Simulation of the Working Volume Reduction through the Bioconversion Model (BioModel) and Its Validation Using Biogas Plant Data for the Prediction of the Optimal Reactor Cleaning Period
Abstract
:1. Introduction
2. Materials and Methods
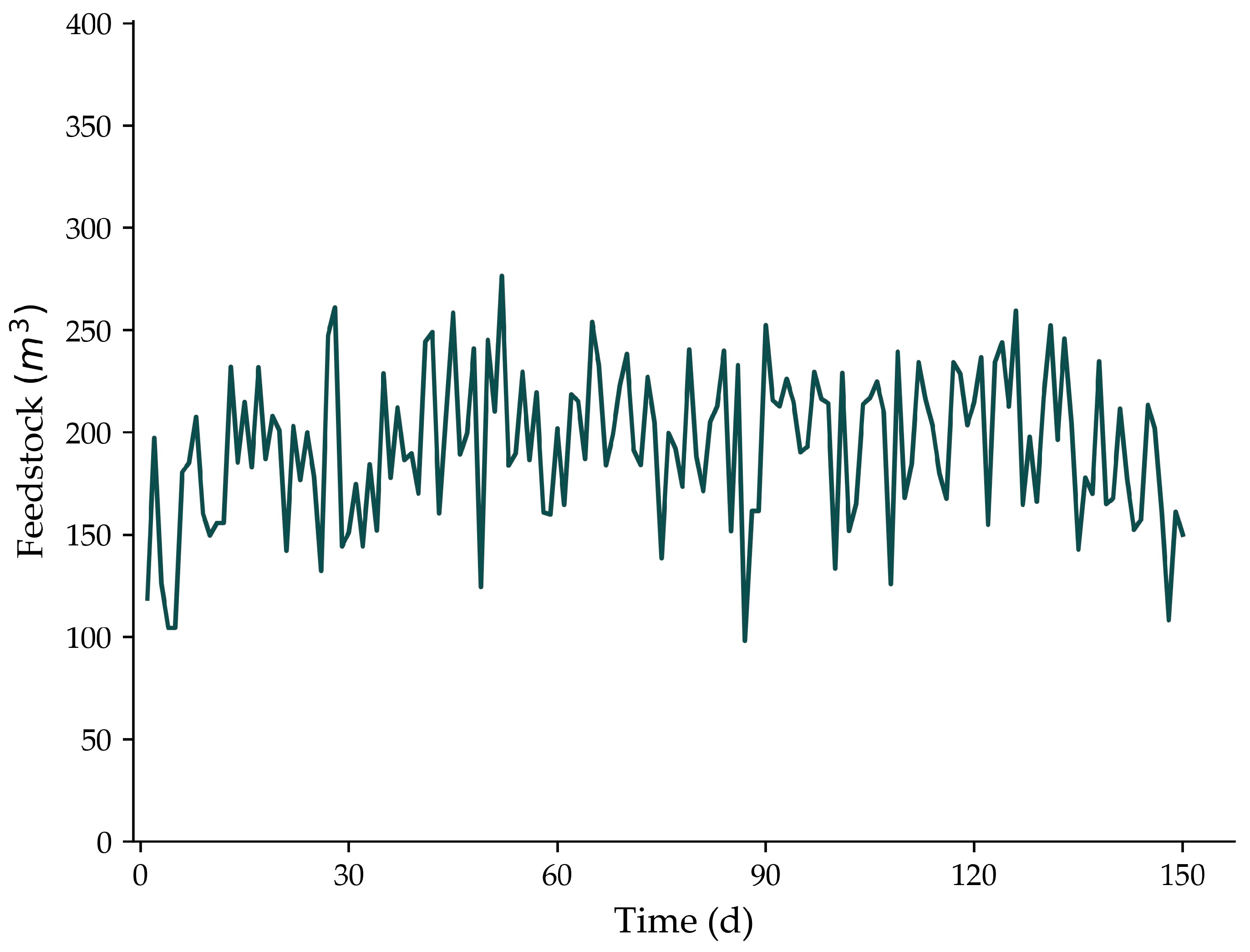
2.1. Full-Scale Biogas Plant Description
2.2. BioModel Description
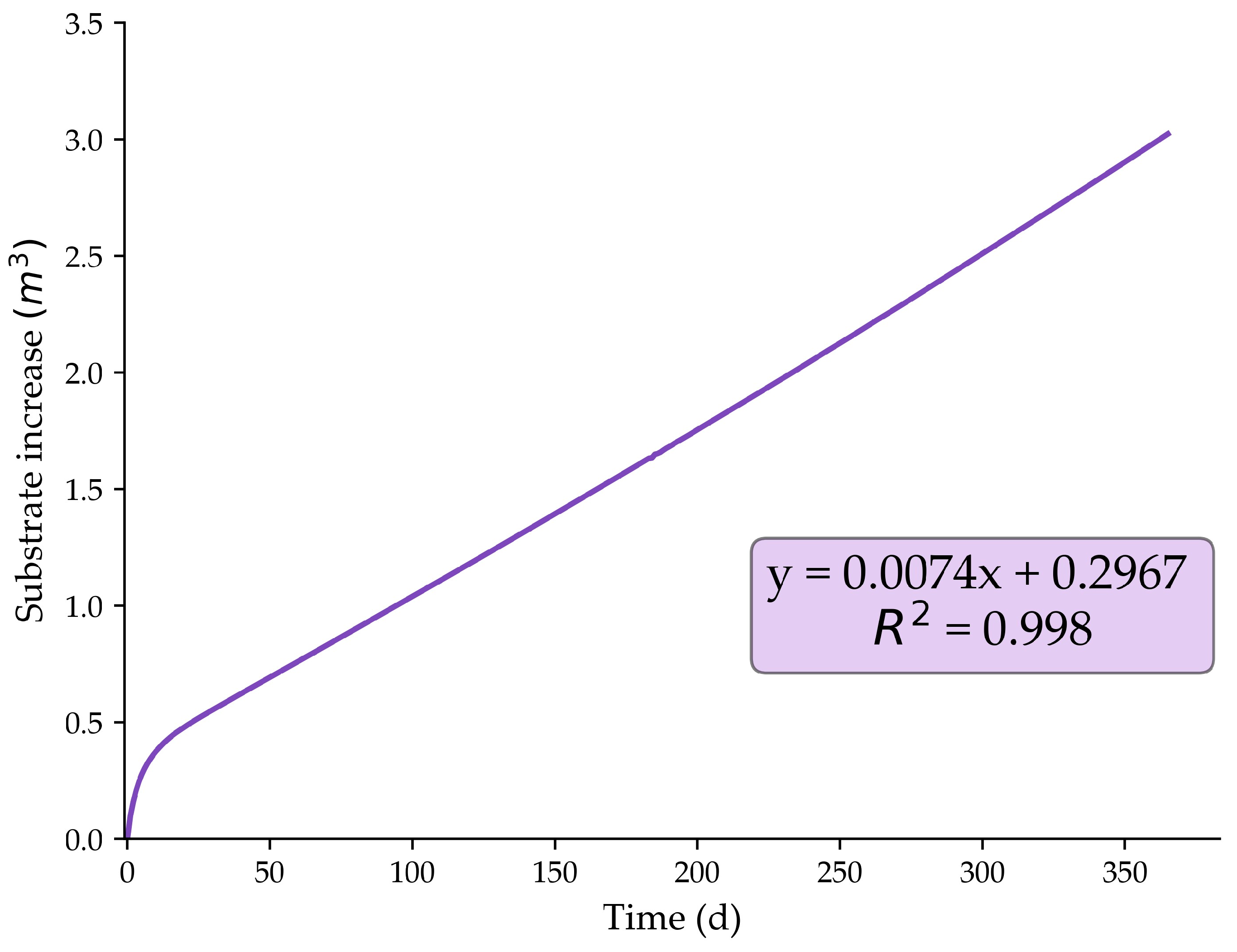
2.3. Working Volume Reduction
2.4. BioModel’s Input Characterization
2.4.1. Parameter Estimation
2.4.2. Composition of Inoculum
2.5. Model’s Validation
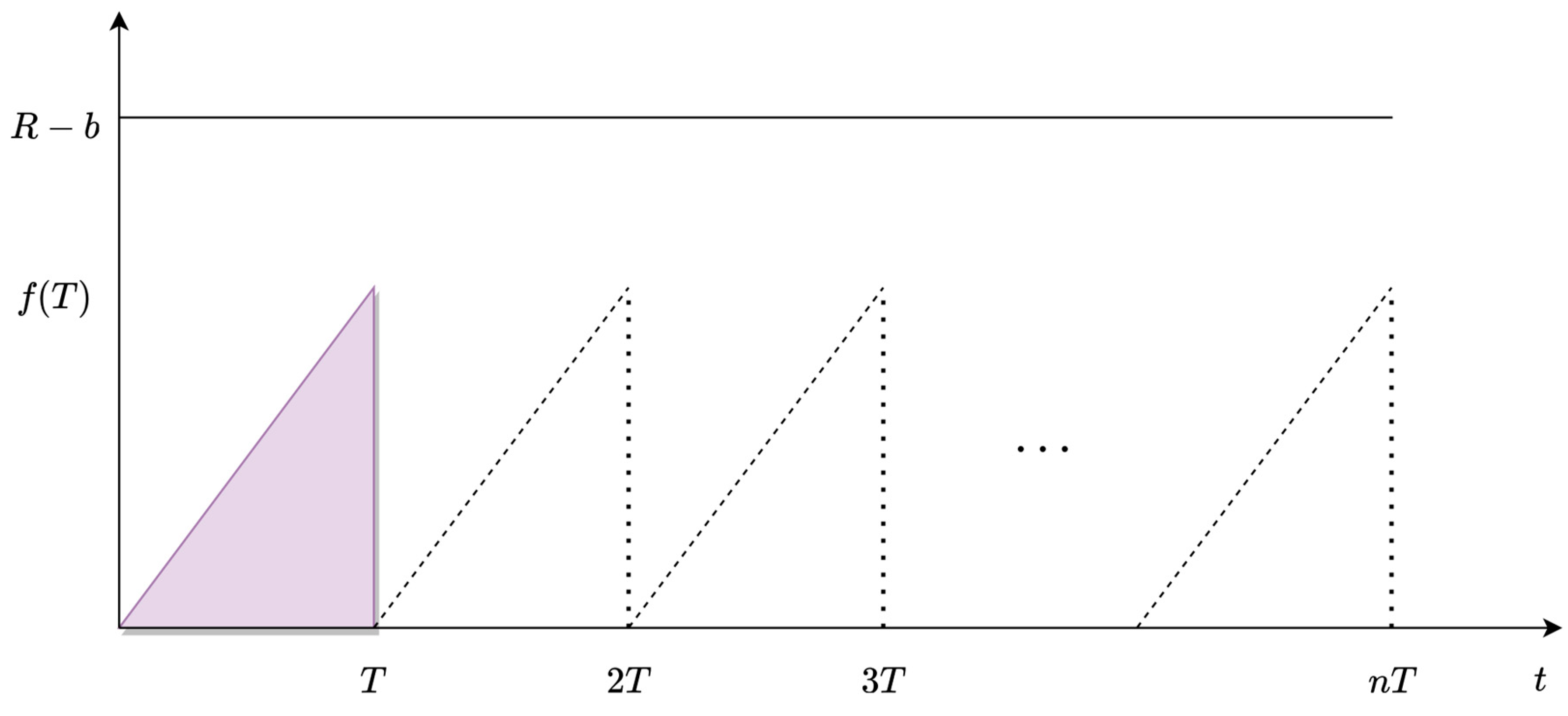
2.6. Prediction of the Optimal Period for Reactor’s Cleaning
3. Results & Discussion
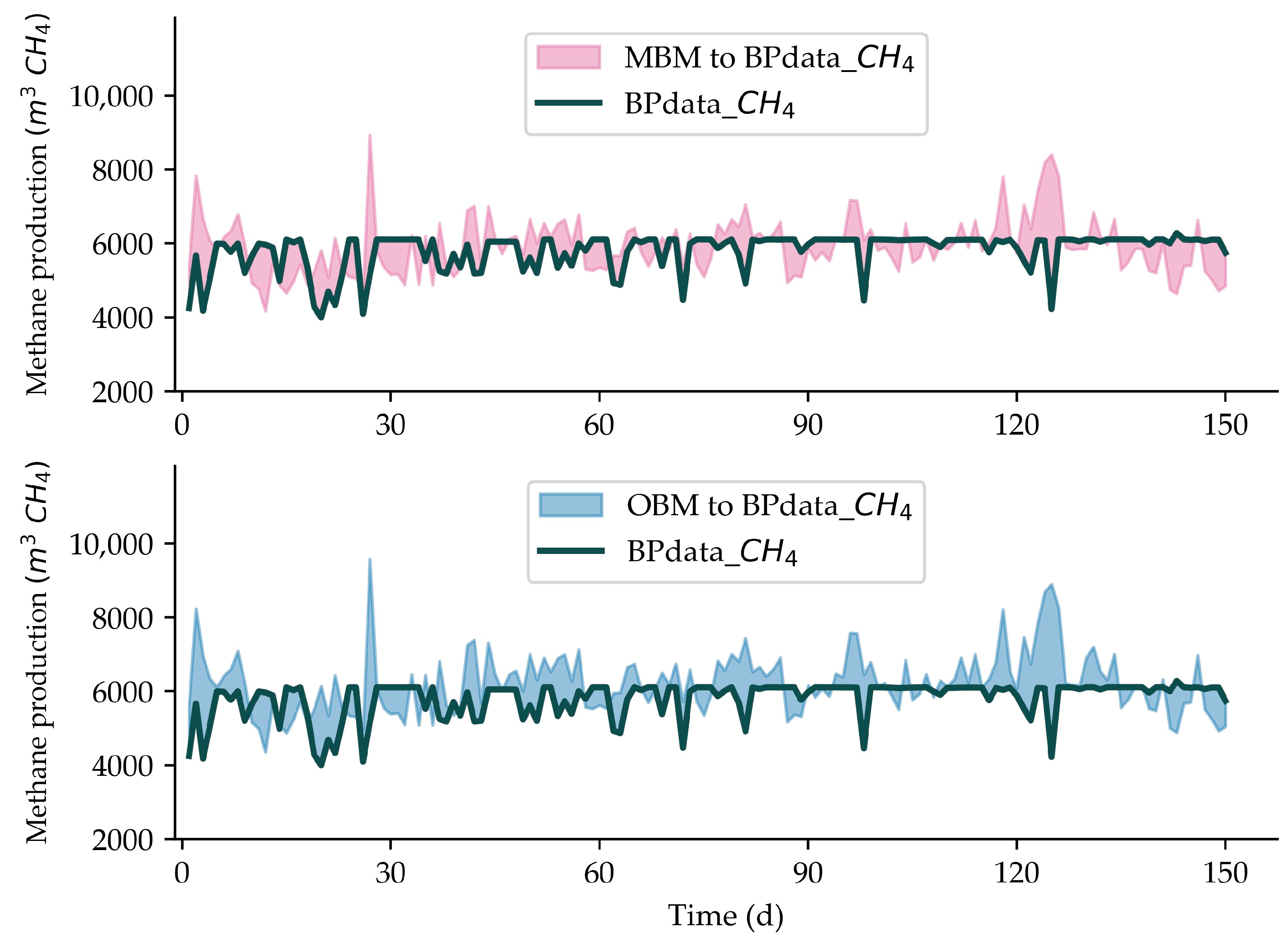
3.1. Model’s Validation
3.2. MBM & Developed T-Prediction Equation: A Combined Method as a Tool for the Prediction of Optimal Reactor Cleaning Period
3.2.1. Discovering the Function of the Increased Cost through the MBM
3.2.2. Applying the T-Prediction Equation
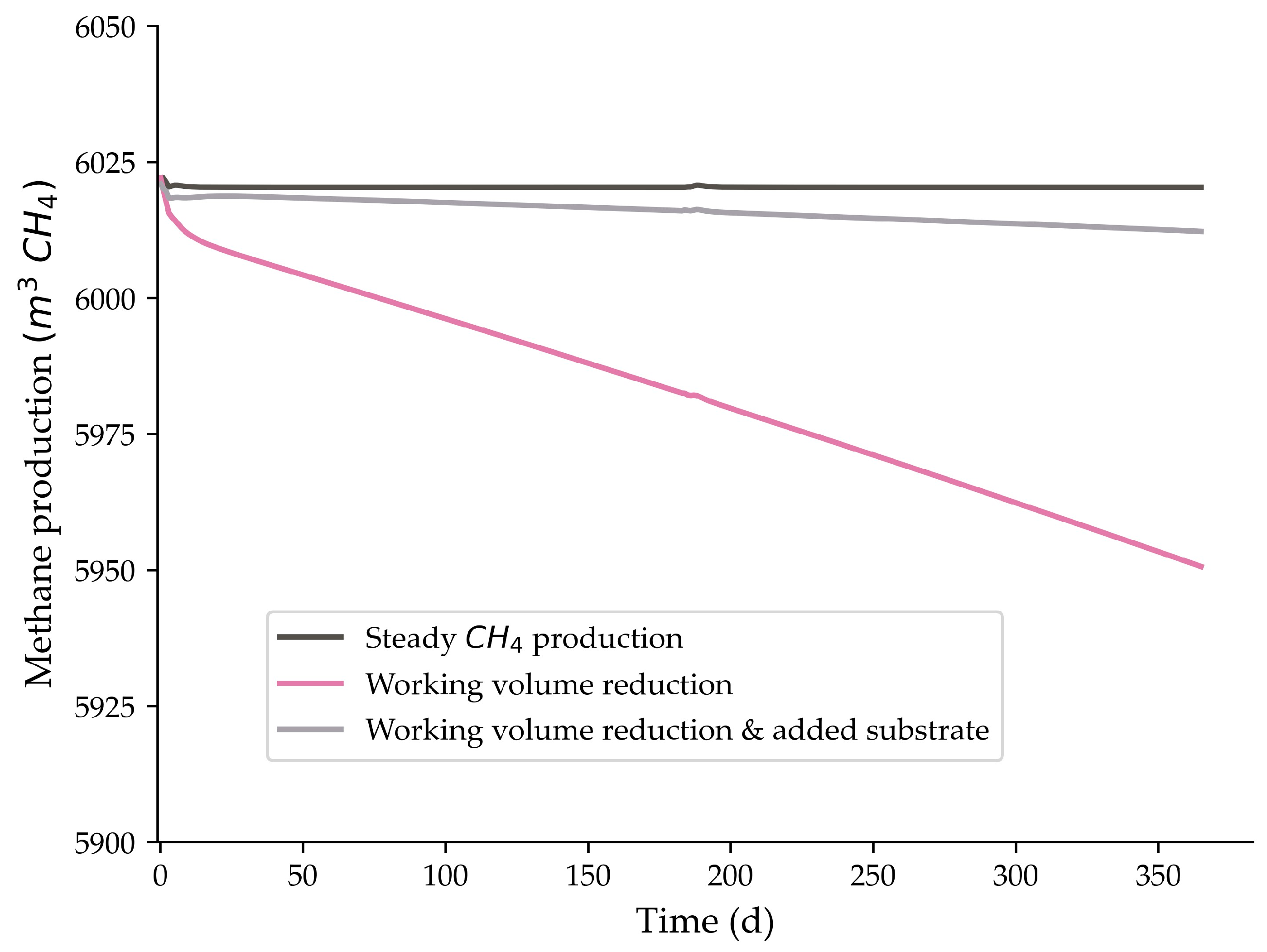
3.3. Significance of the Volume Reduction Coefficient (α)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| BioModel | Bioconversion Model | t | Time |
| GHG | Greenhouse Gas | qin | Inlet flow rate (m3 d−1) |
| AD | Anaerobic Digestion | qout | Outlet flow rate (m3 d−1) |
| CO2 | Carbon dioxide | CH4 production | Daily methane production (m3 d−1) |
| CH4 | Methane | DEP | Daily electricity production (kWh d−1) |
| H2S | Hydrogen sulfide | η | CHP’s electrical efficiency |
| H2 | Hydrogen | LCV | Lower Calorific Value of CH4 (kWh m−3) |
| O2 | Oxygen | PE | Percentage Error (%) |
| N2 | Nitrogen | Xsimulated | Model’s output |
| CHP | Combined Heat and Power engine | Xmeasured | Data from the biogas plant |
| ML | Machine Learning | P | Profit of the plant |
| ADM1 | Anaerobic Digestion Model No. 1 | R | Revenues of the plant |
| IWA | International Water Association | b | Fixed costs of the plant |
| COD | Chemical Oxygen Demand | n | Number of cleanings in time t |
| CSTR | Continuous Stirred Tank Reactor | K | Cost of cleaning the reactor & replacement of the membrane (if necessary) |
| OLR | Organic Loading Rate (kgVS m−3reactor d−1) | T | Cleaning period of the reactor |
| HRT | Hydraulic Retention Time (d) | f(t) | Cost increase function as the result of the additional amount of substrate to maintain CH4 production at desirable levels |
| SRT | Solids Retention Time (d) | Total increased cost over the period T | |
| ODE | Ordinary Differential Equations | BPdata | Data from the biogas plant |
| VFA | Volatile Fatty Acids (kg m−3) | MBM | Simulation with decreasing reactor working volume (Modified_BioModel) |
| LCFA | Long Chain Fatty Acids (kg m−3) | OBM | Simulation with constant reactor working volume (Original_BioModel) |
| VS | Volatile Solids (kg m−3) | BPdata_CH4 | CH4 production of the plant |
| d, h | Days, hours | kgVS | kilograms of Volatile Solids |
| V | Current working volume (m3) | a, b | Slope, y-intercept |
| V0 | Initial working volume (m3) | R2 | Coefficient of Determination |
| α | Volume reduction coefficient per unit time (m3 d−1) or as a percentage (%) of the reactor’s working volume per year | c | Acquisition cost of substrates |
References
- Bumharter, C.; Bolonio, D.; Amez, I.; Martínez, M.J.G.; Ortega, M.F. New opportunities for the European Biogas industry: A review on current installation development, production potentials and yield improvements for manure and agricultural waste mixtures. J. Clean. Prod. 2023, 388, 135867. [Google Scholar] [CrossRef]
- Kougias, P.G.; Angelidaki, I. Biogas and its opportunities—A review. Front. Environ. Sci. Eng. 2018, 12, 14. [Google Scholar] [CrossRef]
- Kapoor, R.; Ghosh, P.; Tyagi, B.; Vijay, V.K.; Vijay, V.; Thakur, I.S.; Kamyab, H.; Nguyen, D.D.; Kumar, A. Advances in biogas valorization and utilization systems: A comprehensive review. J. Clean. Prod. 2020, 273, 123052. [Google Scholar] [CrossRef]
- Nsair, A.; Cinar, S.O.; Alassali, A.; Abu Qdais, H.; Kuchta, K. Operational Parameters of Biogas Plants: A Review and Evaluation Study. Energies 2020, 13, 3761. [Google Scholar] [CrossRef]
- Li, D.; Lee, I.; Kim, H. Application of the linearized ADM1 (LADM) to lab-scale anaerobic digestion system. J. Environ. Chem. Eng. 2021, 9, 105193. [Google Scholar] [CrossRef]
- Kalamaras, S.D.; Vitoulis, G.; Christou, M.L.; Sfetsas, T.; Tziakas, S.; Fragos, V.; Samaras, P.; Kotsopoulos, T.A. The Effect of Ammonia Toxicity on Methane Production of a Full-Scale Biogas Plant—An Estimation Method. Energies 2021, 14, 5031. [Google Scholar] [CrossRef]
- Roopnarain, A.; Rama, H.; Ndaba, B.; Bello-Akinosho, M.; Bamuza-Pemu, E.; Adeleke, R. Unravelling the anaerobic digestion ‘black box’: Biotechnological approaches for process optimization. Renew. Sustain. Energy Rev. 2021, 152, 111717. [Google Scholar] [CrossRef]
- Wu, D.; Peng, X.; Li, L.; Yang, P.; Peng, Y.; Liu, H.; Wang, X. Commercial biogas plants: Review on operational parameters and guide for performance optimization. Fuel 2021, 303, 121282. [Google Scholar] [CrossRef]
- Nielsen, H.B.; Angelidaki, I. Codigestion of manure and industrial organic waste at centralized biogas plants: Process imbalances and limitations. Water Sci. Technol. 2008, 58, 1521–1528. [Google Scholar] [CrossRef]
- Eskicioglu, C.; Ghorbani, M. Effect of inoculum/substrate ratio on mesophilic anaerobic digestion of bioethanol plant whole stillage in batch mode. Process. Biochem. 2011, 46, 1682–1687. [Google Scholar] [CrossRef]
- Cheng, Q.; Huang, W.; Xu, C.; Fan, G.; Yan, J.; Chai, B.; Zhang, Y.; Zhang, Y.; Zhang, S.; Song, G. Challenges of anaerobic digestion in China. Int. J. Environ. Sci. Technol. 2021, 18, 3685–3696. [Google Scholar] [CrossRef]
- Fitamo, T.; Boldrin, A.; Dorini, G.; Boe, K.; Angelidaki, I.; Scheutz, C. Optimising the anaerobic co-digestion of urban organic waste using dynamic bioconversion mathematical modelling. Water Res. 2016, 106, 283–294. [Google Scholar] [CrossRef]
- Biernacki, P.; Steinigeweg, S.; Borchert, A.; Uhlenhut, F.; Brehm, A. Application of Anaerobic Digestion Model No. 1 for describing an existing biogas power plant. Biomass Bioenergy 2013, 59, 441–447. [Google Scholar] [CrossRef]
- Abilmazhinov, Y.; Shakerkhan, K.; Meshechkin, V.; Shayakhmetov, Y.; Nurgaliyev, N.; Suychinov, A. Mathematical Modeling for Evaluating the Sustainability of Biogas Generation through Anaerobic Digestion of Livestock Waste. Sustainability 2023, 15, 5707. [Google Scholar] [CrossRef]
- Kegl, T.; Kralj, A.K. An enhanced anaerobic digestion BioModel calibrated by parameters optimization based on measured biogas plant data. Fuel 2022, 312, 122984. [Google Scholar] [CrossRef]
- Fezzani, B.; Cheikh, R.B. Implementation of IWA anaerobic digestion model No. 1 (ADM1) for simulating the thermophilic anaerobic co-digestion of olive mill wastewater with olive mill solid waste in a semi-continuous tubular digester. Chem. Eng. J. 2008, 141, 75–88. [Google Scholar] [CrossRef]
- Weinrich, S.; Nelles, M. Basics of Anaerobic Digestion: Biochemical Conversion and Process Modelling; Deutsches Biomasseforschungszentrum Gemeinnützige GmbH: Leipzig, Germany, 2021. [Google Scholar]
- Enitan, A.M.; Adeyemo, J.; Swalaha, F.M.; Kumari, S.; Bux, F. Optimization of biogas generation using anaerobic digestion models and computational intelligence approaches. Rev. Chem. Eng. 2017, 33, 309–335. [Google Scholar] [CrossRef]
- Satpathy, P.; Biernacki, P.; Cypionka, H.; Steinigeweg, S. Modelling anaerobic digestion in an industrial biogas digester: Application of lactate-including ADM1 model (Part II). J. Environ. Sci. Health Part A 2016, 51, 1226–1232. [Google Scholar] [CrossRef]
- Ersahin, M.E. Modeling the dynamic performance of full-scale anaerobic primary sludge digester using Anaerobic Digestion Model No. 1 (ADM1). Bioprocess Biosyst. Eng. 2018, 41, 1539–1545. [Google Scholar] [CrossRef]
- Emebu, S.; Pecha, J.; Janáčová, D. Review on anaerobic digestion models: Model classification & elaboration of process phenomena. Renew. Sustain. Energy Rev. 2022, 160, 112288. [Google Scholar] [CrossRef]
- Walid, F.; El Fkihi, S.; Benbrahim, H.; Tagemouati, H. Modeling and Optimization of Anaerobic Digestion: A Review. E3S Web Conf. 2021, 229, 01022. [Google Scholar] [CrossRef]
- Paladino, O. Data Driven Modelling and Control Strategies to Improve Biogas Quality and Production from High Solids Anaerobic Digestion: A Mini Review. Sustainability 2022, 14, 16467. [Google Scholar] [CrossRef]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavlostathis, S.G.; Rozzi, A.; Sanders, W.T.M.; Siegrist, H.A.; Vavilin, V.A. The IWA Anaerobic Digestion Model No 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ellegaard, L.; Ahring, B.K. A mathematical model for dynamic simulation of anaerobic digestion of complex substrates: Focusing on ammonia inhibition. Biotechnol. Bioeng. 1993, 42, 159–166. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ellegaard, L.; Ahring, B.K. A comprehensive model of anaerobic bioconversion of complex substrates to biogas. Biotechnol. Bioeng. 1999, 63, 363–372. [Google Scholar] [CrossRef]
- Kovalovszki, A.; Alvarado-Morales, M.; Fotidis, I.A.; Angelidaki, I. A systematic methodology to extend the applicability of a bioconversion model for the simulation of various co-digestion scenarios. Bioresour. Technol. 2017, 235, 157–166. [Google Scholar] [CrossRef]
- Christou, M.; Vasileiadis, S.; Kalamaras, S.; Karpouzas, D.; Angelidaki, I.; Kotsopoulos, T. Ammonia-induced inhibition of manure-based continuous biomethanation process under different organic loading rates and associated microbial community dynamics. Bioresour. Technol. 2020, 320, 124323. [Google Scholar] [CrossRef]
- Donoso-Bravo, A.; Sadino-Riquelme, M.C.; Valdebenito-Rolack, E.; Paulet, D.; Gómez, D.; Hansen, F. Comprehensive ADM1 Extensions to Tackle Some Operational and Metabolic Aspects in Anaerobic Digestion. Microorganisms 2022, 10, 948. [Google Scholar] [CrossRef]
- Hartley, D.; Starr, S. Verification and Validation. In Estimating Impact: A Handbook of Computational Methods and Models for Anticipating Economic, Social, Political and Security Effects in International Interventions; Kott, A., Citrenbaum, G., Eds.; Springer US: Boston, MA, USA, 2010; pp. 311–336. [Google Scholar]
- Donoso-Bravo, A.; Mailier, J.; Martin, C.; Rodríguez, J.; Aceves-Lara, C.A.; Wouwer, A.V. Model selection, identification and validation in anaerobic digestion: A review. Water Res. 2011, 45, 5347–5364. [Google Scholar] [CrossRef]
- Yu, L.; Wensel, P.C.; Chen, J.M.A.S. Mathematical modeling in anaerobic digestion (AD). J. Bioremed. Biodeg. 2013, S4, 49–66. [Google Scholar] [CrossRef]
- Panaro, D.; Mattei, M.; Esposito, G.; Steyer, J.; Capone, F.; Frunzo, L. A modelling and simulation study of anaerobic digestion in plug-flow reactors. Commun. Nonlinear Sci. Numer. Simul. 2021, 105, 106062. [Google Scholar] [CrossRef]
- Wiedemann, L.; Conti, F.; Janus, T.; Sonnleitner, M.; Zörner, W.; Goldbrunner, M. Mixing in Biogas Digesters and Development of an Artificial Substrate for Laboratory-Scale Mixing Optimization. Chem. Eng. Technol. 2016, 40, 238–247. [Google Scholar] [CrossRef]
- Kaparaju, P.; Buendia, I.; Ellegaard, L.; Angelidakia, I. Effects of mixing on methane production during thermophilic anaerobic digestion of manure: Lab-scale and pilot-scale studies. Bioresour. Technol. 2008, 99, 4919–4928. [Google Scholar] [CrossRef]
- Hou, Y.; Xia, Q.; Yang, F.; Zhang, B.; Bian, Y.; Lei, Z.; Huang, W. Biogas circulation for improving the promotive effect of zero-valent iron on anaerobic digestion of swine manure. Bioresour. Technol. Rep. 2023, 21, 101319. [Google Scholar] [CrossRef]
- Azizi, S.M.M.; Zakaria, B.S.; Haffiez, N.; Niknejad, P.; Dhar, B.R. A critical review of prospects and operational challenges of microaeration and iron dosing for in-situ biogas desulfurization. Bioresour. Technol. Rep. 2022, 20, 101265. [Google Scholar] [CrossRef]
- Kegl, T. Consideration of biological and inorganic additives in upgraded anaerobic digestion BioModel. Bioresour. Technol. 2022, 355, 127252. [Google Scholar] [CrossRef]
- Flores-Alsina, X.; Solon, K.; Mbamba, C.K.; Tait, S.; Gernaey, K.V.; Jeppsson, U.; Batstone, D.J. Modelling phosphorus (P), sulfur (S) and iron (Fe) interactions for dynamic simulations of anaerobic digestion processes. Water Res. 2016, 95, 370–382. [Google Scholar] [CrossRef]
- Momoh, O.L.Y.; Anyata, B.U.; Saroj, D.P. Development of simplified anaerobic digestion models (SADM’s) for studying anaerobic biodegradability and kinetics of complex biomass. Biochem. Eng. J. 2013, 79, 84–93. [Google Scholar] [CrossRef]
- Lovato, G.; Alvarado-Morales, M.; Kovalovszki, A.; Peprah, M.; Kougias, P.G.; Rodrigues, J.A.D.; Angelidaki, I. In-situ biogas upgrading process: Modeling and simulations aspects. Bioresour. Technol. 2017, 245, 332–341. [Google Scholar] [CrossRef]
- Lovato, G.; Kovalovszki, A.; Alvarado-Morales, M.; Jéglot, A.T.A.; Rodrigues, J.A.D.; Angelidaki, I. Modelling bioaugmentation: Engineering intervention in anaerobic digestion. Renew. Energy 2021, 175, 1080–1087. [Google Scholar] [CrossRef]
- Tsapekos, P.; Alvarado-Morales, M.; Kougias, P.G.; Konstantopoulos, K.; Angelidaki, I. Co-digestion of municipal waste biopulp with marine macroalgae focusing on sodium inhibition. Energy Convers. Manag. 2019, 180, 931–937. [Google Scholar] [CrossRef]
- Tsapekos, P.; Kougias, P.; Alvarado-Morales, M.; Kovalovszki, A.; Corbière, M.; Angelidaki, I. Energy recovery from wastewater microalgae through anaerobic digestion process: Methane potential, continuous reactor operation and modelling aspects. Biochem. Eng. J. 2018, 139, 1–7. [Google Scholar] [CrossRef]
- Razaviarani, V.; Buchanan, I.D. Calibration of the Anaerobic Digestion Model No. 1 (ADM1) for steady-state anaerobic co-digestion of municipal wastewater sludge with restaurant grease trap waste. Chem. Eng. J. 2015, 266, 91–99. [Google Scholar] [CrossRef]
- Blumensaat, F.; Keller, J. Modelling of two-stage anaerobic digestion using the IWA Anaerobic Digestion Model No. 1 (ADM1). Water Res. 2005, 39, 171–183. [Google Scholar] [CrossRef]
- Nabaterega, R.; Nazyab, B.; Eskicioglu, C. Modification and calibration of anaerobic digestion model 1 to simulate volatile fatty acids production during fermentation of municipal sludge. Biochem. Eng. J. 2023, 194, 108886. [Google Scholar] [CrossRef]
- Sun, H.; Yang, Z.; Shi, G.; Arhin, S.G.; Papadakis, V.G.; Goula, M.A.; Zhou, L.; Zhang, Y.; Liu, G.; Wang, W. Methane production from acetate, formate and H2/CO2 under high ammonia level: Modified ADM1 simulation and microbial characterization. Sci. Total Environ. 2021, 783, 147581. [Google Scholar] [CrossRef]
- Bond, T.; Templeton, M.R. History and future of domestic biogas plants in the developing world. Energy Sustain. Dev. 2011, 15, 347–354. [Google Scholar] [CrossRef]
- Plugge, C.M. Biogas. Microb. Biotechnol. 2017, 10, 1128–1130. [Google Scholar] [CrossRef]
- Rafiee, A.; Khalilpour, K.R.; Prest, J.; Skryabin, I. Biogas as an energy vector. Biomass Bioenergy 2020, 144, 105935. [Google Scholar] [CrossRef]
- Wu, Y.; Kovalovszki, A.; Pan, J.; Lin, C.; Liu, H.; Duan, N.; Angelidaki, I. Early warning indicators for mesophilic anaerobic digestion of corn stalk: A combined experimental and simulation approach. Biotechnol. Biofuels 2019, 12, 106. [Google Scholar] [CrossRef]
- Gaspari, M.; Alvarado-Morales, M.; Tsapekos, P.; Angelidaki, I.; Kougias, P. Simulating the performance of biogas reactors co-digesting ammonia and/or fatty acid rich substrates. Biochem. Eng. J. 2023, 190, 108741. [Google Scholar] [CrossRef]
- Lindmark, J.; Thorin, E.; Bel Fdhila, R.; Dahlquist, E. Problems and Possibilities with the Implementation of Simulation and Modeling at a Biogas Plant. In Proceedings of the International Conference on Applied Energy ICAE 2012, Suzhou, China, 5–8 July 2012. [Google Scholar]


| Feedstock Characteristics | Units | Cow Manure | Chicken Manure | Corn Silage | Cow Manure (Solid) | Pig Manure | Cheese Whey | Glycerin | Olive Mill Wastewater | Sugar Beet | Soap Paste | Food Waste |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average daily feeding | tons d−1 | 101.94 (±35.04) | 25.00 (±5.99) | 5.91 (±2.48) | 9.13 (±4.99) | 23.53 (±9.58) | 42.33 (±18.96) | 2.41 (±0.91) | 19.78 (±1.84) | 13.07 (±4.87) | 20.65 (±0.95) | 16.84 (±13.72) |
| Volatile solids (VS) | kg m−3 | 125.31 | 72.02 | 395.31 | 1019.94 | 39.60 | 27.02 | 627.24 | 159.12 | 164.48 | 59.68 | 196.09 |
| Insoluble carbohydrates | kg m−3 | 21.80 | 14.70 | 72.20 | 56.8 | 5.00 | 3.00 | 3.00 | 94.80 | 9.80 | 3.00 | 5.80 |
| Inert carbohydrates | kg m−3 | 41.55 | 0.00 | 0.00 | 359.97 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Soluble carbohydrates (Glucose) | kg m−3 | 38.35 | 12.12 | 270.31 | 332.28 | 5.40 | 11.12 | 609.64 | 22.02 | 137.08 | 0.08 | 169.89 |
| Insoluble proteins | kg m−3 | 21.60 | 37.40 | 41.70 | 256.20 | 25.20 | 12.10 | 5.30 | 16.00 | 16.40 | 8.00 | 19.40 |
| Inert proteins | kg m−3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Soluble proteins (Amino acids) | kg m−3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Soluble lipids (GTO) | kg m−3 | 2.00 | 7.80 | 11.10 | 14.00 | 4.00 | 0.8 | 9.30 | 26.30 | 1.20 | 48.60 | 1.00 |
| Long-chain fatty acids (LCFA) | kg m−3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Volatile fatty acids (VFA) | kg m−3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Total ammonium nitrogen | kg m−3 | 0.70 | 1.84 | 0.08 | 7.25 | 3.20 | 0.07 | 0.00 | 0.00 | 0.00 | 0.06 | 0.31 |
| Dissolved CH4 | kg m−3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Total inorganic carbon (CO2) | kg m−3 | 2.88 | 2.88 | 2.88 | 2.88 | 2.88 | 2.88 | 2.88 | 2.88 | 2.88 | 2.88 | 2.88 |
| Total H2S | kg m−3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| H2 | kg m−3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cations | kg m−3 | 6.75 | 6.75 | 6.75 | 6.75 | 6.75 | 6.75 | 6.75 | 6.75 | 6.75 | 6.75 | 6.75 |
| Total phosphoric acid | kg m−3 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
| Anions | kg m−3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Period | Duration | Average CH4 Production (m3) | PE (%) | Goodness of Fit | ||||
|---|---|---|---|---|---|---|---|---|
| MBM | BPdata | OBM | MBM to BPdata | OBM to BPdata | MBM to BPdata | OBM to BPdata | ||
| 1 | 30 d | 5630.10 | 5411.45 | 5899.57 | 4.04 | 9.02 | 0.96 | 0.90 |
| 2 | 30 d | 5932.93 | 5757.57 | 6226.52 | 3.05 | 8.14 | 0.97 | 0.91 |
| 3 | 30 d | 5923.13 | 5847.77 | 6236.80 | 1.29 | 6.65 | 0.98 | 0.93 |
| 4 | 30 d | 6086.63 | 6013.02 | 6419.60 | 1.22 | 6.76 | 0.98 | 0.93 |
| 5 | 30 d | 5999.49 | 5971.68 | 6344.19 | 0.47 | 6.24 | 0.99 | 0.93 |
| α (% of the Working Volume per Year) | CH4 % Difference over a Year | T (Years) |
|---|---|---|
| 4.7% | 0.59% | 7.3 |
| 9.1% * | 1.17% * | 5.1 * |
| 9.5% | 1.23% | 5.0 |
| 14.1% | 1.93% | 4.0 |
| 18.9% | 2.72% | 3.4 |
| 23.8% | 3.65% | 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsitsimpikou, M.-A.; Kalamaras, S.D.; Lithourgidis, A.A.; Mitsopoulos, A.; Ellegaard, L.; Angelidaki, I.; Kotsopoulos, T.A. Simulation of the Working Volume Reduction through the Bioconversion Model (BioModel) and Its Validation Using Biogas Plant Data for the Prediction of the Optimal Reactor Cleaning Period. Sustainability 2023, 15, 16157. https://doi.org/10.3390/su152316157
Tsitsimpikou M-A, Kalamaras SD, Lithourgidis AA, Mitsopoulos A, Ellegaard L, Angelidaki I, Kotsopoulos TA. Simulation of the Working Volume Reduction through the Bioconversion Model (BioModel) and Its Validation Using Biogas Plant Data for the Prediction of the Optimal Reactor Cleaning Period. Sustainability. 2023; 15(23):16157. https://doi.org/10.3390/su152316157
Chicago/Turabian StyleTsitsimpikou, Maria-Athina, Sotirios D. Kalamaras, Antonios A. Lithourgidis, Anastasios Mitsopoulos, Lars Ellegaard, Irini Angelidaki, and Thomas A. Kotsopoulos. 2023. "Simulation of the Working Volume Reduction through the Bioconversion Model (BioModel) and Its Validation Using Biogas Plant Data for the Prediction of the Optimal Reactor Cleaning Period" Sustainability 15, no. 23: 16157. https://doi.org/10.3390/su152316157







