Powering the Future: Progress and Hurdles in Developing Proton Exchange Membrane Fuel Cell Components to Achieve Department of Energy Goals—A Systematic Review
Abstract
:1. Introduction

2. U.S. DOE
3. Recent Developments in Fuel Cell Technologies
3.1. Advancements in MEA
3.2. Advancements on GDL
3.3. Advancements in Bipolar Plates
4. Current Status and Technical Barriers
5. Enhancing PEMFC Lifetime for Commercial Viability
6. Challenges and Solutions for Meeting U.S. DOE’s 2035/2050 PEMFC Targets
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vardhan, R.V.; Mahalakshmi, R.; Anand, R.; Mohanty, A. A review on green hydrogen: Future of green hydrogen in india. In Proceedings of the 2022 6th International Conference on Devices, Circuits and Systems (ICDCS), Coimbatore, India, 21–22 April 2022; IEEE: Piscataway, NJ, USA. [Google Scholar]
- Gardner, R. The Outlook for Energy: A View to 2040. In Proceedings of the 2015 EDI, Kiawah Island, SC, USA, 12–15 April 2015. [Google Scholar]
- Economics. BP Energy Outlook; BP plc: London, UK, 2018. [Google Scholar]
- Dudley, B. BP Energy Outlook; Report–BP Energy Economics: London, UK, 2018; Volume 9. [Google Scholar]
- Duraisamy, B.; Velmurugan, K.; Venkatachalapathy, V.K.; Madheswaran, D.K.; Varuvel, E.G. Biodiesel from Biomass Waste Feedstock Prosopis Juliflora as a Fuel Substitute for Diesel and Enhancement of Its Usability in Diesel Engines Using Decanol. Energy Technol. 2023, 11, 2300346. [Google Scholar] [CrossRef]
- Alternative Fuels Data Center, US Department of Energy. Electric Vehicle Charging Station Locations; US Department of Energy: Washington, DC, USA, 2017.
- Sundén, B. Hydrogen, Batteries and Fuel Cells; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Ou, X.; Yan, X.; Zhang, X.; Liu, Z. Life-cycle analysis on energy consumption and GHG emission intensities of alternative vehicle fuels in China. Appl. Energy 2012, 90, 218–224. [Google Scholar] [CrossRef]
- Raj, J.; Rajasree, S. A Microgrid with Hybrid Solar-Fuel Cell System With CHP Application. In Proceedings of the 2022 IEEE 2nd International Conference on Sustainable Energy and Future Electric Transportation (SeFeT), Hyderabad, India, 4–6 August 2022; IEEE: Piscataway, NJ, USA, 2022. [Google Scholar]
- Luo, Y.; Wu, Y.; Li, B.; Mo, T.; Li, Y.; Feng, S.-P.; Qu, J.; Chu, P.K. Development and application of fuel cells in the automobile industry. J. Energy Storage 2021, 42, 103124. [Google Scholar] [CrossRef]
- Ağbulut, Ü.; Bakir, H. The investigation on economic and ecological impacts of tendency to electric vehicles instead of internal combustion engines. Düzce Üniv. Bilim Ve Teknol. Derg. 2019, 7, 25–36. [Google Scholar] [CrossRef]
- Ahmadi, P. Environmental impacts and behavioral drivers of deep decarbonization for transportation through electric vehicles. J. Clean. Prod. 2019, 225, 1209–1219. [Google Scholar] [CrossRef]
- Jayakumar, A.; Madheswaran, D.K.; Kannan, A.; Sureshvaran, U.; Sathish, J. Can hydrogen be the sustainable fuel for mobility in India in the global context? Int. J. Hydrogen Energy 2022, 47, 33571–33596. [Google Scholar] [CrossRef]
- Gao, Z.; Lin, Z.; LaClair, T.J.; Liu, C.; Li, J.-M.; Birky, A.K.; Ward, J. Battery capacity and recharging needs for electric buses in city transit service. Energy 2017, 122, 588–600. [Google Scholar] [CrossRef]
- Hua, T.; Ahluwalia, R.; Eudy, L.; Singer, G.; Jermer, B.; Asselin-Miller, N.; Wessel, S.; Patterson, T.; Marcinkoski, J. Status of hydrogen fuel cell electric buses worldwide. J. Power Sources 2014, 269, 975–993. [Google Scholar] [CrossRef]
- Shimpalee, S.; Lilavivat, V.; Van Zee, J.W.; McCrabb, H.; Lozano-Morales, A. Understanding the effect of channel tolerances on performance of PEMFCs. Int. J. Hydrogen Energy 2011, 36, 12512–12523. [Google Scholar] [CrossRef]
- Mahjoubi, C.; Olivier, J.-C.; Skander-Mustapha, S.; Machmoum, M.; Slama-Belkhodja, I. An improved thermal control of open cathode proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2019, 44, 11332–11345. [Google Scholar] [CrossRef]
- Zhao, J.; Jian, Q.; Luo, L.; Huang, B.; Cao, S.; Huang, Z. Dynamic behavior study on voltage and temperature of proton exchange membrane fuel cells. Appl. Therm. Eng. 2018, 145, 343–351. [Google Scholar] [CrossRef]
- Subramaniam, U.; Reddy, K.S.; Kaliyaperumal, D.; Sailaja, V.; Bhargavi, P.; Likhith, S. A MIMO–ANFIS-Controlled Solar-Fuel-Cell-Based Switched Capacitor Z-Source Converter for an Off-Board EV Charger. Energies 2023, 16, 1693. [Google Scholar] [CrossRef]
- Akinyele, D.; Olabode, E.; Amole, A. Review of fuel cell technologies and applications for sustainable microgrid systems. Inventions 2020, 5, 42. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Samsun, R.C.; Rex, M.; Antoni, L.; Stolten, D. Deployment of fuel cell vehicles and hydrogen refueling station infrastructure: A global overview and perspectives. Energies 2022, 15, 4975. [Google Scholar] [CrossRef]
- Hao, H.; Wang, H.; Song, L.; Li, X.; Ouyang, M. Energy consumption and GHG emissions of GTL fuel by LCA: Results from eight demonstration transit buses in Beijing. Appl. Energy 2010, 87, 3212–3217. [Google Scholar] [CrossRef]
- Drive, U. Fuel Cell Technical Team Roadmap; US Drive Partnership: New York, NY, USA, 2013; pp. 1–26.
- Schmidt, O.; Hawkes, A.; Gambhir, A.; Staffell, I. The future cost of electrical energy storage based on experience rates. Nat. Energy 2017, 2, 17100. [Google Scholar] [CrossRef]
- Moreno, N.G.; Molina, M.C.; Gervasio, D.; Robles, J.F.P. Approaches to polymer electrolyte membrane fuel cells (PEMFCs) and their cost. Renew. Sustain. Energy Rev. 2015, 52, 897–906. [Google Scholar] [CrossRef]
- Pollet, B.G.; Kocha, S.S.; Staffell, I. Current status of automotive fuel cells for sustainable transport. Curr. Opin. Electrochem. 2019, 16, 90–95. [Google Scholar] [CrossRef]
- Houchins, C.; Kleen, G.J.; Spendelow, J.S.; Kopasz, J.; Peterson, D.; Garland, N.L.; Ho, D.L.; Marcinkoski, J.; Martin, K.E.; Tyler, R. US DOE progress towards developing low-cost, high performance, durable polymer electrolyte membranes for fuel cell applications. Membranes 2012, 2, 855–878. [Google Scholar] [CrossRef]
- Wang, G.; Yu, Y.; Liu, H.; Gong, C.; Wen, S.; Wang, X.; Tu, Z. Progress on design and development of polymer electrolyte membrane fuel cell systems for vehicle applications: A review. Fuel Process. Technol. 2018, 179, 203–228. [Google Scholar] [CrossRef]
- Miller, E.L.; Papageorgopoulos, D.; Stetson, N.; Randolph, K.; Peterson, D.; Cierpik-Gold, K.; Wilson, A.; Trejos, V.; Gomez, J.C.; Rustagi, N. US Department of energy hydrogen and fuel cells program: Progress, challenges and future directions. MRS Adv. 2016, 1, 2839–2855. [Google Scholar] [CrossRef]
- None, N. DOE Hydrogen and Fuel Cells Program 2017 Annual Merit Review and Peer Evaluation Report; EERE Publication and Product Library: Washington, DC, USA, 2017.
- Bethoux, O. Hydrogen fuel cell road vehicles: State of the art and perspectives. Energies 2020, 13, 5843. [Google Scholar] [CrossRef]
- U.S. DOE. The Fuel Cell Technologies Office Multi-Year Research, Development, and Demonstration Plan; US Department of Energy: Washington, DC, USA, 2016.
- Shao, M.; Chang, Q.; Dodelet, J.-P.; Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef]
- Pollet, B.G.; Ashokkumar, M.; Pollet, B.G.; Ashokkumar, M. Fundamental and applied aspects of ultrasonics and sonochemistry. In Introduction to Ultrasound, Sonochemistry and Sonoelectrochemistry; Springer: Cham, Switzerland, 2019; pp. 1–19. [Google Scholar]
- Ahluwalia, R.; Wang, X.; Osmieri, L.; Peng, J.; Chung, H.; Neyerlin, K. Activity of atomically dispersed (AD) Fe-CN ORR catalyst in polymer electrolyte fuel cell environment. J. Electrochem. Soc 2019, 166, F1096–F1104. [Google Scholar] [CrossRef]
- Zelenay, P.; Myers, D. ElectroCat (Electrocatalysis Consortium); US Department of Energy: Arington, MA, USA, 2019.
- Jayakumar, A.; Madheswaran, D.K.; Kumar, N.M. A critical assessment on functional attributes and degradation mechanism of membrane electrode assembly components in direct methanol fuel cells. Sustainability 2021, 13, 13938. [Google Scholar] [CrossRef]
- Banham, D.; Ye, S.; Pei, K.; Ozaki, J.-i.; Kishimoto, T.; Imashiro, Y. A review of the stability and durability of non-precious metal catalysts for the oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 2015, 285, 334–348. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Cheng, T.; Fortunelli, A.; Chen, C.-Y.; Yu, R.; Zhang, Q.; Gu, L.; Merinov, B.V.; Lin, Z. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 2016, 354, 1414–1419. [Google Scholar] [CrossRef]
- Chong, L.; Wen, J.; Kubal, J.; Sen, F.G.; Zou, J.; Greeley, J.; Chan, M.; Barkholtz, H.; Ding, W.; Liu, D.-J. Ultralow-loading platinum-cobalt fuel cell catalysts derived from imidazolate frameworks. Science 2018, 362, 1276–1281. [Google Scholar] [CrossRef]
- Shao, Y. Highly Active and Durable PGM-Free ORR Electrocatalysts through the Synergy of Active Sites; US DOE: Washington, DC, USA, 2020.
- James, B.D.; Huya-Kouadio, J.M.; Houchins, C.; DeSantis, D.A. Mass Production Cost Estimation of Direct H2 PEM Fuel Cell Systems for Transportation Applications: 2012 Update; under Award Number DEEE0005236 for the US Department of Energy; Strategic Analysis Inc.: Arlington, VA, USA, 2012; p. 18. [Google Scholar]
- Kojima, K.; Fukazawa, K. Current status and future outlook of fuel cell vehicle development in Toyota. ECS Trans. 2015, 69, 213. [Google Scholar] [CrossRef]
- Yoshida, T.; Kojima, K. Toyota MIRAI fuel cell vehicle and progress toward a future hydrogen society. Electrochem. Soc. Interface 2015, 24, 45. [Google Scholar] [CrossRef]
- James, B. Cost projections of PEM fuel cell systems for automobiles and medium-duty vehicles. In Proceedings of the Fuel Cell Technologies Office Webinar, Online, 25 April 2018; p. 27. [Google Scholar]
- O’Hayre, R. Next Generation Catalyst Engineering via Support Modification; Colorado School of Mines Golden United States: Golden, CO, USA, 2016. [Google Scholar]
- Tu, W.; Luo, W.; Chen, C.; Chen, K.; Zhu, E.; Zhao, Z.; Wang, Z.; Hu, T.; Zai, H.; Ke, X. Tungsten as “adhesive” in Pt2CuW0. 25 ternary alloy for highly durable oxygen reduction electrocatalysis. Adv. Funct. Mater. 2020, 30, 1908230. [Google Scholar] [CrossRef]
- Ganesan, A.; Narayanasamy, M. Ultra-low loading of platinum in proton exchange membrane-based fuel cells: A brief review. Mater. Renew. Sustain. Energy 2019, 8, 18. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Priest, C.; Shi, Q.; Wu, G. Atomically dispersed metal–nitrogen–carbon catalysts for fuel cells: Advances in catalyst design, electrode performance, and durability improvement. Chem. Soc. Rev. 2020, 49, 3484–3524. [Google Scholar] [CrossRef]
- Wang, X.X.; Swihart, M.T.; Wu, G. Achievements, challenges and perspectives on cathode catalysts in proton exchange membrane fuel cells for transportation. Nat. Catal. 2019, 2, 578–589. [Google Scholar] [CrossRef]
- Borup, R.L.; Kusoglu, A.; Neyerlin, K.C.; Mukundan, R.; Ahluwalia, R.K.; Cullen, D.A.; More, K.L.; Weber, A.Z.; Myers, D.J. Recent developments in catalyst-related PEM fuel cell durability. Curr. Opin. Electrochem. 2020, 21, 192–200. [Google Scholar] [CrossRef]
- Li, S.; Peng, C.; Shen, Q.; Wang, C.; Cheng, Y.; Yang, G. Impact of membrane phosphoric acid doping level on transport phenomena and performance in high temperature PEM fuel cells. Membranes 2021, 11, 817. [Google Scholar] [CrossRef]
- Bae, J.W.; Cho, Y.-H.; Sung, Y.-E.; Shin, K.; Jho, J.Y. Performance enhancement of polymer electrolyte membrane fuel cell by employing line-patterned Nafion membrane. J. Ind. Eng. Chem. 2012, 18, 876–879. [Google Scholar] [CrossRef]
- Kongkanand, A. High-Activity Dealloyed Catalysts; General Motors LLC: Warren, MI, USA, 2014. [Google Scholar]
- Han, B.; Carlton, C.E.; Kongkanand, A.; Kukreja, R.S.; Theobald, B.R.; Gan, L.; O’Malley, R.; Strasser, P.; Wagner, F.T.; Shao-Horn, Y. Record activity and stability of dealloyed bimetallic catalysts for proton exchange membrane fuel cells. Energy Environ. Sci. 2015, 8, 258–266. [Google Scholar] [CrossRef]
- Carcadea, E.; Varlam, M.; Marinoiu, A.; Raceanu, M.; Ismail, M.; Ingham, D. Influence of catalyst structure on PEM fuel cell performance–A numerical investigation. Int. J. Hydrogen Energy 2019, 44, 12829–12841. [Google Scholar] [CrossRef]
- Gagliardi, G.G.; Ibrahim, A.; Borello, D.; El-Kharouf, A. Composite polymers development and application for polymer electrolyte membrane technologies—A review. Molecules 2020, 25, 1712. [Google Scholar] [CrossRef]
- Haragirimana, A.; Ingabire, P.B.; Zhu, Y.; Lu, Y.; Li, N.; Hu, Z.; Chen, S. Four-polymer blend proton exchange membranes derived from sulfonated poly (aryl ether sulfone) s with various sulfonation degrees for application in fuel cells. J. Membr. Sci. 2019, 583, 209–219. [Google Scholar] [CrossRef]
- Suzuki, T.; Tanaka, H.; Hayase, M.; Tsushima, S.; Hirai, S. Investigation of porous structure formation of catalyst layers for proton exchange membrane fuel cells and their effect on cell performance. Int. J. Hydrogen Energy 2016, 41, 20326–20335. [Google Scholar] [CrossRef]
- Kang, T.; Kim, M.; Kim, J.; Sohn, Y.-J. Numerical modeling of the degradation rate for membrane electrode assemblies in high temperature proton exchange membrane fuel cells and analyzing operational effects of the degradation. Int. J. Hydrogen Energy 2015, 40, 5444–5455. [Google Scholar] [CrossRef]
- Sutradhar, S.C.; Rahman, M.M.; Ahmed, F.; Ryu, T.; Yoon, S.; Lee, S.; Kim, J.; Lee, Y.; Jin, Y.; Kim, W. Thermally and chemically stable poly (phenylenebenzophenone) membranes for proton exchange membrane fuel cells by Ni (0) catalyst. J. Ind. Eng. Chem. 2019, 76, 233–239. [Google Scholar] [CrossRef]
- Neethu, B.; Bhowmick, G.; Ghangrekar, M. A novel proton exchange membrane developed from clay and activated carbon derived from coconut shell for application in microbial fuel cell. Biochem. Eng. J. 2019, 148, 170–177. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Peng, L.; Zhang, J.; Shao, Z.; Huang, J.; Sun, C.; Ouyang, M.; He, X. Recent progress on the key materials and components for proton exchange membrane fuel cells in vehicle applications. Energies 2016, 9, 603. [Google Scholar] [CrossRef]
- Lee, D.; Yang, H.; Park, S.; Kim, W. Nafion/graphene oxide composite membranes for low humidifying polymer electrolyte membrane fuel cell. J. Membr. Sci. 2014, 452, 20–28. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Kim, A.R.; Yoo, D.J. Sulfonated graphene oxide/Nafion composite membranes for high temperature and low humidity proton exchange membrane fuel cells. RSC Adv. 2018, 8, 7494–7508. [Google Scholar] [CrossRef]
- Sahu, A.K.; Ketpang, K.; Shanmugam, S.; Kwon, O.; Lee, S.; Kim, H. Sulfonated graphene–nafion composite membranes for polymer electrolyte fuel cells operating under reduced relative humidity. J. Phys. Chem. C 2016, 120, 15855–15866. [Google Scholar] [CrossRef]
- Su, H.; Pasupathi, S.; Bladergroen, B.; Linkov, V.; Pollet, B.G. Optimization of gas diffusion electrode for polybenzimidazole-based high temperature proton exchange membrane fuel cell: Evaluation of polymer binders in catalyst layer. Int. J. Hydrogen Energy 2013, 38, 11370–11378. [Google Scholar] [CrossRef]
- Muthukumar, M.; Karthikeyan, P.; Vairavel, M.; Loganathan, C.; Praveenkumar, S.; Kumar, A.S. Numerical studies on PEM fuel cell with different landing to channel width of flow channel. Procedia Eng. 2014, 97, 1534–1542. [Google Scholar] [CrossRef]
- Chen, T.; Liu, S.; Zhang, J.; Tang, M. Study on the characteristics of GDL with different PTFE content and its effect on the performance of PEMFC. Int. J. Heat Mass Transf. 2019, 128, 1168–1174. [Google Scholar] [CrossRef]
- Wei, J.; Ning, F.; Bai, C.; Zhang, T.; Lu, G.; Wang, H.; Li, Y.; Shen, Y.; Fu, X.; Li, Q. An ultra-thin, flexible, low-cost and scalable gas diffusion layer composed of carbon nanotubes for high-performance fuel cells. J. Mater. Chem. A 2020, 8, 5986–5994. [Google Scholar] [CrossRef]
- Chun, J.H.; Park, K.T.; Jo, D.H.; Kim, S.G.; Kim, S.H. Numerical modeling and experimental study of the influence of GDL properties on performance in a PEMFC. Int. J. Hydrogen Energy 2011, 36, 1837–1845. [Google Scholar] [CrossRef]
- Kitahara, T.; Nakajima, H.; Okamura, K. Gas diffusion layers coated with a microporous layer containing hydrophilic carbon nanotubes for performance enhancement of polymer electrolyte fuel cells under both low and high humidity conditions. J. Power Sources 2015, 283, 115–124. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, C.; Ling, C.-Y.; Han, M.; Yong, R.-Y.; Sun, D.; Chen, J. Review on current research of materials, fabrication and application for bipolar plate in proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2020, 45, 29832–29847. [Google Scholar] [CrossRef]
- Lakshminarayanan, V.R.; Karthikeyan, P.; Aswin, C.; Charandeep Singh, D.S. Parametric analysis of proton exchange membrane fuel cell (PEMFC) performed by the Taguchi method. Trans. FAMENA 2015, 39, 79–88. [Google Scholar]
- Karthikeyan, P.; Muthukumar, M.; Shanmugam, S.V.; Kumar, P.P.; Murali, S.; Kumar, A.S. Optimization of operating and design parameters on proton exchange membrane fuel cell by using Taguchi method. Procedia Eng. 2013, 64, 409–418. [Google Scholar] [CrossRef]
- Krishna, R.; Sreenivasan, M.; Dhass, A.; Sivakumar, E.; Teja, P.S.; Kumar, M.D.; Anand, S.R. Structural and thermoelectric behavior of nanostructured porous graphite for thermoelectric power generator application. Mater. Today Proc. 2020, 33, 179–182. [Google Scholar] [CrossRef]
- Afshari, E.; Mosharaf-Dehkordi, M.; Rajabian, H. An investigation of the PEM fuel cells performance with partially restricted cathode flow channels and metal foam as a flow distributor. Energy 2017, 118, 705–715. [Google Scholar] [CrossRef]
- Tanaka, S.; Bradfield, W.W.; Legrand, C.; Malan, A.G. Numerical and experimental study of the effects of the electrical resistance and diffusivity under clamping pressure on the performance of a metallic gas-diffusion layer in polymer electrolyte fuel cells. J. Power Sources 2016, 330, 273–284. [Google Scholar] [CrossRef]
- Fu, Y.; Lin, G.; Hou, M.; Wu, B.; Shao, Z.; Yi, B. Carbon-based films coated 316L stainless steel as bipolar plate for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2009, 34, 405–409. [Google Scholar] [CrossRef]
- Xiao, L.; Yin, Z.; Bian, M.; Bevilacqua, N.; Zeis, R.; Yuan, J.; Sui, P.-C. Microstructure reconstruction using fiber tracking technique and pore-scale simulations of heterogeneous gas diffusion layer. Int. J. Hydrogen Energy 2022, 47, 20218–20231. [Google Scholar] [CrossRef]
- Leng, Y.; Ming, P.; Yang, D.; Zhang, C. Stainless steel bipolar plates for proton exchange membrane fuel cells: Materials, flow channel design and forming processes. J. Power Sources 2020, 451, 227783. [Google Scholar] [CrossRef]
- Alo, O.A.; Otunniyi, I.O.; Pienaar, H. Manufacturing methods for metallic bipolar plates for polymer electrolyte membrane fuel cell. Mater. Manuf. Process. 2019, 34, 927–955. [Google Scholar] [CrossRef]
- De las Heras, A.; Vivas, F.; Segura, F.; Andújar, J. From the cell to the stack. A chronological walk through the techniques to manufacture the PEFCs core. Renew. Sustain. Energy Rev. 2018, 96, 29–45. [Google Scholar] [CrossRef]
- Haye, E.; Deschamps, F.; Caldarella, G.; Piedboeuf, M.-L.; Lafort, A.; Cornil, H.; Colomer, J.-F.; Pireaux, J.-J.; Job, N. Formable chromium nitride coatings for proton exchange membrane fuel cell stainless steel bipolar plates. Int. J. Hydrogen Energy 2020, 45, 15358–15365. [Google Scholar] [CrossRef]
- Pozio, A.; Zaza, F.; Masci, A.; Silva, R. Bipolar plate materials for PEMFCs: A conductivity and stability study. J. Power Sources 2008, 179, 631–639. [Google Scholar] [CrossRef]
- Lin, K.; Li, X.; Dong, H.; Du, S.; Lu, Y.; Ji, X.; Gu, D. Surface modification of 316 stainless steel with platinum for the application of bipolar plates in high performance proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2017, 42, 2338–2348. [Google Scholar] [CrossRef]
- Rajaei, V.; Rashtchi, H.; Raeissi, K.; Shamanian, M. The study of Ni-based nano-crystalline and amorphous alloy coatings on AISI 304 stainless steel for PEM fuel cell bipolar plate application. Int. J. Hydrogen Energy 2017, 42, 14264–14278. [Google Scholar] [CrossRef]
- Manso, A.; Marzo, F.; Garicano, X.; Alegre, C.; Lozano, A.; Barreras, F. Corrosion behavior of tantalum coatings on AISI 316L stainless steel substrate for bipolar plates of PEM fuel cells. Int. J. Hydrogen Energy 2020, 45, 20679–20691. [Google Scholar] [CrossRef]
- Park, Y.-C.; Lee, S.-H.; Kim, S.-K.; Lim, S.; Jung, D.-H.; Choi, S.-Y.; Kim, J.-H.; Peck, D.-H. Effects of CrN/Cr coating layer on durability of metal bipolar plates under a fuel recirculation system of direct methanol fuel cells. Int. J. Hydrogen Energy 2013, 38, 10567–10576. [Google Scholar] [CrossRef]
- Netwall, C.J.; Gould, B.D.; Rodgers, J.A.; Nasello, N.J.; Swider-Lyons, K.E. Decreasing contact resistance in proton-exchange membrane fuel cells with metal bipolar plates. J. Power Sources 2013, 227, 137–144. [Google Scholar] [CrossRef]
- Jayakumar, A.; Madheswaran, D.K.; Velu, R. Metal additive manufacturing of PEM fuel cell flow field plates and the scope of nanomaterials for its fabrication. Nanotechnol.-Based Addit. Manuf. Prod. Des. Prop. Appl. 2023, 1, 103–129. [Google Scholar]
- Wu, S.; Yang, W.; Yan, H.; Zuo, X.; Cao, Z.; Li, H.; Shi, M.; Chen, H. A review of modified metal bipolar plates for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2021, 46, 8672–8701. [Google Scholar] [CrossRef]
- Husby, H. Carbon Based Coatings for Metallic Bipolar Plates in PEM Fuel Cells. Master’s Thesis, Institutt for materialteknologi, Norwegian University of Science and Technology, Trondheim, Norway, 2013. [Google Scholar]
- Susanna, G.; Salamandra, L.; Brown, T.M.; Di Carlo, A.; Brunetti, F.; Reale, A. Airbrush spray-coating of polymer bulk-heterojunction solar cells. Solar Energy Mater. Sol. Cells 2011, 95, 1775–1778. [Google Scholar] [CrossRef]
- Gago, A.; Ansar, A.; Wagner, N.; Arnold, J.; Friedrich, K.A. Titanium coatings deposited by thermal spraying for bipolar plates of PEM electrolysers. In Proceedings of the 4th European PEFC and H2 Forum, Lucerne, Switzerland, 2–5 July 2013. [Google Scholar]
- Bi, F.; Peng, L.; Yi, P.; Lai, X. Multilayered Zr–C/aC film on stainless steel 316L as bipolar plates for proton exchange membrane fuel cells. J. Power Sources 2016, 314, 58–65. [Google Scholar] [CrossRef]
- Madheswaran, D.K.; Jayakumar, A.; Velu, R.; Raj, R.; Varuvel, E.G. Polymer based flow field plates for polymer electrolyte membrane fuel cell and the scope of additive manufacturing: A techno-economic review. Int. J. Energy Res. 2022, 46, 19737–19761. [Google Scholar] [CrossRef]
- Gerçel, H.F.; Tosun, Ç.G.; Akyalçın, L. Polymer electrolyte membrane fuel cell performance of a sulfonated poly (arylene ether benzimidazole) copolymer membrane. Adv. Mater. Sci. Eng. 2016, 2016, 6123213. [Google Scholar] [CrossRef]
- Mitzel, J.; Friedrich, K.A. 11 Hydrogen fuel cell applications. In Utilization of Hydrogen for Sustainable Energy and Fuels; Walter de Gruyter: Berlin, Germany, 2021; p. 367. [Google Scholar]
- Seselj, N.; Alfaro, S.M.; Bompolaki, E.; Cleemann, L.N.; Torres, T.; Azizi, K. Catalyst Development for High Temperature Polymer Electrolyte Membrane Fuel Cell (HT-PEMFC) Applications. Adv. Mater. 2023, 35, 2302207. [Google Scholar] [CrossRef]
- Madheswaran, D.K.; Thangavelu, P.; Krishna, R.; Thangamuthu, M.; Joseph Chandran, A.; Colak, I. Carbon-based materials in proton exchange membrane fuel cells: A critical review on performance and application. Carbon Lett. 2023, 33, 1495–1518. [Google Scholar] [CrossRef]
- Zhang, J.; Aili, D.; Lu, S.; Li, Q. Advancement toward polymer electrolyte membrane fuel cells at elevated temperatures. Research 2020, 2020, 9089405. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Aldave, S.; Andreoli, E. Fundamentals of gas diffusion electrodes and electrolysers for carbon dioxide utilisation: Challenges and opportunities. Catalysts 2020, 10, 713. [Google Scholar] [CrossRef]
- Matsumoto, M.; Masui, K.; Fukushige, S.; Kondoh, S. Sustainability through Innovation in Product Life Cycle Design; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Madheswaran, D.K.; Jayakumar, A. Recent advancements on non-platinum based catalyst electrode material for polymer electrolyte membrane fuel cells: A mini techno-economic review. Bull. Mater. Sci. 2021, 44, 287. [Google Scholar] [CrossRef]
- Wang, Y.; Diaz, D.F.R.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells–a review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Jaafar, A.; Turpin, C.; Roboam, X.; Bru, E.; Rallières, O. Energy management of a hybrid system based on a fuel cell and a Lithium Ion battery: Experimental tests and integrated optimal design. Math. Comput. Simul. 2017, 131, 21–37. [Google Scholar] [CrossRef]
- Ehsani, M.; Gao, Y.; Longo, S.; Ebrahimi, K. Modern Electric, Hybrid Electric, and Fuel Cell Vehicles; CRC press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Mohideen, M.M.; Subramanian, B.; Sun, J.; Ge, J.; Guo, H.; Radhamani, A.V.; Ramakrishna, S.; Liu, Y. Techno-economic analysis of different shades of renewable and non-renewable energy-based hydrogen for fuel cell electric vehicles. Renew. Sustain. Energy Rev. 2023, 174, 113153. [Google Scholar] [CrossRef]
- Ishikawa, H.; Sugawara, Y.; Tsuji, Y. Development of Residential Fuel Cell System of Panasonic and Approaches to Enhance Durability. In Electrochemical Society Meeting Abstracts 225; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2014. [Google Scholar]
- Chugh, S.; Meenakshi, S.; Sonkar, K.; Sharma, A.; Kapur, G.; Ramakumar, S. Performance evaluation of PEM fuel cell stack on hydrogen produced in the oil refinery. Int. J. Hydrogen Energy 2020, 45, 5491–5500. [Google Scholar] [CrossRef]
- Madheswaran, D.K.; Vengatesan, S.; Varuvel, E.G.; Praveenkumar, T.; Jegadheeswaran, S.; Pugazhendhi, A.; Arulmozhivarman, J. Nanofluids as a coolant for polymer electrolyte membrane fuel cells: Recent trends, challenges, and future perspectives. J. Clean. Prod. 2023, 424, 138763. [Google Scholar] [CrossRef]
- Chen, H.; Song, Z.; Zhao, X.; Zhang, T.; Pei, P.; Liang, C. A review of durability test protocols of the proton exchange membrane fuel cells for vehicle. Appl. Energy 2018, 224, 289–299. [Google Scholar] [CrossRef]
- Xing, Y.; Li, H.; Avgouropoulos, G. Research progress of proton exchange membrane failure and mitigation strategies. Materials 2021, 14, 2591. [Google Scholar] [CrossRef]
- Dirkes, S.; Leidig, J.; Fisch, P.; Pischinger, S. Prescriptive Lifetime Management for PEM fuel cell systems in transportation applications, Part II: On-board operando feature extraction, condition assessment and lifetime prediction. Energy Convers. Manag. 2023, 283, 116943. [Google Scholar] [CrossRef]
- Pei, P.; Meng, Y.; Chen, D.; Ren, P.; Wang, M.; Wang, X. Lifetime prediction method of proton exchange membrane fuel cells based on current degradation law. Energy 2023, 265, 126341. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Han, J.; Nguyen, X.L.; Yu, S.; Goo, Y.-M.; Le, D.D. Review of the durability of polymer electrolyte membrane fuel cell in long-term operation: Main influencing parameters and testing protocols. Energies 2021, 14, 4048. [Google Scholar] [CrossRef]
- Vasilyev, A.; Andrews, J.; Dunnett, S.J.; Jackson, L.M. Dynamic reliability assessment of PEM fuel cell systems. Reliab. Eng. Syst. Saf. 2021, 210, 107539. [Google Scholar] [CrossRef]
- Fan, L.; Tu, Z.; Chan, S.H. Recent development of hydrogen and fuel cell technologies: A review. Energy Rep. 2021, 7, 8421–8446. [Google Scholar] [CrossRef]
- McQueen, S.; Stanford, J.; Satyapal, S.; Miller, E.; Stetson, N.; Papageorgopoulos, D.; Rustagi, N.; Arjona, V.; Adams, J.; Randolph, K. Department of Energy Hydrogen Program Plan; US Department of Energy (USDOE): Washington, DC, USA, 2020.
- Yue, M.; Jemei, S.; Zerhouni, N.; Gouriveau, R. Proton exchange membrane fuel cell system prognostics and decision-making: Current status and perspectives. Renew. Energy 2021, 179, 2277–2294. [Google Scholar] [CrossRef]
- Whiston, M.M.; Azevedo, I.L.; Litster, S.; Whitefoot, K.S.; Samaras, C.; Whitacre, J.F. Expert assessments of the cost and expected future performance of proton exchange membrane fuel cells for vehicles. Proc. Natl. Acad. Sci. USA 2019, 116, 4899–4904. [Google Scholar] [CrossRef]
- Cullen, D.A.; Neyerlin, K.; Ahluwalia, R.K.; Mukundan, R.; More, K.L.; Borup, R.L.; Weber, A.Z.; Myers, D.J.; Kusoglu, A. New roads and challenges for fuel cells in heavy-duty transportation. Nat. Energy 2021, 6, 462–474. [Google Scholar] [CrossRef]
- Lindorfer, J.; Rosenfeld, D.C.; Böhm, H. Fuel cells: Energy conversion technology. In Future Energy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 495–517. [Google Scholar]
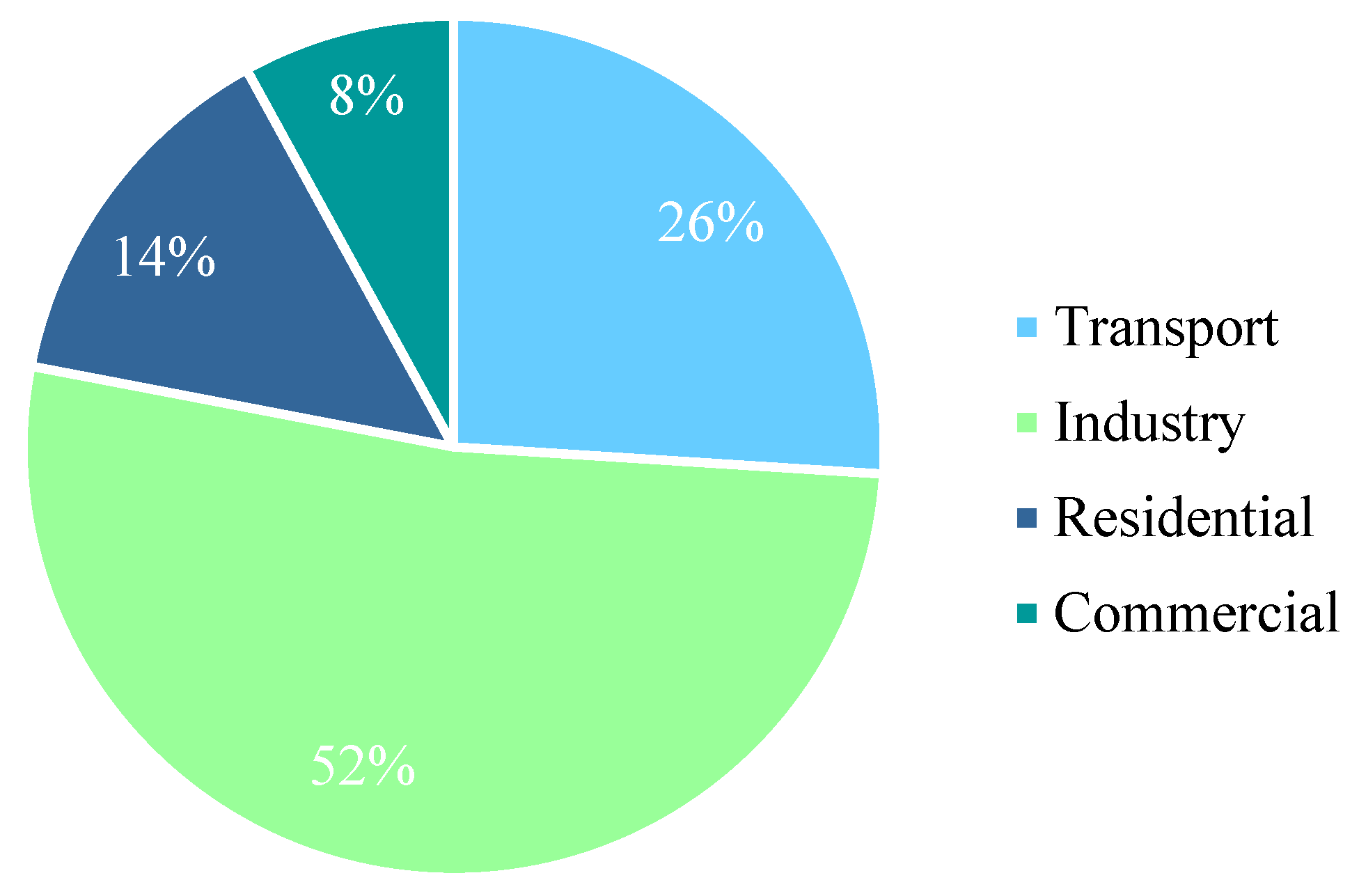
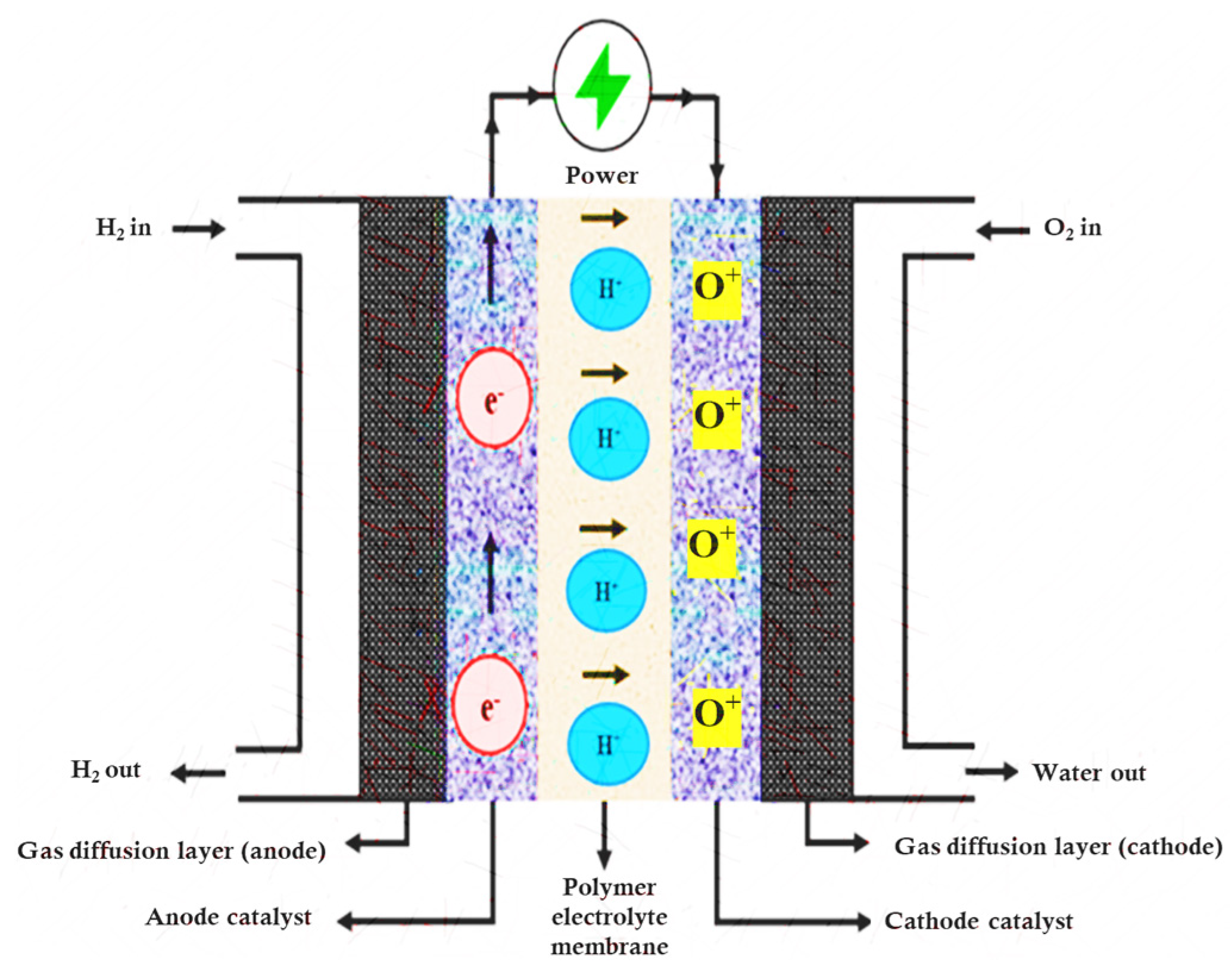

| Characteristics | Units | Status | Aim for 2025 |
|---|---|---|---|
| Pt group metal (PGM) | rated G/kW | 0.125:1.05 (150, 250 kPa) | ≤0.10 |
| Durability with cycling | hrs | 4100 | 8000 |
| Performance at 0.8 V | mW/cm2 | 306 | 300 |
| Rated power | 890, 1190 (150, 250 kPa) | 1800 | |
| Demise in catalytic activity | % | 40 | ≤40% initial loss |
| Deficiency at 0.8 A/cm2 | mV | 20 | ≤30 |
| Constancy of electrocatalyst support | % | Not Available | ≤40 |
| Deficiency at 1.5 A/cm2 | mV | >500 | ≤30 |
| Mass activity | mgpgm at 900 more-free | 0.6 | 0.44 |
| Free catalyst action of PGM | A/cm2 at 900 more-free A | 0.021 | 0.044 |
| Characteristics | Units | Status | Aim for 2025 |
|---|---|---|---|
| Max. working temperature | °C | 120 | 120 |
| Area-specific proton resistance at 120 °C and water partial pressure of 40 kPa | Ω cm2 | 0.054 (40 kPa) 0.019 (80 kPa) | 0.02 |
| Area-specific proton resistance at 95 °C and water partial pressure of 25 kPa | Ω cm2 | 0.027 (25 kPa) (at 80 °C 0.02 at 25 kPa, 0.008 at 45 kPa) | 0.02 |
| Area-specific proton resistance at 30 °C and water partial pressure of 4 kPa | Ω cm2 | 0.018 | 0.03 |
| Area-specific proton resistance at −20 °C | Ω cm2 | 0.2 | 0.2 |
| Max. H2 and O2 crossover | mA/cm2 | 1.9 and 0.6 | 2 and 2 |
| Min. electrical resistance | Ω cm2 | 1635 | 1000 |
| Mechanical durability | Cycles w/<10 sccm crossover | 24,000 | 20,000 |
| Chemical stability | Hours with <5 mA/cm2 crossover or <20% loss in OCV | 614 | 500 |
| Characteristics | Units | Present Status | 2025 |
|---|---|---|---|
| Weight of plate | kg/kW | <0.4 | 0.18 |
| Plate H2 permeation | Std cm3/sec.cm2Pa @ 80 °C, 3 atm 100% relative humidity (RH) | <2 × 10−6 | 2 × 10−6 |
| Corrosion at anode | µA/cm2 | Not reaching the peak | <1 and no active peak |
| Corrosion at cathode | µA/cm2 | <0.1 | <1 |
| Conductivity | S/cm | >100 | >100 |
| Flexural modulus | MPa | >34 (carbon plate) | >40 |
| Elongation | % | 20–40 | 40 |
| Characteristics | Units | 2020 Targets | Current Status | 2025 Targets |
|---|---|---|---|---|
| Total content of PGM | g/kW rated | 0.125 | 0.125 | ≤0.10 |
| Durability with cycling | Hours | 5000 | 4100 | 8000 |
| Performance at 0.8 V | mW/cm2 | 300 | 306 | 300 |
| Performance at peak power | mW/cm2 | 1000 | 890 | 1800 |
| Mass loss | % | <40 | 40 | ≤40% loss of initial |
| Performance loss | mV | <30 | 20 | ≤30 |
| Electrocatalyst support stability | % mass activity loss | <40 | 40 | ≤40 |
| Loss in performance | mV | <30 | >500 | ≤30 |
| Activity of mass | mgpgm @ 900 mVIR-free | 0.44 | 0.6 | 0.44 |
| Free catalyst activity of PGM | A/cm2 @ 900 mVIR-free A | 0.044 | 0.012 | 0.044 |
| Characteristics | Units | 2020 Targets | Current Status | 2025 Targets |
|---|---|---|---|---|
| Max. operating temperature | °C | 120 | 120 | 120 |
| Area-specific proton resistance at 120 °C and water partial pressure of 40 kPa | Ω cm2 | 0.02 | 0.054 | 0.02 |
| Area-specific proton resistance at 95 °C and water partial pressure of 25 kPa | Ω cm2 | 0.02 | 0.027 | 0.02 |
| Area-specific proton resistance at 30 °C and water partial pressure of 4 kPa | Ω cm2 | 0.03 | 0.018 | 0.03 |
| Area-specific proton resistance at −20 °C | Ω cm2 | 0.2 | 0.2 | 0.2 |
| Max. O2 crossover | mA/cm2 | 2 | 0.6 | 2 |
| Max. H2 crossover | mA/cm2 | 2 | 1.9 | 2 |
| Min. electrical resistance | Ω cm2 | 1000 | 1635 | 1000 |
| Mechanical durability | Cycles w/<10 sccm crossover | 20,000 | 24,000 | 20,000 |
| Chemical stability | Hours with <5 mA/cm2 crossover or <20% loss in OCV | >500 | 614 | 500 |
| Characteristics | Units | 2020 Targets | Current Status | 2025 Targets |
|---|---|---|---|---|
| Weight of the plate | kg/kW | 0.4 | <0.4 | 0.18 |
| H2 permeation in the plate | Std cm3/sec.cm2.Pa @ 80 °C, 3 atm 100% Relative Humidity (RH) | <1.3 × 10−14 | <2 × 10−6 | 2 × 10−6 |
| Corrosion anode | µA/cm2 | <1 and no active peak | no active peak | <1 and no active peak |
| Corrosion cathode | µA/cm2 | <1 | <0.1 | <1 |
| Conductivity | S/cm | >100 | >100 | >100 |
| Flexural modulus | MPa | >25 | >34 | >40 |
| Authors | Components | Observations | Conclusions |
|---|---|---|---|
| Su et al. [68] | GDL | At an operating voltage of 0.6 V and a temperature of 160 °C, the power density reached 0.61 W/cm2, with a corresponding current density of 0.52 A/cm2. | The obtained power and current density value of the GDL are higher than the 2020 and 2025 DOE targets. |
| Gago et al. [96] | Bipolar plate | An Icorr value of 1.10 × 10 A/cm2 was achieved. | The obtained Icorr value was higher than the DOE technical targets. |
| Lee et al. [65] | Membrane | A current density of 1.27 A/cm2 at 100% RH was achieved. | The obtained current density value was higher than the DOE technical targets of 2020 and 2025. |
| Muthukumar et al. [69] | GDL | The power and current density are 0.4761 W/cm2 and 1.1902 A/cm2 at 1.4 bar pressure and 0.4 V. | The obtained power and current density value of the GDL are higher than the 2020 and 2025 DOE targets. |
| Gercel et al. [99] | Membrane | The membrane’s power density was assessed at 23.7 mW/cm2 when tested at 80 °C. | The obtained power density value of the membrane does not meet U.S. DOE 2020 and 2025 targets. |
| Vinothkannan et al. [66] | Membrane | A power density of 300 mW/cm2 was achieved at 70 °C. | The obtained power density values met U.S. DOE 2020 and 2025 targets. |
| Bi et al. [97] | Bipolar plate | An ICR value of 3.63 µ/cm2 and corrosion current value of 0.49 mA/cm2 were achieved for Zr-C/a-C film. | The ICR value of the bipolar plate met the DOE 2020 targets. |
| He et al. [50] | Catalyst | A current density of 0.004 A/cm2 at 0.87 ViR-free was achieved. | The obtained current density value did not meet DOE targets of 2020 and 2025. |
| Li et al. [53] | Membrane | A current density of 1.372 A/cm2 at a cell voltage of 3 V was achieved. | The obtained value met the DOE targets of 2020 and 2025. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madheswaran, D.K.; Thangamuthu, M.; Gnanasekaran, S.; Gopi, S.; Ayyasamy, T.; Pardeshi, S.S. Powering the Future: Progress and Hurdles in Developing Proton Exchange Membrane Fuel Cell Components to Achieve Department of Energy Goals—A Systematic Review. Sustainability 2023, 15, 15923. https://doi.org/10.3390/su152215923
Madheswaran DK, Thangamuthu M, Gnanasekaran S, Gopi S, Ayyasamy T, Pardeshi SS. Powering the Future: Progress and Hurdles in Developing Proton Exchange Membrane Fuel Cell Components to Achieve Department of Energy Goals—A Systematic Review. Sustainability. 2023; 15(22):15923. https://doi.org/10.3390/su152215923
Chicago/Turabian StyleMadheswaran, Dinesh Kumar, Mohanraj Thangamuthu, Sakthivel Gnanasekaran, Suresh Gopi, Tamilvanan Ayyasamy, and Sujit S. Pardeshi. 2023. "Powering the Future: Progress and Hurdles in Developing Proton Exchange Membrane Fuel Cell Components to Achieve Department of Energy Goals—A Systematic Review" Sustainability 15, no. 22: 15923. https://doi.org/10.3390/su152215923
APA StyleMadheswaran, D. K., Thangamuthu, M., Gnanasekaran, S., Gopi, S., Ayyasamy, T., & Pardeshi, S. S. (2023). Powering the Future: Progress and Hurdles in Developing Proton Exchange Membrane Fuel Cell Components to Achieve Department of Energy Goals—A Systematic Review. Sustainability, 15(22), 15923. https://doi.org/10.3390/su152215923









