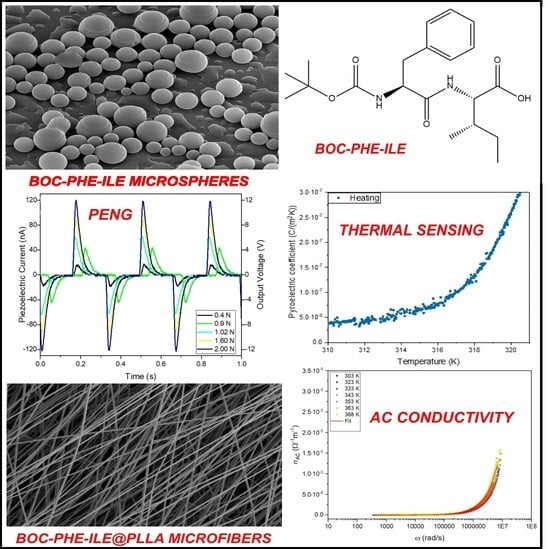
Electrospun Microstructured Biopolymer Fibers Containing the Self-Assembled Boc–Phe–Ile Dipeptide: Dielectric and Energy Harvesting Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymeric Solutions and Electrospinning Microfibers
2.3. Scanning Electron Microscopy (SEM)
2.4. Optical Absortion and Photoluminescence
2.5. Dynamic Light Scattering (DLS)
2.6. Dielectric Spectroscopy
2.7. Pyroelectric Coefficient
2.8. Piezoelectric Measurements
3. Results and Discussion
3.1. Morphology of Self-Assembled Dipeptides in Solution and in Fibers
3.2. Optical Absorption and Photoluminescence of Dipeptide Self-Assemblies
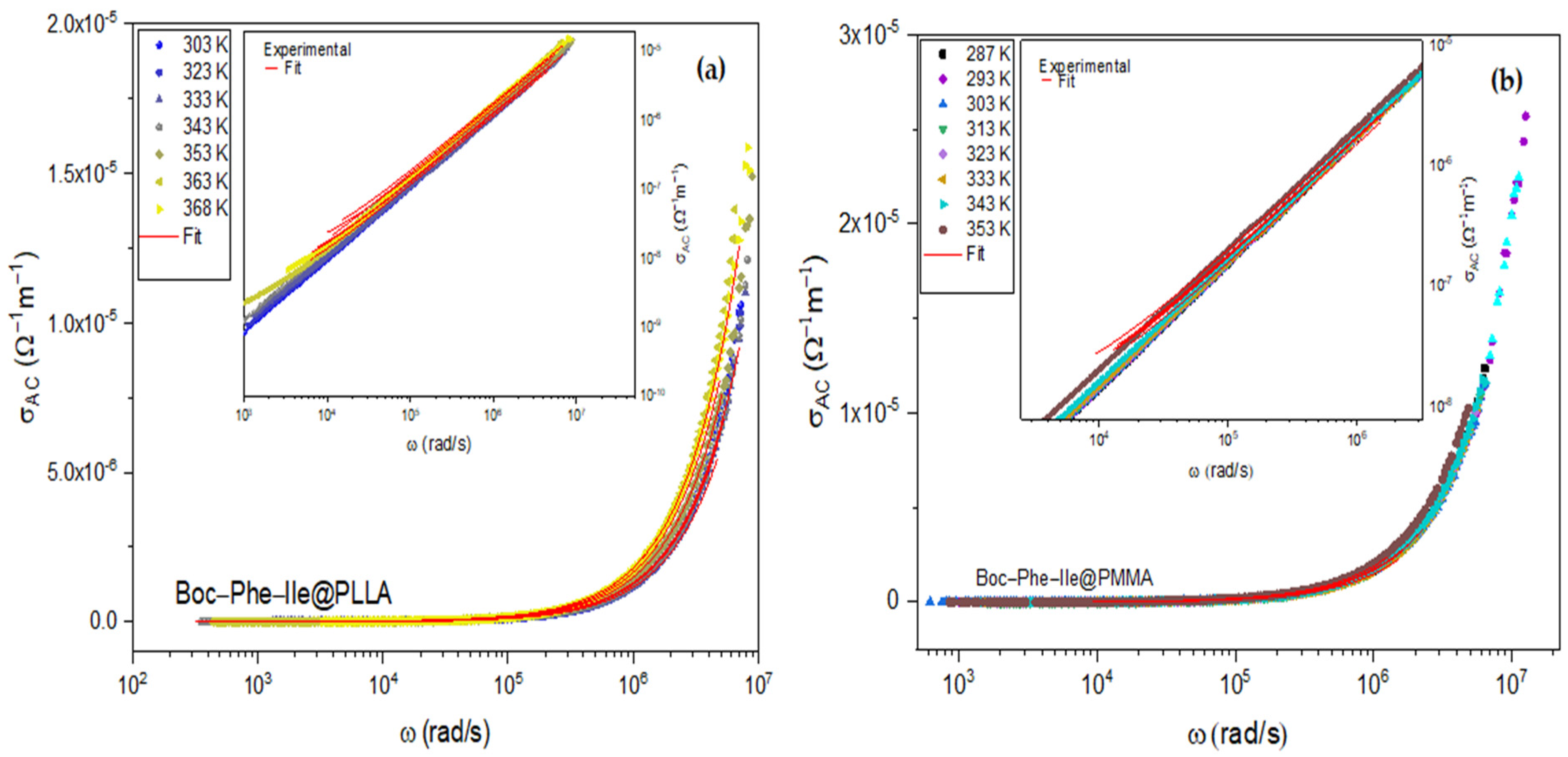
3.3. Dieletric Properties
3.4. Pyroelectricity in Fibers
3.5. Effective Piezoelectric Coefficient
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shin, D.-M.; Hong, S.W.; Hwang, Y.-H. Recent advances in organic piezoelectric biomaterials for energy and biomedical applications. Nanomaterials 2020, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Bystrov, V.; Bdikin, I.; Singh, B. Piezoelectric and ferroelectric properties of various amino acids and dipeptides tubular nanostructures: Molecular modeling. Nanomater. Sci. Eng. 2020, 2, 11–24. [Google Scholar]
- Guerin, S.; Stapleton, A.; Chovan, D.; Mouras, R.; Gleeson, M.; McKeown, C.; Noor, M.R.; Silien, C.; Rhen, F.M.F.; Kholkin, A.L.; et al. Control of piezoelectricity in amino acids by supramolecular packing. Nat. Mater. 2018, 17, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, S.; Zelenovskiy, P.; Vasileva, D.; Nuraeva, A.; Shur, V.Y.; Kholkin, A.L. Piezoelectric properties of diphenylalanine microtubes prepared from the solution. J. Phys. Chem. Solids 2016, 93, 68–72. [Google Scholar] [CrossRef]
- Pandey, G.; Datta, D. Chapter 6—Peptide-based nanomaterials: Applications and challenges. In De Novo Peptide Design; Ramakrishnan, V., Patel, K., Goyal, R., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 133–171. [Google Scholar]
- Subbalakshmi, C.; Basak, P.; Nagaraj, R. Self-assembly of t-butyloxycarbonyl protected dipeptide methyl esters composed of leucine, isoleucine, and valine into highly organized structures from alcohol and aqueous alcohol mixtures. Pept. Sci. 2017, 108, e23033. [Google Scholar] [CrossRef]
- Kumaraswamy, P.; Lakshmanan, R.; Sethuraman, S.; Krishnan, U.M. Self-assembly of peptides: Influence of substrate, pH and medium on the formation of supramolecular assemblies. Soft Matter 2011, 7, 2744–2754. [Google Scholar] [CrossRef]
- Amdursky, N.; Molotskii, M.; Gazit, E.; Rosenman, G. Elementary Building Blocks of Self-Assembled Peptide Nanotubes. J. Am. Chem. Soc. 2010, 132, 15632–15636. [Google Scholar] [CrossRef]
- Amdursky, N.; Molotskii, M.; Gazit, E.; Rosenman, G. Self-assembled bioinspired quantum dots: Optical properties. Appl. Phys. Lett. 2009, 94, 261907. [Google Scholar] [CrossRef]
- Adler-Abramovich, L.; Aronov, D.; Beker, P.; Yevnin, M.; Stempler, S.; Buzhansky, L.; Rosenman, G.; Gazit, E. Self-assembled arrays of peptide nanotubes by vapour deposition. Nat. Nanotechnol. 2009, 4, 849. Available online: https://www.nature.com/articles/nnano.2009.298#supplementary-information (accessed on 10 May 2022). [CrossRef]
- Reches, M.; Gazit, E. Molecular self-assembly of peptide nanostructures: Mechanism of association and potential uses. Curr. Nanosci. 2006, 2, 105–111. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Controlled patterning of aligned self-assembled peptide nanotubes. Nat. Nanotechnol. 2006, 1, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Görbitz, C.H. The structure of nanotubes formed by diphenylalanine, the core recognition motif of Alzheimer’s β-amyloid polypeptide. Chem. Commun. 2006, 22, 2332–2334. [Google Scholar] [CrossRef]
- Gazit, E. Self-assembled peptide nanostructures: The design of molecular building blocks and their technological utilization. Chem. Soc. Rev. 2007, 36, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Zhu, R.; Jenkins, K.; Yang, R. Self-assembly of diphenylalanine peptide with controlled polarization for power generation. Nat. Commun. 2016, 7, 13566. Available online: https://www.nature.com/articles/ncomms13566#supplementary-information (accessed on 22 May 2022). [CrossRef] [PubMed]
- Agarwal, S.; Greiner, A.; Wendorff, J.H. Functional materials by electrospinning of polymers. Prog. Polym. Sci. 2013, 38, 963–991. [Google Scholar] [CrossRef]
- Frenot, A.; Chronakis, I.S. Polymer nanofibers assembled by electrospinning. Curr. Opin. Colloid Interface Sci. 2003, 8, 64–75. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Santos, D.; Baptista, R.M.F.; Handa, A.; Almeida, B.; Rodrigues, P.V.; Torres, A.R.; Machado, A.; Belsley, M.; de Matos Gomes, E. Bioinspired Cyclic Dipeptide Functionalized Nanofibers for Thermal Sensing and Energy Harvesting. Materials 2023, 16, 2477. [Google Scholar] [CrossRef]
- Santos, D.; Baptista, R.M.F.; Handa, A.; Almeida, B.; Rodrigues, P.V.; Castro, C.; Machado, A.; Rodrigues, M.J.L.F.; Belsley, M.; de Matos Gomes, E. Nanostructured Electrospun Fibers with Self-Assembled Cyclo-L-Tryptophan-L-Tyrosine Dipeptide as Piezoelectric Materials and Optical Second Harmonic Generators. Materials 2023, 16, 4993. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Lim, T.-C.; Ma, Z. An Introduction to Electrospinning and Nanofibers; World Scientific: Singapore, 2005; p. 396. [Google Scholar]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Singh, G.; Bittner, A.M.; Loscher, S.; Malinowski, N.; Kern, K. Electrospinning of diphenylalanine nanotubes. Adv. Mater. 2008, 20, 2332–2336. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Basavalingappa, V.; Bera, S.; Xue, B.; O’Donnell, J.; Guerin, S.; Cazade, P.-A.; Yuan, H.; Haq, E.u.; Silien, C.; Tao, K.; et al. Diphenylalanine-Derivative Peptide Assemblies with Increased Aromaticity Exhibit Metal-like Rigidity and High Piezoelectricity. ACS Nano 2020, 14, 7025–7037. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, K.; Kelly, S.; Nguyen, V.; Wu, Y.; Yang, R. Piezoelectric diphenylalanine peptide for greatly improved flexible nanogenerators. Nano Energy 2018, 51, 317–323. [Google Scholar] [CrossRef]
- Yan, X.; Zhu, P.; Li, J. Self-assembly and application of diphenylalanine-based nanostructures. Chem. Soc. Rev. 2010, 39, 1877–1890. [Google Scholar] [CrossRef]
- Lee, J.-H.; Heo, K.; Schulz-Schönhagen, K.; Lee, J.H.; Desai, M.S.; Jin, H.-E.; Lee, S.-W. Diphenylalanine Peptide Nanotube Energy Harvesters. ACS Nano 2018, 12, 8138–8144. [Google Scholar] [CrossRef]
- Baptista, R.M.F.; Lopes, P.E.; Rodrigues, A.R.O.; Cerca, N.; Belsley, M.S.; de Matos Gomes, E. Self-assembly of Boc-p-nitro-l-phenylalanyl-p-nitro-l-phenylalanine and Boc-l-phenylalanyl-l-tyrosine in solution and into piezoelectric electrospun fibers. Mater. Adv. 2022, 3, 2934–2944. [Google Scholar] [CrossRef]
- Baptista, R.M.F.; de Matos Gomes, E.; Raposo, M.M.M.; Costa, S.P.G.; Lopes, P.E.; Almeida, B.; Belsley, M.S. Self-assembly of dipeptide Boc-diphenylalanine nanotubes inside electrospun polymeric fibers with strong piezoelectric response. Nanoscale Adv. 2019, 1, 4339–4346. [Google Scholar] [CrossRef]
- Chandrudu, S.; Simerska, P.; Toth, I. Chemical Methods for Peptide and Protein Production. Molecules 2013, 18, 4373–4388. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Porter, S.L.; Coulter, S.M.; Pentlavalli, S.; Laverty, G. Pharmaceutical Formulation and Characterization of Dipeptide Nanotubes for Drug Delivery Applications. Macromol. Biosci. 2020, 20, 2000115. [Google Scholar] [CrossRef] [PubMed]
- Böhmer, R.; Kremer, F. Dielectric spectroscopy and multidimensional NMR—A comparison. In Broadband Dielectric Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2003; pp. 625–684. [Google Scholar]
- Kremer, F.; Schönhals, A. Broadband Dielectric Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Abu Hassan Shaari, H.; Ramli, M.M.; Mohtar, M.N.; Abdul Rahman, N.; Ahmad, A. Synthesis and Conductivity Studies of Poly(Methyl Methacrylate) (PMMA) by Co-Polymerization and Blending with Polyaniline (PANi). Polymers 2021, 13, 1939. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Cheng, Z.; Yang, S.; Sun, X.; Xie, L.; Zheng, Q. Extensional flow-induced conductive nanohybrid shish in poly(lactic acid) nanocomposites toward pioneering combination of high electrical conductivity, strength, and ductility. Compos. Part B Eng. 2020, 203, 108467. [Google Scholar] [CrossRef]
- Poplavko, Y. 2—Broadband dielectric spectroscopy. In Dielectric Spectroscopy of Electronic Materials; Poplavko, Y., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 41–73. [Google Scholar]
- Fukushima, K.; Kimura, Y. Stereocomplexed polylactides (Neo-PLA) as high-performance bio-based polymers: Their formation, properties, and application. Polym. Int. 2006, 55, 626–642. [Google Scholar] [CrossRef]
- Nguyen, H.T.H.; Qi, P.; Rostagno, M.; Feteha, A.; Miller, S.A. The quest for high glass transition temperature bioplastics. J. Mater. Chem. A 2018, 6, 9298–9331. [Google Scholar] [CrossRef]
- Huang, C.-C.; Liu, C.-Y. Peculiar α-β relaxations of Syndiotactic-Poly (methyl methacrylate). Polymer 2021, 225, 123760. [Google Scholar] [CrossRef]
- Fukao, K.; Uno, S.; Miyamoto, Y.; Hoshino, A.; Miyaji, H. Dynamics of α and β processes in thin polymer films: Poly (vinyl acetate) and poly (methyl methacrylate). Phys. Rev. E 2001, 64, 051807. [Google Scholar] [CrossRef]
- Wubbenhorst, M.; Murray, C.; Forrest, J.; Dutcher, J. Dielectric relaxations in ultra-thin films of PMMA: Assessing the length scale of cooperativity in the dynamic glass transition. In Proceedings of the 11th International Symposium on Electrets, Melbourne, Australia, 3 October 2002; pp. 401–406. [Google Scholar]
- Dionísio, M.; Fernandes, A.C.; Mano, J.F.; Correia, N.T.; Sousa, R.C. Relaxation studies in PEO/PMMA blends. Macromolecules 2000, 33, 1002–1011. [Google Scholar] [CrossRef]
- El Bachiri, A.; Bennani, F.; Bousselamti, M. Dielectric and electrical properties of LiNbO3 ceramics. J. Asian Ceram. Soc. 2016, 4, 46–54. [Google Scholar] [CrossRef]
- Macdonald, J.R. Comparison of the universal dynamic response power-law fitting model for conducting systems with superior alternative models. Solid. State Ion. 2000, 133, 79–97. [Google Scholar] [CrossRef]
- Bombile, J.H.; Janik, M.J.; Milner, S.T. Polaron formation mechanisms in conjugated polymers. Phys. Chem. Chem. Phys. 2018, 20, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Franchini, C.; Reticcioli, M.; Setvin, M.; Diebold, U. Polarons in materials. Nat. Rev. Mater. 2021, 6, 560–586. [Google Scholar] [CrossRef]
- Udovč, L.; Spreitzer, M.; Vukomanović, M. Towards hydrophilic piezoelectric poly-L-lactide films: Optimal processing, post-heat treatment and alkaline etching. Polym. J. 2020, 52, 299–311. [Google Scholar] [CrossRef]












| Nanogenerator | deff pCN−1 | Force/Area (Nm−2) | geff (VmN−1) | Power Density (μWcm−2) | Ref. |
|---|---|---|---|---|---|
| Boc–Phe–Ile@PLLA (fiber mat) | 56 | 2 × 103 | 3.9 | 0.12 | This work |
| Boc–Phe–Ile@PMMA (fiber mat) | 20 | 4 × 103 | 1.1 | 0.07 | This work |
| Cyclo(L-Trp–L-Trp)@PLLA | 57 | 3 × 103 | 4.7 | 0.18 | [19] |
| Boc-PhePhe@PLLA (fiber mat) | 8.4 | 4 × 103 | 0.3 | 2.3 | [29,30] |
| Boc-PheTyr@PLLA (fiber mat) | 7 | 4 × 103 | 0.3 | 1.0 | [29] |
| Boc-pNPhepNPhe@PLLA (fiber mat) | 16 | 4 × 103 | 0.6 | 9.0 | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Handa, A.; Baptista, R.M.F.; Santos, D.; Silva, B.; Rodrigues, A.R.O.; Oliveira, J.; Almeida, B.; de Matos Gomes, E.; Belsley, M. Electrospun Microstructured Biopolymer Fibers Containing the Self-Assembled Boc–Phe–Ile Dipeptide: Dielectric and Energy Harvesting Properties. Sustainability 2023, 15, 16040. https://doi.org/10.3390/su152216040
Handa A, Baptista RMF, Santos D, Silva B, Rodrigues ARO, Oliveira J, Almeida B, de Matos Gomes E, Belsley M. Electrospun Microstructured Biopolymer Fibers Containing the Self-Assembled Boc–Phe–Ile Dipeptide: Dielectric and Energy Harvesting Properties. Sustainability. 2023; 15(22):16040. https://doi.org/10.3390/su152216040
Chicago/Turabian StyleHanda, Adelino, Rosa M. F. Baptista, Daniela Santos, Bruna Silva, Ana Rita O. Rodrigues, João Oliveira, Bernardo Almeida, Etelvina de Matos Gomes, and Michael Belsley. 2023. "Electrospun Microstructured Biopolymer Fibers Containing the Self-Assembled Boc–Phe–Ile Dipeptide: Dielectric and Energy Harvesting Properties" Sustainability 15, no. 22: 16040. https://doi.org/10.3390/su152216040