Layered-Defect Perovskite K3Bi2X9 (X = I, Br, and Cl) Thin Films for CO2 Photoreduction: An Analysis of Their Pseudocatalytic Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of K3Bi2X9 (X = I, Br, and Cl)
2.3. Application of Stability Strategies
- (a)
- Intrinsic approach
- (b)
- Extrinsic approach
- Growth of K3Bi2I9 in earth-abundant mica substrates: The adherence and stability of the films was improved via the substitution of the substrates using flexible (and natural) mica. These substrates were cut to the same size as the previously used glass substrates (7.6–1.3 cm), and their frames were covered with mildly adhesive tape to prevent leakage through the edges. After these preparation steps, the spin-coating method was performed as stated above (2000 rpm; 30 s).
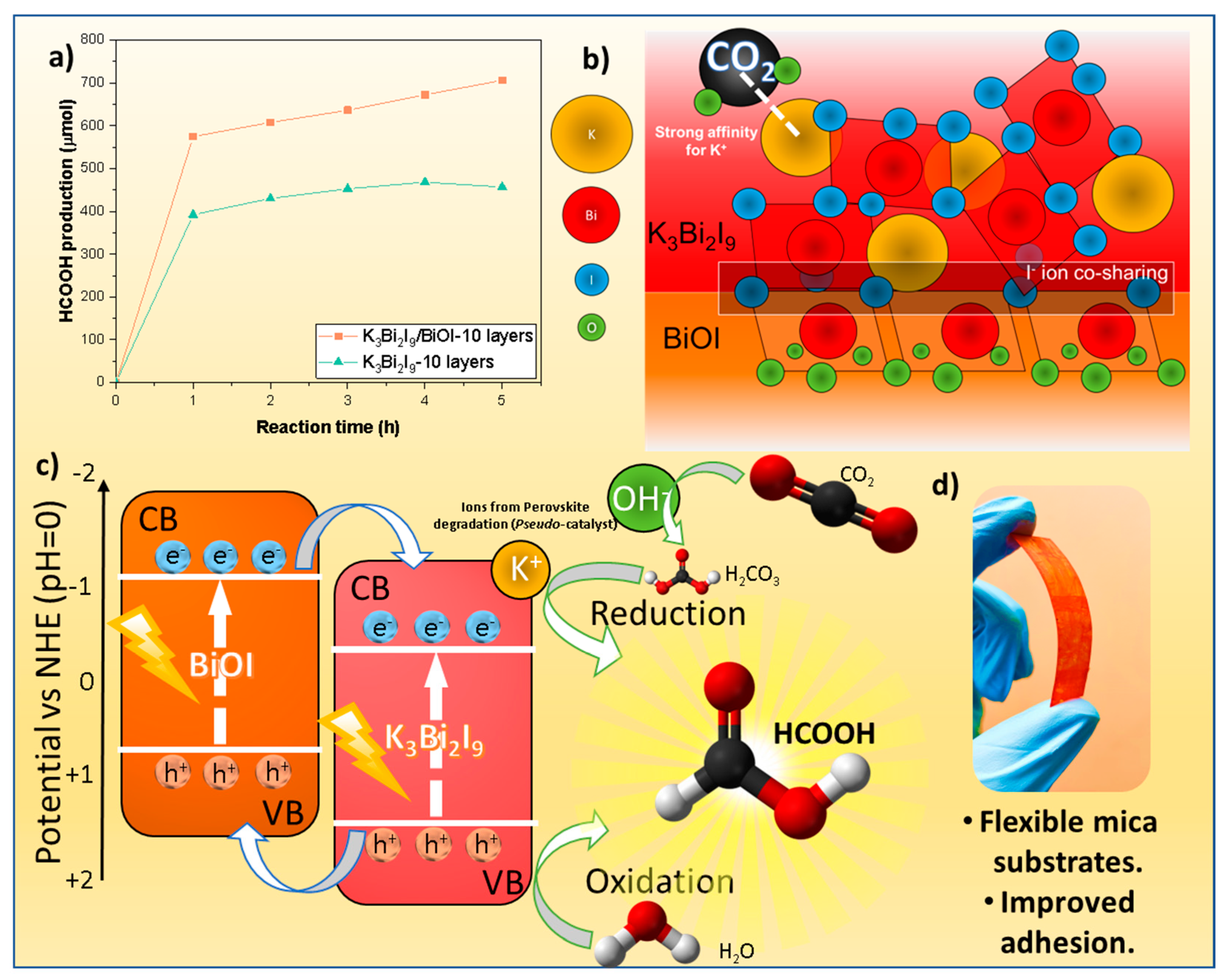
- Formation of m-BiOI/K3Bi2I9 heterostructures: Based on the hypothesis that a heterostructure of BiOI with the K3Bi2I9 perovskite would cause self-assembled co-sharing of the I− ions in both materials (thereby enhancing the film adhesion), a microwave-assisted methodology was carried out. For this purpose, the BiOI film was grown on mica substrates using a microwave–hydrothermal method, as previously reported by our research group [36]. The utilized equipment was a Mars 6® synthesis research microwave into which a Teflon reactor was introduced. This reactor contained a single mica film, BiI3 and Bi(NO3)3 as reactants, and a solvent mixture of isopropyl alcohol and glycerol. The solvents and reactants were added separately in a 2:1 v/v ratio to each other. The reaction conditions were 600 W of power, a temperature of 180 °C, a reaction time of 30 min, and a heating step of 10 °C/min. Finally, the K3Bi2I9 spin-coating deposition procedure was performed on this film, as described previously.
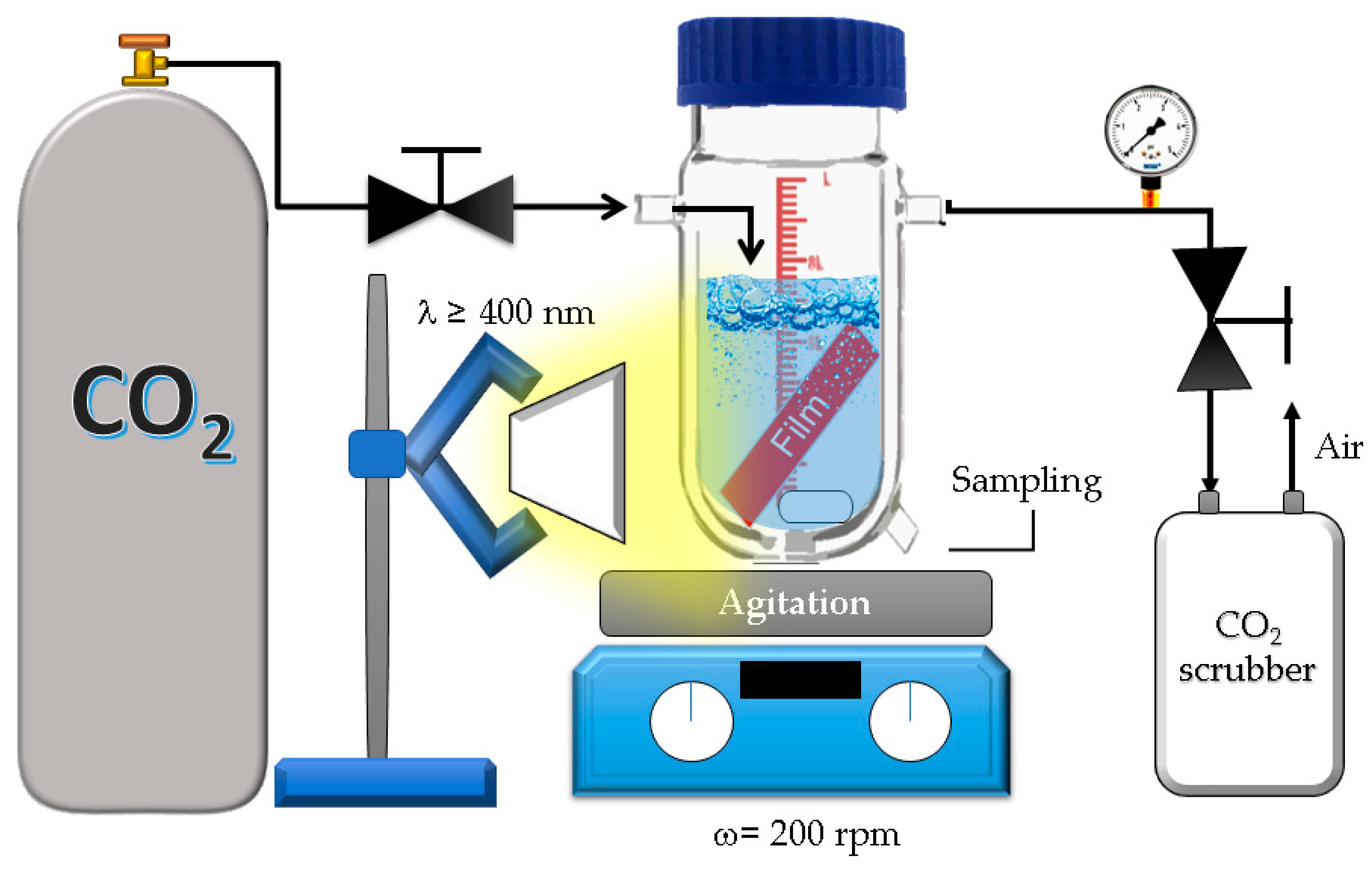
2.4. CO2 Photoreduction Experiments
2.5. Characterization Techniques
3. Results and Discussion
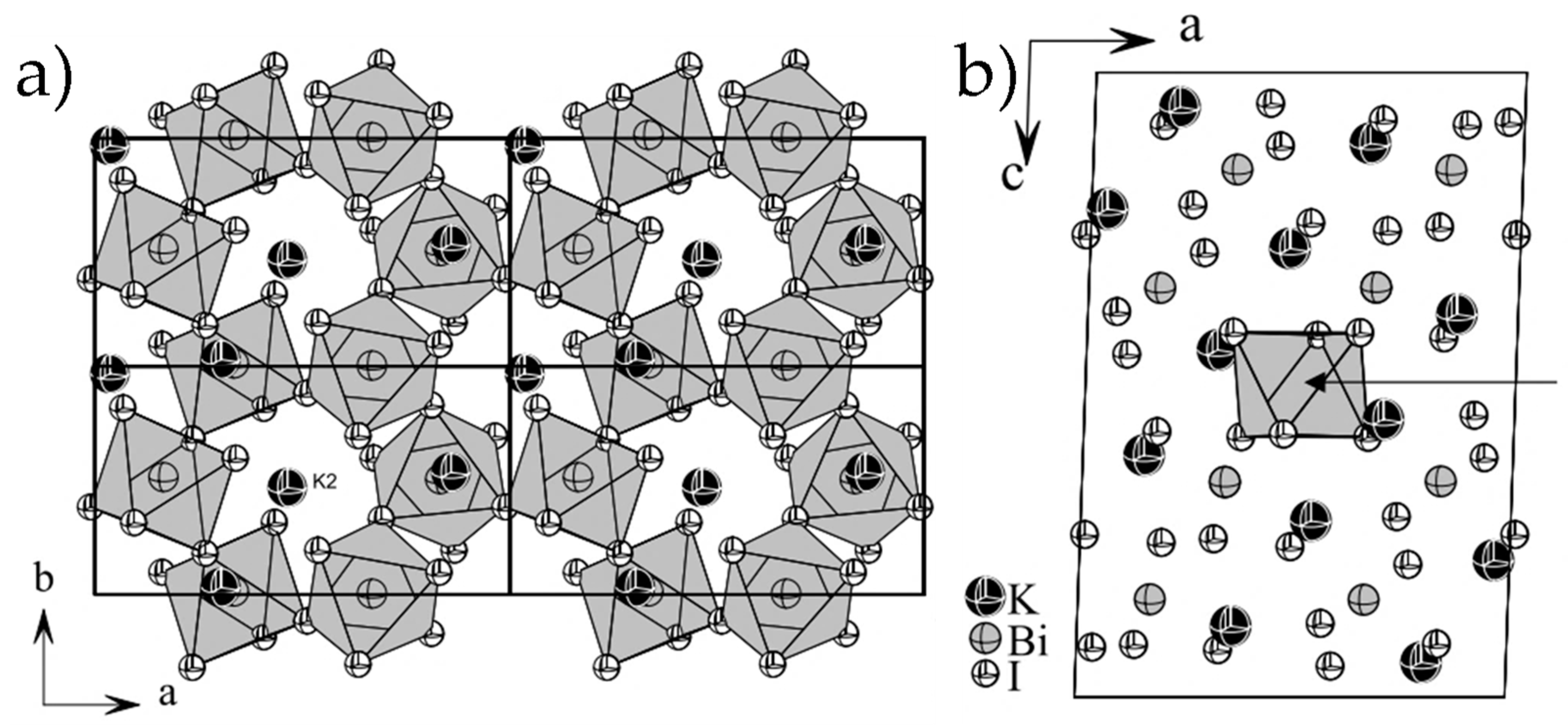
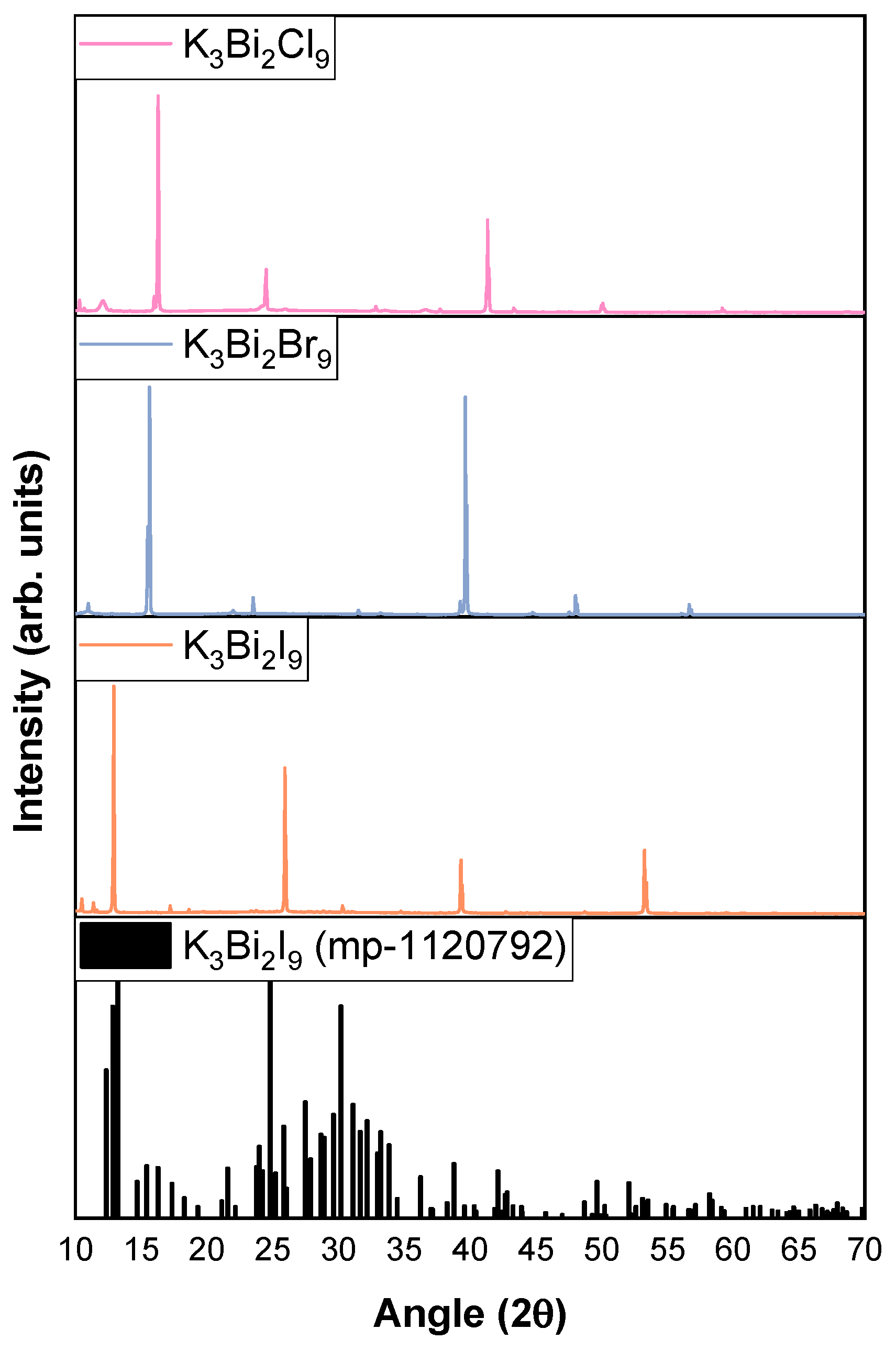
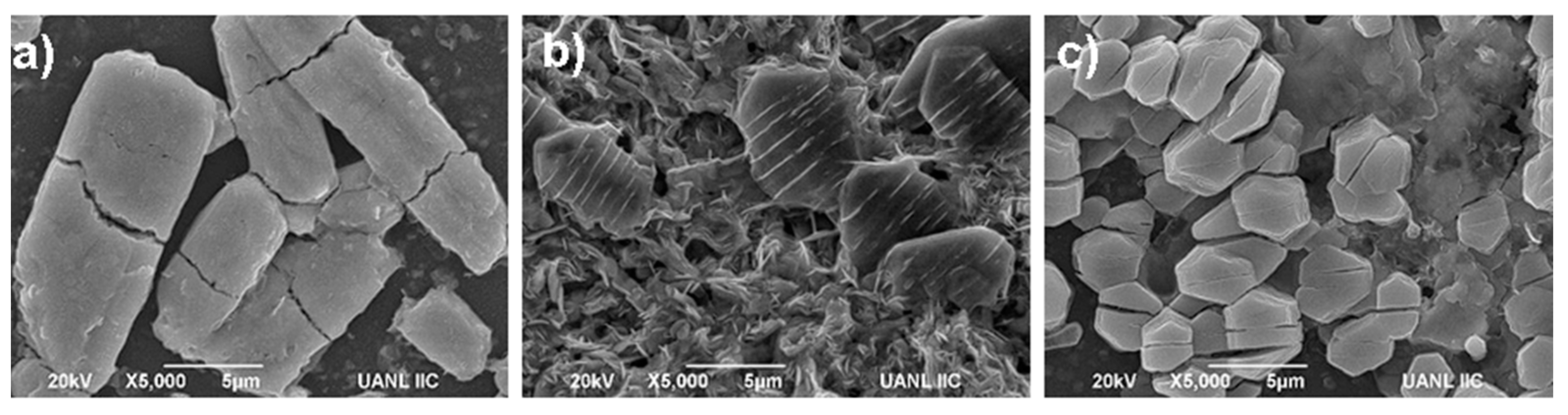
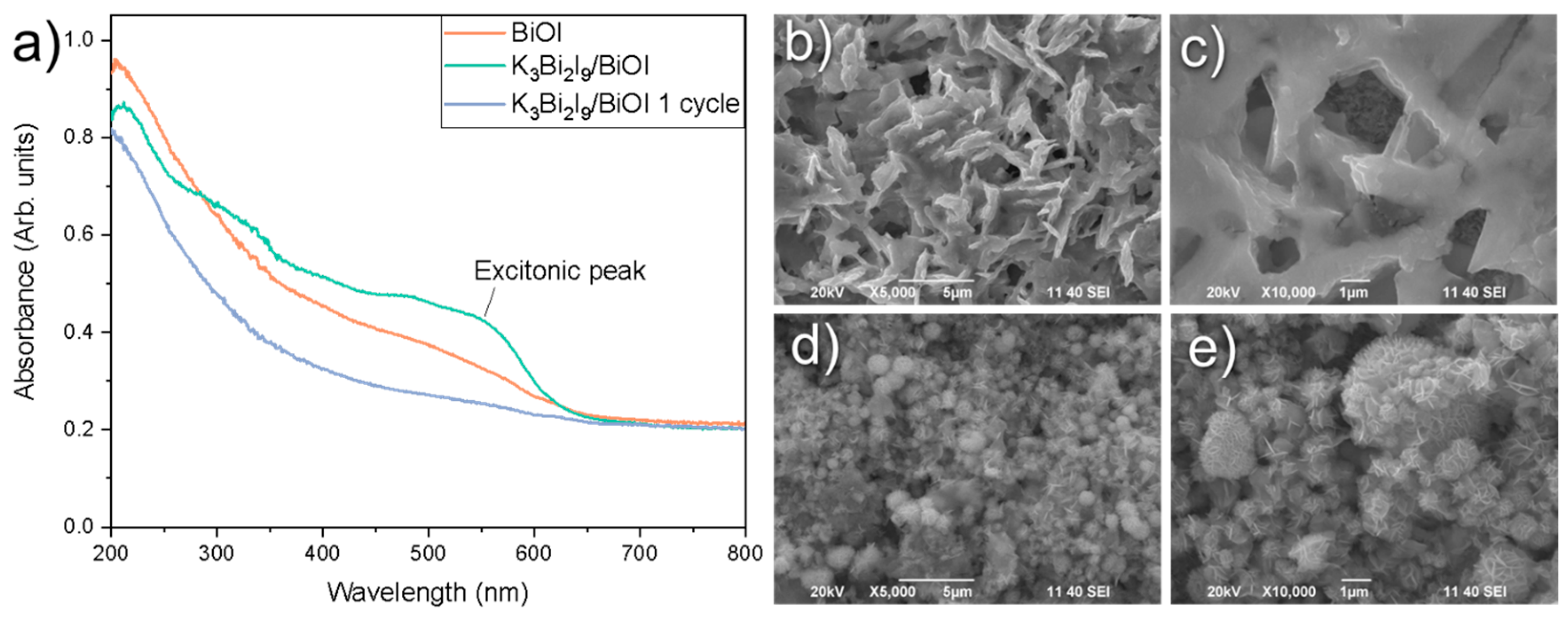
3.1. Characterization of K3Bi2X9 (X = I, Br, and Cl)
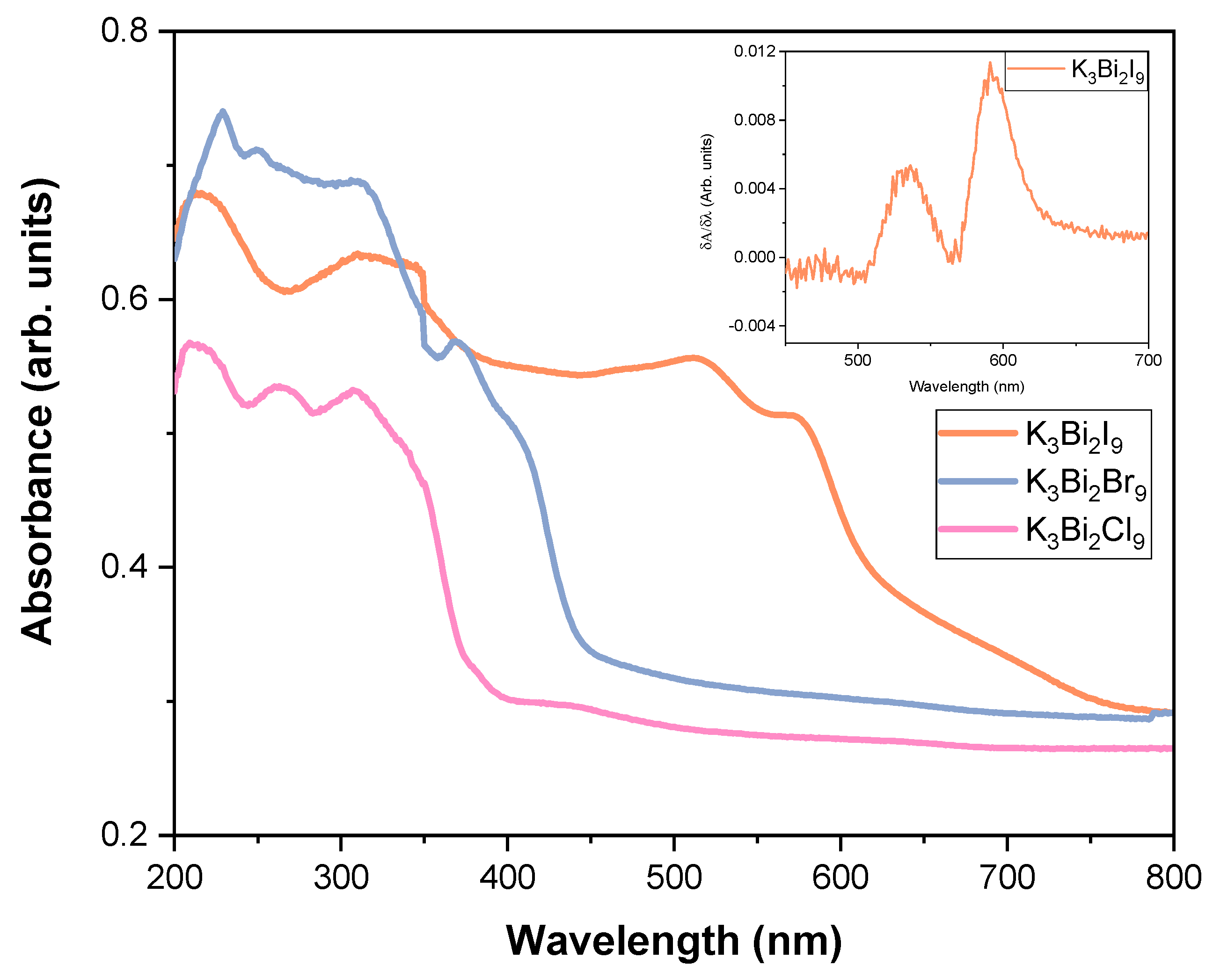
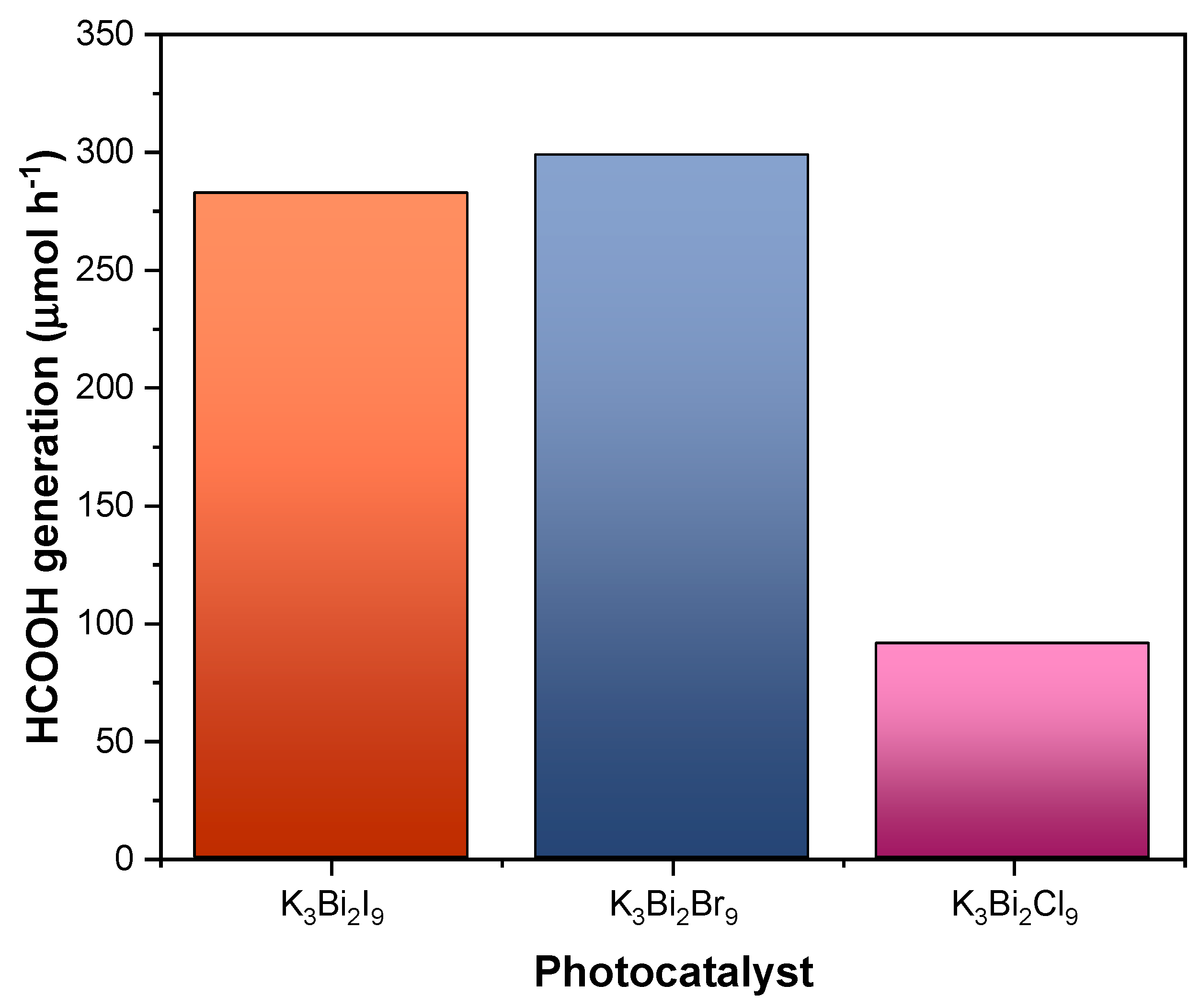
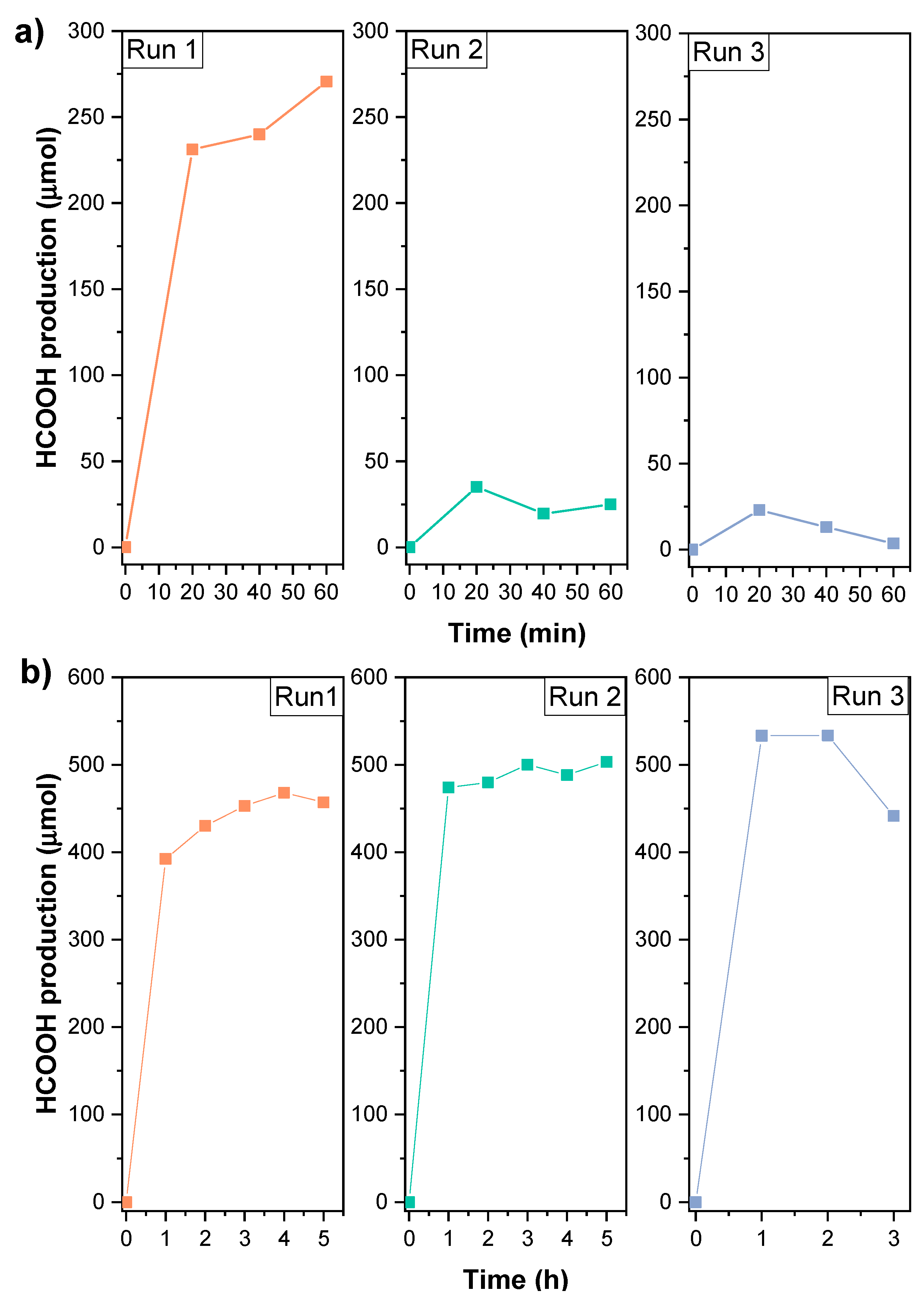
3.2. Preliminary Study of the Photocatalytic Activity of K3Bi2X9 (X = I, Br, and Cl)
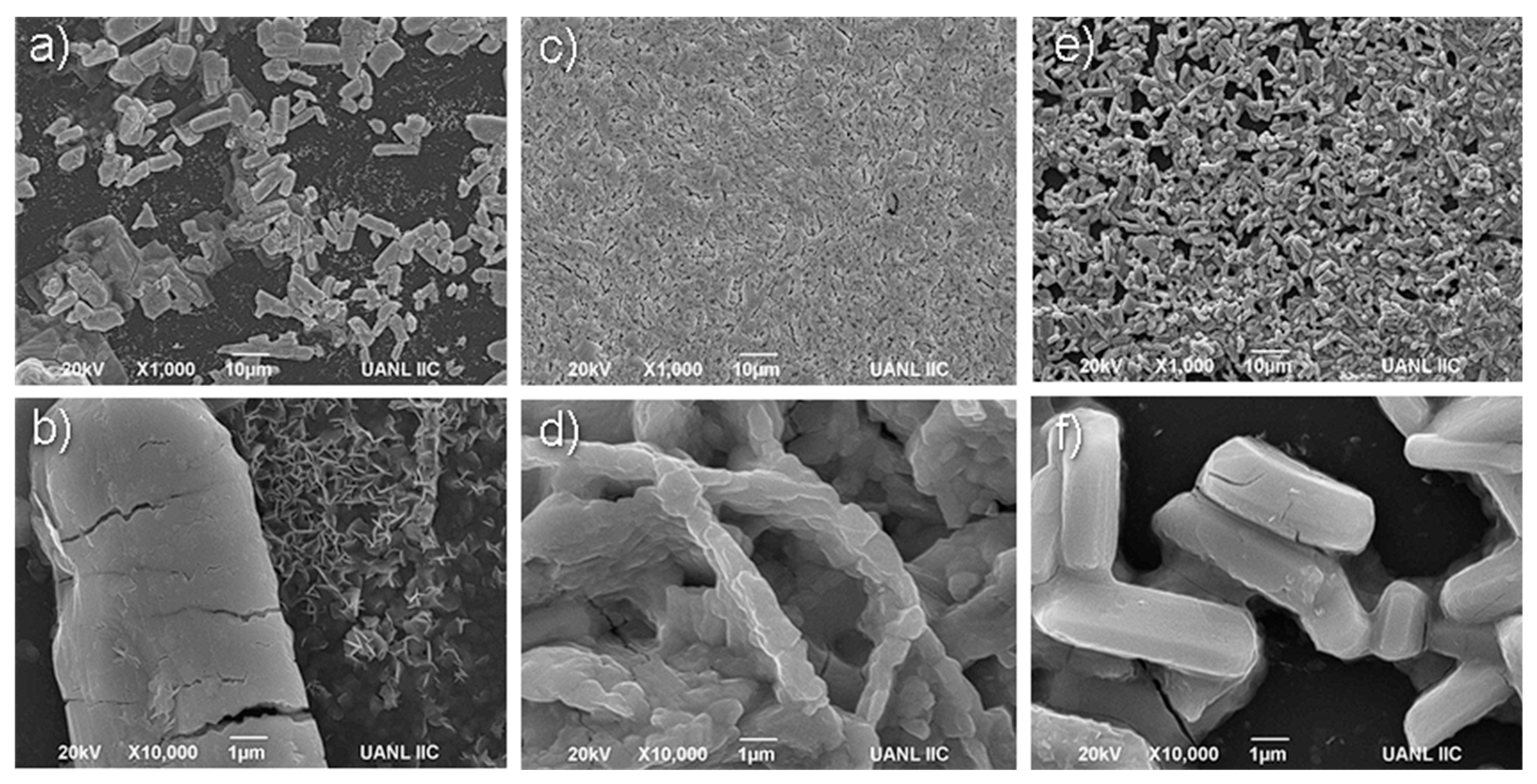
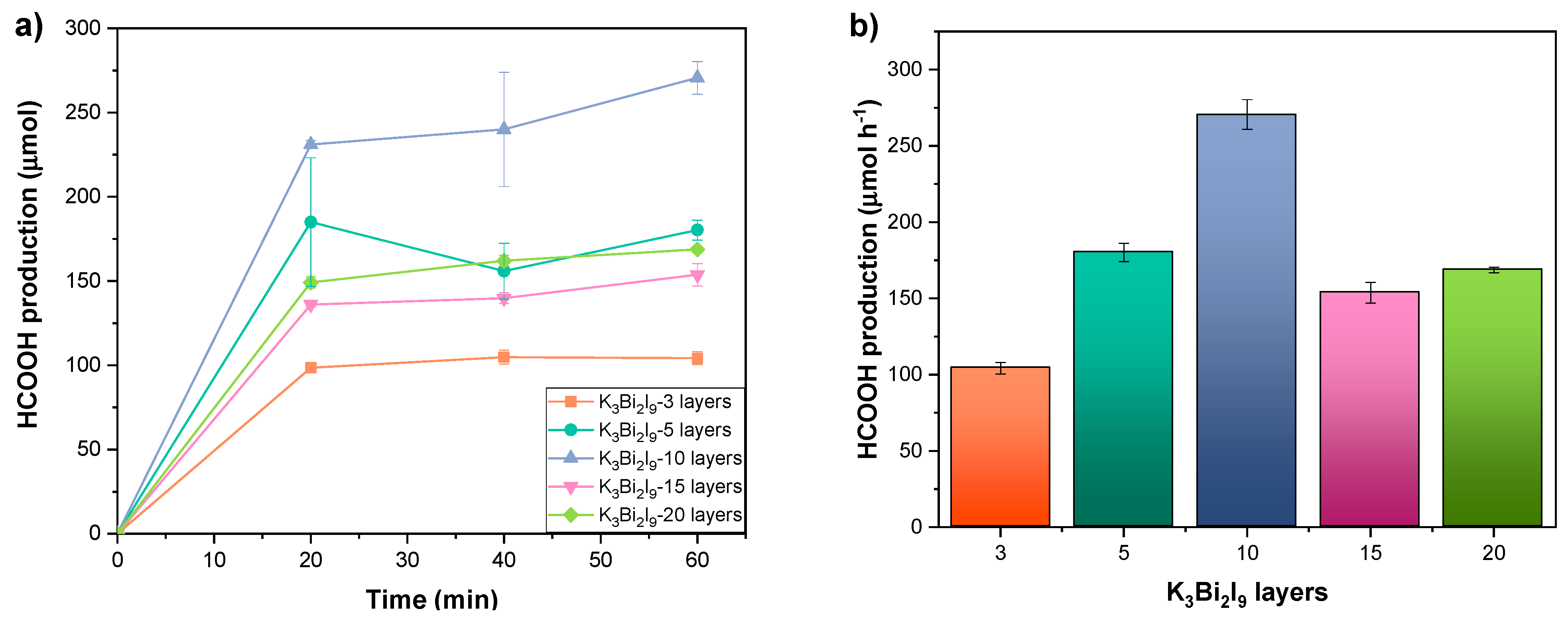
3.3. Implementation of Intrinsic and Extrinsic Strategies for the K3Bi2I9 Perovskite
Strategies 1 and 2: Recrystallization and Substrate Modification
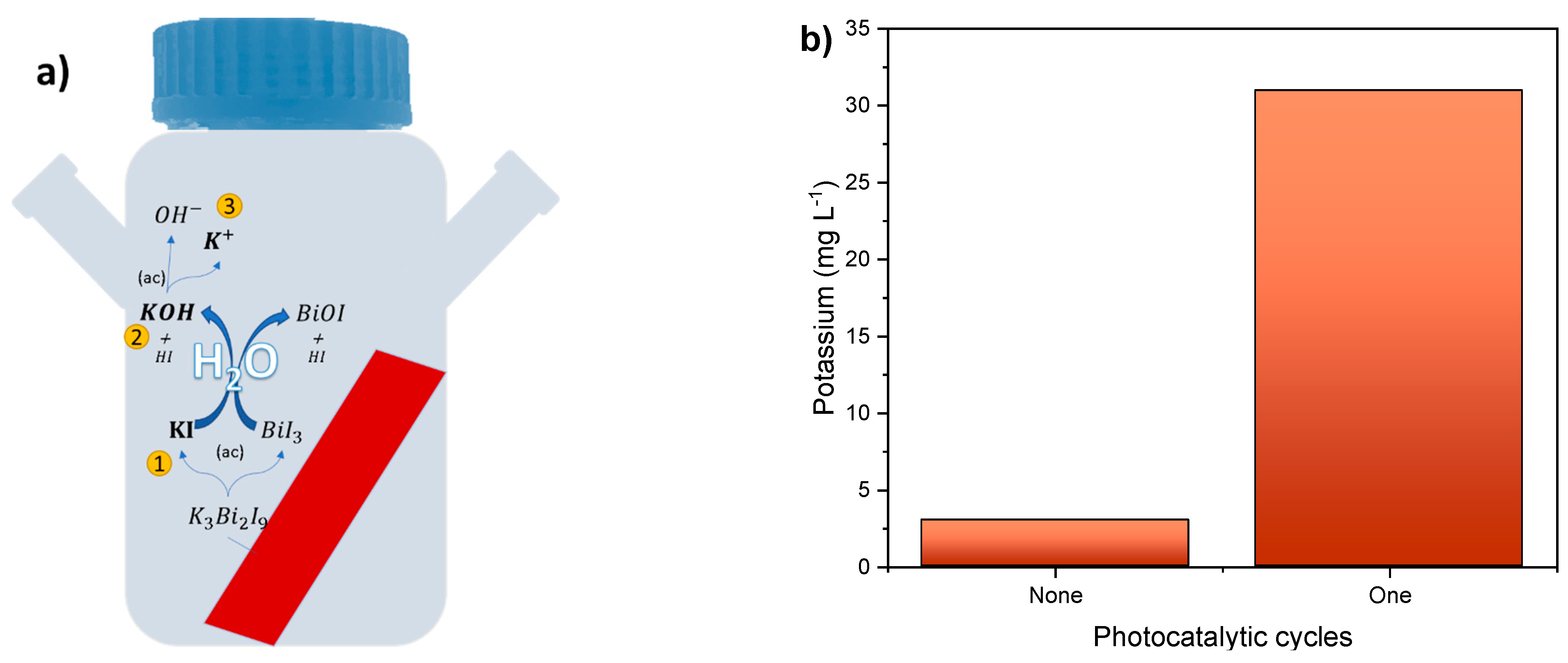
3.4. Stability
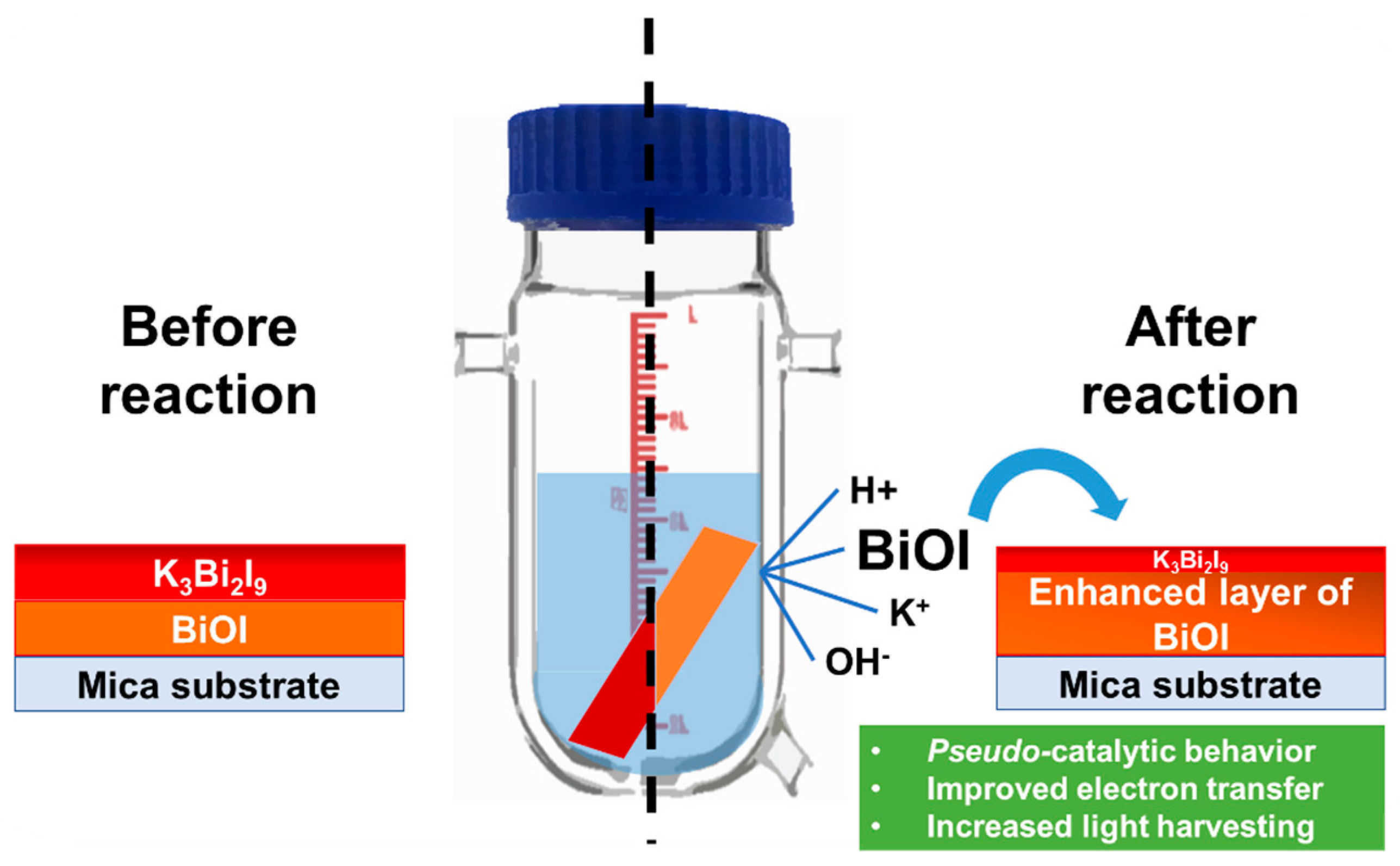
3.5. Discussion of the Photocatalytic Mechanism
Strategy 3: Heterojunction Engineering
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Overview of Greenhouse Gases. Available online: https://www.epa.gov/ghgemissions/overview-greenhouse-gases (accessed on 20 June 2021).
- Zhang, Y.; Xia, B.; Ran, J.; Davey, K.; Qiao, S.Z. Atomic-Level Reactive Sites for Semiconductor-Based Photocatalytic CO2 Reduction. Adv. Energy Mater. 2020, 10, 1903879:1–1903879:85. [Google Scholar] [CrossRef]
- Eskander, S.; Fankhauser, S. Reduction in greenhouse gas emissions from national climate legislation. Nat. Clim. Chang. 2020, 10, 750–756. [Google Scholar] [CrossRef]
- Gabrielli, P.; Gazzani, M.; Mazzotti, M. The Role of Carbon Capture and Utilization, Carbon Capture and Storage, and Biomass to Enable a Net-Zero-CO2 Emissions Chemical Industry. Ind. Eng. Chem. Res. 2020, 59, 7033–7045. [Google Scholar] [CrossRef]
- Qazi, A.; Fayaz, H.; Rahim, N.A.; Hardaker, G.; Alghazzawi, D.; Shaban, K.; Haruna, K. Towards Sustainable Energy: A Systematic review of renewable energy sources, technologies, and public opinions. IEEE Access 2019, 7, 63837–63851. [Google Scholar] [CrossRef]
- Zhu, Q. Developments on CO2-utilization technologies. Clean Energy 2019, 3, 85–100. [Google Scholar] [CrossRef]
- Ješić, D.; Jurković, D.L.; Pohar, A.; Suhadolnik, L.; Likozar, B. Engineering photocatalytic and photoelectrocatalytic CO2 reduction reactions: Mechanisms, intrinsic kinetics, mass transfer resistances, reactors and multi-scale modelling simulations. Chem. Eng. J. 2021, 407, 126799:1–126799:118. [Google Scholar] [CrossRef]
- Gong, E.; Ali, S.; Hiragond, C.B.; Kim, H.S.; Powar, N.S.; Kim, D.; Kim, H.; In, S.I. Solar fuels: Research and development strategies to accelerate photocatalytic CO2 conversion into hydrocarbon fuels. Energy Environ. Sci. 2022, 15, 880–937. [Google Scholar] [CrossRef]
- Huang, H.; Song, H.; Kou, J.; Lu, C.; Ye, J. Atomic-level insights into surface engineering of semiconductors for photocatalytic CO2 reduction. J. Energy Chem. 2022, 67, 309–341. [Google Scholar] [CrossRef]
- Miao, Y.F.; Guo, R.T.; Gu, J.W.; Liu, Y.; Wu, G.L.; Duan, C.P.; Zhang, X.; Pan, W. Fabrication of β-In2S3/NiAl-LDH heterojunction photocatalyst with enhanced separation of charge carriers for efficient CO2 photocatalytic reduction. Appl. Surf. Sci. 2020, 527, 146792. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Davey, K.; Mullins, C.B. Tuning the intrinsic properties of carbon nitride for high quantum yield photocatalytic hydrogen production. Adv. Sci. 2018, 5, 1800820:1–1800820:14. [Google Scholar] [CrossRef]
- Jia, Q.; Iwase, A.; Kudo, A. BiVO4–Ru/SrTiO3:Rh composite Z-scheme photocatalyst for solar water splitting. Chem. Sci. 2014, 5, 1513:1–1513:7. [Google Scholar] [CrossRef]
- Shi, J.; Guo, L. ABO3-based photocatalysts for water splitting. Prog. Nat. Sci. Mater. Int. 2012, 22, 592–615. [Google Scholar] [CrossRef]
- Li, P.; Ouyang, S.; Xi, G.; Kako, T.; Ye, J. The Effects of Crystal Structure and Electronic Structure on Photocatalytic H2 Evolution and CO2 Reduction over Two Phases of Perovskite-Structured NaNbO3. J. Phys. Chem. C. 2012, 116, 7621–7628. [Google Scholar] [CrossRef]
- Nakanishi, H.; Iizuka, K.; Takayama, T.; Iwase, A.; Kudo, A. Highly Active NaTaO3-Based Photocatalysts for CO2 Reduction to Form CO Using Water as the Electron Donor. ChemSusChem 2016, 10, 112–118. [Google Scholar] [CrossRef]
- Li, D.; Ouyang, S.; Xu, H.; Lu, D.; Zhao, M.; Zhang, X.L.; Ye, J. Synergistic effect of Au and Rh on SrTiO3 in significantly promoting visible-light-driven syngas production from CO2 and H2O. Chem. Commun. 2016, 52, 5989–5992. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Li, J.; Fang, W.; Song, H.; Li, Q.; Tang, Y. Visible-light-induced photocatalyst based on C-doped LaCoO3 synthesized by novel microorganism chelate method. Catal. Commun. 2009, 10, 1230–1234. [Google Scholar] [CrossRef]
- Han, C.; Zhu, X.; Martin, J.S.; Lin, Y.; Spears, S.; Yan, Y. Recent progress in engineering metal halide perovskites for efficient Visible-Light-Driven photocatalysis. ChemSusChem 2020, 13, 4005–4025. [Google Scholar] [CrossRef]
- Trivedi, S.; Prochowicz, D.; Kalam, A.; Tavakoli, M.M.; Yadav, P. Development of all-inorganic lead halide perovskites for carbon dioxide photoreduction. Renew. Sustain. Energy Rev. 2021, 145, 111047:1–111047:11. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, M.; Chen, B.; Wang, X.; Chen, L.; Kuang, D.B.; Su, C.Y. A CsPbBr3 Perovskite Quantum Dot/Graphene Oxide Composite for Photocatalytic CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 5660–5663. [Google Scholar] [CrossRef]
- Connor, B.A.; Leppert, L.; Smith, M.D.; Neaton, J.B.; Karunadasa, H.I. Layered Halide Double Perovskites: Dimensional Reduction of Cs2AgBiBr6. J. Am. Chem. Soc. 2018, 140, 5235–5240. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Y. Chemical stability and instability of inorganic halide perovskites. Energy Environ. Sci. 2019, 12, 1495–1511. [Google Scholar] [CrossRef]
- Ahmad, K. Bismuth Halide Perovskites for Photovoltaic Applications; IntechOpen EBooks: London, UK, 2020. [Google Scholar] [CrossRef]
- Sheng, J.; He, Y.; Li, J.; Yuan, C.; Huang, H.; Wang, S.; Sun, Y.; Wang, Z. Identification of Halogen-Associated Active Sites on Bismuth-Based Perovskite Quantum Dots for Efficient and Selective CO2-to-CO Photoreduction. ACS Nano 2020, 14, 13103–13114. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.S.; Kharade, A.K.; Jokar, E.; Fathi, A.; Chang, S.M.; Diau, E.W.G. Mechanism of Photocatalytic CO2 Reduction by Bismuth-Based Perovskite Nanocrystals at the Gas–Solid Interface. J. Am. Chem. Soc. 2019, 141, 20434–20442. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.X.; Dong, G.X.; Su, K.; Liu, Z.; Zhang, W.; Zhang, M.; Lu, T.B. Self-template-oriented synthesis of lead-free perovskite Cs3Bi2I9 nanosheets for boosting photocatalysis of CO2 reduction over Z-scheme heterojunction Cs3Bi2I9/CeO2. J. Energy Chem. 2022, 69, 348–355. [Google Scholar] [CrossRef]
- Preitschaft, C. Ternäre und Quaternäre Materialien mit Komplexen Thio-, Selenido- und Halogenid-Anionen. Synthese, Strukturen und Physikalische Eigenschaften. Ph.D. Thesis, Regensburg University, Regensburg, Germany, 2006. Available online: https://epub.uni-regensburg.de/10371/ (accessed on 20 October 2023).
- Nair, S.; Bhorde, A.; Kulkarni, R.; Bade, B.; Punde, A.; Vairale, P.; Hase, Y.; Waghmare, A.; Waykar, R.; Deshpande, M.; et al. Synthesis and characterization of inorganic K3Bi2I9 perovskite thin films for lead-free solution processed solar cells. Mater. Today Process. 2021, 34, 684–689. [Google Scholar] [CrossRef]
- Huang, H.; Pradhan, B.; Hofkens, J.; Roeffaers, M.B.J.; Steele, J.A. Solar-Driven Metal Halide Perovskite Photocatalysis: Design, Stability, and performance. ACS Energy Lett. 2020, 5, 1107–1123. [Google Scholar] [CrossRef]
- Giuri, A.; Rolston, N.; Colella, S.; Listorti, A.; Corcione, C.E.; Elmaraghi, H.; Lauciello, S.; Dauskardt, R.H.; Rizzo, A. Robust, High-Performing Maize–Perovskite-Based Solar Cells with Improved Stability. ACS Appl. Energy Mater. 2021, 4, 11194–11203. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Ju, M.G.; Garces, H.F.; Carl, A.D.; Ono, L.K.; Hawash, Z.; Zhang, Y.; Shen, T.; Qi, Y.; Grimm, R.L.; et al. Highly stable and efficient all-inorganic lead-free perovskite solar cells with native-oxide passivation. Nat. Commun. 2019, 10, 16:1–16:8. [Google Scholar] [CrossRef]
- Chang, J.; Yu, T.; Li, F.; Bao, C.; Gao, H.; Yi, Y.; Yang, J.; Gao, F.P.; Zhou, X.; Zou, Z. A facile, solvent vapor–fumigation-induced, self-repair recrystallization of CH3NH3PbI3 films for high-performance perovskite solar cells. Nanoscale 2015, 7, 5427–5434. [Google Scholar] [CrossRef]
- Kulbak, M.; Gupta, S.; Kedem, N.; Levine, I.; Bendikov, T.; Hodes, G.; Cahen, D. Cesium enhances Long-Term stability of lead bromide Perovskite-Based solar cells. J. Phys. Chem. Lett. 2015, 7, 167–172. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y. Junction Engineering for Photocatalytic and Photoelectrocatalytic CO2 Reduction. Sol. RRL 2020, 5, 2000430:1–2000430:49. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Liao, J.; Chen, B.; Chen, L.; Kuang, D.B. Amorphous-TiO2 -Encapsulated CsPbBr3 Nanocrystal Composite Photocatalyst with Enhanced Charge Separation and CO2 Fixation. Adv. Mater. Interfaces 2018, 5, 1801015. [Google Scholar] [CrossRef]
- Luévano-Hipólito, E.; Torres-Alvarez, D.A.; Torres-Martínez, L.M. Flexible BiOI thin films photocatalysts toward renewable solar fuels production. J. Environ. Chem. Eng. 2023, 11, 109557. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; D’Innocenzo, V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L. Tuning the optical properties of cesium lead halide perovskite nanocrystals by anion exchange reactions. J. Am. Chem. Soc. 2015, 137, 10276–10281. [Google Scholar] [CrossRef] [PubMed]
- Muniz, F.T.L.; Miranda, M.A.R.; Santos, C.; Sasaki, J.M. The Scherrer equation and the dynamical theory of X-ray diffraction. Acta Crystallogr. 2016, 72, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Ünlü, F.; Kulkarni, A.; Lê, K.; Bohr, C.; Bliesener, A.; Öz, S.D.; Jena, A.K.; Ando, Y.; Miyasaka, T.; Kirchartz, T.; et al. Single- or double A-site cations in A3Bi2I9 bismuth perovskites: What is the suitable choice? J. Mater. Res. 2021, 36, 1794–1804. [Google Scholar] [CrossRef]
- Shafir, O.; Shopin, A.; Grinberg, I. Band-Gap Engineering of Mo- and W-Containing Perovskite Oxides Derived from Barium Titanate. Phys. Rev. Appl. 2020, 13, 034066:1–034066:13. [Google Scholar] [CrossRef]
- Bai, F.; Hu, Y.; Hu, Y.; Qiu, T.; Miao, X.; Zhang, S. Lead-free, air-stable ultrathin Cs3Bi2I9 perovskite nanosheets for solar cells. Sol. Energy Mater. Sol. Cells. 2018, 184, 15–21. [Google Scholar] [CrossRef]
- Bae, H.Y.; Choi, G.M. Electrical and reducing gas sensing properties of ZnO and ZnO–CuO thin films fabricated by spin coating method. Sens. Actuators B Chem. 1999, 55, 47–54. [Google Scholar] [CrossRef]
- Su, T.; Tian, H.; Qin, Z.; Ji, H. Preparation and characterization of Cu modified BiYO3 for carbon dioxide reduction to formic acid. Appl. Cat. B Environ. 2017, 202, 364–373. [Google Scholar] [CrossRef]
- Bafaqeer, A.; Tahir, M.N.; Amin, N.A.S. Synergistic effects of 2D/2D ZnV2O6/RGO nanosheets heterojunction for stable and high performance photo-induced CO2 reduction to solar fuels. Chem. Eng. J. 2018, 334, 2142–2153. [Google Scholar] [CrossRef]
- Aguirre-Astrain, A.; Luévano-Hipólito, E.; Torres-Martínez, L.M. Integration of 2D printing technologies for AV2O6 (A=Ca, Sr, Ba)-MO (M=Cu, Ni, Zn) photocatalyst manufacturing to solar fuels production using seawater. Int. J. Hydrogen Energy 2021, 46, 37294–37310. [Google Scholar] [CrossRef]
- Yoshitomi, F.; Sekizawa, K.; Maeda, K.; Ishitani, O. Selective Formic Acid Production via CO2 Reduction with Visible Light Using a Hybrid of a Perovskite Tantalum Oxynitride and a Binuclear Ruthenium(II) Complex. ACS Appl. Mater. Int. 2015, 7, 13092–13097. [Google Scholar] [CrossRef]
- Luévano-Hipólito, E.; Quintero-Lizárraga, O.L.; Torres-Martínez, L.M. A Critical Review of the use of bismuth halide perovskites for CO2 photoreduction: Stability challenges and Strategies Implemented. Catalyst 2022, 12, 1410. [Google Scholar] [CrossRef]
- Jain, A.; Hautier, G.; Ong, S.P.; Moore, C.; Fischer, C.R.; Persson, K.A.; Ceder, G. Formation enthalpies by mixing GGA and GGA+U calculations. Phys. Rev. B 2011, 84, 045115:1–045115:10. [Google Scholar] [CrossRef]
- Tenuta, E.; Zheng, C.; Rubel, O. Thermodynamic origin of instability in hybrid halide perovskites. Sci. Rep. 2016, 6, 37654. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wang, Y.; Shen, J.; Scott, S.; Riley, B.J.; Vienna, J.D.; Lian, J. Cs3Bi2I9-hydroxyapatite composite waste forms for cesium and iodine immobilization. J. Adv. Ceram. 2022, 11, 712–728. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, R.R.; Mu, Y.F.; Feng, Y.X.; Dong, G.X.; Zhang, M.; Lu, T.B. In Situ Construction of Lead-Free Perovskite Direct Z-Scheme Heterojunction Cs3Bi2I9 /Bi2WO6 for Efficient Photocatalysis of CO2 Reduction. Sol. RRL 2021, 5, 2000691:1–2000691:9. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. Compendium of Chemical Terminology: IUPAC Recommendations; Wiley-Blackwell: New York, NY, USA, 1997. [Google Scholar]
- Shende, P.; Kasture, P.; Gaud, R. Nanoflowers: The future trend of nanotechnology for multi-applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 413–422. [Google Scholar] [CrossRef]
- Eguchi, R.; Uchida, S.; Mizuno, N. Inverse and high CO2/C2H2 sorption selectivity in flexible Organic-Inorganic ionic crystals. Angew. Chem. 2012, 51, 1635–1639. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, J.; Wang, R.; Li, W.; Zhao, C.; Li, C.; Zhang, J. Recent advances in potassium-based adsorbents for CO2 capture and separation: A review. Carbon Capture Sci. Technol. 2021, 1, 100011:1–100011:23. [Google Scholar] [CrossRef]
- Liu, P.; Liu, H.; Zhang, S.; Wang, J. A general strategy for obtaining BiOX nanoplates derived Bi nanosheets as efficient CO2 reduction catalysts by enhancing CO2•− adsorption and electron transfer. J. Colloid Interface Sci. 2021, 602, 740–747. [Google Scholar] [CrossRef] [PubMed]

| Properties | Diethyl Ether (DE) | Isopropyl Alcohol (IPA) |
|---|---|---|
| Polarity index | 2.8 | 3.9 |
| Boiling point (°C) | 35 | 82 |
| Viscosity (cP) | 0.32 | 2.3 |
| Miscibility with DMF | Miscible | Miscible |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintero-Lizárraga, O.L.; Luévano-Hipólito, E.; Ibarra-Rodríguez, L.I.; Torres-Martínez, L.M. Layered-Defect Perovskite K3Bi2X9 (X = I, Br, and Cl) Thin Films for CO2 Photoreduction: An Analysis of Their Pseudocatalytic Behavior. Sustainability 2023, 15, 16835. https://doi.org/10.3390/su152416835
Quintero-Lizárraga OL, Luévano-Hipólito E, Ibarra-Rodríguez LI, Torres-Martínez LM. Layered-Defect Perovskite K3Bi2X9 (X = I, Br, and Cl) Thin Films for CO2 Photoreduction: An Analysis of Their Pseudocatalytic Behavior. Sustainability. 2023; 15(24):16835. https://doi.org/10.3390/su152416835
Chicago/Turabian StyleQuintero-Lizárraga, Oscar L., Edith Luévano-Hipólito, Luz I. Ibarra-Rodríguez, and Leticia M. Torres-Martínez. 2023. "Layered-Defect Perovskite K3Bi2X9 (X = I, Br, and Cl) Thin Films for CO2 Photoreduction: An Analysis of Their Pseudocatalytic Behavior" Sustainability 15, no. 24: 16835. https://doi.org/10.3390/su152416835






