Harnessing the Synergy of the Cyanobacteria-Plant Growth Promoting Bacteria for Improved Maize (Zea mays) Growth and Soil Health
Abstract
:1. Introduction
2. Materials and Methods
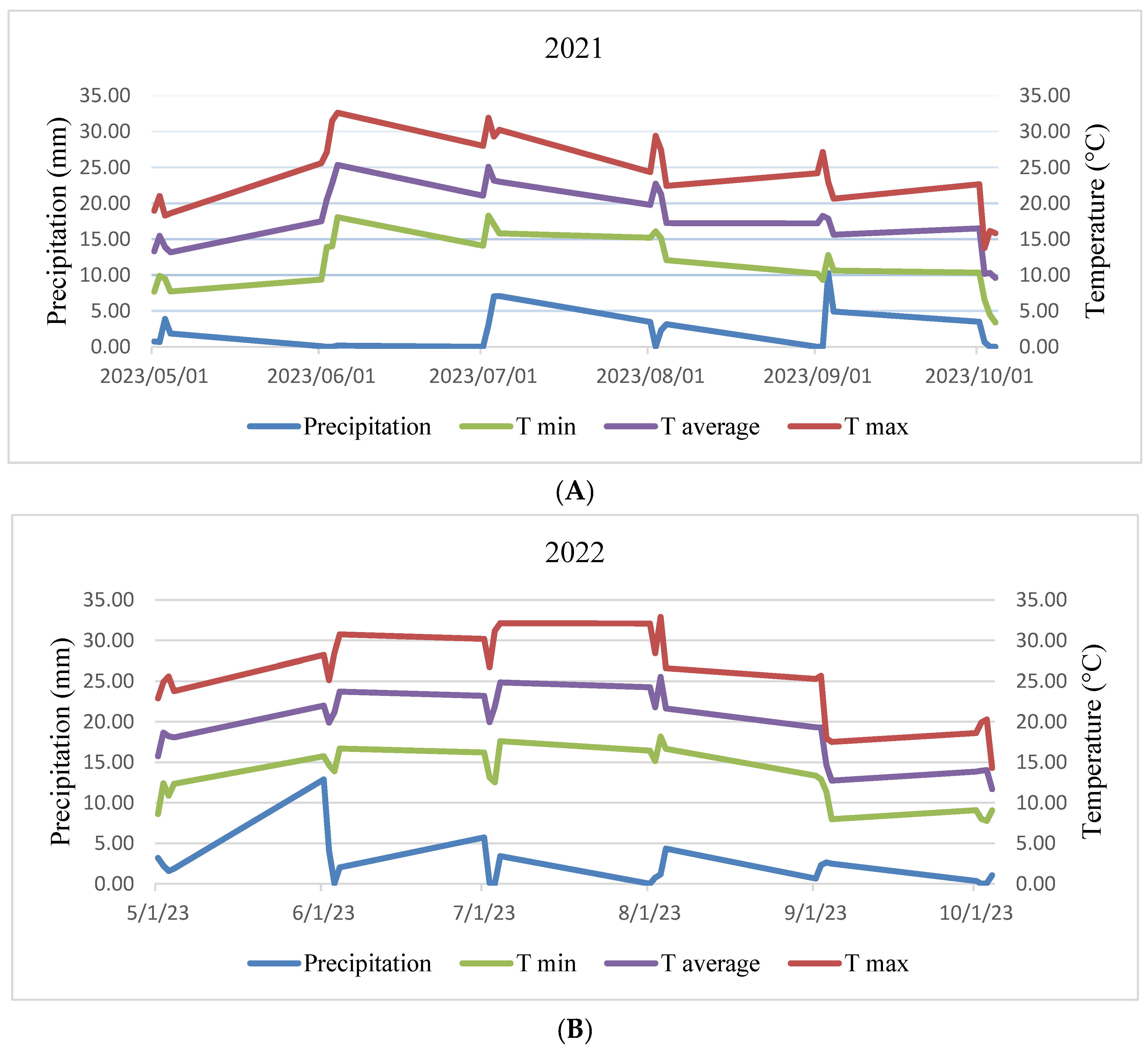
2.1. Experimental Site and Design
2.2. Plant Yield Attributes
2.3. Soil Collection and Analysis
2.4. Soil Microbial Biomass Evaluation
2.5. Statistical Analysis
3. Results
3.1. Plant Yield Parameters
3.2. Soil Chemical Properties
3.3. Microbial Activity of the Soil
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.; Jagota, N.; Kaur, H.; Kaur, R.; Kaur, G.; Sandhu, S.; Sharma, A. Deciphering behavioral changes in maize plants in a quest to identify species specific plant growth promoting rhizobacteria. Total. Environ. Res. Themes 2023, 6, 100043. [Google Scholar] [CrossRef]
- Grote, U.; Fasse, A.; Nguyen, T.T.; Erenstein, O. food security and the dynamics of wheat and maize value chains in Africa and Asia. Front. Sustain. Food Syst. 2021, 4, 617009. [Google Scholar] [CrossRef]
- Poole, N.; Donovan, J.; Erenstein, O. Viewpoint: Agri-nutrition research: Revisiting the contribution of maize and wheat to human nutrition and health. Food Policy 2021, 100, 101976. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.P.; Yadav, G.S.; Jhariya, M.K.; Jangir, C.K.; et al. Impact of agrochemicals on soil microbiota and management: A review. Land 2020, 9, 34. [Google Scholar] [CrossRef]
- Mahmud, K.; Missaoui, A.; Lee, K.; Ghimire, B.; Presley, H.W.; Makaju, S. Rhizosphere microbiome manipulation for sustainable crop production. Curr. Plant Biol. 2021, 27, 100210. [Google Scholar] [CrossRef]
- Sharma, S.; Kumawat, K.C. Role of rhizospheric microbiome in enhancing plant attributes and soil health for sustainable agriculture. In Core Microbiome; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; pp. 139–162. [Google Scholar] [CrossRef]
- Jaiswal, S.K.; Mohammed, M.; Ibny, F.Y.I.; Dakora, F.D. Rhizobia as a source of plant growth-promoting molecules: Potential applications and possible operational mechanisms. Front. Sustain. Food Syst. 2021, 4, 619676. [Google Scholar] [CrossRef]
- Chen, C.; Wang, M.; Zhu, J.; Tang, Y.; Zhang, H.; Zhao, Q.; Jing, M.; Chen, Y.; Xu, X.; Jiang, J.; et al. Long-term effect of epigenetic modification in plant–microbe interactions: Modification of DNA methylation induced by plant growth-promoting bacteria mediates promotion process. Microbiome 2022, 10, 36. [Google Scholar] [CrossRef]
- Khalil, A.T.; Shinwari, Z.K. Utilization of plant growth-promoting bacteria (pgpb) against phytopathogens. In Antifungal Metabolites of Rhizobacteria for Sustainable Agriculture; Sayyed, R.Z., Singh, A., Ilyas, N., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 53–63. [Google Scholar] [CrossRef]
- Eman, E.; Magdi, A.E.A.; Hassan, E.F. Perspective chapter: Cyanobacteria—A futuristic effective tool in sustainable agriculture. In Cyanobacteria; Archana, T., Ed.; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar] [CrossRef]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 2016, 7, 529. [Google Scholar] [CrossRef]
- Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Megharaj, M. Soil microalgae and cyanobacteria: The biotechnological potential in the maintenance of soil fertility and health. Crit. Rev. Biotechnol. 2019, 39, 981–998. [Google Scholar] [CrossRef]
- Vinoth, M.; Sivasankari, S.; Ahamed, A.K.K.; Al-Arjani, A.-B.F.; Abd_Allah, E.F.; Baskar, K. Biological soil crust (BSC) is an effective biofertilizer on Vigna mungo (L.). Saudi J. Biol. Sci. 2020, 27, 2325–2332. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Subramanian, J.; Gopalsamy, J.; Jayasingam, P.; Arumugam, A.; Kannadasan, S.; Sampathkumar, P. The impact of using microalgae as biofertilizer in maize (Zea mays L.). Waste Biomass- Valorization 2019, 10, 1101–1110. [Google Scholar] [CrossRef]
- Ramakrishnan, B.; Maddela, N.R.; Venkateswarlu, K.; Megharaj, M. Potential of microalgae and cyanobacteria to improve soil health and agricultural productivity: A critical view. Environ. Sci. Adv. 2023, 2, 586–611. [Google Scholar] [CrossRef]
- Glaser, K.; Albrecht, M.; Baumann, K.; Overmann, J.; Sikorski, J. Biological soil crust from mesic forests promote a specific bacteria community. Front. Microbiol. 2022, 13, 769767. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; Peyton, B.M.; Gardner, R.D. Microalgae, soil and plants: A critical review of microalgae as renewable resources for agriculture. Algal Res. 2021, 54, 102200. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, M.; Shim, C.; Bae, S.; Jang, S. Potential of algae–bacteria synergistic effects on vegetable production. Front. Plant Sci. 2021, 12, 656662. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Bo, Y.; Feng, Y.; Tan, Y.; Zhou, C.; Yan, X.; Ruan, R.; Xu, Q.; Cheng, P. Potential applications for multifunctional microalgae in soil improvement. Front. Environ. Sci. 2022, 10, 1035332. [Google Scholar] [CrossRef]
- Lee, S.-M.; Ryu, C.-M. Algae as New Kids in the Beneficial Plant Microbiome. Front. Plant Sci. 2021, 12, 599742. [Google Scholar] [CrossRef]
- Berthon, J.-Y.; Michel, T.; Wauquier, A.; Joly, P.; Gerbore, J.; Filaire, E. Seaweed and microalgae as major actors of blue biotechnology to achieve plant stimulation and pest and pathogen biocontrol—A review of the latest advances and future prospects. J. Agric. Sci. 2021, 159, 523–534. [Google Scholar] [CrossRef]
- Reed, L.; Glick, B.R. The recent use of plant-growth-promoting bacteria to promote the growth of agricultural food crops. Agriculture 2023, 13, 1089. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Tavasolee, A.; Ghassemi-Golezani, K.; Torabian, S.; Monirifar, H.; Rahmani, H.A. Growth-promoting bacteria and natural regulators mitigate salt toxicity and improve rapeseed plant performance. Protoplasma 2020, 257, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Barua, N.; Clouse, K.M.; Diaz, D.A.R.; Wagner, M.R.; Platt, T.G.; Hansen, R.R. Screening the maize rhizobiome for consortia that improve Azospirillum brasilense root colonization and plant growth outcomes. Front. Sustain. Food Syst. 2023, 7, 1106528. [Google Scholar] [CrossRef]
- Khan, M.Y.; Nadeem, S.M.; Sohaib, M.; Waqas, M.R.; Alotaibi, F.; Ali, L.; Zahir, Z.A.; Al-Barakah, F.N.I. Potential of plant growth promoting bacterial consortium for improving the growth and yield of wheat under saline conditions. Front. Microbiol. 2022, 13, 958522. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mei, S.; Salles, J.F. Inoculated microbial consortia perform better than single strains in living soil: A meta-analysis. Appl. Soil Ecol. 2023, 190, 105011. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, L.M.; De-Bashan, L.E. The potential of microalgae–bacteria consortia to restore degraded soils. Biology 2023, 12, 693. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.L.; Garbayo, I.; Cuaresma, M.; Montero, Z.; González-Del-Valle, M.; Vílchez, C. Impact of microalgae-bacteria interactions on the production of algal biomass and associated compounds. Mar. Drugs 2016, 14, 100. [Google Scholar] [CrossRef]
- González-González, L.M.; De-Bashan, L.E. Toward the enhancement of microalgal metabolite production through microalgae–bacteria consortia. Biology 2021, 10, 282. [Google Scholar] [CrossRef]
- Solomon, W.; Mutum, L.; Janda, T.; Molnár, Z. Potential benefit of microalgae and their interaction with bacteria to sustainable crop production. Plant Growth Regul. 2023, 101, 53–65. [Google Scholar] [CrossRef]
- Amin, S.A.; Hmelo, L.R.; Van Tol, H.M.; Durham, B.P.; Carlson, L.T.; Heal, K.R.; Morales, R.L.; Berthiaume, C.T.; Parker, M.S.; Djunaedi, B.; et al. Interaction and signalling between a cosmopolitan phytoplankton and associated bacteria. Nature 2015, 522, 98–101. [Google Scholar] [CrossRef]
- Kim, B.-H.; Ramanan, R.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S. Role of rhizobium, a plant growth promoting bacterium, in enhancing algal biomass through mutualistic interaction. Biomass- Bioenergy 2014, 69, 95–105. [Google Scholar] [CrossRef]
- Bunbury, F.; Deery, E.; Sayer, A.P.; Bhardwaj, V.; Harrison, E.L.; Warren, M.J.; Smith, A.G. Exploring the onset of B12 -based mutualisms using a recently evolved Chlamydomonas auxotroph and B12-producing bacteria. Environ. Microbiol. 2022, 24, 3134–3147. [Google Scholar] [CrossRef]
- Smith, M.J.; Francis, M.B. A Designed A. vinelandii–S. elongatus coculture for chemical photoproduction from air, water, phosphate, and trace metals. ACS Synth. Biol. 2016, 5, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Chittora, D.; Meena, M.; Barupal, T.; Swapnil, P.; Sharma, K. Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem. Biophys. Rep. 2020, 22, 100737. [Google Scholar] [CrossRef]
- Prasanna, R.; Hossain, F.; Babu, S.; Bidyarani, N.; Adak, A.; Verma, S.; Shivay, Y.S.; Nain, L. Prospecting cyanobacterial formulations as plant-growth-promoting agents for maize hybrids. S. Afr. J. Plant Soil 2015, 32, 199–207. [Google Scholar] [CrossRef]
- Ördög, V.; Stirk, W.; Takács, G.; Pőthe, P.; Illés, A..; Bojtor, C.; Széles, A.; Tóth, B.; van Staden, J.; Nagy, J. Plant biostimulating effects of the cyanobacterium Nostoc piscinale on maize (Zea mays L.) in field experiments. S. Afr. J. Bot. 2021, 140, 153–160. [Google Scholar] [CrossRef]
- Sharma, V.; Prasanna, R.; Hossain, F.; Muthusamy, V.; Nain, L.; Das, S.; Shivay, Y.S.; Kumar, A. Priming maize seeds with cyanobacteria enhances seed vigour and plant growth in elite maize inbreds. 3 Biotech 2020, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- Santini, G.; Biondi, N.; Rodolfi, L.; Tredici, M.R. Plant biostimulants from cyanobacteria: An emerging strategy to improve yields and sustainability in agriculture. Plants 2021, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Florio, A.; Pommier, T.; Gervaix, J.; Bérard, A.; Le Roux, X. Soil C and N statuses determine the effect of maize inoculation by plant growth-promoting rhizobacteria on nitrifying and denitrifying communities. Sci. Rep. 2017, 7, 8411. [Google Scholar] [CrossRef] [PubMed]
- Walker, V.; Couillerot, O.; Von Felten, A.; Bellvert, F.; Jansa, J.; Maurhofer, M.; Bally, R.; Moënne-Loccoz, Y.; Comte, G. Variation of secondary metabolite levels in maize seedling roots induced by inoculation with Azospirillum, Pseudomonas and Glomus consortium under field conditions. Plant Soil 2012, 356, 151–163. [Google Scholar] [CrossRef]
- Mohanty, P.; Singh, P.K.; Chakraborty, D.; Mishra, S.; Pattnaik, R. Insight into the role of pgpr in sustainable agriculture and environment. Front. Sustain. Food Syst. 2021, 5, 667150. [Google Scholar] [CrossRef]
- Jiang, S.; Li, J.; Wang, Q.; Yin, C.; Zhan, Y.; Yan, Y.; Lin, M.; Ke, X. Maize growth promotion by inoculation with an engineered ammonium-excreting strain of nitrogen-fixing pseudomonasstutzeri. Microorganisms 2022, 10, 1986. [Google Scholar] [CrossRef]
- Ördög, V. Apparatus for Laboratory Algal Bioassay. Int. Revue ges. Hydrobiol. 1982, 67, 127–136. [Google Scholar]
- Takács, G.; Stirk, W.; Gergely, I.; Molnár, Z.; van Staden, J.; Ördög, V. Biostimulating effects of the cyanobacterium Nostoc piscinale on winter wheat in field experiments. S. Afr. J. Bot. 2019, 126, 99–106. [Google Scholar] [CrossRef]
- Tamiya, H. Mass culture of algae. Annu. Rev. Plant Physiol. 1957, 8, 309–334. [Google Scholar] [CrossRef]
- Pansu, M.A.; Pansu, M.B. Handbook of soil analysis of the chapter. In Book Handbook of Soil Analysis; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- ISO 13395:1996; Water Quality—Determination of Nitrite Nitrogen and Nitrate Nitrogen and the Sum of Both by Flow Analysis (CFA and FIA) and Spectrometric Detection. ISO Central Secretariat: Geneva, Switzerland, 1996. Available online: https://infostore.saiglobal.com/en-gb/standards/iso-13395-1996-613022_saig_iso_iso_1406298/ (accessed on 20 August 2023).
- Clark, F.E. Agar-plate method for total microbial count. In Methods of Soil Analysis; Chemical and Microbiological Properties: Madson, NY, USA, 1965; pp. 1460–1466. [Google Scholar] [CrossRef]
- Olsen, R.A.; Bakken, L.R. Viability of soil bacteria: Optimization of plate-counting technique and comparison between total counts and plate counts within different size groups. Microb. Ecol. 1987, 13, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.T.; Goodfellow, M.; Alderson, G.; Wellington, E.M.H.; Sneath, P.H.A.; Sackin, M.J. Numerical classification of streptomyces and related genera. Microbiology 1983, 129, 1743–1813. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M. Most-probable-number method for microbial populations. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1965, 9, 1467–1472. [Google Scholar] [CrossRef]
- R Core Team, R. R: A language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Mendiburu, F.E.D. Agricolae: Statistical Procedures for Agricultural Research. 2023, (Version 1.3-7). Available online: https://cran.r-project.org/web/packages/agricolae/agricolae.pdf (accessed on 24 October 2023).
- FAO. World Food and Agriculture—Statistical Yearbook 2022; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2022. [Google Scholar]
- Alves, D.K.M.; Teixeira, M.B.; Cunha, F.N.; Filho, F.R.C.; Cunha, G.N.; Andrade, C.L.L.D. Grain yield of maize crops under nitrogen fertigation using wastewater from swine and fish farming. Agronomy 2023, 13, 1834. [Google Scholar] [CrossRef]
- Hungria, M.; Barbosa, J.Z.; Rondina, A.B.L.; Nogueira, M.A. Improving maize sustainability with partial replacement of N fertilizers by inoculation with Azospirillum brasilense. Agron. J. 2022, 114, 2969–2980. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Y.; Jiang, X. Seed soaking with bacillus sp. Strain hx-2 alleviates negative effects of drought stress on maize seedlings. Chil. J. Agric. Res. 2019, 79, 396–404. [Google Scholar] [CrossRef]
- Pandey, C.; Dheeman, S.; Prabha, D.; Negi, Y.K.; Maheshwari, D.K. Plant growth-promoting bacteria: Effective tools for increasing nutrient use efficiency and yield of crops. In Endophytes: Mineral Nutrient Management; Maheshwari, D.K., Dheeman, S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; Volume 3, pp. 293–313. [Google Scholar] [CrossRef]
- Di Benedetto, N.A.; Corbo, M.R.; Campaniello, D.; Cataldi, M.P.; Bevilacqua, A.; Sinigaglia, M.; Flagella, Z. The role of plant growth promoting bacteria in improving nitrogen use efficiency for sustainable crop production: A focus on wheat. AIMS Microbiol. 2017, 3, 413–434. [Google Scholar] [CrossRef]
- Múnera-Porras, L.M.; García-Londoño, S.; Ríos-Osorio, L.A. Ríos-Osorio. Action mechanisms of plant growth promoting cyanobacteria in crops in situ: A systematic review of literature. Int. J. Agron. 2020, 2020, 2690410. [Google Scholar] [CrossRef]
- Singh, S.K.; Wu, X.; Shao, C.; Zhang, H. Microbial enhancement of plant nutrient acquisition. Stress Biol. 2022, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Saia, S.; Rappa, V.; Ruisi, P.; Abenavoli, M.R.; Sunseri, F.; Giambalvo, D.; Frenda, A.S.; Martinelli, F. Soil inoculation with symbiotic microorganisms promotes plant growth and nutrient transporter genes expression in durum wheat. Front. Plant Sci. 2015, 6, 815. [Google Scholar] [CrossRef] [PubMed]
- Gavilanes, F.Z.; Andrade, D.S.; Zucareli, C.; Horácio, E.H.; Yunes, J.S.; Barbosa, A.P.; Alves, L.A.R.; Cruzatty, L.G.; Maddela, N.R.; de Fátima Guimarães, M. Co-inoculation of Anabaena cylindrica with Azospirillum brasilense increases grain yield of maize hybrids. Rhizosphere 2020, 15, 100224. [Google Scholar] [CrossRef]
- Vuolo, F.; Novello, G.; Bona, E.; Gorrasi, S.; Gamalero, E. Impact of plant-beneficial bacterial inocula on the resident bacteriome: Current knowledge and future perspectives. Microorganisms 2022, 10, 2462. [Google Scholar] [CrossRef]
- Singh, J.S. Cyanobacteria: A vital bio-agent in eco-restoration of degraded lands and sustainable agriculture. Clim. Change Environ. Sustain. 2014, 2, 133–137. [Google Scholar]
- Mutale-Joan, C.; Sbabou, L.; Hicham, E.A. Microalgae and cyanobacteria: How exploiting these microbial resources can address the underlying challenges related to food sources and sustainable agriculture: A review. J. Plant Growth Regul. 2023, 42, 1–20. [Google Scholar] [CrossRef]
- Prasanna, R.; Hossain, F.; Saxena, G.; Singh, B.; Kanchan, A.; Simranjit, K.; Ramakrishnan, B.; Ranjan, K.; Muthusamy, V.; Shivay, Y.S. Analyses of genetic variability and genotype x cyanobacteria interactions in biofortified maize (Zea mays L.) for their responses to plant growth and physiological attributes. Eur. J. Agron. 2021, 130, 126343. [Google Scholar] [CrossRef]
- Mutum, L.; Janda, T.; Ördög, V.; Molnár, Z. Biologia Futura: Potential of different forms of microalgae for soil improvement. Biol. Futur. 2022, 73, 1–8. [Google Scholar] [CrossRef]
- Kumar, S.; Diksha; Sindhu, S.S.; Kumar, R. Biofertilizers: An ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. [Google Scholar] [CrossRef]
- Schoebitz, M.; Mengual, C.; Roldán, A. Combined effects of clay immobilized Azospirillum brasilense and Pantoea dispersa and organic olive residue on plant performance and soil properties in the revegetation of a semiarid area. Sci. Total. Environ. 2014, 466–467, 67–73. [Google Scholar] [CrossRef]
- Ranjan, K.; Priya, H.; Ramakrishnan, B.; Prasanna, R.; Venkatachalam, S.; Thapa, S.; Tiwari, R.; Nain, L.; Singh, R.; Shivay, Y.S. Cyanobacterial inoculation modifies the rhizosphere microbiome of rice planted to a tropical alluvial soil. Appl. Soil Ecol. 2016, 108, 195–203. [Google Scholar] [CrossRef]
- Uzoh, I.M.; Babalola, O.O. Rhizosphere biodiversity as a premise for application in bio-economy. Agric. Ecosyst. Environ. 2018, 265, 524–534. [Google Scholar] [CrossRef]
- Alobwede, E.; Cotton, A.; Leake, J.R.; Pandhal, J. The fate and distribution of microalgal nitrogen when applied as an agricultural soil fertiliser and its effect on soil microbial communities. Phycology 2022, 2, 297–318. [Google Scholar] [CrossRef]


| Key Assigned to Each Treatment | Treatments |
|---|---|
| MACC0 + B0 | Control or untreated |
| MACC0 + BA | Untreated with N. piscinale, MACC-612 + A. lipoferum |
| MACC0 + BP | Untreated with N. piscinale, MACC-612 + P. fluorescens |
| MACC1 + B0 | 0.3 g/L of N. piscinale, MACC-612 + untreated with bacteria |
| MACC1 + BA | 0.3 g/L of N. piscinale, MACC-612 + A. lipoferum |
| MACC1 + BP | 0.3 g/L of N. piscinale, MACC-612 + P. fluorescens |
| MACC2 + B0 | 1.0 g/L of N. piscinale, MACC-612 + untreated with bacteria |
| MACC2 + BA | 1.0 g/L of N. piscinale, MACC-612 + A. lipoferum |
| MACC2 + BP | 1.0 g/L of N. piscinale, MACC-612 + P. fluorescens |
| Soil Parameters | 2021 | 2022 |
|---|---|---|
| pH (KCl) | 7.29 ± 0.01 | 7.33 ± 0.02 |
| Humus (m/m%) | 2.06 ± 0.47 | 1.91 ± 0.23 |
| (NO3− + NO2−)-N (mg/kg) | 9.81 ± 1.12 | 10.24 ± 0.89 |
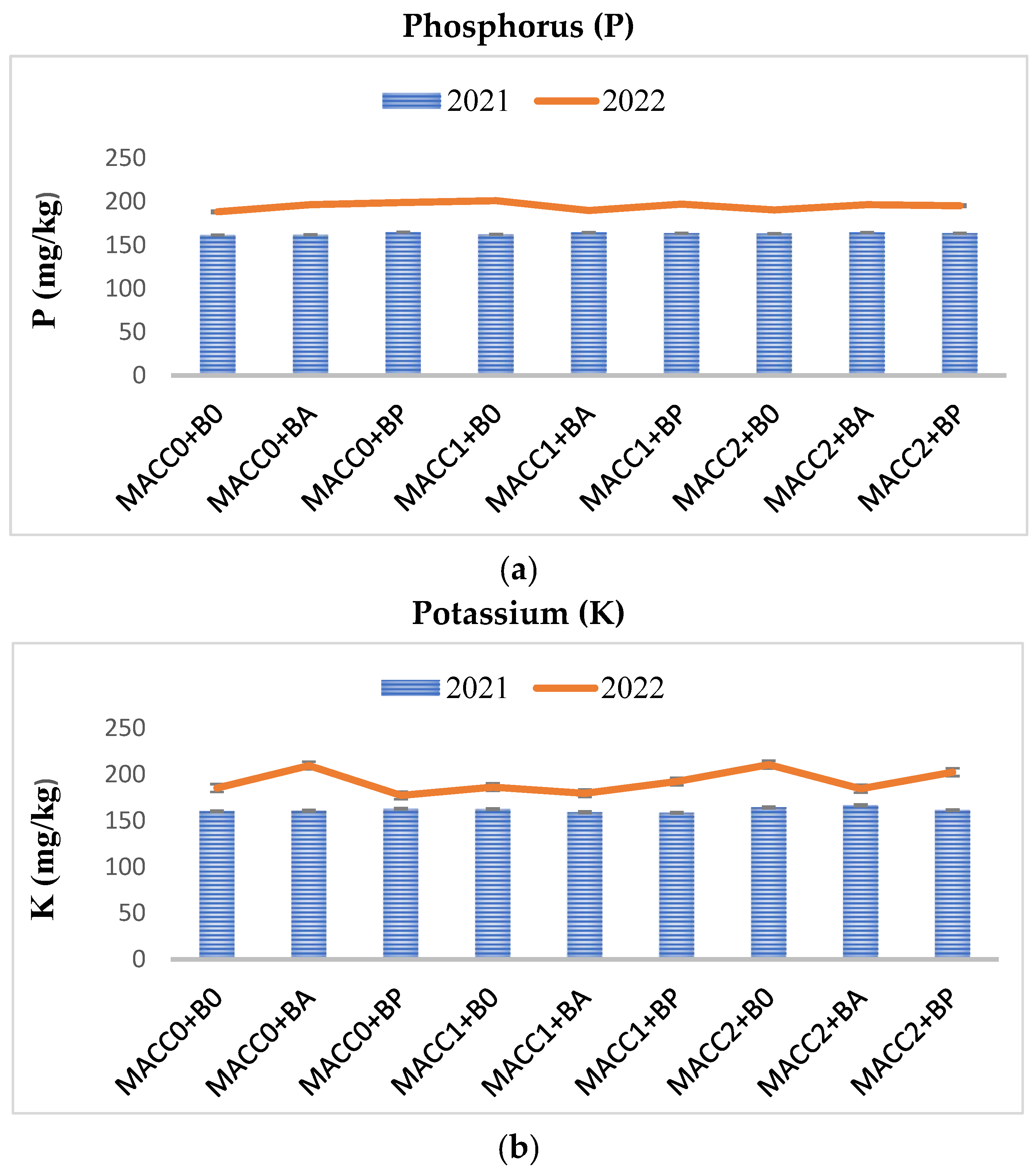
| P (mg/kg) | 158 ± 24.6 | 187 ± 17.55 |
| K (mg/kg) | 176 ± 10.89 | 193 ± 16.7 |
| Treatment | Plant Height (cm) | Number of Seeds/Ears | Thousand Kernel Weight (kg) | Yield (ton/ha) | ||||
|---|---|---|---|---|---|---|---|---|
| 2021 | 2022 | 2021 | 2022 | 2021 | 2022 | 2021 | 2022 | |
| MACC0 + B0 | 213.15 ± 5.96 | 171.75 ± 8.22 | 385.10 ± 35.05 b | 400.34 ± 14.91 d | 0.26 ± 0.02 b | 0.35 ± 0.01 h | 5.20 ± 0.29 d | 5.93 ± 1.21 b |
| MACC0 + BA | 214.40 ± 6.56 | 178.98 ± 5.08 | 473.47 ± 54.57 a | 476.50 ± 15.73 bc | 0.43 ± 0.08 ab | 0.57 ± 0.01 f | 5.97 ± 0.42 bcd | 7.16 ± 0.73 ab |
| MACC0 + BP | 216.84 ± 4.17 | 180.03 ± 8.15 | 472.68 ± 23.62 a | 441.33 ± 49.72 cd | 0.41 ± 0.05 ab | 0.52 ± 0.04 g | 5.78 ± 0.54 cd | 6.85 ± 1.07 ab |
| MACC1 + B0 | 217.00 ± 5.50 | 182.03 ± 9.03 | 472.68 ± 25.27 a | 435.80 ± 20.30 cd | 0.38 ± 0.03 ab | 0.61 ± 0.01 e | 6.03 ± 0.41 bcd | 7.13 ± 0.55 ab |
| MACC1 + BA | 217.95 ± 3.55 | 184.69 ± 9.79 | 517.05 ± 19.33 a | 548.60 ± 6.00 a | 0.77 ± 0.41 a | 0.85 ± 0.02 a | 7.27 ± 0.45 a | 8.15 ± 0.41 a |
| MACC1 + BP | 218.03 ± 5.63 | 181.75 ± 2.74 | 514.88 ± 20.90 a | 510.20 ± 11.25 ab | 0.72 ± 0.23 a | 0.72 ± 0.02 b | 7.09 ± 0.69 ab | 7.71 ± 0.70 a |
| MACC2 + B0 | 214.40 ± 5.84 | 182.81 ± 9.67 | 482.53 ± 46.09 a | 504.67 ± 24.65 ab | 0.47 ± 0.09 ab | 0.68 ± 0.01 c | 6.02 ± 0.43 bcd | 7.33 ± 0.32 ab |
| MACC2 + BA | 215.70 ± 8.99 | 183.63 ± 5.58 | 461.90 ± 28.02 ab | 508.50 ± 6.31 ab | 0.67 ± 0.15 a | 0.73 ± 0.01 b | 7.07 ± 0.67 ab | 7.96 ± 0.22 a |
| MACC2 + BP | 218.10 ± 7.24 | 180.31 ± 9.10 | 475.08 ± 19.46 a | 472.50 ± 14.92 bc | 0.64 ± 0.06 ab | 0.64 ± 0.01 d | 6.75 ± 0.41 abc | 7.03 ± 0.25 ab |
| Treatment | Bacteria | Actinomycete | ||
|---|---|---|---|---|
| 2021 | 2022 | 2021 | 2022 | |
| MACC0 + B0 | 6.06 ± 0.42 d | 6.15 ± 0.29 d | 5.27 ± 0.29 b | 5.68 ± 0.30 bc |
| MACC0 + BA | 6.64 ± 0.22 cd | 7.51 ± 0.28 bc | 5.51 ± 0.28 ab | 6.18 ± 0.43 abc |
| MACC0 + BP | 6.54 ± 0.38 cd | 7.16 ± 0.77 bcd | 5.46 ± 0.45 ab | 5.97 ± 0.62 abc |
| MACC1 + B0 | 6.22 ± 0.21 d | 6.73 ± 0.76 cd | 5.63 ± 0.07 ab | 6.26 ± 0.42 ab |
| MACC1 + BA | 7.05 ± 0.05 bc | 8.93 ± 0.32 a | 5.71 ± 0.04 ab | 6.52 ± 0.03 ab |
| MACC1 + BP | 7.55 ± 0.35 ab | 7.74 ± 0.78 abc | 5.73 ± 0.04 ab | 6.51 ± 0.05 ab |
| MACC2 + B0 | 7.30 ± 0.05 ab | 7.27 ± 0.34 bcd | 5.93 ± 0.15 a | 6.21 ± 0.75 ab |
| MACC2 + BA | 7.84 ± 0.13 a | 8.21 ± 0.25 ab | 5.76 ± 0.13 ab | 6.56 ± 0.06 a |
| MACC2 + BP | 7.77 ± 0.04 a | 8.21 ± 0.64 ab | 5.84 ± 0.03 a | 6.57 ± 0.03 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solomon, W.; Mutum, L.; Rakszegi, M.; Janda, T.; Molnár, Z. Harnessing the Synergy of the Cyanobacteria-Plant Growth Promoting Bacteria for Improved Maize (Zea mays) Growth and Soil Health. Sustainability 2023, 15, 16660. https://doi.org/10.3390/su152416660
Solomon W, Mutum L, Rakszegi M, Janda T, Molnár Z. Harnessing the Synergy of the Cyanobacteria-Plant Growth Promoting Bacteria for Improved Maize (Zea mays) Growth and Soil Health. Sustainability. 2023; 15(24):16660. https://doi.org/10.3390/su152416660
Chicago/Turabian StyleSolomon, Wogene, Lamnganbi Mutum, Mariann Rakszegi, Tibor Janda, and Zoltán Molnár. 2023. "Harnessing the Synergy of the Cyanobacteria-Plant Growth Promoting Bacteria for Improved Maize (Zea mays) Growth and Soil Health" Sustainability 15, no. 24: 16660. https://doi.org/10.3390/su152416660
APA StyleSolomon, W., Mutum, L., Rakszegi, M., Janda, T., & Molnár, Z. (2023). Harnessing the Synergy of the Cyanobacteria-Plant Growth Promoting Bacteria for Improved Maize (Zea mays) Growth and Soil Health. Sustainability, 15(24), 16660. https://doi.org/10.3390/su152416660









