Synthesis and Evaluation of Geopolymer Mixtures Containing Chronologically Aged Basic Oxygen Furnace Slags
Abstract
:1. Introduction
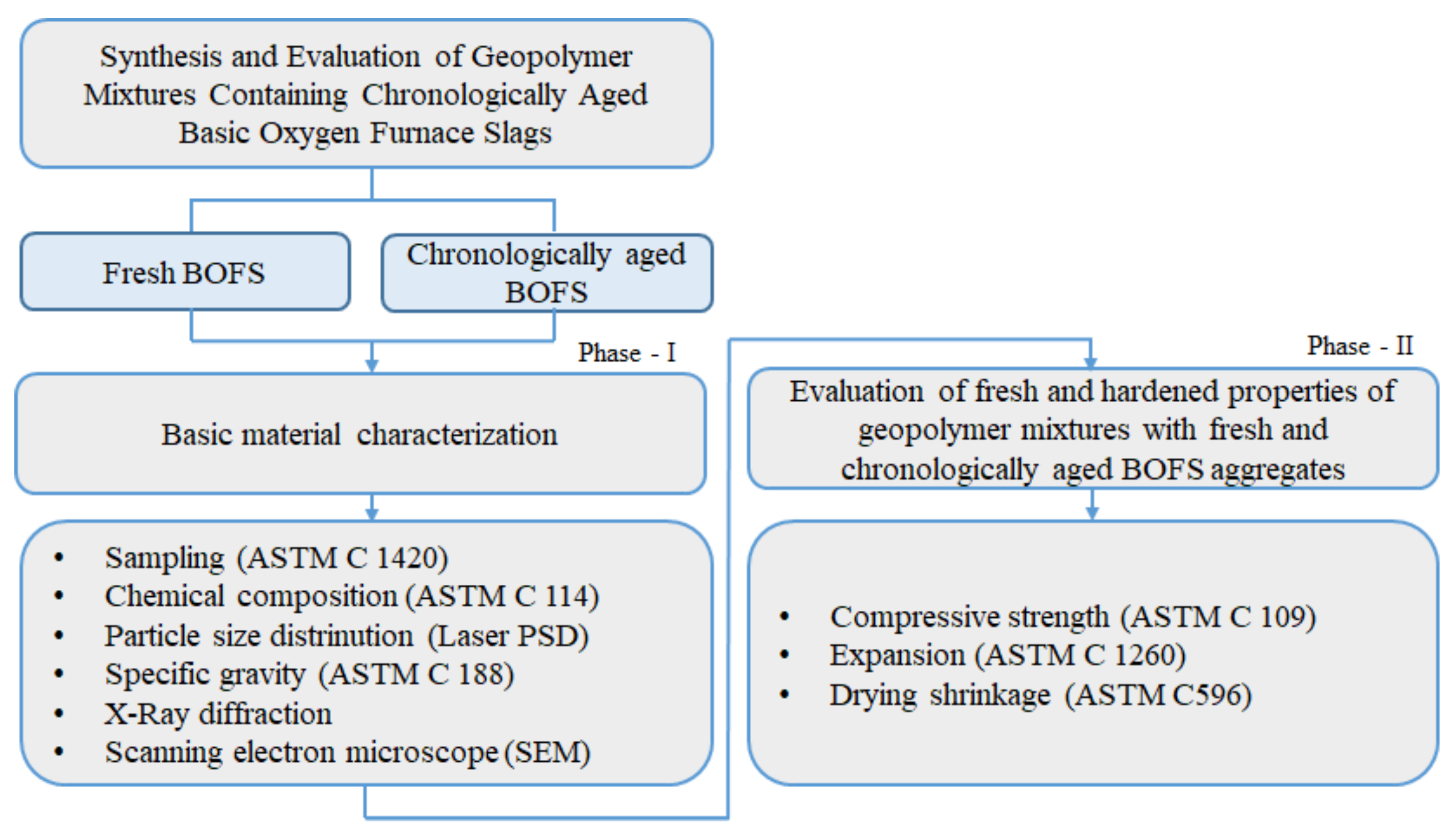
2. Research Objective and Scope
3. Experimental Programs
3.1. Sampling of Stockpiled BOFS Materials
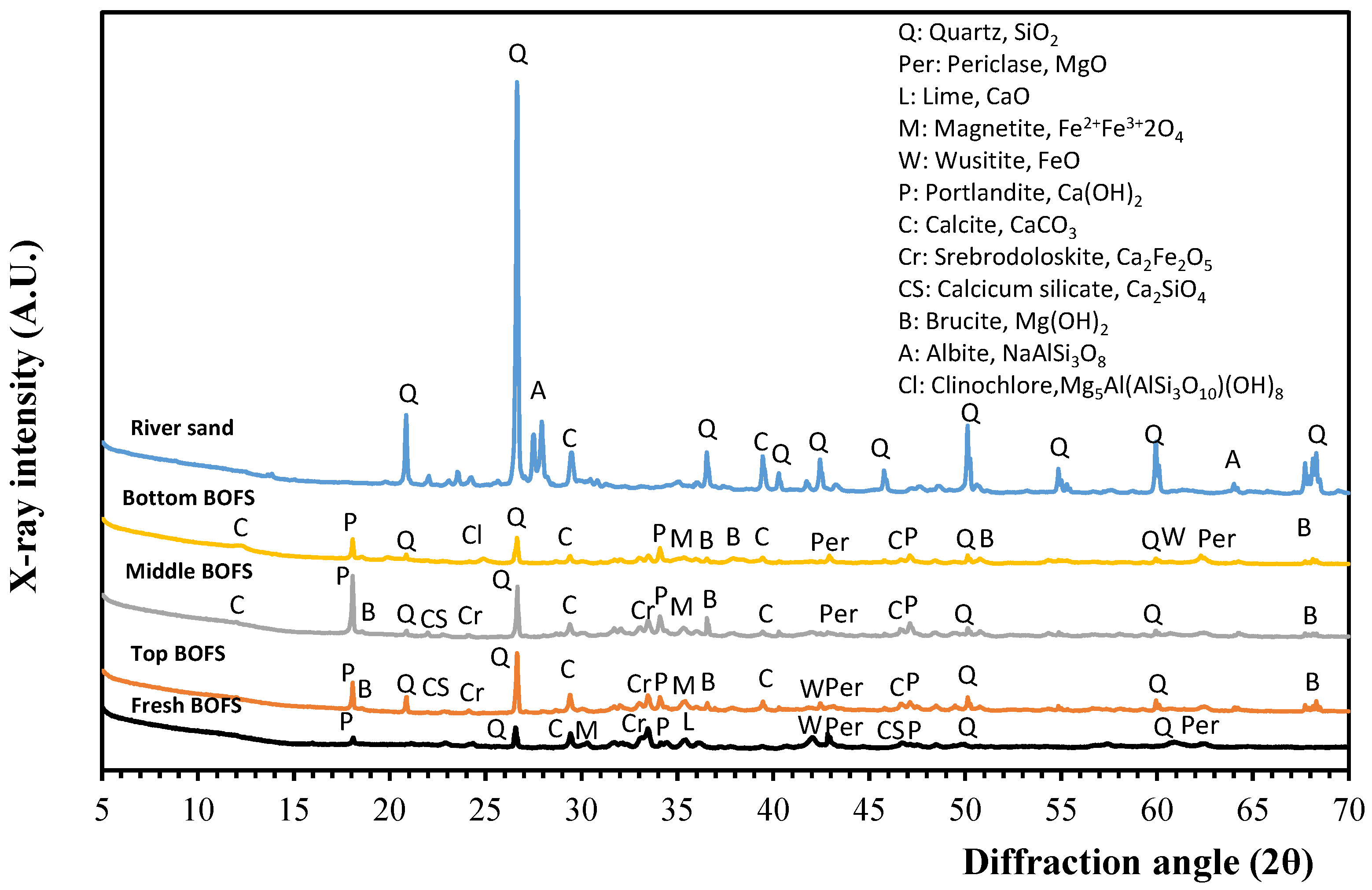
3.2. Material Characteristics
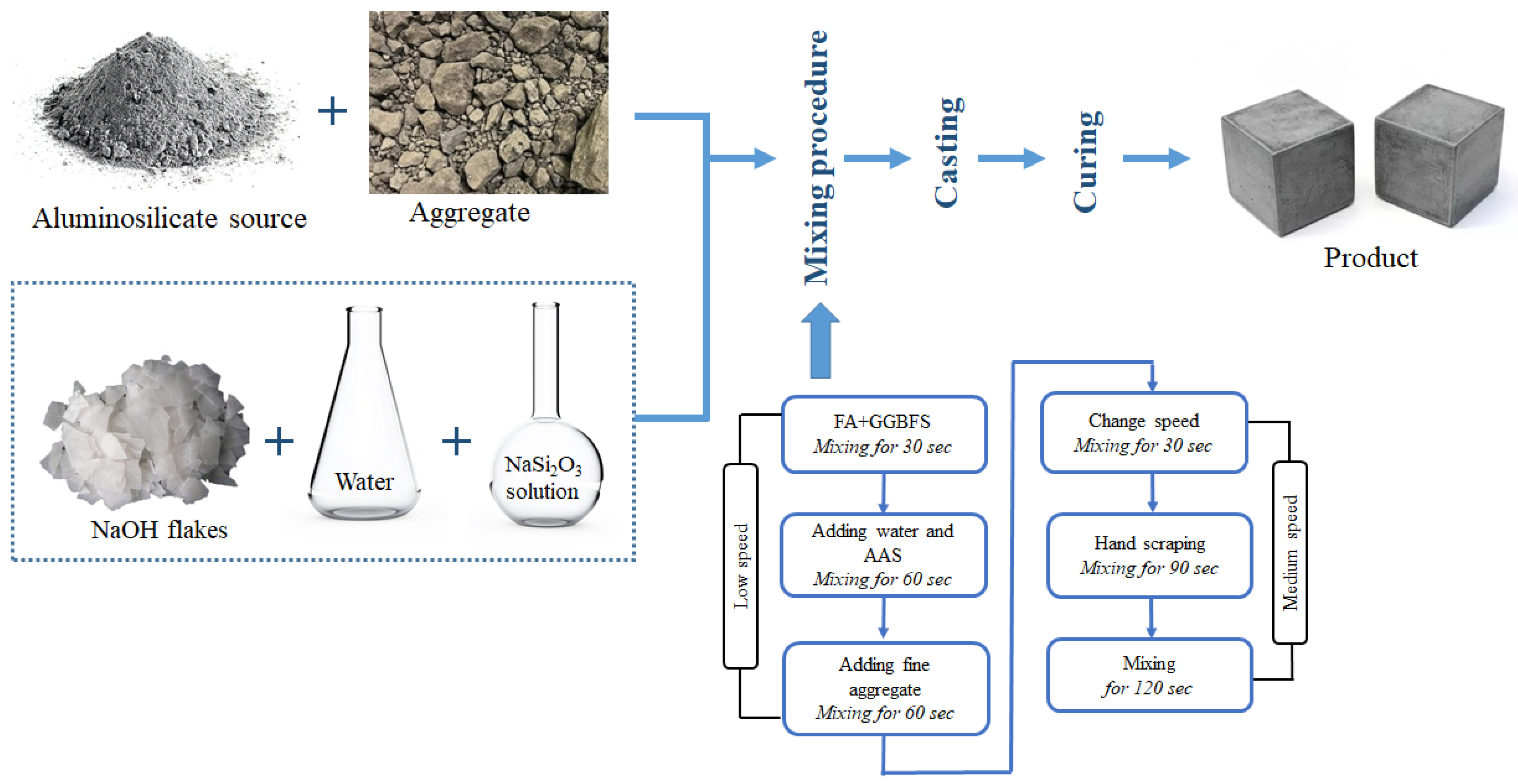
3.3. Mixture Proportions and Sample Preparation
3.4. Test Methods
4. Test Results and Discussions
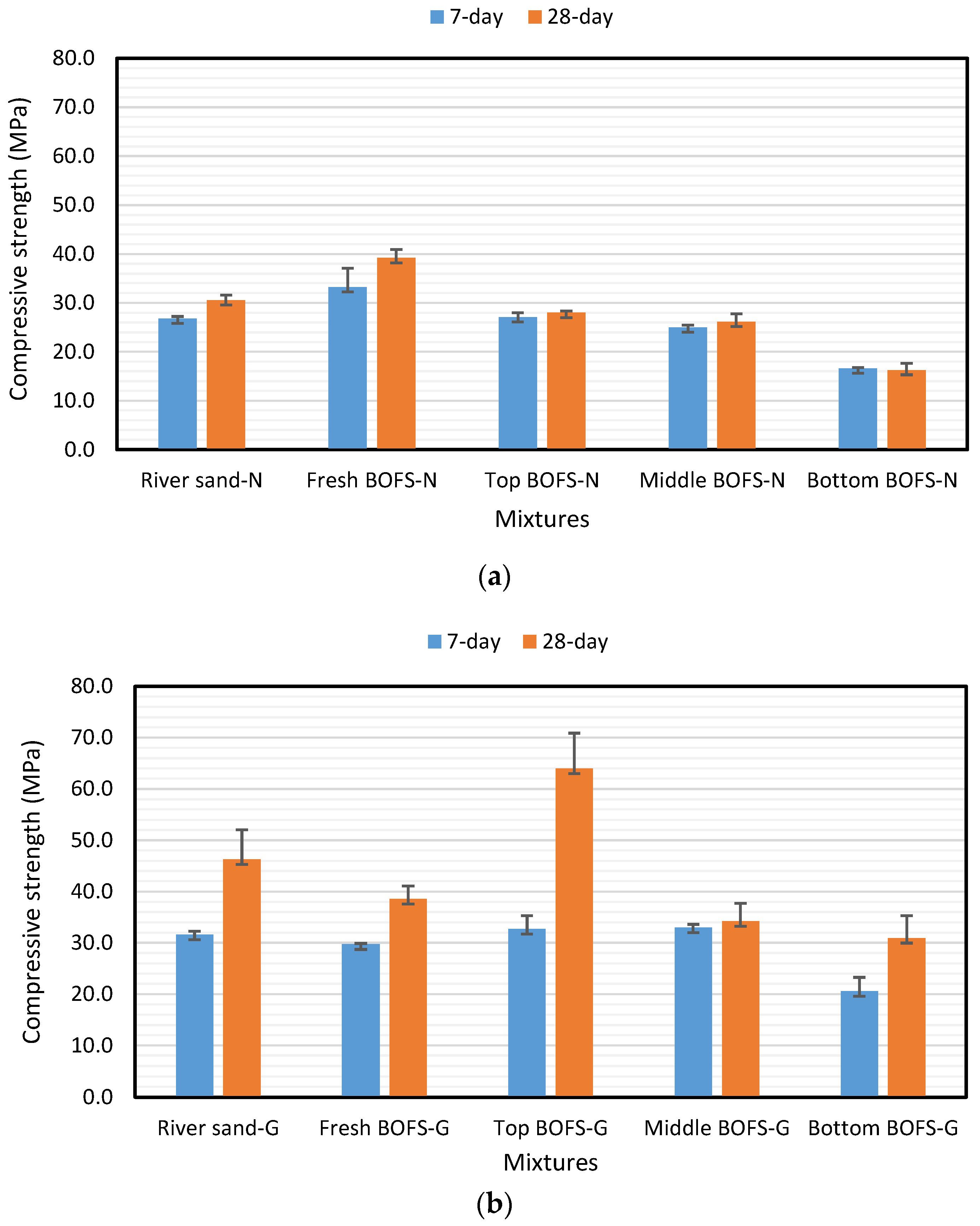
4.1. Compressive Strength
4.2. Expansion Characteristics of Mortar Bar
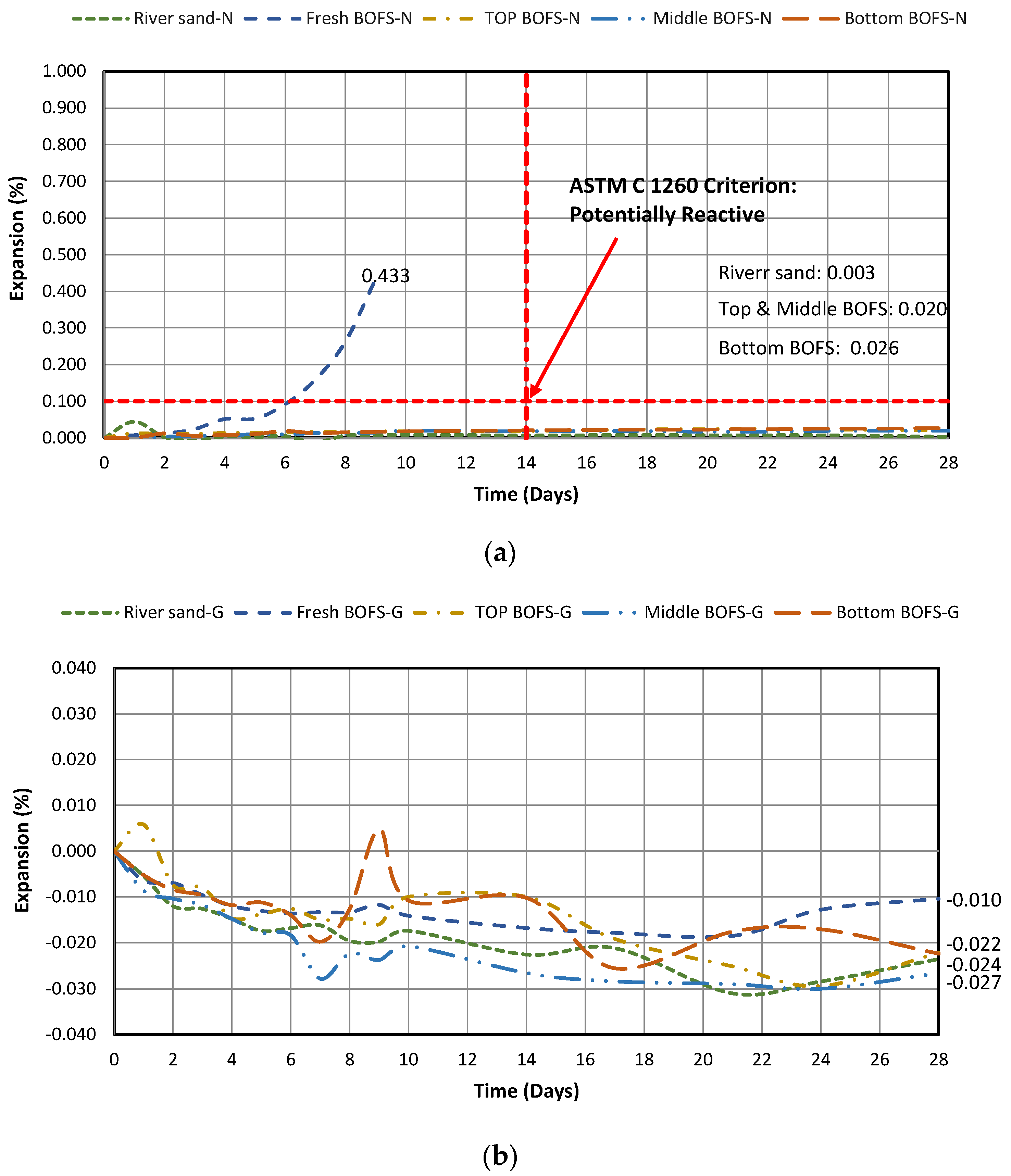
4.2.1. Expansion in the Water
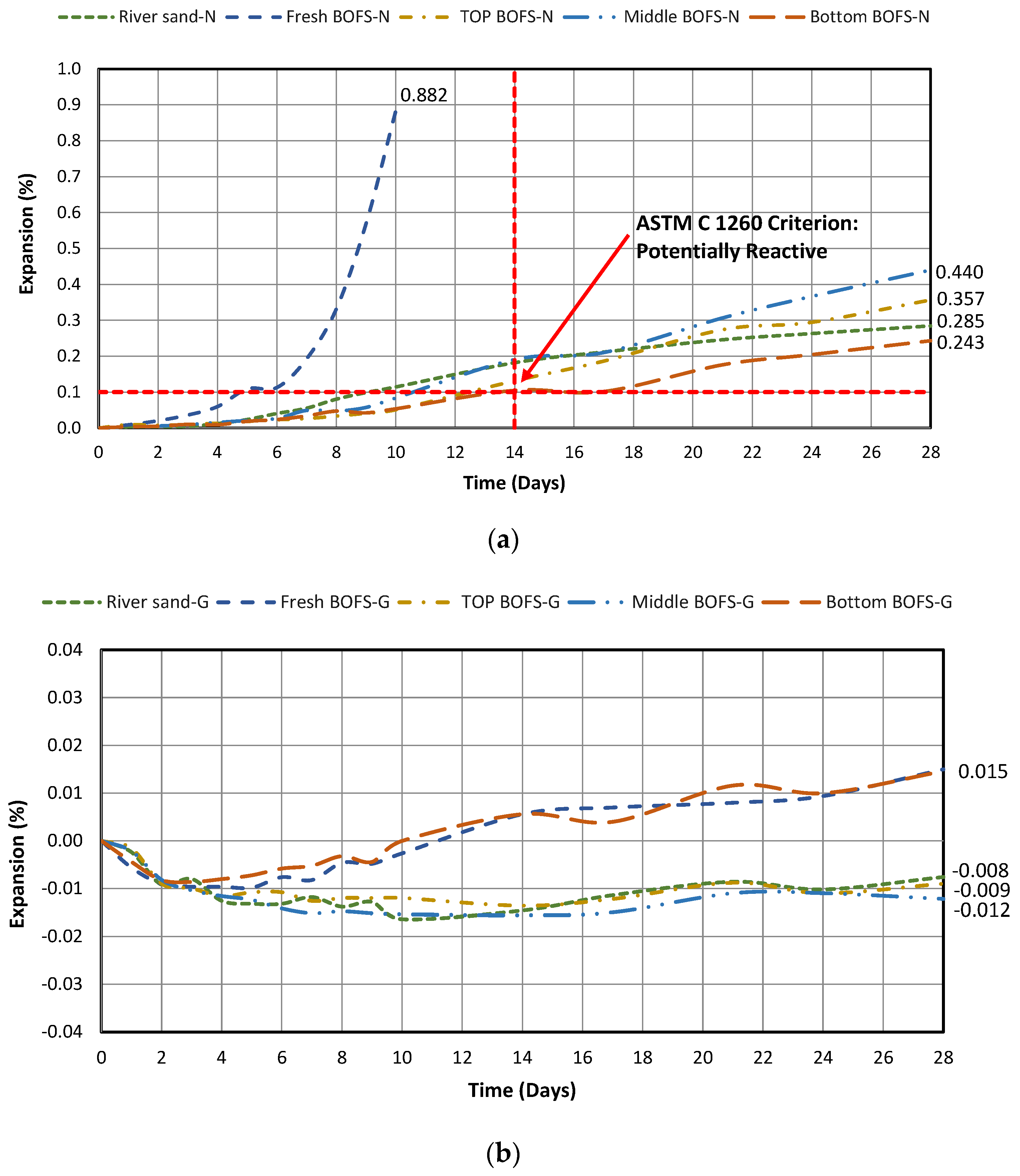
4.2.2. Expansion in 1 M NaOH Solution
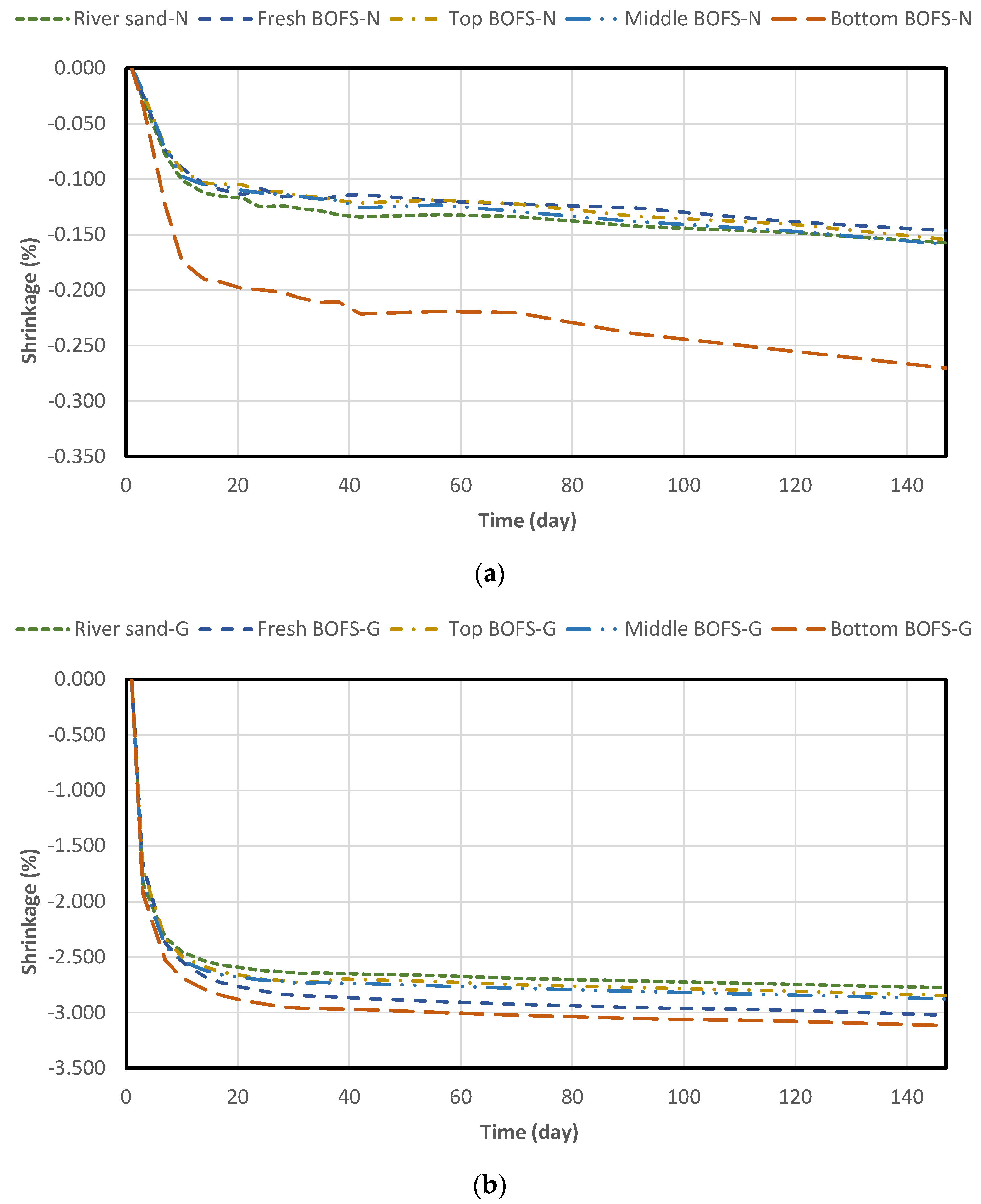
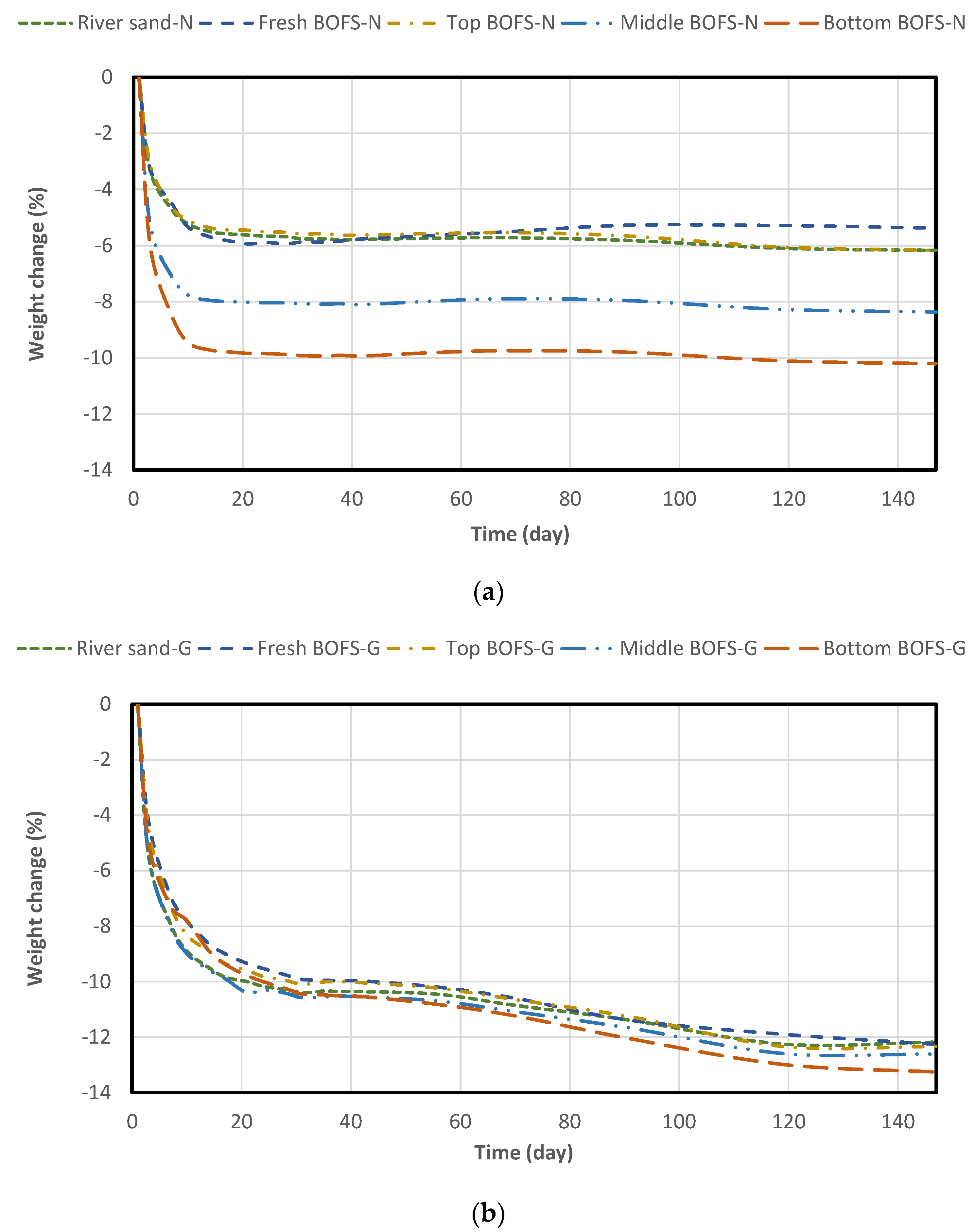
4.3. Drying Shrinkage and Mass Change
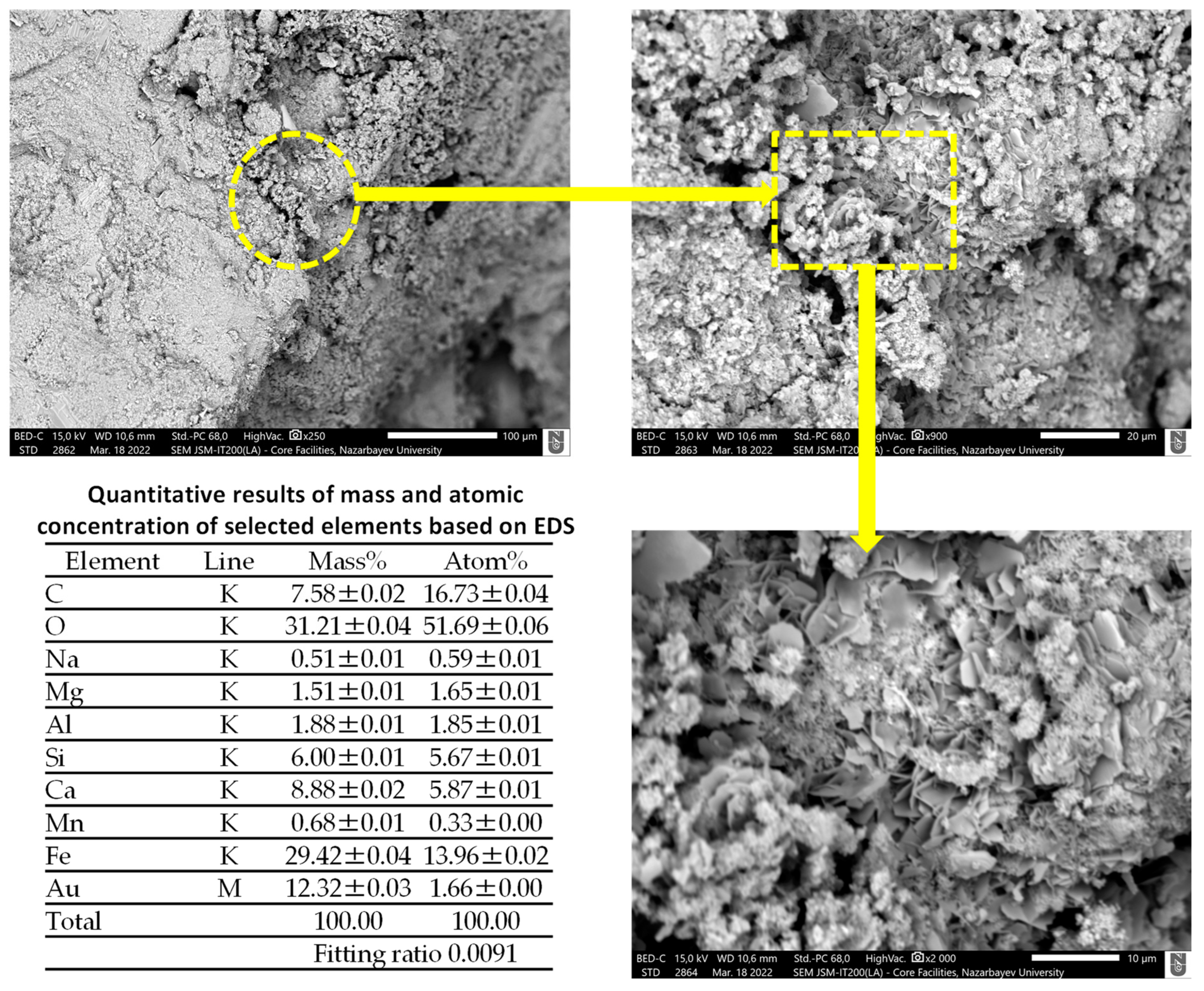
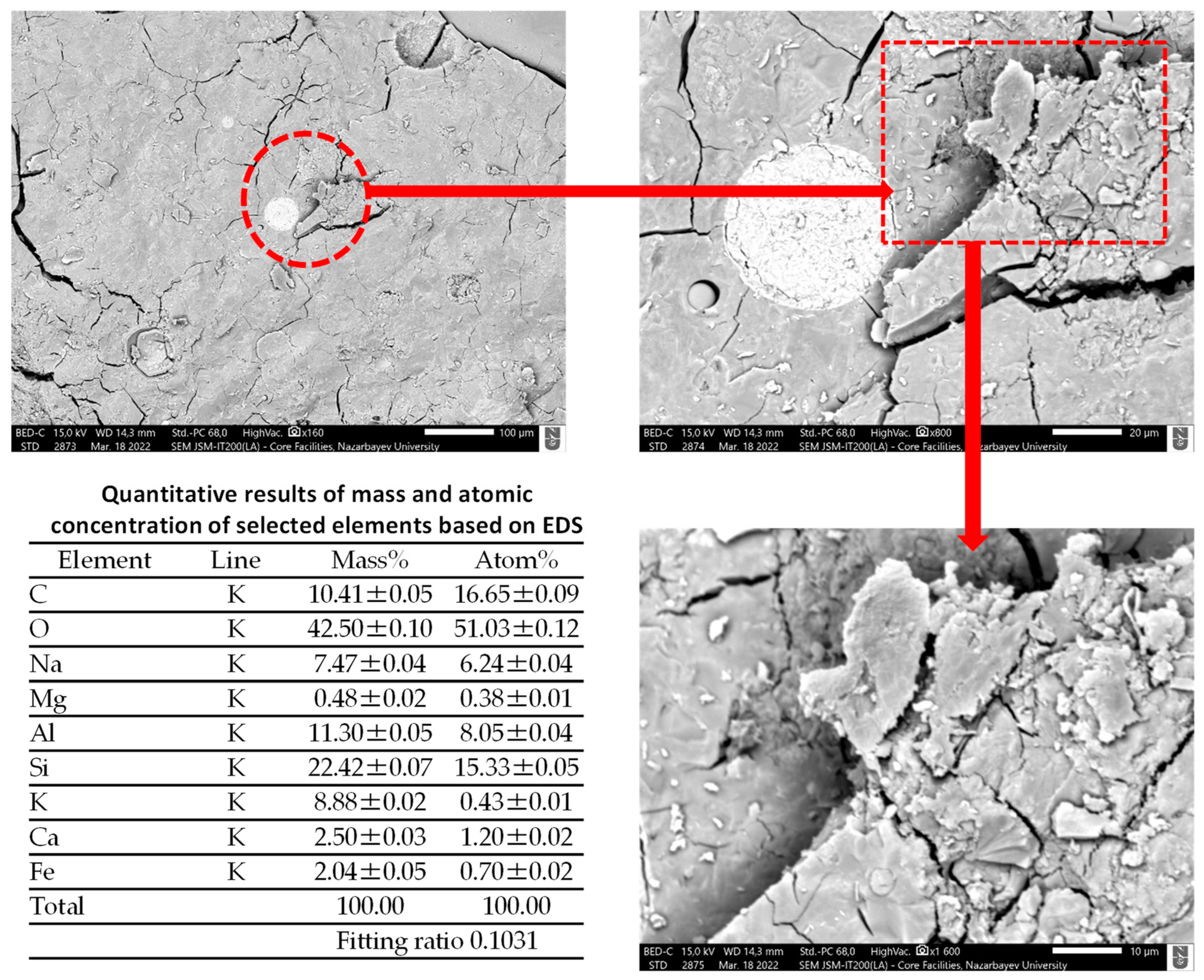
4.4. Scanning Electron Microscopy (SEM) Analysis
5. Discussion
6. Conclusions
- Generally, geopolymer mixture samples showed higher compressive strength. However, it was affected by many factors, such as the appropriate ratio of GGBFS to FFA and AAS concentration, etc. The mixtures with aged BOFS aggregate had lower compressive strength regardless of mixture type. It might be explained by the fact that the free oxides in the aggregate were already consumed and formatted portlandite that was not involved in strength development but might produce expansion in the mixture.
- Regardless of the test conditions (water and 1 M NaOH submersion at 80 °C), a normal OPC mixture containing fresh BOFS aggregate demonstrated the highest expansion compared with the mixtures with chronologically aged BOFS aggregates since fresh BOFS had the highest amount of free oxides and reactive silica that could induce the formation of Ca(OH)2 and ASR gel.
- Implementing geopolymerization technology can reduce the expansion behavior under both water and 1 M NaOH test conditions. Even the expansion of the mixture containing fresh BOFS aggregate mixture can be stabilized.
- The drying shrinkage and weight loss of the geopolymer mixture were more intensive than those of the normal OPC mixture, irrespective of aggregate type. In geopolymer mixtures with BOFS aggregate, aging duration did not significantly affect the reduction in drying shrinkage.
- SEM/EDS analysis supported the expansion characteristics of the mixtures containing BOFS aggregate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joshi, S.V.; Kadu, M.S. Role of alkaline activator in development of eco-friendly fly ash based geopolymer concrete. Int. J. Environ. Sci. Dev. 2012, 3, 417–422. [Google Scholar] [CrossRef]
- Malhotra, V.M. Introduction: Sustainable development and concrete technology. Concr. Int. 2002, 24, 22. [Google Scholar]
- Thomas, B.S.; Yang, J.; Bahurudeen, A.; Chinnu, S.N.; Abdalla, J.A.; Hawileh, R.A.; Siddika, A.; Hamada, H.M. Geopolymer concrete incorporating recycled aggregates: A comprehensive review. Clean. Mater. 2022, 3, 100056. [Google Scholar] [CrossRef]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S.K. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, Z.; Liu, X. Comprehensive Understanding of Aluminosilicate Phosphate Geopolymers: A Critical Review. Materials 2022, 15, 5961. [Google Scholar] [CrossRef]
- Akhtar, N.; Ahmad, T.; Husain, D.; Majdi, A.; Alam, M.T.; Husain, N.; Wayal, A.K.S. Ecological footprint and economic assessment of conventional and geopolymer concrete for sustainable construction. J. Clean. Prod. 2022, 380, 134910. [Google Scholar] [CrossRef]
- Figiela, B.; Korniejenko, K. The possibility of using waste materials as raw materials for the production of geopolymers. Acta Innov. 2020, 36, 48–56. [Google Scholar] [CrossRef]
- Figiela, B.; Brudny, K.; Lin, W.-T.; Korniejenko, K. Investigation of Mechanical Properties and Microstructure of Construction- and Demolition-Waste-Based Geopolymers. J. Compos. Sci. 2022, 6, 191. [Google Scholar] [CrossRef]
- Salas, D.A.; Ramirez, A.D.; Ulloa, N.; Baykara, H.; Boero, A.J. Life cycle assessment of geopolymer concrete. Constr. Build. Mater. 2018, 190, 170–177. [Google Scholar] [CrossRef]
- Zhang, P.; Han, X.; Guo, J.; Hu, S. High-temperature behavior of geopolymer mortar containing nano-silica. Constr. Build. Mater. 2023, 364, 129983. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers and geopolymeric materials. J. Therm. Anal. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W. Compressive strength and microstructure of fly ash based geopolymer blended with silica fume under thermal cycle. Cem. Concr. Compos. 2017, 78, 108–119. [Google Scholar] [CrossRef]
- Singh, N.B. Fly ash-based geopolymer binder: A future construction material. Minerals 2018, 8, 299. [Google Scholar] [CrossRef]
- Luhar, I.; Luhar, S. A Comprehensive Review on Fly Ash-Based Geopolymer. J. Compos. Sci. 2022, 6, 219. [Google Scholar] [CrossRef]
- Bakri, A.M.M.A.; Kamarudin, H.; Bnhussain, M.; Nizar, I.K.; Mastura, W.I.W. Mechanism and chemical reaction of fly ash geopolymer cement—A Review. J. Asian Sci. Res. 2011, 1, 247–253. Available online: https://archive.aessweb.com/index.php/5003/article/view/3292 (accessed on 28 October 2023).
- Aziz, I.H.; Abdullah, M.M.A.B.; Yong, H.C.; Ming, L.Y.; Hussin, K.; Kadir, A.A.; Azimi, E.A. Manufacturing of fire resistance geopolymer: A review. MATEC Web Conf. 2016, 78, 01023. [Google Scholar] [CrossRef]
- Mathivet, V.; Jouin, J.; Gharzouni, A.; Sobrados, I.; Celerier, H.; Rossignol, S.; Parlier, M. Acid-based Geopolymers: Understanding of the Structural Evolutions During Consolidation and After Thermal Treatments. J. Non-Cryst. Solids 2019, 512, 90–97. [Google Scholar] [CrossRef]
- Guo, X.; Xiong, G. Resistance of fiber-reinforced fly ash-steel slag based geopolymer mortar to sulfate attack and drying-wetting cycles. Constr. Build. Mater. 2021, 269, 121326. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Bakri, A.M.; Kamarudin, H.; Binhussain, M.; Nizar, I.K.; Rafiza, A.R.; Zarina, Y. Comparison of geopolymer fly ash and ordinary portland cement to the strength of concrete. Adv. Sci. Lett. 2013, 19, 3592–3595. [Google Scholar] [CrossRef]
- Sagoe-Crentsil, K.; Brown, T.; Taylor, A. Drying shrinkage and creep performance of geopolymer concrete. J. Sustain. Cem. 2013, 2, 35–42. [Google Scholar] [CrossRef]
- Aiken, T.A.; Sha, W.; Kwasny, J.; Soutsos, M.N. Resistance of geopolymer and Portland cement based systems to silage effluent attack. Cem. Concr. Res. 2017, 92, 56–65. [Google Scholar] [CrossRef]
- Zeng, Q.; Gao, P.; Li, K.; Dong, G.; Jin, G.; Sun, X.; Zhao, J.; Chen, L. Experimental research on the properties and formulation of fly ash based geopolymer grouting material. Buildings 2022, 12, 503. [Google Scholar] [CrossRef]
- Jindal, B.B. Investigations on the properties of geopolymer mortar and concrete with mineral admixtures: A review. Constr. Build. Mater. 2019, 227, 116644. [Google Scholar] [CrossRef]
- Provis, J.L. Introduction and scope. In Alkali Activated Materials; Springer: Dordrecht, Germany, 2014; pp. 1–9. [Google Scholar]
- Lahoti, M.; Tan, K.H.; Yang, E.H. A Critical Review of Geopolymer Properties for Structural Fire-Resistance Applications. Constr. Build. Mater. 2019, 221, 514–526. [Google Scholar] [CrossRef]
- Available online: https://egov.kz/cms/en/articles/ecology/waste_reduction_recycling_and_reuse (accessed on 28 October 2023).
- Siddique, R. Utilization of industrial by-products in concrete. Procedia Eng. 2014, 95, 335–347. [Google Scholar] [CrossRef]
- Kaja, A.M.; Melzer, S.; Brouwers, H.J.H.; Yu, Q. On the optimization of BOF slag hydration kinetics. Cem. Concr. Compos. 2021, 124, 104262. [Google Scholar] [CrossRef]
- Gao, W.; Zhou, W.; Lyu, X.; Liu, X.; Su, H.; Li, C.; Wang, H. Comprehensive utilization of steel slag: A review. Powder Technol. 2023, 422, 118449. [Google Scholar] [CrossRef]
- Pasetto, M.; Baldo, N. Experimental evaluation of high performance base course and road base asphalt concrete with electric arc furnace steel slags. J. Hazard. Mater. 2010, 181, 938–948. [Google Scholar] [CrossRef]
- Carvalho, S.Z.; Vernilli, F.; Almeida, B.; Demarco, M.; Silva, S.N. The recycling effect of BOF slag in the portland cement properties. Resour. Conserv. Recycl. 2017, 127, 216–220. [Google Scholar] [CrossRef]
- Dhoble, Y.N.; Ahmed, S. Review on the innovative uses of steel slag for waste minimization. J. Mater. Cycles Waste Manag. 2018, 20, 1373–1382. [Google Scholar] [CrossRef]
- Altun, I.A.; Yılmaz, İ. Study on steel furnace slags with high MgO as additive in Portland cement. Cem. Concr. Res. 2002, 32, 1247–1249. [Google Scholar] [CrossRef]
- Jiang, Y.; Ling, T.C.; Shi, C.; Pan, S.Y. Characteristics of steel slags and their use in cement and concrete—A review. Resour. Conserv. Recycl. 2018, 136, 187–197. [Google Scholar] [CrossRef]
- Yildirim, I.Z.; Prezzi, M. Chemical, mineralogical, and morphological properties of steel slag. Adv. Civ. Eng. 2011, 2011, 463638. [Google Scholar] [CrossRef]
- Shi, C. Steel slag—Its production, processing, characteristics, and cementitious properties. J. Mater. Civ. Eng. 2004, 16, 230–236. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P. Hydration properties of basic oxygen furnace steel slag. Constr. Build. Mater. 2010, 24, 1134–1140. [Google Scholar] [CrossRef]
- Feng, P.; Li, Z.; Zhang, S.; Yang, J.Q. Steel slag aggregate concrete filled-in FRP tubes: Volume expansion effect and axial compressive behaviour. Constr. Build. Mater. 2022, 318, 125961. [Google Scholar] [CrossRef]
- Tukaziban, A.; Shon, C.-S.; Orynbassarov, I.; Sandybay, S.; Syzdykov, D.; Zhang, D.; Kim, J.R. Mechanical, swelling, and thermal properties of geopolymer mixture containing basic oxygen furnace slag aggregates. IOP Conf. Ser. Earth Environ. Sci. 2022, 1050, 012021. [Google Scholar] [CrossRef]
- Zago, S.C.; Vernilli, F.; Cascudo, O. The reuse of basic oxygen furnace slag as concrete aggregate to achieve sustainable development: Characteristics and limitations. Buildings 2023, 13, 1193. [Google Scholar] [CrossRef]
- Su, T.-H.; Yang, H.-J.; Shau, Y.-H.; Takazawa, E.; Lee, Y.-C. CO2 sequestration utilizing basic-oxygen furnace slag: Controlling factors, reaction mechanisms and V–Cr concerns. J. Environ. Sci. 2016, 41, 99–111. [Google Scholar] [CrossRef]
- Yasipourtehrani, S.; Tian, S.; Strezov, V.; Kan, T.; Evans, T. Development of robust CaO-based sorbents from blast furnace slag for calcium looping CO2 capture. Chem. Eng. J. 2020, 387, 124140. [Google Scholar] [CrossRef]
- Lee, W.H.; Cheng, T.W.; Lin, K.Y.; Lin, K.L.; Wu, C.C.; Tsai, C.T. Geopolymer technologies for stabilization of basic oxygen furnace slags and sustainable application as construction materials. Sustainability 2020, 12, 5002. [Google Scholar] [CrossRef]
- Wang, W.C. Feasibility of stabilizing expanding property of furnace slag by autoclave method. Constr. Build. Mater. 2014, 68, 552–557. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Li, H.H.; Wang, Y.C.; Xu, D.L. Geopolymer microstructure and hydration mechanism of alkali-activated fly ash-based geopolymer. Adv. Mat. Res. 2012, 374–377, 1481–1484. [Google Scholar] [CrossRef]
- Lazorenko, G.; Kasprzhitskii, A. Geopolymer additive manufacturing: A review. Addit. Manuf. 2022, 55, 102782. [Google Scholar] [CrossRef]
- ASTM C596; Standard Test Method for Drying Shrinkage of Mortar Containing Hydraulic Cement. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM C188; Standard Test Method for Density of Hydraulic Cement. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM C1420; Standard Guide for Selection, Removal, and Shipment of Manufactured Masonry Units Placed in Usage. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM C114; Standard Test Method for Chemical Analysis of Hydraulic Cement. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM C109/C109M; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens). ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM C1260; Standard Test Method for Potential Alkali Reactivity of Aggregates (Mortar-Bar Method). ASTM International: West Conshohocken, PA, USA, 2023.
- Tuyan, M.; Andiç-Çakir, Ö.; Ramyar, K. Effect of alkali activator concentration and curing condition on strength and microstructure of waste clay brick powder-based geopolymer. Compos. B Eng. 2018, 135, 242–252. [Google Scholar] [CrossRef]
- Mashifana, T.; Sithole, N. Utilization of fly ash: Basic oxygen furnace slag as a raw material in geopolymerization. IOP Conf. Ser. Mater. Sci. Eng. 2019, 652, 012060. [Google Scholar] [CrossRef]
- Memon, F.A.; Nuruddin, M.F.; Khan, S.; Shafiq, N.A.S.I.R.; Ayub, T. Effect of sodium hydroxide concentration on fresh properties and compressive strength of self-compacting geopolymer concrete. J. Eng. Sci. Technol. 2013, 8, 44–56. [Google Scholar]
- Doğan-Sağlamtimur, N.; Öz, H.Ö.; Bilgil, A.; Vural, T.; Süzgeç, E. The effect of alkali activation solutions with different water glass/NaOH solution ratios on geopolymer composite materials. IOP Conf. Ser. Mater. Sci. Eng. 2019, 660, 012003. [Google Scholar] [CrossRef]
- ASTM C305; Standard Practice for Mechanical Mixing of Hydraulic Cement Pastes and Mortars of Plastic Consistency. ASTM International: West Conshohocken, PA, USA, 2020.
- Zhang, H.Y.; Kodur, V.; Wu, B.; Yan, J.; Yuan, Z.S. Effect of temperature on bond characteristics of geopolymer concrete. Constr. Build. Mater. 2018, 163, 277–285. [Google Scholar] [CrossRef]
- Ding, Y.; Dai, J.G.; Shi, C.J. Mechanical properties of alkali-activated concrete: A state-of-the-art review. Constr. Build. Mater. 2016, 127, 68–79. [Google Scholar] [CrossRef]
- Atiş, C.D.; Görür, E.B.; Karahan, O.; Bilim, C.; İlkentapar, S.; Luga, E. Very high strength (120 MPa) class F fly ash geopolymer mortar activated at different NaOH amount, heat curing temperature and heat curing duration. Constr. Build. Mater. 2015, 96, 673–678. [Google Scholar] [CrossRef]
- Kuszhanova, A.; Raiymbek, M.; Abzal, A.; Shon, C.-S.; Sandybay, S.; Tukaziban, A.; Kim, J.R. Compressive strength and expansion characteristics of mortar mixtures incorporating chronologically aged BOFS aggregates blended with GGBFS and fly ash. Mater. Sci. Forum. 2022, 1077, 237–242. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Lu, M.; Chen, B.; Gao, S.; Bai, J.; Zhang, H.; Yang, Y. Study on the Effect of Calcium and Sulfur Content on the Properties of Fly Ash-Based Geopolymer. Constr. Build. Mater. 2022, 314, 125650. [Google Scholar] [CrossRef]
- Lee, J.-C.; Jang, B.-K.; Shon, C.-S.; Kim, J.-H.; Chung, C.-W. Potential use of borosilicate glass to make neutron shielding mortar: Enhancement of thermal neutron shielding and strength development and mitigation of alkali-silica reaction. J. Clean. Prod. 2019, 210, 638–645. [Google Scholar] [CrossRef]
- Shon, C.-S.; Tugelbayev, A.; Shaimakhanov, R.; Karatay, K.; Zhang, D.; Kim, J.R. Use of Off-ASTM class F fly ash and waste limestone powder in mortar mixtures containing waste glass sand. Sustainability 2022, 14, 75. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Puertas, F. The alkali–silica reaction in alkali-activated granulated slag mortars with reactive aggregate. Cem. Concr. Res. 2002, 32, 1019–1024. [Google Scholar] [CrossRef]
- Kozhageldi, N.; Shon, C.-S.; Kareken, G.; Tukaziban, A.; Mardenov, M.; Zhang, D.; Kim, J.R. Properties of geopolymer mortar mixtures containing waste glass aggregates and river sand. Key Eng. Mater. 2023, 945, 93–99. [Google Scholar] [CrossRef]
- Roy, D.M. Hydration, structure, and properties of blast furnace slag cements, mortars, and concrete. ACI J. 1982, 79, 444–457. [Google Scholar] [CrossRef]
- Shi, C. Strength, pore structure and permeability of alkali-activated slag mortars. Cem. Concr. Res. 1996, 26, 1789–1799. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Alkali activation of Australian slag cements. Cem. Concr. Res. 1999, 29, 113–120. [Google Scholar] [CrossRef]
- Ban, J.; Sun, K.; Lu, J.-X.; Ali, H.A.; Yao, J.; Sunahara, G.; Poon, C.-S. Effect of air pollution-controlled residue of a sewage sludge incinerator on the drying shrinkage and the pore structure of alkali-activated materials. Waste Manage. 2023, 161, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhu, H.; Feng, P.; Zhang, P. A review on shrinkage-reducing methods and mechanisms of alkali-activated/geopolymer systems: Effects of chemical additives. J. Build. Eng. 2022, 49, 104056. [Google Scholar] [CrossRef]
- Deb, P.S.; Nath, P.; Sarker, P.K. Drying shrinkage of slag blended fly ash geopolymer concrete cured at room temperature. Procedia Eng. 2015, 125, 594–600. [Google Scholar] [CrossRef]
- Özkan, Ö.; Sarıbıyık, M. Alkali silica reaction of BOF and BFS wastes combination in cement. J. Civ. Eng. Manag. 2013, 19, 113–120. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Puertas, F.; Sobrados, I.; Sanz, J. Structure of calcium silicate hydrates formed in alkaline-activated slag: Influence of the type of alkaline activator. Am. Ceram. Soc. 2003, 86, 1389–1394. [Google Scholar] [CrossRef]
- Brand, A.S.; Fanijo, E.O. A Review of the influence of steel furnace slag type on the properties of cementitious composites. Appl. Sci. 2020, 10, 8210. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Q.; Fang, K. Optimization of f-MgO/f-CaO phase in ladle furnace slag by air rapidly cooling. Mater. Lett. 2020, 280, 128528. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Li, R. Simulation and property prediction of MgO-FeO-MnO solid solution in steel slag. Mater. Lett. 2020, 273, 127930. [Google Scholar] [CrossRef]
- Li, Y.; Mehdizadeh, H.; Kim, H.M.; Ling, T.-C. Co-utilization of aqueous carbonated basic oxygen furnace slag (BOFS) and carbonated filtrate in cement pastes considering reaction duration effect. Cem. Concr. Compos. 2023, 138, 104988. [Google Scholar] [CrossRef]
- Vollprecht, D.; Wohlmuth, D. Mineral carbonation of basic oxygen furnace slags. Recycling 2022, 7, 84. [Google Scholar] [CrossRef]
- Kuo, W.-T.; Gao, Y.-S.; Juang, C.-U. Influence of BOF and GGBFS based alkali activated materials on the properties of porous concrete. Materials 2019, 12, 2214. [Google Scholar] [CrossRef] [PubMed]



| Chemical Compositions | |||||||||
| CaO | SiO2 | Fe2O3 | MgO | Al2O3 | P2O5 | MnO | TiO2 | Cr2O3 | |
| River sand | 12.01 | 64.46 | 4.26 | 1.51 | 11.38 | 0.39 | 0.44 | 0.37 | - |
| Fresh BOFS | 40.28 | 8.03 | 36.93 | 2.63 | 0.61 | 2.75 | 5.35 | 0.94 | 0.39 |
| Top BOFS | 41.17 | 22.83 | 19.64 | 6.67 | 3.22 | 2.68 | 1.97 | 0.39 | 0.39 |
| Middle BOFS | 44.02 | 19.79 | 19.00 | 7.33 | 2.19 | 3.75 | 2.18 | 0.39 | 0.43 |
| Bottom BOFS | 27.73 | 33.76 | 10.57 | 6.49 | 16.60 | 1.47 | 1.10 | 0.66 | 0.27 |
| Aggregate Properties | |||||||||
| Specific Gravity (%) | Absorption Capacity (%) | Size of Aggregate (Sieve Number) | Mass (%) | ||||||
| River sand | 2.68 | 2.87 | 2.36 mm (#8) | 10% | |||||
| Fresh BOFS | 3.33 | 5.08 | 1.18 mm (#16) | 25% | |||||
| Top BOFS | 2.91 | 3.28 | 600 μm (#30) | 25% | |||||
| Middle BOFS | 2.86 | 5.27 | 300 μm (#50) | 25% | |||||
| Bottom BOFS | 2.60 | 10.22 | 150 μm (#100) | 15% | |||||
| Chemical Compositions (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | MgO | K2O | TiO2 | MnO | |
| OPC | 69.19 | 17.41 | 4.36 | 2.96 | 2.61 | 2.05 | 0.95 | 0.20 | 0.12 |
| FFA | 1.86 | 65.34 | 24.85 | 4.01 | 0.31 | 0.53 | 0.68 | 1.10 | 0.07 |
| GGBFS | 40.51 | 32.25 | 11.40 | 0.33 | 2.00 | 10.46 | 0.88 | 1.41 | 0.31 |
| OPC Mixtures (kg/m3) | ||||||||||||
| Water | Cement | River sand | BOFS | |||||||||
| River sand-N | 222.0 | 555.0 | 1608.8 | - | ||||||||
| Fresh BOFS-N | 222.0 | 555.0 | - | 2001.8 | ||||||||
| Top BOFS-N | 222.0 | 555.0 | - | 1748.1 | ||||||||
| Middle BOFS-N | 222.0 | 555.0 | - | 1719.6 | ||||||||
| Bottom BOFS-N | 222.0 | 555.0 | - | 1563.7 | ||||||||
| Geopolymer Mixtures (kg/m3) | ||||||||||||
| Water | FFA | GGBFS | River sand | BOFS | AAS | |||||||
| River sand-G | 122.6 | 522.3 | 522.3 | 313.4 | - | 417.8 | ||||||
| Fresh BOFS-G | 125.4 | 534.5 | 534.5 | - | 320.7 | 427.6 | ||||||
| Top BOFS-G | 123.7 | 527.3 | 527.2 | 316.3 | 421.7 | |||||||
| Middle BOFS-G | 123.5 | 526.2 | 526.2 | - | 315.7 | 420.9 | ||||||
| Bottom BOFS-G | 122.1 | 520.4 | 520.4 | - | 312.3 | 416.3 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tukaziban, A.; Shon, C.-S.; Zhang, D.; Kim, J.R.; Kim, J.-H.; Chung, C.-W. Synthesis and Evaluation of Geopolymer Mixtures Containing Chronologically Aged Basic Oxygen Furnace Slags. Sustainability 2023, 15, 16934. https://doi.org/10.3390/su152416934
Tukaziban A, Shon C-S, Zhang D, Kim JR, Kim J-H, Chung C-W. Synthesis and Evaluation of Geopolymer Mixtures Containing Chronologically Aged Basic Oxygen Furnace Slags. Sustainability. 2023; 15(24):16934. https://doi.org/10.3390/su152416934
Chicago/Turabian StyleTukaziban, Aizhan, Chang-Seon Shon, Dichuan Zhang, Jong Ryeol Kim, Ji-Hyun Kim, and Chul-Woo Chung. 2023. "Synthesis and Evaluation of Geopolymer Mixtures Containing Chronologically Aged Basic Oxygen Furnace Slags" Sustainability 15, no. 24: 16934. https://doi.org/10.3390/su152416934
APA StyleTukaziban, A., Shon, C.-S., Zhang, D., Kim, J. R., Kim, J.-H., & Chung, C.-W. (2023). Synthesis and Evaluation of Geopolymer Mixtures Containing Chronologically Aged Basic Oxygen Furnace Slags. Sustainability, 15(24), 16934. https://doi.org/10.3390/su152416934