Evaluation of Kunkur Fines for Utilization in the Production of Ternary Blended Cements
Abstract
:1. Introduction
2. Materials and Experimental Methods
2.1. Materials
2.2. Methods
2.2.1. Chemical Composition
2.2.2. Mineralogical Composition of Raw Materials and Reacted Mixtures
2.2.3. Mixture Design
2.2.4. Compressive Strength
2.2.5. DoE Model Validation
2.2.6. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Compressive Strength
3.2. DoE Model Validation
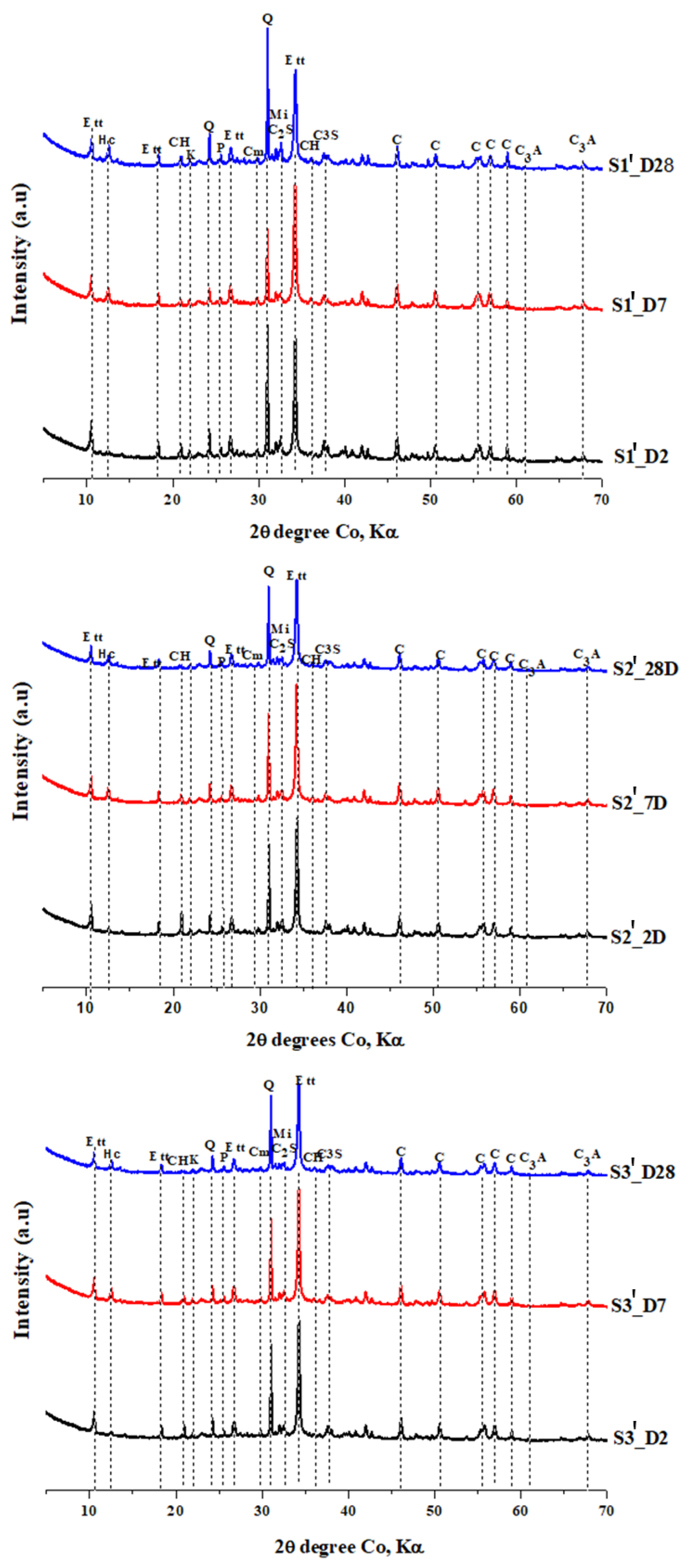
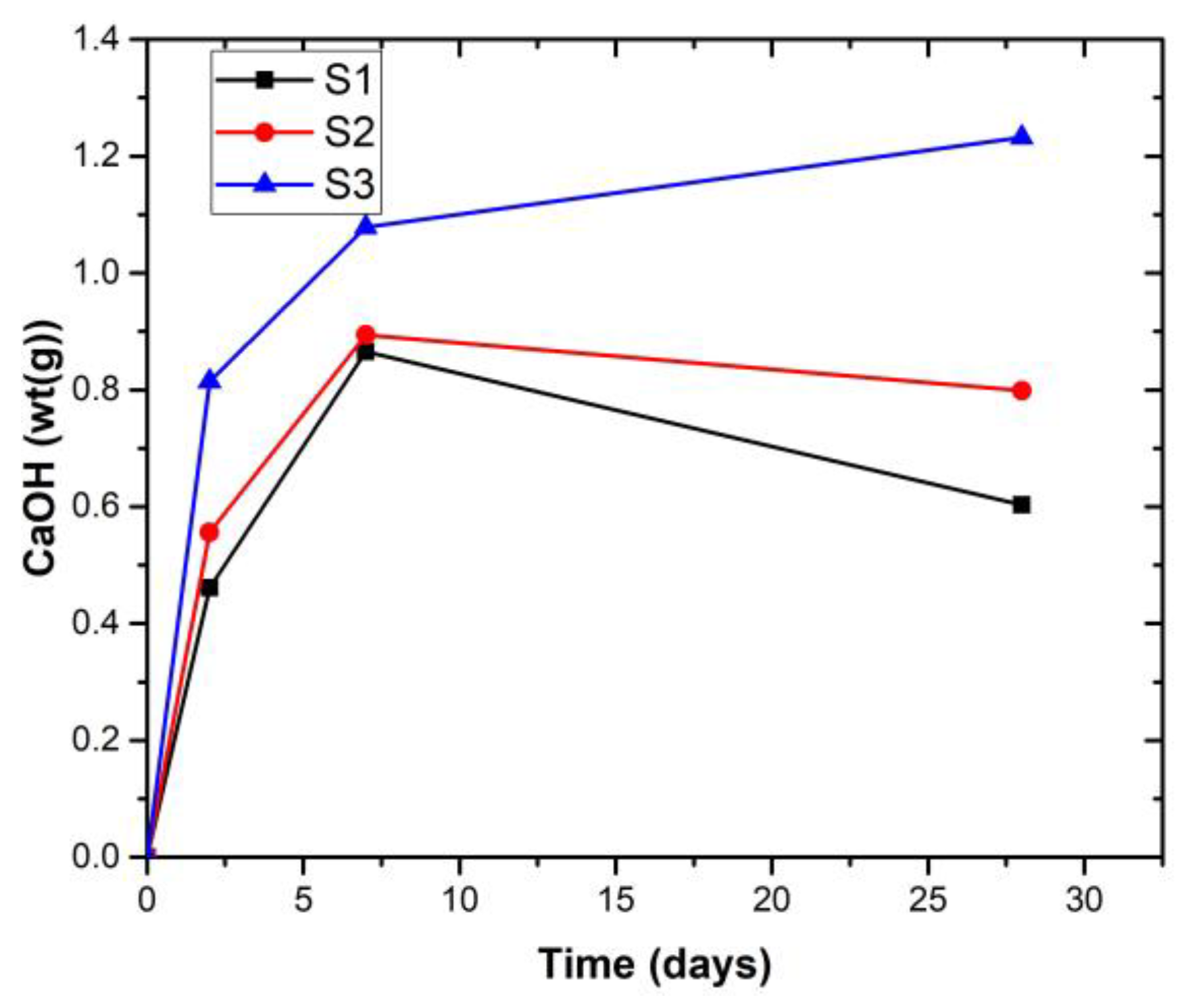
3.3. XRD Analysis of the Hardened Pastes
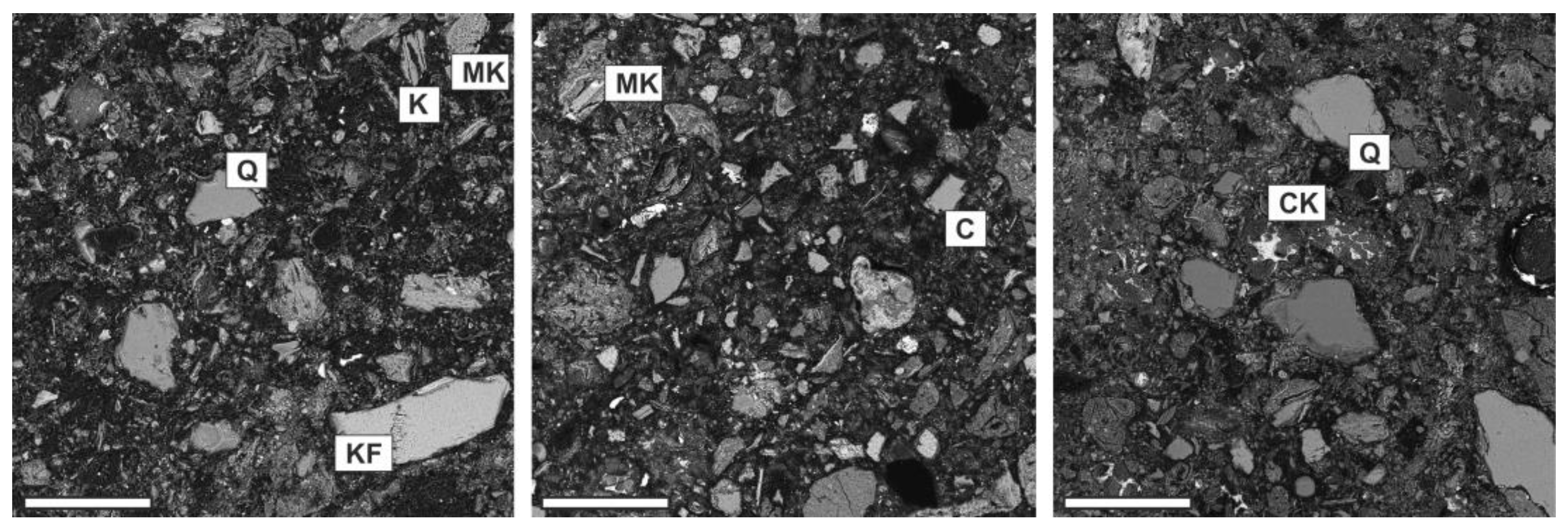
3.4. Microstructural and Microchemical Analyses of Hardened Pastes
4. Conclusions
- A high level of clinker substitution with calcined clay and kunkur fines was studied. The highest compressive strength for the ternary blends was 30 MPa at day 28. This was a cement blend with a composition consisting of 40% clinker + 5% gypsum + 40% calcined clay + 15% kunkur fines.
- Kunkur fines can possibly replace primary limestone in ternary blends, owing to their relatively high calcite content. The partial reactivity of this material is testified by the formation of hemicarboaluminate. However, the presence of kaolinite in the fines may hinder the overall performance, e.g., by increasing the water demand in the mix.
- The presence of kunkur fines in the ternary blends leads to similar hydration products as those occurring in LC3 containing limestone.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UN Environment; Scrivener, K.; John, V.M.; Gartner, E.M. Eco-efficient Cements: Potential Economically Viable Solutions for a Low-CO2 Cement-based Materials Industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Tennis, P.D.; Thomas, M.D.A.; Weiss, W.J. State-of-the-Art Report on Use of Limestone in Cements at Levels of up to 15%; PCA R&D SN3148; Portland Cement Association: Skokie, IL, USA, 2011. [Google Scholar]
- Scrivener, K.L. Options for the Future of Cement. Indian Concr. J. 2014, 88, 11–21. [Google Scholar]
- Machner, A.; Zajac, M.; Haha, M.B.; Kjellsen, K.O.; Geiker, M.R.; De Weerdt, K. Limitations of the Hydrotalcite Formation in Portland Composite Cement Pastes Containing Dolomite and Metakaolin. Cem. Concr. Res. 2018, 105, 1–17. [Google Scholar] [CrossRef]
- Oey, T.; Kumar, A.; Bullard, J.W.; Neithalath, N.; Sant, G. The Filler Effect: The Influence of Filler Content and Surface Area on Cementitious Reaction Rates. J. Am. Ceram. Soc. 2013, 96, 1978–1990. [Google Scholar] [CrossRef]
- Imbabi, M.S.; Carrigan, C.; McKenna, S. Trends and Developments in Green Cement and Concrete Technology. Int. J. Sustain. Built Environ. 2012, 1, 194–216. [Google Scholar] [CrossRef]
- Błaszczyński, T.; Król, M. Usage of Green Concrete Technology in Civil Engineering. Procedia Eng. 2015, 122, 296–301. [Google Scholar] [CrossRef]
- Habert, G.; Miller, S.A.; John, V.M.; Provis, J.L.; Favier, A.; Horvath, A.; Scrivener, K.L. Environmental Impacts and Decarbonization Strategies in the Cement and Concrete Industries. Nat. Rev. Earth Environ. 2020, 1, 559–573. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined Clay Limestone Cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Dhandapani, Y.; Sakthivel, T.; Santhanam, M.; Gettu, R.; Pillai, R.G. Mechanical Properties and Durability Performance of Concretes with Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2018, 107, 136–151. [Google Scholar] [CrossRef]
- Hanein, T.; Thienel, K.-C.; Zunino, F.; Marsh, A.; Maier, M.; Wang, B.; Canut, M.; Juenger, M.C.; Ben Haha, M.; Avet, F. Clay Calcination Technology: State-of-the-Art Review by the RILEM TC 282-CCL. Mater. Struct. 2022, 55, 3. [Google Scholar] [CrossRef]
- Medepalli, S.; Shah, V.; Bishnoi, S. Production of Lab Scale Limestone Calcined Clay Cements Using Low Grade Limestone. In Proceedings of the 7th International Conference on Sustainable Built Environment, Kandy, Sri Lanka, 16–18 December 2016. [Google Scholar]
- Zolfagharnasab, A.; Ramezanianpour, A.A.; Bahman-Zadeh, F. Investigating the Potential of Low-Grade Calcined Clays to Produce Durable LC3 Binders against Chloride Ions Attack. Constr. Build. Mater. 2021, 303, 124541. [Google Scholar] [CrossRef]
- Krishnan, S.; Gopala Rao, D.; Bishnoi, S. Why Low-Grade Calcined Clays Are the Ideal for the Production of Limestone Calcined Clay Cement (LC3). In Calcined Clays for Sustainable Concrete; Springer: Berlin/Heidelberg, Germany, 2020; pp. 125–130. [Google Scholar]
- Krishnan, S.; Emmanuel, A.C.; Shah, V.; Parashar, A.; Mishra, G.; Maity, S.; Bishnoi, S. Industrial Production of Limestone Calcined Clay Cement: Experience and Insights. Green Mater. 2019, 7, 15–27. [Google Scholar] [CrossRef]
- Emmanuel, A.C.; Haldar, P.; Maity, S.; Bishnoi, S. Second Pilot Production of Limestone Calcined Clay Cement in India: The Experience. Indian Concr. J. 2016, 90, 57–63. [Google Scholar]
- Danner, T.; Justnes, H.; Norden, G.; Østnor, T. Feasibility of Calcined Marl as an Alternative Pozzolanic Material. In Calcined Clays for Sustainable Concrete; Springer: Berlin/Heidelberg, Germany, 2015; pp. 67–73. [Google Scholar]
- Beuntner, N.; Thienel, K.C. Properties of Calcined Lias Delta Clay—Technological Effects, Physical Characteristics and Reactivity in Cement. In Calcined Clays for Sustainable Concrete; Springer: Berlin/Heidelberg, Germany, 2015; pp. 43–50. [Google Scholar]
- Bullerjahn, F.; Zajac, M.; Pekarkova, J.; Nied, D. Novel SCM Produced by the Co-Calcination of Aluminosilicates with Dolomite. Cem. Concr. Res. 2020, 134, 106083. [Google Scholar] [CrossRef]
- Marangu, J.M. Physico-Chemical Properties of Kenyan Made Calcined Clay-Limestone Cement (LC3). Case Stud. Constr. Mater. 2020, 12, e00333. [Google Scholar] [CrossRef]
- Marangu, J.M.; Latif, E.; Maddalena, R. Evaluation of the Reactivity of Selected Rice Husk Ash-Calcined Clay Mixtures for Sustainable Cement Production; Maddalena, R., Wright-Syed, M., Eds.; Cardiff University: Cardiff, Wales, 2021; pp. 81–84. [Google Scholar]
- Rahhal, V.; Pavlík, Z.; Trezza, M.; Castellano, C.; Tironi, A.; Kulovaná, T.; Pokorný, J.; Černý, R.; Irassar, E.F. Red Ceramic Wastes: A Calcined Clay Pozzolan. In Calcined Clays for Sustainable Concrete; Springer: Berlin/Heidelberg, Germany, 2015; pp. 179–187. [Google Scholar]
- Bentz, D.P.; Ferraris, C.F. Rheology and Setting of High Volume Fly Ash Mixtures. Cem. Concr. Compos. 2010, 32, 265–270. [Google Scholar] [CrossRef]
- Castellano, C.C.; Bonavetti, V.L.; Donza, H.A.; Irassar, E.F. The Effect of w/b and Temperature on the Hydration and Strength of Blastfurnace Slag Cements. Constr. Build. Mater. 2016, 111, 679–688. [Google Scholar] [CrossRef]
- Bonavetti, V.L.; Rahhal, V.F.; Irassar, E.F. Studies on the Carboaluminate Formation in Limestone Filler-Blended Cements. Cem. Concr. Res. 2001, 31, 853–859. [Google Scholar] [CrossRef]
- De Weerdt, K.; Kjellsen, K.O.; Sellevold, E.; Justnes, H. Synergy between Fly Ash and Limestone Powder in Ternary Cements. Cem. Concr. Compos. 2011, 33, 30–38. [Google Scholar] [CrossRef]
- Moesgaard, M.; Herfort, D.; Steenberg, M.; Kirkegaard, L.F.; Yue, Y. Physical Performances of Blended Cements Containing Calcium Aluminosilicate Glass Powder and Limestone. Cem. Concr. Res. 2011, 41, 359–364. [Google Scholar] [CrossRef]
- Cost, V.T.; Aci, F. Concrete Sustainability versus Constructability—Closing the Gap. In Proceedings of the 2011 International Concrete Sustainability Conference, Boston, MA, USA, 9–11 August 2011. [Google Scholar]
- Costa, E.B.C.; Cardoso, F.A.; John, V.M. Influence of High Contents of Limestone Fines on Rheological Behaviour and Bond Strength of Cement-Based Mortars. Constr. Build. Mater. 2017, 156, 1114–1126. [Google Scholar] [CrossRef]
- Sato, T.; Beaudoin, J.J. Effect of Nano-CaCO3 on Hydration of Cement Containing Supplementary Cementitious Materials. Adv. Cem. Res. 2011, 23, 33–43. [Google Scholar] [CrossRef]
- Ferrari, G.; Brocchi, A.; Castiglioni, F.; Bravo, A.; Moretti, E.; Salvioni, D.; Squinzi, M.; Artioli, G.; Dalconi, M.C.; Valentini, L. A New Multifunctional Additive for Blended Cements. Constr. Build. Mater. 2022, 354, 129086. [Google Scholar] [CrossRef]
- Nair, N.; Haneefa, K.M.; Santhanam, M.; Gettu, R. A Study on Fresh Properties of Limestone Calcined Clay Blended Cementitious Systems. Constr. Build. Mater. 2020, 254, 119326. [Google Scholar] [CrossRef]
- Sposito, R.; Beuntner, N.; Thienel, K.-C. Characteristics of Components in Calcined Clays and Their Influence on the Efficiency of Superplasticizers. Cem. Concr. Compos. 2020, 110, 103594. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Wang, Z. Optimizing Design of High Strength Cement Matrix with Supplementary Cementitious Materials. Constr. Build. Mater. 2016, 120, 123–136. [Google Scholar] [CrossRef]
- Fernández, Á.; Calvo, J.G.; Alonso, M.C. Ordinary Portland Cement Composition for the Optimization of the Synergies of Supplementary Cementitious Materials of Ternary Binders in Hydration Processes. Cem. Concr. Compos. 2018, 89, 238–250. [Google Scholar] [CrossRef]
- Eren, M. Caliche Formation and Features. Jeol. Mühendisliği Derg. 2006, 30, 1–8. [Google Scholar]
- Gareth, H. Mapping calcretes in Inhambane province, Mozambique, for use in road construction. In Bulletin of Engineering Geology and the Environment; Springer: Berlin/Heidelberg, Germany, 2015; pp. 405–426. Available online: https://link.springer.com/article/10.1007/s10064-014-0688-3 (accessed on 19 February 2023).
- Netterberg, F. Low-Cost Local Road Materials in Southern Africa. Geotech. Geol. Eng. 1994, 12, 35–42. [Google Scholar] [CrossRef]
- Reeves, C.C.; Suggs, J.D. Caliche of Central and Southern Llano Estacado, Texas. J. Sediment. Res. 1964, 34, 669–672. [Google Scholar] [CrossRef]
- Mutua, M.G. Investigation of Matisaa Gray Rock as a Potential Raw Material for the Manufacture of Cement. Master’s Thesis, Maasai Mara University, Narok, Kenya, 2020. [Google Scholar]
- Geoffrey, M.; Isaac, M.; Fredrick, O. Parametric Study of Matisaa Gray Rock as a Potential Clinker Material. Asian J. Adv. Res. Rep. 2020, 14, 22–29. [Google Scholar] [CrossRef]
- EN 196-1:2016; Methods of Testing Cement—Part 1: Determination of Strength. European Committee for Standardization: Brussels, Belgium, 2016.
- KS EAS 148-2:2017; Cement—Test Methods Part 2:Chemical Composition. Kenya Bureau of Standards: Nairobi, Kenya, 2017.
- Leardi, R. Experimental Design in Chemistry: A Tutorial. Anal. Chim. Acta 2009, 652, 161. [Google Scholar] [CrossRef]
- KS EAS 18-1:2017; Cement Part 1: Composition, Specification and Conformity Criteria for Common Cements. Kenya Bureau of Standards: Nairobi, Kenya, 2017.
- KS EAS 18-2:2017; Cement Part 2:Conformity Evaluation. Kenya Bureau of Standards: Nairobi, Kenya, 2017.
- Sui, H.; Hou, P.; Liu, Y.; Sagoe-Crentsil, K.; de Souza, F.B.; Duan, W. Limestone Calcined Clay Cement: Mechanical Properties, Crystallography, and Microstructure Development. J. Sustain. Cem.-Based Mater. 2022, 12, 427–440. [Google Scholar] [CrossRef]
- Snell, L. Caliche: Nature’s Natural Concrete. Concr. Int. 2016, 38, 70–71. [Google Scholar]
- Gameiro, A.; Silva, A.S.; Faria, P.; Grilo, J.; Branco, T.; Veiga, R.; Velosa, A. Physical and Chemical Assessment of Lime–Metakaolin Mortars: Influence of Binder: Aggregate Ratio. Cem. Concr. Compos. 2014, 45, 264–271. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Q.; Chen, Q.; Wang, D.; Guo, H.; Liu, L.; Yang, Q. Hydration and Strength Development in Blended Cement with Ultrafine Granulated Copper Slag. PLoS ONE 2019, 14, e0215677. [Google Scholar] [CrossRef]
- Zunino, F.; Scrivener, K. The Reaction between Metakaolin and Limestone and Its Effect in Porosity Refinement and Mechanical Properties. Cem. Concr. Res. 2021, 140, 106307. [Google Scholar] [CrossRef]
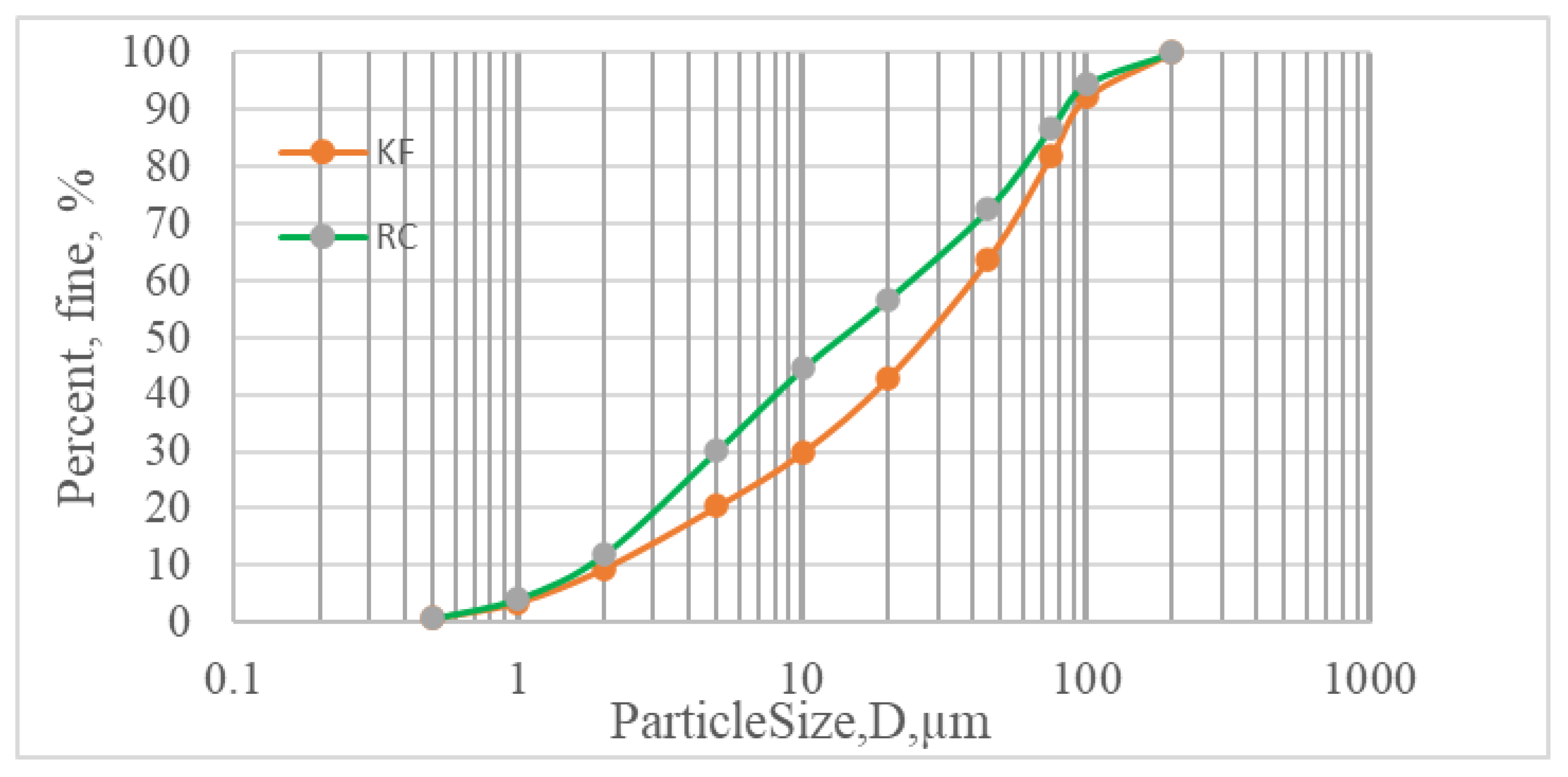
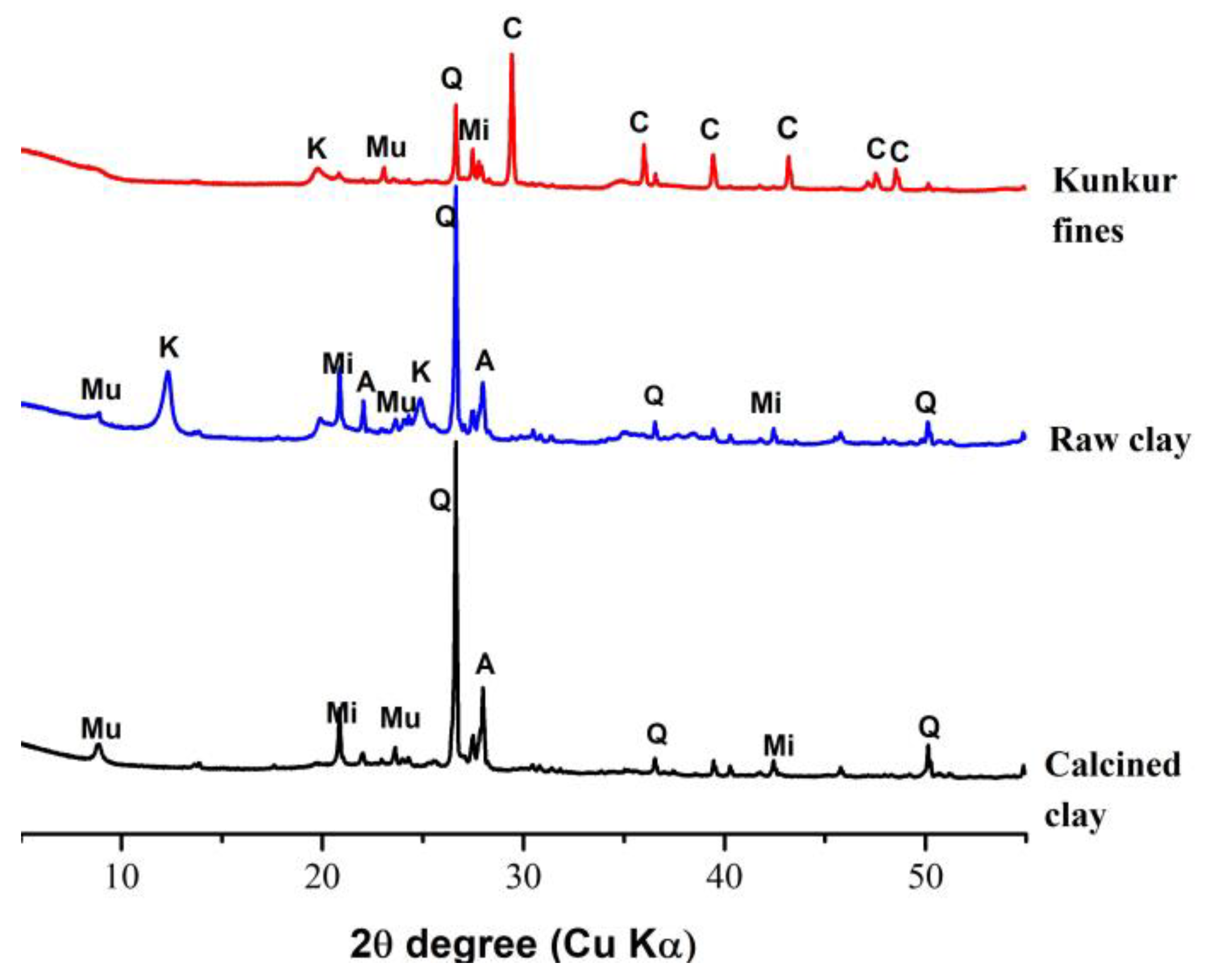
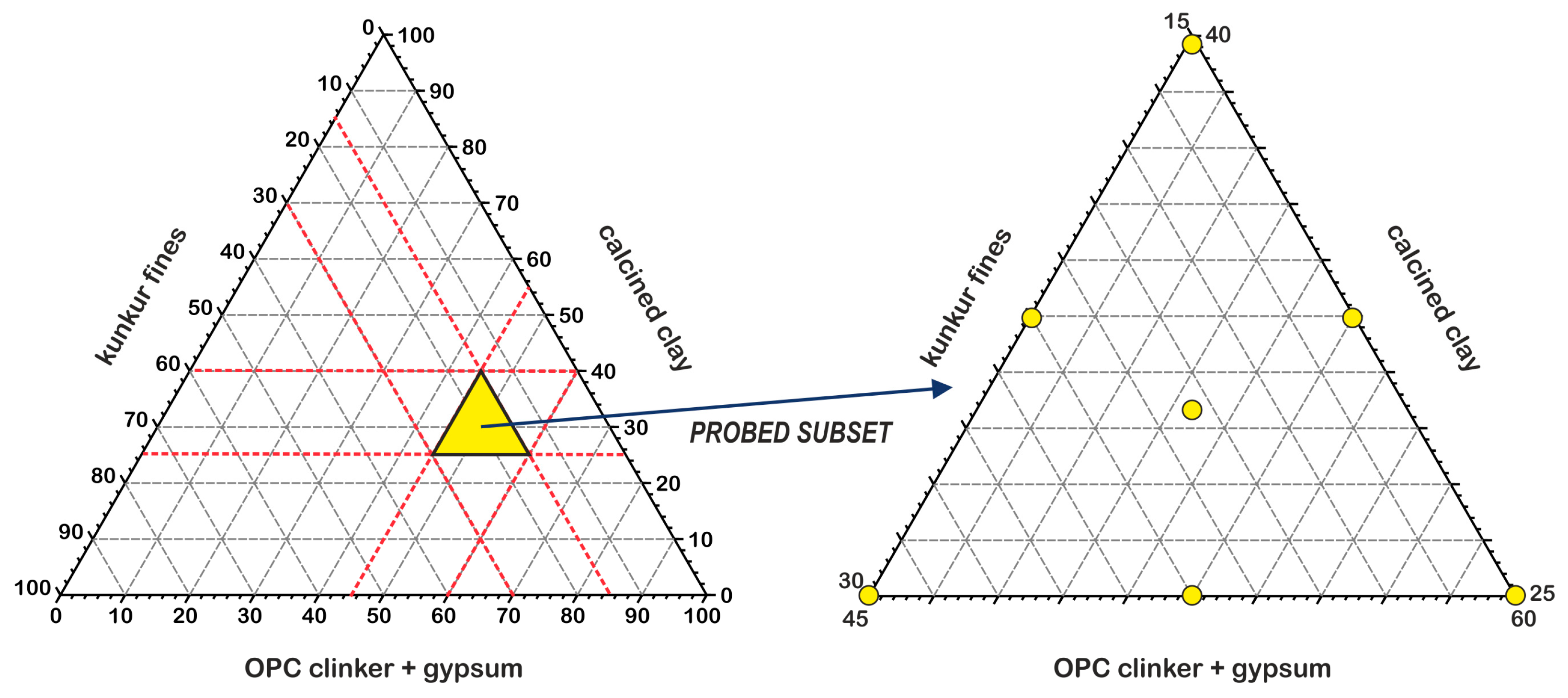

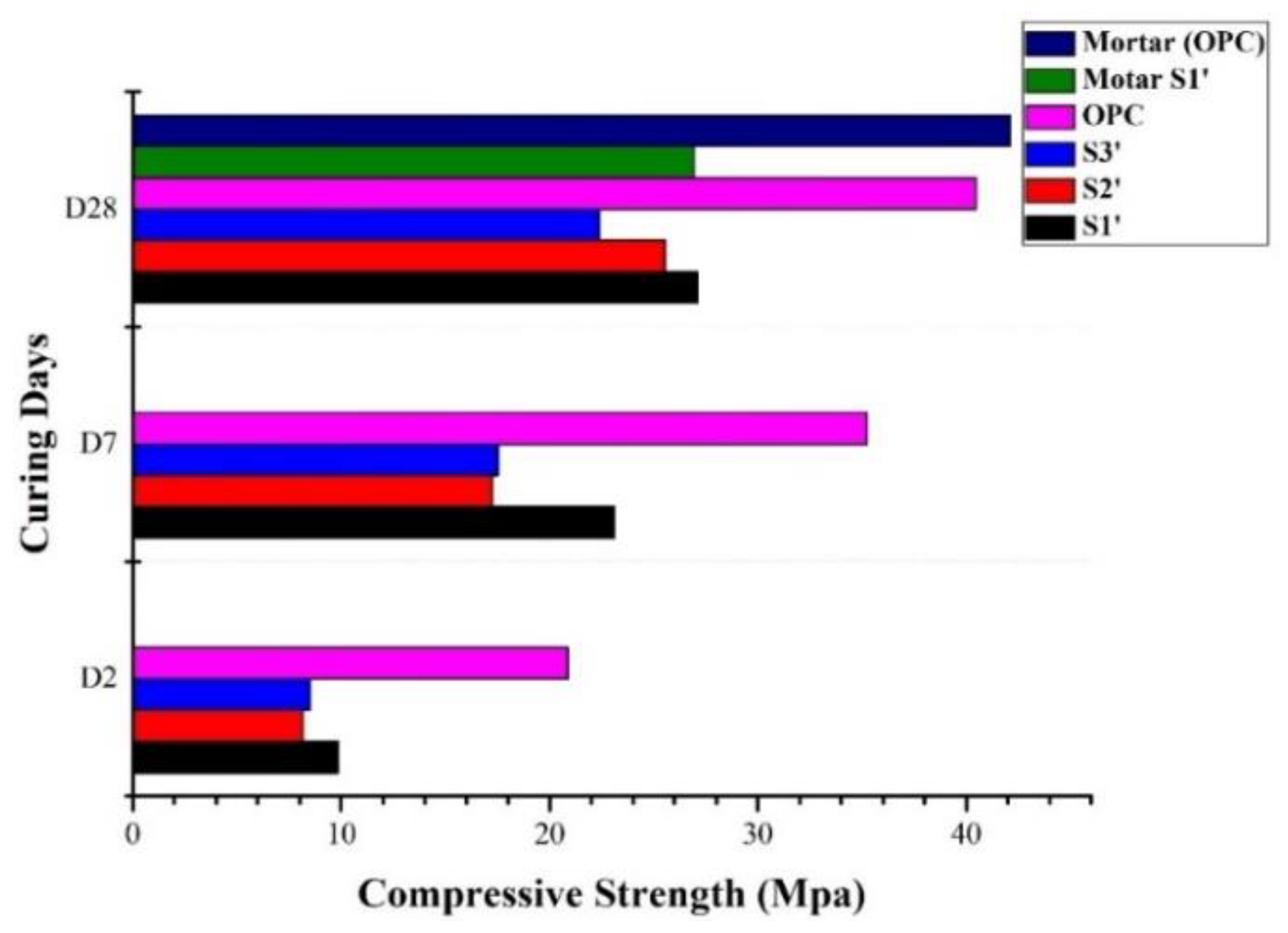

| Chemical Composition (w%) | Clinker | Calcined Clay | Gypsum | Kunkur Fines |
|---|---|---|---|---|
| SiO2 | 21.3 | 59.3 | 7.2 | 52.3 |
| Al2O3 | 6.3 | 29.5 | 1.3 | 9.6 |
| Fe2O3 | 3.7 | 4.8 | 0.8 | 7.9 |
| CaO | 62.2 | 0.7 | 29.5 | 14.5 |
| SO3 | 1.5 | - | 40.4 | 0.2 |
| MgO | 3.9 | 2.1 | 0.3 | 3.22 |
| K2 O | 1.0 | 2.4 | 0.34 | 1.12 |
| Na2O | 0.4 | 1.2 | - | - |
| Cl | - | - | - | 0.01 |
| L.O.I. | 0.8 |
| Mix | Clinker (%) | KFs (%) | CC (%) | GY (%) |
|---|---|---|---|---|
| S1 | 55.0 | 15.0 | 25.0 | 5.0 |
| S2 | 40.0 | 15.0 | 40.0 | 5.0 |
| S3 | 40.0 | 30.0 | 25.0 | 5.0 |
| S4 | 40.0 | 22.5 | 32.5 | 5.0 |
| S5 | 47.5 | 22.5 | 25.0 | 5.0 |
| S6 | 47.5 | 15.0 | 32.5 | 5.0 |
| S7 | 45.0 | 20.0 | 30.0 | 5.0 |
| S8 | 95.0 | - | - | 5.0 |
| Mix | Clinker (%) | KFs (%) | CC (%) | GY (%) |
|---|---|---|---|---|
| S1′ | 41.0 | 16.0 | 38.0 | 5.0 |
| S2′ | 42.0 | 26.0 | 27.0 | 5.0 |
| S3′ | 45.0 | 18.0 | 32.0 | 5.0 |
| OPC | 95.0 | 5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutai, V.K.; Marangu, J.M.; M’Thiruaine, C.M.; Valentini, L. Evaluation of Kunkur Fines for Utilization in the Production of Ternary Blended Cements. Sustainability 2023, 15, 16453. https://doi.org/10.3390/su152316453
Mutai VK, Marangu JM, M’Thiruaine CM, Valentini L. Evaluation of Kunkur Fines for Utilization in the Production of Ternary Blended Cements. Sustainability. 2023; 15(23):16453. https://doi.org/10.3390/su152316453
Chicago/Turabian StyleMutai, Victor Kiptoo, Joseph Mwiti Marangu, Cyprian Muturia M’Thiruaine, and Luca Valentini. 2023. "Evaluation of Kunkur Fines for Utilization in the Production of Ternary Blended Cements" Sustainability 15, no. 23: 16453. https://doi.org/10.3390/su152316453
APA StyleMutai, V. K., Marangu, J. M., M’Thiruaine, C. M., & Valentini, L. (2023). Evaluation of Kunkur Fines for Utilization in the Production of Ternary Blended Cements. Sustainability, 15(23), 16453. https://doi.org/10.3390/su152316453









