Influence of Cutting Intervals and Transition Periods on Chemical Composition Variability of Selected Tropical Grasses under Flooded Savanna Conditions of Arauca, Colombian Orinoquia
Abstract
1. Introduction
2. Materials and Methods
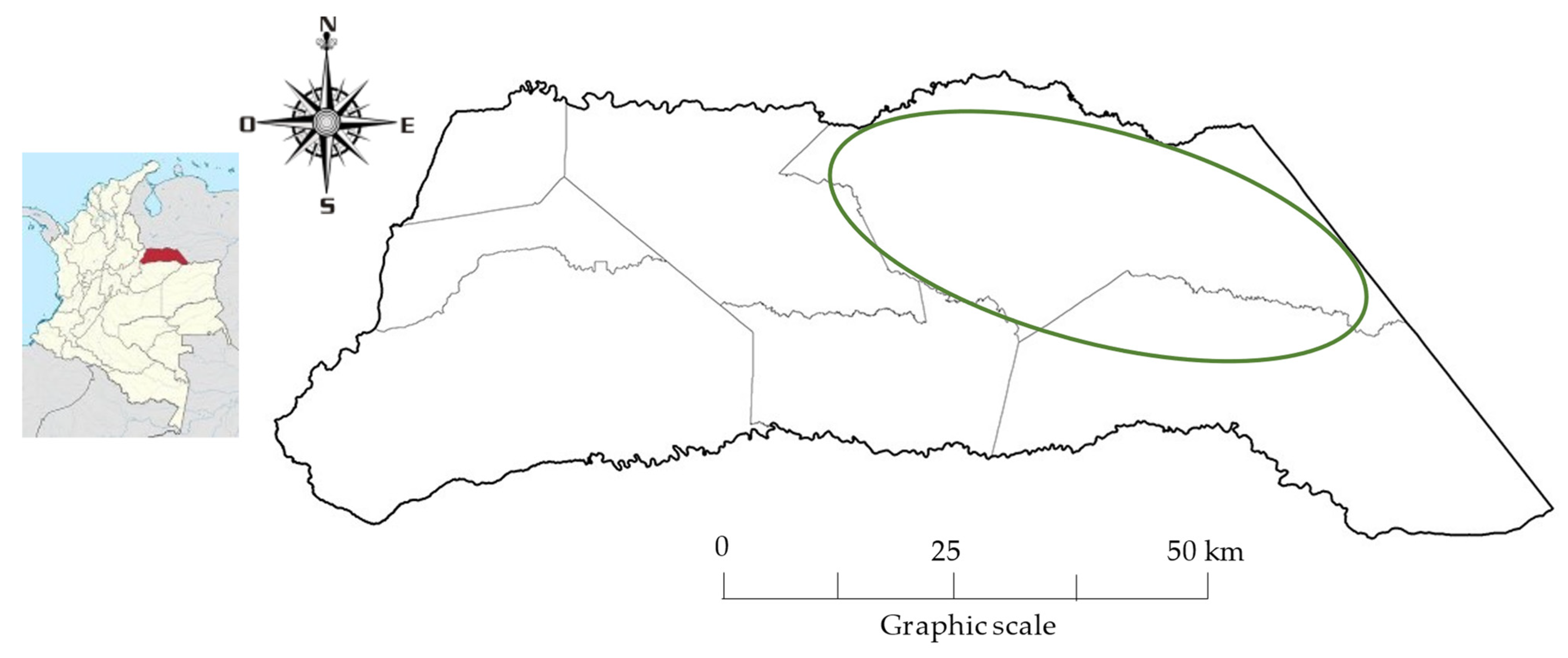
2.1. Study Region
2.2. Evaluated Grass Species
2.3. Forage Sampling Process
2.4. Statistical Analysis
3. Results
Influence of Transition Periods and Cutting Intervals on Chemical Composition Variables
4. Discussion
4.1. Grasses’ Productive and Chemical Composition Characteristics
4.2. Chemical Composition Tendencies among “Bank” and “Low” Physiographic Position Grasses
4.3. Influence of Transition Periods on Grasses’ Chemical Compositions
4.4. Influence of Cutting Intervals on Grasses’ Chemical Compositions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moscovici Joubran, A.; Pierce, K.M.; Garvey, N.; Shalloo, L.; O’Callaghan, T.F. Invited review: A 2020 perspective on pasture-based dairy systems and products. J. Dairy Sci. 2021, 104, 7364–7382. [Google Scholar] [CrossRef]
- Wróbel, B.; Zielewicz, W.; Staniak, M. Challenges of Pasture Feeding Systems—Opportunities and Constraints. Agriculture 2023, 13, 974. [Google Scholar] [CrossRef]
- Boval, M.; Dixon, R.M. The importance of grasslands for animal production and other functions: A review on management and methodological progress in the tropics. Animal 2012, 6, 748–762. [Google Scholar] [CrossRef]
- Paul, K.P.; Koge, J.; Maass, B.L.; Notenbaert, A.; Peters, M.; Groot, J.C.J.; Tittonell, P. Tropical forage technologies can deliver multiple benefits in Sub-Saharan Africa. A meta-analysis. Agron. Sustain. Devel. 2020, 40, 22. [Google Scholar] [CrossRef]
- Oosting, S.; van der Lee, J.; Verdegem, M.; De Vries, M.; Vernooij, A.; Bonilla-Cedrez, C.; Kabir, K. Farmed animal production in tropical circular food systems. Food Secur. 2022, 14, 273–292. [Google Scholar] [CrossRef]
- Peñuela, L.; Fernández, A.P.; Castro, F.; Ocampo, A. Uso y Manejo de Forrajes Nativos en la Sabana Inundable de la Orinoquia; Convenio de Cooperación Interinstitucional; The Nature Conservancy; Fundación Horizonte Verde; Fundación Biodiversidad de España & Corporación Autónoma Regional de la Orinoquia; Universidad de los Llanos: Villavicencio, Colombia, 2011. [Google Scholar]
- ICA. Instituto Colombiano Agropecuario. Censos Pecuarios Nacional. 2023. Available online: https://www.ica.gov.co/areas/pecuaria/servicios/epidemiologia-veterinaria/censos-2016/censo-2018 (accessed on 13 September 2023).
- Mora-Fernández, C.; Peñuela-Recio, L.; Castro-Lima, F. Estado del conocimiento de los ecosistemas de las sabanas inundables en la Orinoquia Colombiana. Rev. Orinoquia 2015, 19, 253–271. [Google Scholar] [CrossRef]
- Sousa Feitosa, O.; Costa Leite, R.; Alexandrino, E.; Saboia Pires, T.J.; Taverny de Oliveira, L.B.; Paula Neto, J.J.; Dos Santos, A.C. Forage performance and cattle production as a function of the seasonality of a Brazilian tropical region. Acta Scient. Anim. Sci. 2022, 44, e53779. [Google Scholar] [CrossRef]
- Salamanca-Carreño, A.; Vélez-Terranova, M.; Vargas-Corzo, O.M.; Pérez-López, O.; Castillo-Pérez, A.F.; Parés-Casanova, P.M. Relationship of Physiographic Position to Physicochemical Characteristics of Soils of the Flooded-Savannah Agroecosystem, Colombia. Agriculture 2023, 13, 220. [Google Scholar] [CrossRef]
- Salamanca-Carreño, A.; Vélez-Terranova, M.; Vargas-Corzo, O.M.; Parés-Casanova, P.M.; Bentez-Molano, J. Productive and Nutritional Characteristics of Native Grasses from the Floodplain Banks Ecosystem in the Colombian Orinoquia. Sustainability 2022, 14, 15151. [Google Scholar] [CrossRef]
- Vélez-Terranova, M.; Salamanca-Carreño, A.; Vargas-Corzo, O.M.; Parés-Casanova, P.M.; Arias-Landazábal, J.N. Chemical Composition and In Vitro Ruminal Fermentation Characteristics of Native Grasses from the Floodplain Lowlands Ecosystem in the Colombian Orinoquia. Animals 2023, 13, 2760. [Google Scholar] [CrossRef]
- Pérez Bona, R.A.; Vargas Corzo, O.M. Características de la Sabana Nativa y su Potencial de Producción Bovina en la Llanura Inundable de Arauca; Boleín Técnico N° 25; Programa Regional de Investigación Pecuaria, Corpoica: Arauca, Arauca, 2001. [Google Scholar]
- USDA. United States Department of Agriculture. Oxisols. In Soil Taxonomy. A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; Soil Survey Staff: Washington, DC, USA, 1999; Chapter 17; pp. 655–695. [Google Scholar]
- Rangel-Ch, J.O.; Celis, V. Suelos del territorio sabanas y humedales de Arauca, Colombia. In Col. Div. Biótica XX: Suelos Sabanas y Humedales de Arauca; Instituto de Ciencias Naturales, Universidad Nacional de Colombia: Bogotá, Colombia, 2019; pp. 171–197. [Google Scholar]
- Holdridge, L.R. Ecología Basada en Zonas de Vida; IICA Biblioteca Venezuela: San Jose de Costa Rica, Costa Rica, 1987; p. 216. [Google Scholar]
- Schweizer Lassaga, S. Muestreo y Análisis de Suelos Para Diagnóstico de Fertilidad; Instituto Nacional de Innovación y Transferencia en Tecnología Agropecuaria: San José, Costa Rica, 2011. [Google Scholar]
- UN. Universidad Nacional de Colombia, Orinoquía. Guía para el Usuario de los Servicios del Laboratorio de Suelos, Aguas y Foliares; Arauca, Colombia. 2021. Available online: https://bit.ly/3HiscdG (accessed on 14 September 2021).
- ICONTEC. Instituto Colombiano de Normas Técnicas y Certificación. Sistema Nacional de la Calidad. 2021. Available online: https://bit.ly/3P8u8ax (accessed on 14 September 2021).
- Cerdas, R. Programa de fertilización de forrajes. Desarrollo de un módulo práctico para técnicos y estudiantes de ganadería de Guanacaste, Costa Rica. InterSedes 2011, 12, 109–128. [Google Scholar]
- Ariza-Nieto, C.M.O.L.; Mojica, B.; Parra, D.; Afanador-Tellez, G. Use of LOCAL algorithm with near infrared spectroscopy in forage resources for grazing systems in Colombia. J. Near Infrared Spectr. 2018, 26, 44–52. [Google Scholar] [CrossRef]
- InfoStat. Software Estadistico, Versión 30/04/2020; Grupo InfoStat, FCA. Universidad Nacional de Córdoba: Córdoba, Argentina, 2020.
- Ocampo, A.; Peñuela, L. Manejo y nutrición en sabana inundable como eje de la producción y reproducción de la ganadería de cría. In Fortalecimiento Institucional y de Política Para Incrementar la Conservación de la Biodiversidad en Predios Privados en Colombia; Red Colombiana de Reservas Naturales de la Sociedad; Fundación Natura; World Wildlife Fund; The Nature Conservancy; Parques Nacionales Naturales de Colombia: Bogotá, Colombia, 2014. [Google Scholar]
- Reyes-Pérez, J.J.; Méndez-Martínez, Y.; Verdecia, D.M.; Luna-Murillo, R.A.; Hernández Montiel, L.G.; Herrera, R.S. Components of the yield and bromatological composition of three Brachiaria varieties in El Empalme area, Ecuador. Cuban J. Agricult. Sc. 2018, 52, 435–445. [Google Scholar]
- Meale, S.J.; Chaves, A.V.; Baah, J.; McAllister, T.A. Methane Production of Different Forages in In vitro Ruminal Fermentation. Asian-Aust. J. Anim. Sci. 2012, 25, 86–91. [Google Scholar] [CrossRef]
- Méndez-Martínez, Y.; Verdecia, D.M.; Reyes-Pérez, J.J.; Luna-Murillo, R.A.; Rivero-Herrada, M.; Montenegro-Vivas, L.B.; Herrera, R.S. Quality of three Megathyrsus maximus cultivars in the Empalme area, Ecuador. Cuban J. Agricult. Sci. 2018, 52, 423–433. [Google Scholar]
- Gaviria-Uribe, X.; Bolívar-Vergara, D.M.; Chirinda, N.; Molina-Botero, I.C.; Mazabel, J.; Barahona-Rosales, R.; Arango, J. In vitro methane production and ruminal fermentation parameters of tropical grasses and grass-legume associations commonly used for cattle feeding in the tropics. Livest. Res. Rural Devel. 2022, 34, 1–17. [Google Scholar]
- Cruz-Hernández, A.; Hernández-Garay, A.; Aranda-Ibañez, E.; Chay-Canul, A.; Márquez-Quiroz, C.; Rojas-Garcia, A.L.; Gómez-Vázquez, A. Nutritive value of Mulato grass under dierent grazing strategies. Esosist. Recur. Agropec. 2017, 4, 65–72. [Google Scholar] [CrossRef][Green Version]
- Cuadrado, H.; Torregroza, L.; Garcés, J. Producción de Carne con Machos de Ceba en Pastoreo de Pasto Híbrido Mulato y Brachiaria decumbens en el Valle del Sinú. MVZ-Córdoba 2005, 10, 573–580. [Google Scholar]
- Cruz Hernández, A.; Hernández Garay, A.; Chay Canul, A.J.; Mendoza Pedroza, S.I.; Ramírez Vera, S.; Rojas García, A.R.; Ventura Ríos, J. Components of the yield and nutritional value of Brachiaria humidicola cv Chetumal to different grazing strategies. Rev. Mex. Cienc. Agríc. 2017, 8, 599–610. [Google Scholar]
- Van Soest, P.J. Development of comprehensive system of feed analysis and its application to forages. J. Anim. Sc. 1967, 26, 119–128. [Google Scholar] [CrossRef]
- Leng, R.A. Evaluation of Tropical Feed Resources for Ruminant Livestock; Tropical Feeds and Feeding Systems; FAO: Rome, Italy, 1995; Available online: https://www.fao.org/ag/aga/agap/frg/econf95/pdf/evalu.pdf (accessed on 20 September 2022).
- Lee, M.A. A global comparison of the nutritive values of forage plants grown in contrasting environments. J. Plant Res. 2018, 131, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Mlay, P.S.; Pereka, A.; Phiri, E.; Balthazary, S.; Igusti, J.; Hvelplund, T.; Weisbjerg, M.R.; Madsen, J. Feed value of selected tropical grasses, legumes and concentrates. Veterinarski Arhiv 2006, 76, 53–63. [Google Scholar]
- Jayanegara, A.; Ridla, M.; Nahrowi; Laconi, E.B. Estimation and validation of total digestible nutrient values of forage and concentrate feedstuffs. IOP Conf. Series: Mater. Sci. Engin. 2019, 546, 1–5. [Google Scholar] [CrossRef]
- Horne da Cruz, C.; Alvarenga Santos, S.; Pinto de Carvalho, G.G.; Gomes Azevedo, J.A.; Detmann, E.; Valadares Filho, S.C.; Silva Mariz, L.D.; Sales Pereira, E.; Carvalho Nicory, I.M.; Libânio Tosto, M.S.; et al. Estimating digestible nutrients in diets for small rumiants fed with tropical forages. Livest. Sci. 2021, 249, 104532. [Google Scholar] [CrossRef]
- Harper, K.J.; McNeill, D.M. The Role iNDF in the Regulation of Feed Intake and the Importance of Its Assessment in Subtropical Ruminant Systems (the Role of iNDF in the Regulation of Forage Intake). Agriculture 2015, 5, 778–790. [Google Scholar] [CrossRef]
- Jayasinghe, P.; Ramilan, T.; Donaghy, D.J.; Pembleton, K.G.; Barber, D.G. Comparison of Nutritive Values of Tropical Pasture Species Grown in Different Environments, and Implications for Livestock Methane Production: A Meta-Analysis. Animals 2022, 12, 1806. [Google Scholar] [CrossRef]
- Bogale, S.; Melaku, S.; Yami, A. Influence of rainfall pattern on grass/legume composition and nutritive value of natural pasture in Bale Highlands of Ethiopia. Livest. Res. Rural Develop 2008, 20. Available online: http://www.lrrd.org/lrrd20/3/boga20038.htm (accessed on 20 September 2022).
- Gherardia, L.A.; Sala, O.E. Enhanced precipitation variability decreases grass- and increases shrub-productivity. Proc. Nat. Acad. Sci. USA 2015, 112, 12735–12740. [Google Scholar] [CrossRef]
- Garay, J.R.; Joaquin-Cancino, S.; Zárate-Fortuna, P.; Ibarra-Hinojosa, M.A.; Martínez-González, J.C.; González-Dávila, R.P.; Cienfuegos-Rivas, E.G. Dry matter accumulation and crude protein concentration in Brachiaria spp. cultivars in the humid tropics of Ecuador. Trop. Grasslands-Forrajes Tropicales 2017, 5, 66–76. [Google Scholar] [CrossRef]
- Ramos-Montaño, C.; García-Conde, M.R. Características Ecosistémicas asociadas a la actividad ganadera en Arauca (Colombia): Desafíos frente al cambio climático. Orinoquia 2016, 20, 28–38. [Google Scholar] [CrossRef]
- Melo, C.D.; Maduro Dias, C.S.A.M.; Wallon, S.; Borba, A.E.S.; Madruga, J.; Borges, P.A.V.; Ferreira, M.T.; Elias, R.B. Influence of Climate Variability and Soil Fertility on the Forage Quality and Productivity in Azorean Pastures. Agriculture 2022, 12, 358. [Google Scholar] [CrossRef]
- Fonseca-Pereira, G.; Emerenciano-Neto, J.V.; dos Santos-Difante, G.; Cortes-Assis, L.C.S.L.; de Oliveira- Lima, P. Morphogenic and structural characteristics of tropical forage grasses managed under different regrowth periods in the Brazilian semi-arid region. Semina: Ciênc. Agrá. 2019, 40, 283–292. [Google Scholar]
- Mwendia, S.W.; Ohmstedt, U.; Nyakundi, F.; Notenbaert, A.; Peters, P. Does harvesting Urochloa and Megathyrsus forages at short intervals confer an advantage on cumulative dry matter yields and quality? J. Sci. Food Agric. 2022, 102, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Suttle, N.F. Mineral Nutrition of Livestock, 4th Edition. 2010. Available online: http://www.ucv.ve/fileadmin/user_upload/facultad_agronomia/Producion_Animal/Minerals_in_Animal_Nutrition.pdf (accessed on 3 September 2023).
- Liu, J.; Duan, C.; Zhang, X.; Zhu, Y.; Lu, X. Potential of Leersia hexandra Swartz for phytoextraction of Cr from soil. J. Hazard. Mater. 2011, 188, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, L.; Manoni, M.; Ferrari, L.; Tretola, M.; Cazzola, R.; Givens, I. The Contribution of Dietary Magnesium in Farm Animals and Human Nutrition. Nutrients 2021, 13, 509. [Google Scholar] [CrossRef]


| Grass (Scientific Name) | Vulgar Name | Growth Habit | Origin |
|---|---|---|---|
| “Bank” position | |||
| Paspalum plicatulum Michx. (1803) | Black “bank” grass | Bunch | Native |
| Axonopus compressus (Sw) P. Beauv. (1812) | Gegei grass | Stoloniferous | Native |
| Panicum versicolor (EP Bincknell) Nieuwl. | White “bank” grass | Bunch | Native |
| Paspalum sp. (L.) L. (1762) | Native grama | Rhizomatous | Native |
| Urochloa hybrid cv Mulato I | Mulatto grass | Bunch | Introduced |
| “Low” position | |||
| Leersia hexandra Sw. (1788) | Lambedora grass | Bunch | Native |
| Acroceras zizanioides (Kunth) Dandy (1931) | Black water straw | Stoloniferous | Native |
| Hymenachne amplexicaulis (Rudge) Nees (1829) | Water straw | Stoloniferous | Native |
| Urochloa humidicola (Rendle) Morrone & Zuloaga | Humidicola grass | Stoloniferous | Introduced |
| “Bank” Physiographic Position | |||||
|---|---|---|---|---|---|
| Nutritional Variable | P. plicatulum | A. compressus | P. versicolor | Paspalum sp. | Mulato |
| DMY/ha (kg) | 1967.61 ± 880.94 | 1053.44 ± 272.01 | 1215.28 ± 515.35 | 1198.22 ± 513.28 | 1644.28 ± 646.72 |
| DM (%) | 27.21 ± 4.34 | 33.72 ± 3.17 | 28.19 ± 5.14 | 33.81 ± 4.76 | 23.88 ± 2.84 |
| CP (%) | 8.88 ± 1.88 | 8.96 ± 0.98 | 9.54 ± 1.23 | 7.73 ± 1.18 | 8.09 ± 0.98 |
| Ash (%) | 10.36 ± 1.24 | 9.95 ± 0.41 | 11.02 ± 0.8 | 10.76 ± 0.64 | 9.9 ± 0.86 |
| EE (%) | 1.35 ± 0.24 | 1.47 ± 0.19 | 1.87 ± 0.35 | 1.45 ± 0.2 | 1.74 ± 0.23 |
| NDF (%) | 68.21 ± 2.97 | 68.08 ± 1.88 | 65.22 ± 3.91 | 71.41 ± 1.44 | 68.42 ± 2.17 |
| ADF (%) | 35.57 ± 3.44 | 33.95 ± 1.82 | 35.21 ± 3.68 | 38.14 ± 1.73 | 34.69 ± 2.34 |
| Lignin (%) | 8.42 ± 0.66 | 8.47 ± 0.42 | 8.06 ± 0.87 | 8.78 ± 0.42 | 8.55 ± 0.63 |
| HC (%) | 32.64 ± 2.64 | 34.13 ± 0.45 | 30.01 ± 1.69 | 33.27 ± 1.6 | 33.73 ± 2.72 |
| TDN (%) | 50.51 ± 2.28 | 51.04 ± 1.19 | 51.1 ± 1.81 | 48.91 ± 1.2 | 50.18 ± 1.22 |
| DMD (%) | 55.39 ± 2.47 | 55.97 ± 1.28 | 56.04 ± 1.96 | 53.67 ± 1.29 | 55.04 ± 1.32 |
| ME (Mcal/kg) | 1.78 ± 0.1 | 1.8 ± 0.05 | 1.81 ± 0.08 | 1.71 ± 0.05 | 1.76 ± 0.05 |
| Ca2+ (%) | 0.52 ± 0.09 | 0.31 ± 0.07 | 0.41 ± 0.08 | 0.42 ± 0.07 | 0.53 ± 0.13 |
| P (%) | 0.18 ± 0.02 | 0.17 ± 0.02 | 0.21 ± 0.03 | 0.2 ± 0.02 | 0.2 ± 0.03 |
| Mg2+ (%) | 0.23 ± 0.02 | 0.19 ± 0.01 | 0.24 ± 0.05 | 0.19 ± 0.02 | 0.28 ± 0.03 |
| K (%) | 1.93 ± 0.49 | 1.26 ± 0.16 | 1.97 ± 0.24 | 2.02 ± 0.59 | 1.77 ± 0.45 |
| Na (%) | 0.03 ± 0.01 | 0.03 ± 0 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.0043 |
| S (%) | 0.25 ± 0.05 | 0.22 ± 0.03 | 0.25 ± 0.04 | 0.21 ± 0.05 | 0.22 ± 0.05 |
| “Low” physiographic position | |||||
| Nutritional variable | L. hexandra | A. zizanioides | H. amplexicaulis | U. humidicola | |
| DMY/ha (kg) | 1142.39 ± 342.06 | 1030.44 ± 217.82 | 840.56 ± 283.04 | 1957.83 ± 749.12 | |
| DM (%) | 38.09 ± 8.19 | 29.92 ± 8.26 | 27.37 ± 8.7 | 27.27 ± 3.75 | |
| CP (%) | 14.13 ± 2.84 | 11.33 ± 2.69 | 11.22 ± 2.4 | 6.68 ± 1.43 | |
| Ash (%) | 14.76 ± 1.51 | 12.49 ± 1.51 | 13.39 ± 1.6 | 8.16 ± 0.65 | |
| EE (%) | 2.3 ± 0.22 | 1.76 ± 0.29 | 1.9 ± 0.29 | 1.54 ± 0.15 | |
| NDF (%) | 59.83 ± 2.35 | 63.01 ± 4.54 | 61.98 ± 2.45 | 72.21 ± 2.72 | |
| ADF (%) | 33.54 ± 2.13 | 36.75 ± 1.8 | 35.75 ± 2.21 | 35.78 ± 2.09 | |
| Lignin (%) | 7.52 ± 0.75 | 8.35 ± 0.57 | 8.01 ± 0.66 | 8.64 ± 0.48 | |
| HC (%) | 26.29 ± 1.84 | 26.25 ± 3.64 | 26.23 ± 1.79 | 36.43 ± 3.28 | |
| TDN (%) | 54.98 ± 2.57 | 51.97 ± 2.23 | 52.19 ± 2.34 | 48.82 ± 1.34 | |
| DMD (%) | 60.22 ± 2.78 | 56.98 ± 2.41 | 57.21 ± 2.53 | 53.57 ± 1.45 | |
| ME (Mcal/kg) | 1.98 ± 0.11 | 1.84 ± 0.1 | 1.85 ± 0.1 | 1.71 ± 0.06 | |
| Ca2+ (%) | 0.31 ± 0.06 | 0.34 ± 0.11 | 0.34 ± 0.08 | 0.31 ± 0.07 | |
| P (%) | 0.26 ± 0.04 | 0.24 ± 0.04 | 0.23 ± 0.04 | 0.19 ± 0.03 | |
| Mg2+ (%) | 0.18 ± 0.02 | 0.22 ± 0.04 | 0.2 ± 0.03 | 0.22 ± 0.04 | |
| K (%) | 2.29 ± 0.27 | 2.03 ± 0.43 | 2.25 ± 0.36 | 2.07 ± 0.46 | |
| Na (%) | 0.04 ± 0.0043 | 0.03 ± 0.0047 | 0.04 ± 0.0046 | 0.03 ± 0.01 | |
| S (%) | 0.27 ± 0.04 | 0.26 ± 0.06 | 0.28 ± 0.05 | 0.21 ± 0.03 | |
| Transition Periods | Grass Cutting Intervals | |||||
|---|---|---|---|---|---|---|
| Lambda | Value | Proportion | Cum. Prop. | Value | Proportion | Cum. Prop. |
| 1 | 9.77 | 0.54 | 0.54 | 10.69 | 0.59 | 0.59 |
| 2 | 2.70 | 0.15 | 0.69 | 1.99 | 0.11 | 0.70 |
| 3 | 2.11 | 0.12 | 0.81 | 1.66 | 0.09 | 0.80 |
| 4 | 0.85 | 0.05 | 0.86 | 1.05 | 0.06 | 0.86 |
| 5 | 0.73 | 0.04 | 0.90 | 0.75 | 0.04 | 0.90 |
| 6 | 0.63 | 0.04 | 0.93 | 0.62 | 0.03 | 0.93 |
| 7 | 0.45 | 0.03 | 0.96 | 0.56 | 0.03 | 0.96 |
| 8 | 0.32 | 0.02 | 0.98 | 0.24 | 0.01 | 0.98 |
| 9 | 0.2 | 0.01 | 0.99 | 0.21 | 0.01 | 0.99 |
| 10 | 0.12 | 0.01 | 0.99 | 0.10 | 0.01 | 0.99 |
| 11 | 0.05 | 0.0028 | 1.00 | 0.07 | 0.0039 | 1.00 |
| Transition Periods | Grass Cutting Intervals | |||
|---|---|---|---|---|
| Variable | e1 | e2 | e1 | e2 |
| DMY/ha | −0.21 | 0.06 | −0.19 | 0.19 |
| DM | 0.18 | −0.02 | 0.14 | −0.44 |
| CP | 0.31 | 0.05 | 0.30 | −0.02 |
| Ash | 0.30 | 0.07 | 0.27 | −0.03 |
| EE | 0.28 | −0.10 | 0.27 | 0.12 |
| NDF | −0.30 | −0.06 | −0.29 | −0.06 |
| ADF | −0.16 | 0.49 | −0.15 | −0.05 |
| Lignin | −0.24 | 0.20 | −0.28 | −0.06 |
| HC | −0.24 | −0.35 | −0.27 | −0.05 |
| TDN | 0.31 | −0.11 | 0.29 | −0.01 |
| DMD | 0.31 | −0.11 | 0.29 | −0.01 |
| ME | 0.31 | −0.11 | 0.29 | −0.01 |
| Ca2+ | −0.13 | −0.23 | −0.13 | 0.51 |
| P | 0.23 | 0.31 | 0.27 | 0.07 |
| Mg2+ | −0.10 | −0,32 | −0.07 | 0.58 |
| K | 0.02 | 0.50 | 0.17 | 0.24 |
| Na | 0.16 | −0.14 | 0.14 | 0.22 |
| S | 0.18 | 0.08 | 0.22 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vélez-Terranova, M.; Salamanca-Carreño, A.; Vargas-Corzo, O.M.; Parés-Casanova, P.M.; Pérez-López, O. Influence of Cutting Intervals and Transition Periods on Chemical Composition Variability of Selected Tropical Grasses under Flooded Savanna Conditions of Arauca, Colombian Orinoquia. Sustainability 2023, 15, 16301. https://doi.org/10.3390/su152316301
Vélez-Terranova M, Salamanca-Carreño A, Vargas-Corzo OM, Parés-Casanova PM, Pérez-López O. Influence of Cutting Intervals and Transition Periods on Chemical Composition Variability of Selected Tropical Grasses under Flooded Savanna Conditions of Arauca, Colombian Orinoquia. Sustainability. 2023; 15(23):16301. https://doi.org/10.3390/su152316301
Chicago/Turabian StyleVélez-Terranova, Mauricio, Arcesio Salamanca-Carreño, Oscar Mauricio Vargas-Corzo, Pere M. Parés-Casanova, and Otoniel Pérez-López. 2023. "Influence of Cutting Intervals and Transition Periods on Chemical Composition Variability of Selected Tropical Grasses under Flooded Savanna Conditions of Arauca, Colombian Orinoquia" Sustainability 15, no. 23: 16301. https://doi.org/10.3390/su152316301
APA StyleVélez-Terranova, M., Salamanca-Carreño, A., Vargas-Corzo, O. M., Parés-Casanova, P. M., & Pérez-López, O. (2023). Influence of Cutting Intervals and Transition Periods on Chemical Composition Variability of Selected Tropical Grasses under Flooded Savanna Conditions of Arauca, Colombian Orinoquia. Sustainability, 15(23), 16301. https://doi.org/10.3390/su152316301






