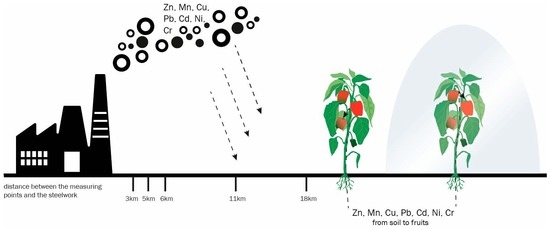
Effect of the Cultivation Method and the Distance from a Steel Mill on the Content of Heavy Metals in Bell Pepper Fruit
Abstract
:1. Introduction
- -
- Does the cultivation method (ground and plastic greenhouses) influence the content of heavy metals in pepper fruits and in the soil? Acronyms: q.cultvat. and q.soil.cultivat. Does the location of the cultivation (the distance from the steel mill) influence the content of heavy metals in pepper fruits and in the soil? Acronyms: q.locat. and q.soil.locat.
- -
- Is there a combined effect of both of these factors on bell pepper fruits and on the soil? Acronyms: q.interact. and q.soil.interact.
- -
- Does the date of the harvest of the bell peppers influence the content of heavy metals in the fruits? The shorter the time it takes for the fruit to grow and ripen, the lower the level of accumulation of metals. Acronyms: q.harvest.date.
2. Materials and Methods
2.1. Study Area
2.2. Field Experiment and Sampling
2.3. Laboratory Analyses
2.4. Statistical Analysis of the Data
3. Results and Discussion
3.1. Content of Heavy Metals in Soil
- -
- It decreased or remained unchanged. This pattern was identified for cadmium, lead, chromium, and zinc. For the first three of these metals, the concentrations at the second farthest (Wa) and farthest (Ru) distance did not statistically differ, while for zinc there were no differences between the second (Po) and third (Wa) distances. Thus, it can be assumed that Ru and perhaps even Wa were the boundaries of the effect of the steel mill. This corresponds to research by Topolska et al., who reported a similar range of impact for this site (about 20 km) for cadmium and zinc in a study of the spleens of bank voles (Clethrionomys glareolus) [41]. González-Miqueo et al. studied the impact of iron works on mosses in the Basque Country, within a range of 2 km. However, they found that this was not the range of their strongest impact [40]. In the vicinity of the industrial centre of Portoscuso in Sardinia, elevated concentrations of metals in the moss Bryum radiculosum were noted at distances of 13 km [42].
- -
- In the second pattern, the distance from the steel mill had no effect on the content of manganese, with a probability of 0.097.
- -
- In the third pattern, the distance from the steel mill did not affect the concentrations of copper and nickel. For Cu, a significant difference was only shown between the concentrations determined at sites Ru and Wa (farthest and second farthest). For nickel, only the concentration in Po (second distance) was statistically significantly different.
3.2. Content of Heavy Metals in Bell Pepper Fruits
Verification of the Hypotheses Regarding the Effect of Cultivation Method and Distance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wiesner-Reinhold, M.; Schreiner, M.; Baldermann, S.; Schwarz, D.; Hanschen, F.S.; Kipp, A.P.; Rowan, D.D.; Bentley-Hewitt, K.L.; McKenzie, M.J. Mechanisms of selenium enrichment and measurement in Brassicaceous vegetables and their application to human health. Front. Plant Sci. 2017, 8, 1365. [Google Scholar] [CrossRef] [PubMed]
- Bvenura, C.; Sivakumar, D. The role of wild fruits and vegetables in delivering a balanced and healthy diet. Food Res. Int. 2017, 99, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Mokganya, M.G.; Tshisikhawe, M.P. Medicinal uses of selected wild edible vegetables consumed by Vhavenda of the Vhembe District Municipality, South Africa. S. Afr. J. Bot. 2019, 122, 184–188. [Google Scholar] [CrossRef]
- Olas, B. Anti-aggregatory potential of selected vegetables-promising dietary components for the prevention and treatment of cardiovascular disease. Adv. Nutr. 2019, 10, 280–290. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. Vegetables from the Cucurbitaceae family and their products: Positive effect on human health. Nutrition 2020, 78, 110788. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.-B.; Xin, G.; Su, Y.-B.; Wang, G. Risk assessment of heavy metals in soils and vegetables around non-ferrous metals mining and smelting sites, Baiyin, China. J. Environ. Sci. 2006, 18, 1124–1134. [Google Scholar] [CrossRef]
- Zhong, T.; Xue, D.; Zhao, L.; Zhang, X. Concentration of heavy metals in vegetables and potential health risk assessment in China. Environ. Geochem. Health 2018, 40, 313–322. [Google Scholar] [CrossRef]
- Qin, G.; Chen, Y.; He, H.; Yang, B.; Zou, K.; Shen, N.; Zuo, B.; Liu, R.; Zhang, W.; Li, Y. Risk assessment of fungicide pesticide residues in vegetables and fruits in the mid-western region of China. J. Food Compos. Anal. 2021, 95, 103663. [Google Scholar] [CrossRef]
- Almasi, A.; Mohammadi, M.; Dargahi, A.; Amirian, F.; Motlagh, Z.J.; Ahmadidoust, G.; Noori, M. Nitrogenous contamination in Iranian vegetables: A review. Pol. J. Environ. Stud. 2018, 27, 2405–2416. [Google Scholar] [CrossRef]
- Elrys, A.S.; Abdo, A.I.E.; Desoky, E.-S.M. Potato tubers contamination with nitrate under the influence of nitrogen fertilizers and spray with molybdenum and salicylic acid. Environ. Sci. Pollut. Res. 2018, 25, 7076–7089. [Google Scholar] [CrossRef]
- Ghasemidehkordi, B.; Malekirad, A.A.; Nazem, H.; Fazilati, M.; Salavati, H.; Shariatifar, N.; Rezaei, M.; Fakhri, Y.; Khaneghah, A.M. Concentration of lead and mercury in collected vegetables and herbs from Markazi province, Iran: A non-carcinogenic risk assessment. Food Chem. Toxicol. 2018, 113, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, J.; Sharma, M.; Wani, K.A. Heavy metals in vegetables and their impact on the nutrient quality of vegetables: A review. J. Plant Nutr. 2018, 113, 204–210. [Google Scholar] [CrossRef]
- Chrysikou, L.; Gemenetzis, G.; Kouras, A.; Manoli, E.; Terzi, E.; Sama, C. Distribution of persistent organic pollutants, polycyclic aromatic hydrocarbons and trace elements in soil and vegetation following a large scale landfill fire in northern Greece. Environ. Int. 2008, 34, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Säumel, I.; Kotsyuk, I.; Hölscher, M.; Lenkereit, C.; Weber, F.; Kowarik, I. How healthy is urban horticulture in high traffic areas? Trace metal concentrations in vegetable crops from plantings within inner city neighbourhoods in Berlin, Germany. Environ. Pollut. 2012, 165, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Rahmdel, S.; Rezaei, M.; Ekhlasi, J.; Zarei, S.H.; Akhlaghi, M.; Abdollahzadeh, S.M.; Sefidkar, R.; Mazloomi, S.M. Heavy metals (Pb, Cd, Cu, Zn, Ni, Co) in leafy vegetables collected from production sites: Their potential health risk to the general population in Shiraz, Iran. Environ. Monit. Assess. 2018, 190, 650. [Google Scholar] [CrossRef] [PubMed]
- Courter, J.W. Plastic greenhouses. Circular 1962, 857, 4–32. [Google Scholar]
- Courter, J.W.; Vandemark, J.S.; Hinton, R.A. The feasibility of growing greenhouse tomatoes in southern Illinois. Circular 1965, 914, 5–23. [Google Scholar]
- Seginer, I.; Kantz, D.; Peiper, U.M.; Levav, N. Transfer coefficient of several polyethylene greenhouse covers. J. Agric. Eng. Res. 1988, 39, 19–37. [Google Scholar] [CrossRef]
- Gil, C.; Boluda, R.; Martin, J.A.R.; Guzmán, M.; del Moral, F.; Ramos-Miras, J. Assessing soil contamination and temporal trends of heavy metal contents in greenhouses on semiarid soil. Land Degrad. Dev. 2018, 29, 3344–3354. [Google Scholar] [CrossRef]
- Sun, J.; Pan, L.; Li, Z.; Zeng, Q.; Wang, L.; Zhu, L. Comparison of greenhouse and open field cultivations across China: Soil characteristics, contamination and microbial diversity. Environ. Pollut. 2018, 243, 1509–1516. [Google Scholar] [CrossRef]
- Purvis, B.; Mao, Y.; Robinson, D. Three pillars of sustainability: In search of conceptual origins. Sustain. Sci. 2019, 14, 681–695. [Google Scholar] [CrossRef]
- Kubiak, R.; Rudek, Z.; Cieszkowski, J.; Garlicki, S. Cytogenetic examinations in biomonitoring residents residing closest to the area near the Sendzimir metallurgy plant in Krakow exposed to environmental pollution. Folia Med. Cracov. 1993, 34, 187–198. [Google Scholar] [PubMed]
- Curzydło, J.; Gambuś, F.; Mundała, P.; Szwalec, A. Zmiany zawartości metali ciężkich w roślinach uprawianych w latach 1983–1994 w sąsiedztwie Kombinatu Metalurgicznego w Nowej Hucie. Zesz. Nauk. AR Krakowie Rol. 1997, 34, 93–103. (In Polish) [Google Scholar]
- Mundała, P.; Szwalec, A.; Petryk, A. Zawartość wybranych pierwiastków śladowych w glebach położonych w sąsiedztwie Kombinatu Metalurgicznego w Nowej Hucie. Inż. Ekol. 2013, 33, 67–76. (In Polish) [Google Scholar] [CrossRef]
- Kicińska, A.; Wikar, J. Ecological risk associated with agricultural production in soils contaminated by the activities of the metal ore and processing industry–example from southern Poland. Soil Tillage Res. 2021, 205, 104817. [Google Scholar] [CrossRef]
- Bieda, B. Life cycle inventory processes of the Mittal Steel Poland (MSP) S.A. in Krakow, Poland-blast furnace pig iron production-a case study. Int. J. Life Cycle Assess. 2012, 17, 787–794. [Google Scholar] [CrossRef]
- Gajdzik, B. The road of Polish steelworks towards market success–changes after restricting process. Metalurgija 2013, 3, 421–424. [Google Scholar]
- Burchart-Korol, D. Life cycle assessment of steel production in Poland: A case study. J. Clean. Prod. 2013, 54, 235–243. [Google Scholar] [CrossRef]
- Damek-Poprawa, M.; Sawicka-Kapusta, K. Damage to the liver, kidney, and testis with reference to burden of heavy metals in yellow-necked mice from areas around steelworks and zinc smelters in Poland. Toxicology 2003, 186, 1–10. [Google Scholar] [CrossRef]
- Gajdzik, B.; Sroka, W. Analytic study of the capital restructuring processes in metallurgical enterprises around the world and in Poland. Metalurgija 2012, 51, 265–268. [Google Scholar]
- Mundała, P.; Szwalec, A.; Siejka, Z.; Kędzior, R.; Lipka, K. Zanieczyszczenie wybranymi metalami ciężkimi osadów dennych Zalewu Nowohuckiego. Acta Agrophys. 2016, 23, 455–465. [Google Scholar]
- Kruczek, Z.; Mazanek, L. Krakow as a tourist metropolitan area. Inpact of turism on the economof the city. Stud. Perieget. 2019, 2, 25–41. [Google Scholar]
- Szwalec, A.; Mundała, P.; Kędzior, R.; Pawlik, J. Monitoring and assessment of cadmium, lead, zinc and copper concentrations in arable roadside soils in terms of different traffic conditions. Environ. Monit. Assess. 2020, 192, 155. [Google Scholar] [CrossRef] [PubMed]
- Adamicki, F.; Nawrocka, B.; Dobrzański, A.; Kosson, R.; Pałczyński, J.; Stepowska, A.; Szwjda, J.; Ślusarski, C. Metodyka Integrowanej Produkcj Papryki; Opracowanie Zbiorowe Zespołu Instytutu Ogrodnictwa w Skierniecicach: Warszawa, Poland, 2020. (In Polish) [Google Scholar]
- Labaz, B.; Kabala, C.; Waroszewski, J. Ambient geochemical baselines for trace elements in Chernozems–approximation of geochemical soil transformation in an agricultural area. Environ. Monit. Assess. 2019, 191, 19. [Google Scholar]
- Lukin, S.V.; Selyukova, S.V.; Chernikov, V.A.; Gaitova, N.A. Ecological estimation of cadmium content in agricultural cenosis of the Central Chernozem Region of Russia. Pollut. Res. 2020, 39, 196–201. [Google Scholar]
- Bezuglova, O.S.; Gorbov, S.N.; Tischenko, S.A.; Aleksikova, A.S.; Tagiverdiev, S.S.; Sherstnev, A.K.; Dubinina, M.N. Accumulation and migration of heavy metals in soils of the Rostov region, south of Russia. J. Soil Sediments 2016, 16, 1203–1213. [Google Scholar] [CrossRef]
- Novykh, L.; Solovyov, A.; Novykh, I.; Chuikova, E.; Tricula, L. Ecological and geochemical features of the distribution of trace elements in virgin and arable Chernozems. In Proceedings of the 18th International Multidisciplinary Scientific Geo Conferences (SGEM 2018): Conference Proceedings, Albena, Bulgaria, 2–8 July 2018. [Google Scholar]
- Wang, J.; Wang, L.; Wang, Y.; Tsang, D.C.W.; Yang, X.; Beiyuan, J.; Yin, M.; Xiao, T.; Jiang, Y.; Lin, W.; et al. Emerging risks of toxic metal(loids)s in soil-vegetables influenced by steel-making activities and isotopic source apportionment. Environ. Int. 2021, 146, 106207. [Google Scholar] [CrossRef]
- González-Miqueo, L.; Elustondo, D.; Lasheras, E.; Santamaría, J.M. Use of native mosses as biomonitors of heavy metals and nitrogen deposition in the surroundings of two steel works. Chemosphere 2010, 78, 965–971. [Google Scholar] [CrossRef]
- Topolska, K.; Sawicka-Kapusta, K.; Cieślik, E. The effect of contamination of the Kraków region on heavy metals content in the organs of bank voles (Clethrionomys glareolus, Schreber, 1780). Pol. J. Environ. Stud. 2004, 13, 103–109. [Google Scholar]
- Schintu, M.; Cogoni, A.; Durante, L.; Cantaluppi, C.; Contu, A. Moss (Bryum radiculosum) as a bioindicator of trace metal deposition around an industrialised area in Sardinia (Italy). Chemosphere 2005, 60, 610–618. [Google Scholar] [CrossRef]
- Jankaitė, A.; Baltrėnas, P.; Kazlauskienė, A. Heavy metal concentrations in roadside soils of Lithuania’s highways. Geologija 2008, 50, 237–245. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Kapusta, P.; Zubek, S.; Stanek, M.; Woch, M.W. Soil organic matter prevails over heavy metal pollution and vegetation as a factor shaping soil microbial communities at historical Zn–Pb mining sites. Chemosphere 2020, 240, 124922. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Impellitteri, C.A.; You, S.-J.; Allen, H.E. The importance of organic matter distribution and extract soil: Solution ratio on the desorption of heavy metals from soils. Sci. Total Environ. 2002, 287, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Gerritse, R.G.; van Driel, W.; Smidle, K.W.; van Luit, B. Uptake of heavy metals by crops in relation to their concentrations in the soil solution. Plant Soil 1983, 75, 393–405. [Google Scholar] [CrossRef]
- Knight, B.; Zaho, F.J.; McGrath, S.P.; Shen, Z.G. Zinc and cadmium uptake by the hyperaccumulator Thlaspi caerulescens in contaminated soils and its effects on the concentration and chemical speciation of metals in soil solution. Plant Soil 1997, 197, 71–78. [Google Scholar] [CrossRef]
- Nassar, O.; Nasr, H.; El-Sayed, M.; Kobisi, A. Heavy metal levels in some popular vegetables from some selected markets in Saudi Arabia. Egypt. J. Bot. 2018, 58, 627–638. [Google Scholar] [CrossRef]
- Osma, E.; Serin, M.; Leblebici, Z.; Aksoy, A. Assessment of heavy metal accumulations (Cd, Cr, Cu, Ni, Pb, and Zn) in vegetables and soils. Pol. J. Environ. Stud. 2013, 22, 1449–1455. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Sharma, A.; Katnoria, J.K.; Nagpal, A.K. Heavy metals in vegetables: Screening health risks involved in cultivation along wastewater drain and irrigating with wastewater. SpringerPlus 2016, 5, 488. [Google Scholar] [CrossRef]
- Shikhova, N.S. Some regularities in the accumulation of lead in urban plants (by example of Vladivostok). Contemp. Prob. Ecol. 2012, 5, 285–294. [Google Scholar] [CrossRef]
- Ahmad, J.U.; Goni, M.A. Heavy metal contamination in water, soil, and vegetables of the industrial areas in Dhaka, Bangladesh. Environ. Monit. Assess. 2010, 166, 347–357. [Google Scholar] [CrossRef]
- Leblebici, Z.; Kar, M.; Başaran, L. Assessment of the heavy metal accumulation of various green vegetables grown in Nevşehir and their risks human health. Environ. Monit. Assess. 2020, 192, 483. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.; Bilal, M.; Asghar, W.; Azeem, M.; Ahmad, M.I.; Abbas, A.; Ahmad, M.Z.; Shahzad, T. Heavy metal accumulation in vegetables and assessment of their potential health risk. J. Environ. Anal. Chem. 2018, 5, 2380–2391. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Ntoupa, P.A.; Spiliopoulou, C.A.; Stefanidou, M.E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020, 945, 1443–1460. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Zarasvand, M.A. Heavy metals in selected edible vegetables and estimation of their daily intake in Sanandaj, Iran. Southeast Asian J. Trop. Med. Public Health 2008, 39, 335–340. [Google Scholar] [PubMed]
- Rahman, M.M.; Asaduzzaman, M.; Naidu, R. Consumption of arsenic and other elements from vegetables and drinking water from an arsenic-contaminated area of Bangladesh. J. Hazard. Mater. 2013, 262, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Pandita, S.; Sidhu, G.P.S.; Sharma, A.; Khanna, K.; Kaur, P.; Bali, A.S.; Setia, R. Copper bioavailability, uptake, toxicity and tolerance in plants: A comprehensive review. Chemosphere 2021, 262, 127810. [Google Scholar] [CrossRef]
- Mohammed, G.A.; Edet, B.E.; Badawi, H.L.; Bala, H.M.; Marte, M.U.; Mohammed, A.; Dauda, A. Appraisal of some heavy metals and elements in irrigated vegetables and fruit cultivated in College Farm, Maiduguri, Nigeria. Int. J. Appl. Agric. Sci. 2017, 3, 78–86. [Google Scholar] [CrossRef]
- Alloway, B.J. (Ed.) Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Springer: Dordrecht, The Netherland, 2013; Volume 22. [Google Scholar]
- Matloob, M.H. Using stripping voltammetry to determine heavy metals in cooking spices used in Iraq. Pol. J. Environ. Stud. 2016, 25, 2057–2070. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in plants: From acquisition to subcellular allocation. Front. Plant Sci. 2020, 26, 300. [Google Scholar] [CrossRef]
- O’Neal, S.L.; Zheng, W. Manganese toxicity upon overexposure: A decade in review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef]
- Islam, M.D.; Hasan, M.M.; Rahaman, A.; Hauge, P.; Islam, M.S.; Rahman, M.M. Translocation and bioaccumulation of trace metals from industrial effluent to locally grown vegetables and assessment of human health risk in Bangladesh. SN Appl. Sci. 2020, 2, 1315. [Google Scholar] [CrossRef]
- Stambulska, U.Y.; Bayliak, M.M.; Lushchak, V.I. Chromium (VI) toxicity in legume plants: Modulation effects of Rhizobial symbiosis. BioMed Res. Int. 2018, 2018, 8031213. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.J.A.; Ramos-Miras, J.J.; Boluda, R.; Gil, C. Spatial relations of heavy metals in arable and greenhouse soils of a Mediterranean environment region (Spain). Geoderma 2013, 200, 180–188. [Google Scholar] [CrossRef]
- Kalkhajeh, K.Y.; Huang, B.; Hu, W.; Ma, C.; Gao, H.; Thompson, M.L.; Hansen, H.C.B. Environmental soil quality and vegetable safety under current greenhouse vegetable production management in China. Agric. Ecosyst. Environ. 2021, 307, 107230. [Google Scholar] [CrossRef]
- Gruda, N. Impact of environmental factors on product quality of greenhouse vegetables for fresh consumption. Crit. Rev. Plant Sci. 2005, 24, 227–247. [Google Scholar] [CrossRef]
- Jalali, M.; Mojahed, J.K. Assessment of the health risks of heavy metals in soils and vegetables from greenhouse production systems in Iran. Int. J. Phytorem. 2020, 22, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Shi, W.; Jin, Z.; Wu, H.; Sheng, G.D. Excessive uptake of heavy metals by greenhouse vegetables. J. Geochem. Explor. 2017, 173, 76–84. [Google Scholar] [CrossRef]
- Cao, C.; Chen, X.-P.; Ma, Z.-B.; Jia, H.-H.; Wang, J.-J. Greenhouse cultivation mitigates metal-ingestion-associated health risk from vegetables in wastewater-irrigated agroecosystems. Sci. Total Environ. 2016, 560, 204–211. [Google Scholar] [CrossRef]
- Song, B.; Lei, M.; Chen, T.; Zheng, Y.; Xie, Y.; Li, X.; Gao, D. Assessing the health risk of heavy metals in vegetables to the general population in Beijing, China. J. Environ. Sci. 2009, 21, 1702–1709. [Google Scholar] [CrossRef]
- Mateos, R.M.; Jiménez, A.; Román, P.; Romojaro, F.; Bacarizo, S.; Leterrier, M.; Gómez, M.; Sevilla, F.; del Rio, L.A.; Corpas, F.J.; et al. Antioxidant systems from pepper (Capsicum annuum L.): Involvement in the response to temperature changes in ripe fruits. Int. J. Mol. Sci. 2013, 14, 9556–9580. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defence response. A review. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef] [PubMed]
- Gratão, P.L.; Polle, A.; Lea, P.J.; Azevedo, R.A. Making the life of heavy metal-stressed plants a little easier. A review. Funct. Plant Biol. 2005, 32, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Fu, Y.; Yan, S.; Yang, Y.; Guo, Y.; Bao, Z. Heavy metal distribution between parent soil an pepper in an unpolluted area, Hainan Island, China. Environ. Earth Sci. 2012, 66, 1083–1089. [Google Scholar] [CrossRef]
- López, A.; Fenoll, J.; Hellín, P.; Flores, P. Cultivation approach for comparing the nutritional quality of two pepper cultivars grown under different agricultural regimes. LWT-Food Sci. Technol. 2014, 58, 299–305. [Google Scholar] [CrossRef]
- Ziernicka-Wojtaszek, A. Summer drought in 2019 on Polish territory—A case study. Atmosphere 2021, 12, 1475. [Google Scholar] [CrossRef]
- Ziernicka-Wojtaszek, A. Pluviothermal regionalization of Poland in light of present-day climate change. Pol. J. Environ. Stud. 2020, 29, 989–996. [Google Scholar] [CrossRef]
- Ziernicka-Wojtaszek, A.; Krużel, J. The diversification of air temperature trends in Poland (1981–2010). Pol. J. Environ. Stud. 2016, 25, 2205–2209. [Google Scholar] [CrossRef]
- Yu, J.; Wu, J. The sustainability of agricultural development in China. The agriculture-environment nexus. Sustainability 2018, 10, 1776. [Google Scholar] [CrossRef]
| Metal, Site, Unit, Mean ± RSD, HSD | Ko | Po | Wa | Ru | ||
|---|---|---|---|---|---|---|
| mg·kg−1 d.w. ± % | ||||||
| Cd | pg | n.a. | 0.92 ± 11 | 0.77 ± 16 | 0.61 ± 15 | 0.55 ± 15 |
| f | 0.353 | 0.89 ± 18 | 0.83 ± 23 | 0.64 ± 11 | 0.60 ± 10 | |
| Pb | pg | n.a. | 35.65 ± 16 | 32.08 ± 12 | 22.88 ± 12 | 22.15 ± 9 |
| f | 0.088 | 33.23 ± 15 | 30.83 ± 17 | 25.09 ± 14 | 23.08 ± 12 | |
| Zn | pg | n.a. | 87.10 ± 15 | 62.09 ± 14 | 57.20 ± 18 | 47.07 ± 9 |
| f | 0.838 | 91.49 ± 10 | 64.89 ± 19 | 57.94 ± 17 | 47.72 ± 12 | |
| Cu | pg | n.a. | 29.51 ± 9 | 30.05 ± 6 | 27.96 ± 11 | 30.28 ± 8 |
| f | 0.233 | 30.43 ± 7 | 30.00 ± 7 | 30.21 ± 7 | 31.07 ± 6 | |
| Ni | pg | 0.005 | 24.47 ± 6 a | 28.49 ± 11 d | 24.69 ± 7 a | 24.15 ± 6 a |
| f | 0.68 | 24.42 ± 6 a | 26.38 ± 6 c | 24.71 ± 7 a | 25.54 ± 4 b | |
| Mn | pg | n.a. | 564.80 ± 6 | 566.60 ± 5 | 582.30 ± 10 | 573.60 ± 6 |
| f | 0.704 | 551.70 ± 6 | 545.90 ± 7 | 566.30 ± 5 | 574.00 ± 4 | |
| Cr | pg | 0.75 | 25.62 ± 9 a | 27.57 ± 10 c | 25.16 ± 8 a | 23.80 ± 5 e |
| f | 0.0013 | 28.53 ± 10 b | 31.91 ± 10 d | 25.40 ± 6 a | 25.32 ± 4 a | |
| Site, Cultivation, Statistics, Metal, and Water Content | mg·kg−1 d.w. + % | % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cd | Pb | Zn | Cu | Ni | Mn | Cr | %H2O | ||
| Ko | mean | 0.366 | 0.11 | 27.39 | 6.94 | 4.13 | 2.35 | 0.26 | 91.84 |
| Greenhouse | RSD | 17 | 18 | 18 | 35 | 34 | 32 | 42 | 0.9 |
| min | 0.211 | 0.07 | 17.89 | 3.69 | 1.89 | 1.25 | 0.11 | 90.48 | |
| max | 0.554 | 0.15 | 37.43 | 12.03 | 7.23 | 4.11 | 0.56 | 94.64 | |
| Ko | mean | 0.384 | 0.12 | 31.61 | 10.19 | 4.25 | 6.59 | 0.20 | 89.78 |
| Field | RSD | 19 | 17 | 22 | 15 | 10 | 18 | 30 | 1.1 |
| min | 0.274 | 0.07 | 20.65 | 6.94 | 3.47 | 4.84 | 0.10 | 88.21 | |
| max | 0.526 | 0.16 | 45.95 | 13.34 | 5.34 | 8.27 | 0.30 | 92.3 | |
| Po | mean | 0.332 | 0.08 | 22.97 | 8.24 | 2.69 | 4.84 | 0.22 | 92.37 |
| Greenhouse | RSD | 20 | 25 | 20 | 21 | 44 | 28 | 45 | 1.1 |
| min | 0.169 | 0.05 | 14.09 | 2.93 | 0.72 | 2.42 | 0.10 | 90.5 | |
| max | 0.538 | 0.12 | 36.34 | 12.39 | 6.69 | 7.54 | 0.45 | 94.7 | |
| Po | mean | 0.346 | 0.08 | 26.36 | 10.01 | 4.26 | 6.76 | 0.17 | 90.14 |
| Field | RSD | 18 | 25 | 20 | 16 | 10 | 18 | 24 | 1.1 |
| min | 0.261 | 0.05 | 16.65 | 6.55 | 3.50 | 4.99 | 0.10 | 88.29 | |
| max | 0.459 | 0.12 | 33.69 | 13.07 | 5.39 | 8.25 | 0.24 | 92.8 | |
| Wa | mean | 0.239 | 0.08 | 18.96 | 4.64 | 2.65 | 6.92 | 0.17 | 91.49 |
| Greenhouse | RSD | 31 | 13 | 23 | 39 | 57 | 47 | 53 | 1.1 |
| min | 0.028 | 0.05 | 12.49 | 2.41 | 0.97 | 3.27 | 0.10 | 89.3 | |
| max | 0.408 | 0.11 | 28.19 | 9.28 | 6.34 | 18.88 | 0.53 | 94 | |
| Wa | mean | 0.293 | 0.08 | 22.03 | 9.11 | 7.00 | 9.94 | 0.18 | 90.23 |
| Field | RSD | 11 | 25 | 14 | 26 | 39 | 30 | 22.2 | 0.9 |
| min | 0.222 | 0.05 | 14.37 | 4.52 | 3.60 | 4.36 | 0.11 | 88.9 | |
| max | 0.356 | 0.12 | 26.99 | 12.34 | 12.35 | 14.26 | 0.27 | 92.2 | |
| Ru | mean | 0.222 | 0.11 | 18.31 | 4.81 | 2.84 | 7.63 | 0.18 | 91.53 |
| Greenhouse | RSD | 31 | 18 | 23 | 38 | 54 | 58 | 56 | 2.3 |
| min | 0.026 | 0.07 | 11.74 | 2.49 | 1.08 | 3.25 | 0.09 | 88.27 | |
| max | 0.371 | 0.16 | 27.34 | 9.76 | 6.86 | 20.46 | 0.58 | 96.92 | |
| mean | 0.262 | 0.12 | 22.53 | 9.31 | 6.96 | 9.69 | 0.22 | 89.78 | |
| Field | RSD | 12 | 17 | 18 | 26 | 38 | 30 | 27 | 1.1 |
| min | 0.215 | 0.07 | 14.10 | 4.38 | 3.51 | 4.32 | 0.11 | 88.21 | |
| max | 0.344 | 0.16 | 30.05 | 12.37 | 12.23 | 13.69 | 0.34 | 92.3 | |
| Metal | Date | Cultivation | Location | Interaction | |||
|---|---|---|---|---|---|---|---|
| Cd | without date | yes | 1.61102 × 10−5 | yes | 2.69543 × 10−35 | no | 0.166127179 |
| first | yes | 0.001057297 | yes | 1.51436 × 10−10 | no | 0.447972664 | |
| second | yes | 1.4973 × 10−7 | yes | 6.03749 × 10−15 | no | 0.195601826 | |
| third | no | 0.573449124 | yes | 2.77247 × 10−13 | yes | 0.011140489 | |
| Pb | without date | yes | 0.005778259 | yes | 4.53459 × 10−32 | no | 0.377992523 |
| first | no | 0.077739309 | yes | 7.70073 × 10−8 | no | 0.969154031 | |
| second | no | 0.137281884 | yes | 6.57555 × 10−11 | no | 0.488248034 | |
| third | no | 0.114373794 | yes | 8.51547 × 10−14 | no | 0.642761923 | |
| Zn | without date | yes | 2.2587 × 10−10 | yes | 3.49283 × 10−29 | no | 0.849134489 |
| first | yes | 4.0469 × 10−7 | yes | 3.95237 × 10−10 | no | 0.70077181 | |
| second | yes | 0.013566398 | yes | 1.52903 × 10−11 | no | 0.9615407 | |
| third | yes | 0.001260023 | yes | 7.16335 × 10−8 | no | 0.79998615 | |
| Cu | without date | yes | 3.64205 × 10−37 | yes | 6.48875 × 10−13 | yes | 7.30079 × 10−5 |
| first | yes | 1.11915 × 10−13 | yes | 8.25647 × 10−5 | no | 0.112520737 | |
| second | yes | 1.62725 × 10−14 | yes | 3.73758 × 10−5 | no | 0.23146229 | |
| third | yes | 9.54922 × 10−11 | yes | 0.002608164 | yes | 0.010834713 | |
| Ni | without date | yes | 7.12524 × 10−30 | yes | 5.40517 × 10−7 | yes | 3.46854 × 10−15 |
| first | yes | 1.01104 × 10−11 | yes | 0.046182502 | yes | 0.003484608 | |
| second | yes | 1.01846 × 10−13 | yes | 0.005144557 | yes | 3.01505 × 10−6 | |
| third | yes | 1.22667 × 10−7 | yes | 0.008438733 | yes | 1.68976 × 10−6 | |
| Mn | without date | yes | 5.33308 × 10−18 | yes | 6.75671 × 10−24 | yes | 0.026878579 |
| first | yes | 3.4776 × 10−18 | yes | 2.10097 × 10−7 | no | 0.601630132 | |
| second | yes | 6.74274 × 10−11 | yes | 3.19018 × 10−9 | no | 0.160592215 | |
| third | no | 0.050745839 | yes | 1.36384 × 10−12 | yes | 0.036573495 | |
| Cr | without date | no | 0.175542097 | yes | 0.000755465 | yes | 0.000118379 |
| first | no | 0.053384005 | yes | 0.031748219 | no | 0.498843733 | |
| second | yes | 0.445841227 | no | 0.139288076 | yes | 0.001191324 | |
| third | yes | 0.00689218 | yes | 0.011037716 | yes | 0.011062122 | |
| Cd [mg·kg −1 d.m.] | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cult./HSD | Without Date | Diff. | First | Diff. | Second | Diff. | Third | Diff. |
| Greenhouse | 0.290 | a | 0.294 | a | 0.254 | a | 0.321 | a |
| Field | 0.321 | b | 0.334 | b | 0.316 | b | 0.314 | a |
| HSD | 0.010 | 0.017 | 0.015 | |||||
| Pb | ||||||||
| Greenhouse | 0.094 | a | 0.089 | a | 0.094 | a | 0.098 | a |
| Field | 0.100 | b | 0.095 | a | 0.101 | a | 0.104 | a |
| HSD | 0.003 | n.i. | n.i. | n.i. | ||||
| Zn | ||||||||
| Greenhouse | 21.91 | a | 21.26 | a | 23.33 | a | 21.14 | a |
| Field | 25.63 | b | 26.53 | b | 25.55 | b | 24.82 | b |
| HSD | 0.79 | 1.35 | 1.24 | 1.55 | ||||
| Cu | ||||||||
| Greenhouse | 6.16 | a | 5.85 | a | 6.11 | a | 6.53 | a |
| Field | 9.66 | b | 9.54 | b | 9.69 | b | 9.74 | b |
| HSD | 0.33 | 0.59 | 0.55 | 0.62 | ||||
| Ni | ||||||||
| Greenhouse | 3.08 | a | 2.73 | a | 2.83 | a | 3.67 | a |
| Field | 5.62 | b | 5.48 | b | 5.57 | b | 5.80 | b |
| HSD | 0.28 | 0.49 | 0.44 | 0.52 | ||||
| Mn | ||||||||
| Greenhouse | 5.43 | a | 3.69 | a | 5.09 | a | 7.52 | a |
| Field | 8.24 | b | 7.85 | b | 8.26 | b | 8.63 | a |
| HSD | 0.42 | 0.53 | 0.60 | n.i. | ||||
| Cr | ||||||||
| Greenhouse | 0.204 | a | 0.157 | a | 0.208 | a | 0.248 | a |
| Field | 0.192 | a | 0.179 | a | 0.197 | a | 0.201 | b |
| HSD | n.i. | n.i. | 0.023 | 0.024 | ||||
| H2O content [%] | ||||||||
| Greenhouse | 91.8 | a | 91.5 | a | 91.6 | a | 92.3 | a |
| Field | 90.0 | b | 89.8 | b | 90.2 | b | 90.0 | b |
| HSD | 0.2 | 0.3 | 0.3 | 0.4 | ||||
| Cd mg·kg −1 d.m. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Site/HSD | All | Diff. | First | Diff. | Second | Diff. | Third | Diff. |
| Ko | 0.375 | a | 0.378 | a | 0.359 | a | 0.389 | a |
| Po | 0.339 | b | 0.339 | a | 0.317 | b | 0.361 | a |
| Wa | 0.266 | c | 0.281 | b | 0.243 | c | 0.275 | b |
| Ru | 0.242 | c | 0.257 | b | 0.221 | c | 0.247 | b |
| HSD | 0.026 | 0.044 | 0.040 | 0.046 | ||||
| Pb | ||||||||
| Ko | 0.114 | a | 0.105 | a | 0.115 | a | 0.121 | a |
| Po | 0.079 | b | 0.076 | b | 0.082 | b | 0.079 | b |
| Wa | 0.082 | b | 0.084 | b | 0.079 | b | 0.084 | b |
| Ru | 0.112 | a | 0.103 | a | 0.114 | a | 0.120 | a |
| HSD | 0.008 | 0.014 | 0.015 | 0.015 | ||||
| Zn | ||||||||
| Ko | 29.50 | a | 29.87 | a | 30.11 | a | 28.53 | a |
| Po | 24.66 | b | 24.36 | b | 25.56 | b | 24.07 | b |
| Wa | 20.49 | c | 20.58 | c | 21.16 | c | 19.74 | c |
| Ru | 20.42 | c | 20.78 | c | 20.92 | c | 19.56 | c |
| HSD | 2.07 | 3.57 | 3.27 | 4.10 | ||||
| Cu | ||||||||
| Ko | 8.57 | a | 8.39 | a | 8.72 | a | 8.59 | a.b |
| Po | 9.13 | a | 9.01 | a | 9.07 | a | 9.31 | a.b |
| Wa | 6.88 | b | 6.60 | b | 6.82 | b | 7.21 | c |
| Ru | 7.06 | b | 6.76 | b | 7.00 | b | 7.43 | a.c |
| HSD | 0.86 | 1.56 | 1.45 | 1.62 | ||||
| Ni | ||||||||
| Ko | 4.19 | a.b | 3.92 | a | 4.15 | a.b | 4.50 | a.b |
| Po | 3.47 | a.b | 3.37 | a | 3.28 | a.b | 3.78 | a.b |
| Wa | 4.83 | a | 4.55 | a | 4.67 | a | 5.26 | a |
| Ru | 4.90 | a | 4.60 | a | 4.70 | a | 5.41 | a |
| HSD | 0.73 | 1.30 | 1.15 | 1.37 | ||||
| Mn | ||||||||
| Ko | 4.47 | a | 4.18 | a | 4.43 | a | 4.79 | a |
| Po | 5.80 | b | 5.00 | a | 5.97 | a | 6.43 | a |
| Wa | 8.43 | c | 7.00 | b | 8.12 | b | 10.17 | b |
| Ru | 8.66 | c | 6.89 | b | 8.18 | b | 10.90 | b |
| HSD | 1.11 | 1.40 | 1.58 | 2.08 | ||||
| Cr | ||||||||
| Ko | 0.23 | a | 0.19 | a | 0.22 | a | 0.27 | a |
| Po | 0.19 | b | 0.15 | b | 0.22 | a | 0.20 | b |
| Wa | 0.18 | b | 0.16 | a.b | 0.17 | a | 0.20 | b.c |
| Ru | 0.20 | a.b | 0.17 | b | 0.20 | a | 0.22 | a.b |
| HSD | 0.03 | 0.04 | n.i. | 0.06 | ||||
| H2O content [%] | ||||||||
| Ko | 90.8 | a.b | 90.5 | a | 91.0 | a.b | 90.9 | a |
| Po | 91.3 | a.b | 90.9 | a | 91.4 | a.b | 91.5 | a |
| Wa | 90.9 | a.b | 90.7 | a | 90.6 | a.b | 91.2 | a |
| Ru | 90.7 | a | 90.3 | a | 90.6 | a | 91.0 | a |
| HSD | 0.5 | n.i. | 0.8 | n.i. | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mundała, P.; Szwalec, A. Effect of the Cultivation Method and the Distance from a Steel Mill on the Content of Heavy Metals in Bell Pepper Fruit. Sustainability 2023, 15, 16400. https://doi.org/10.3390/su152316400
Mundała P, Szwalec A. Effect of the Cultivation Method and the Distance from a Steel Mill on the Content of Heavy Metals in Bell Pepper Fruit. Sustainability. 2023; 15(23):16400. https://doi.org/10.3390/su152316400
Chicago/Turabian StyleMundała, Paweł, and Artur Szwalec. 2023. "Effect of the Cultivation Method and the Distance from a Steel Mill on the Content of Heavy Metals in Bell Pepper Fruit" Sustainability 15, no. 23: 16400. https://doi.org/10.3390/su152316400