands in the Cholistan desert (Pakistan). Sci. Chang. Planétaires Sécheresse 2006, 17, 210–217. [Google Scholar]
- Rafay, M.; Khan, R.A.; Yaqoob, S.; Ahmad, M. Floristic composition of grass species in the degrading rangelands of Cholistan desert. Pak. J. Agric. Sci. 2013, 50, 599–603. [Google Scholar]
- Omar, S.; Al-Mutawa, Y.; Zaman, S. Vegetation of Kuwait; Kuwait Institute for Scientific Research: Kuwait City, Kuwait, 2007. [Google Scholar]
- Bush, E.W.; Wilson, P.; Shepard, D.P.; McClure, G. Enhancement of Seed Germination in Common Carpet Grass and Centipede Grass Seed. HortScience 2000, 35, 769–770. [Google Scholar] [CrossRef]
- Milberg, P.; Andersson, L.; Thompson, K. Large-seeded spices are less dependent on light for germination than small-seeded ones. Seed Sci. Res. 2000, 10, 99–104. [Google Scholar] [CrossRef]
- Viegi, L.; Pieroni, A.; Guarrera, P.M.; Vangelisti, R. A review of plants used in folk veterinary medicine in Italy as basis for a databank. J. Ethnopharmacol. 2003, 89, 221–244. [Google Scholar] [CrossRef] [PubMed]
- Calderoni, M.; Altare, M.; Mastracci, L.; Grillo, F.; Cornara, L.; Pagano, A. Potential risks of plant constituents in dietary supplements: Qualitative and quantitative analysis of Peganum harmala seeds. Molecules 2021, 26, 1368. [Google Scholar] [CrossRef]
- Amartuvshin, N.; Dariimaa, S.; Tserenbaljid, G. Taxonomy of the genus Peganum L. (Peganaceae VAN TIEGHEM) in Mongolia. Mong. J. Biol. Sci. 2006, 4, 9–13. [Google Scholar]
- Danin, A. Plant adaptations to environmental stresses in desert dunes. Plants Desert Dunes 1996, 7, 133–152. [Google Scholar]
- Ghazanfar, S.A.; Böer, B.; Al Khulaidi, A.W.; El-Keblawy, A.; Alateeqi, S. Plants of Sabkha ecosystems of the Arabian Peninsula. Sabkha Ecosyst. 2019, VI, 55–80. [Google Scholar]
- Smith, N.M. Weeds of the Wet/Dry Tropics of Australia—A Field Guide; Environment Centre NT, Inc.: Darwin, Australia, 2002; p. 112. [Google Scholar]
- Mifsud, S. Update on Maltese flora (Central Mediterranean) including very rare species thought to be extinct from mainland Malta or its islands. Cent. Mediterr. Nat. 2009, 5, 7–16. [Google Scholar]
- Moles, A.T.; Ackerly, D.D.; Webb, C.O.; Tweddle, J.C.; Dickie, J.B.; Westoby, M. A brief history of seed size. Science 2005, 307, 576–580. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Nolan, D.G.; Upadhyaya, M.K. Primary seed dormancy in diffuse and spotted knapweed. Can. J. Plant Sci. 1988, 68, 775–783. [Google Scholar] [CrossRef]
- El-Keblawy, A. Impacts of dormancy regulating chemicals on innate and salinity-induced dormancy of four forage grasses native to Arabian deserts. Grass Forage Sci. 2013, 68, 288–296. [Google Scholar] [CrossRef]
- Yi, F.; Wang, Z.; Baskin, C.C.; Baskin, J.M.; Ye, R.; Sun, H.; Huang, Z. Seed germination responses to seasonal temperature and drought stress are species-specific but not related to seed size in a desert steppe: Implications for effect of climate change on community structure. Ecol. Evol. 2019, 9, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- El-Keblawy, A.; Shaltout, K.H.; Lovett-Doust, J.; Ramadan, A. Population dynamic of an Egyptian desert shrub, Thymelaea hirsuta. Can. J. Plant Sci. 1997, 75, 2027–2037. [Google Scholar] [CrossRef]
- Bhatt, A.; Gairola, S.; El-Keblawy, A.A. Seed colour affects light and temperature requirements during germination in two Lotus species (Fabaceae) of the Arabian subtropical deserts. Rev. Biol. Trop. 2016, 64, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Ottewell, K.M.; Bickerton, D.; Lowe, A.J. Can a seed bank provide demographic and genetic rescue in a declining population of the endangered shrub Acacia pinguifolia? Conserv. Genet. 2011, 12, 669–678. [Google Scholar] [CrossRef]
- Abeli, T.; Dixon, K. Translocation ecology: The role of ecological sciences in plant translocation. Plant Ecol. 2016, 217, 123–125. [Google Scholar] [CrossRef][Green Version]
- Sano, N.; Rajjou, L.; North, H.M.; Debeaujon, I.; Marion-Poll, A.; Seo, M. Staying alive: Molecular aspects of seed longevity. Plant Cell Physiol. 2016, 57, 660–674. [Google Scholar] [CrossRef] [PubMed]
- Stavrinides, A.K.; Dussert, S.; Combes, M.C.; Fock-Bastide, I.; Severac, D.; Minier, J.; Joët, T. Seed comparative genomics in three coffee species identify desiccation tolerance mechanisms in intermediate seeds. J. Exp. Bot. 2020, 71, 1418–1433. [Google Scholar] [CrossRef] [PubMed]
- Sallon, S.; Solowey, E.; Cohen, Y.; Korchinsky, R.; Egli, M.; Woodhatch, I.; Kislev, M. Germination, genetics, and growth of an ancient date seed. Science 2008, 320, 1464. [Google Scholar] [CrossRef]
- Groot, S.; Surki, A.A.; Vos, R.; Kodde, J. Seed storage at elevated partial pressure of oxygen, a fast method for analysing seed ageing under dry conditions. Ann. Bot. 2012, 110, 1149–1159. [Google Scholar] [CrossRef]
- Righetti, K.; Ly Vu, J.; Pelletier, S.; Ly Vu, B.; Glaab, E.; Lalanne, D.; Pasha, A.; Patel, R.V.; Provart, N.J.; Verdier, J.; et al. Inference of longevity-related genes from a robust co-expression network of seed maturation identifies new regulators linking seed storability to biotic defense-related pathways. Plant Cell 2015, 27, 2692–2708. [Google Scholar] [PubMed]
- Walck, J.L.; Hidayati, S.N.; Dixon, K.W.; Thompson, K.E.N.; Poschlod, P. Climate change and plant regeneration from seed. Glob. Chang. Biol. 2011, 17, 2145–2161. [Google Scholar] [CrossRef]
- Liu, H.; Abudureheman, B.; Zhang, L.; Baskin, J.M.; Baskin, C.C.; Zhang, D. Seed dormancy-breaking in a cold desert shrub in relation to sand temperature and moisture. AoB Plants 2017, 9, plx003. [Google Scholar] [CrossRef]
- Commander, L.E.; Merritt, D.J.; Rokich, D.P.; Dixon, K.W. The role of after-ripening in promoting germination of arid zone seeds: A study on six Australian species. Bot. J. Linn. Soc. 2009, 161, 411–421. [Google Scholar] [CrossRef][Green Version]
- Jaganathan, G.K.; Yule, K.J.; Biddick, M. Determination of the water gap and the germination ecology of Adenanthera pavonine (Fabaceae, Mimosoideae); the adaptive role of physical dormancy in mimetic seeds. AoB Plants 2018, 10, ply048. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, G.K.; Biddick, M. Experimental warming hastens physical dormancy break and germination in tropical Fabaceae. Front. Plant Sci. 2021, 12, 782706. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Saatkamp, A.; Affre, L.; Dutoit, T.; Poschlod, P. Germination traits explain soil seed persistence across species: The case of Mediterranean annual plants in cereal fields. Ann. Bot. 2011, 107, 415–426. [Google Scholar] [CrossRef]
- Scopel, A.L.; Ballaré, C.L.; Sánchez, R.A. Induction of extreme light sensitivity in buried weed seeds and its role in the perception of soil cultivations. Plant Cell Environ. 1991, 14, 501–508. [Google Scholar] [CrossRef]
- Casal, J.J.; Sánchez, R.A. Phytochromes and seed germination. Seed Sci. Res. 1998, 8, 317–329. [Google Scholar] [CrossRef]
- Silva, L.M.; Fernandes, G.W. Effect of seed storage on germination, seedling growth and survival of Mimosa foliolosa (Fabaceae): Implications for seed banks and restoration ecology. Trop. Ecol. 2014, 55, 385–392. [Google Scholar]
- Rühl, A.T.; Eckstein, R.L.; Otte, A. Distinct germination response of endangered and common arable weeds to reduced water potential. Plant Biol. 2016, 218, 83–90. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).