Microalgae Production on Biogas Digestate in Sub-Alpine Region of Europe—Development of Simple Management Decision Support Tool
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae Cultivation
2.2. Microalgae Inoculum
2.3. Digestate Usage
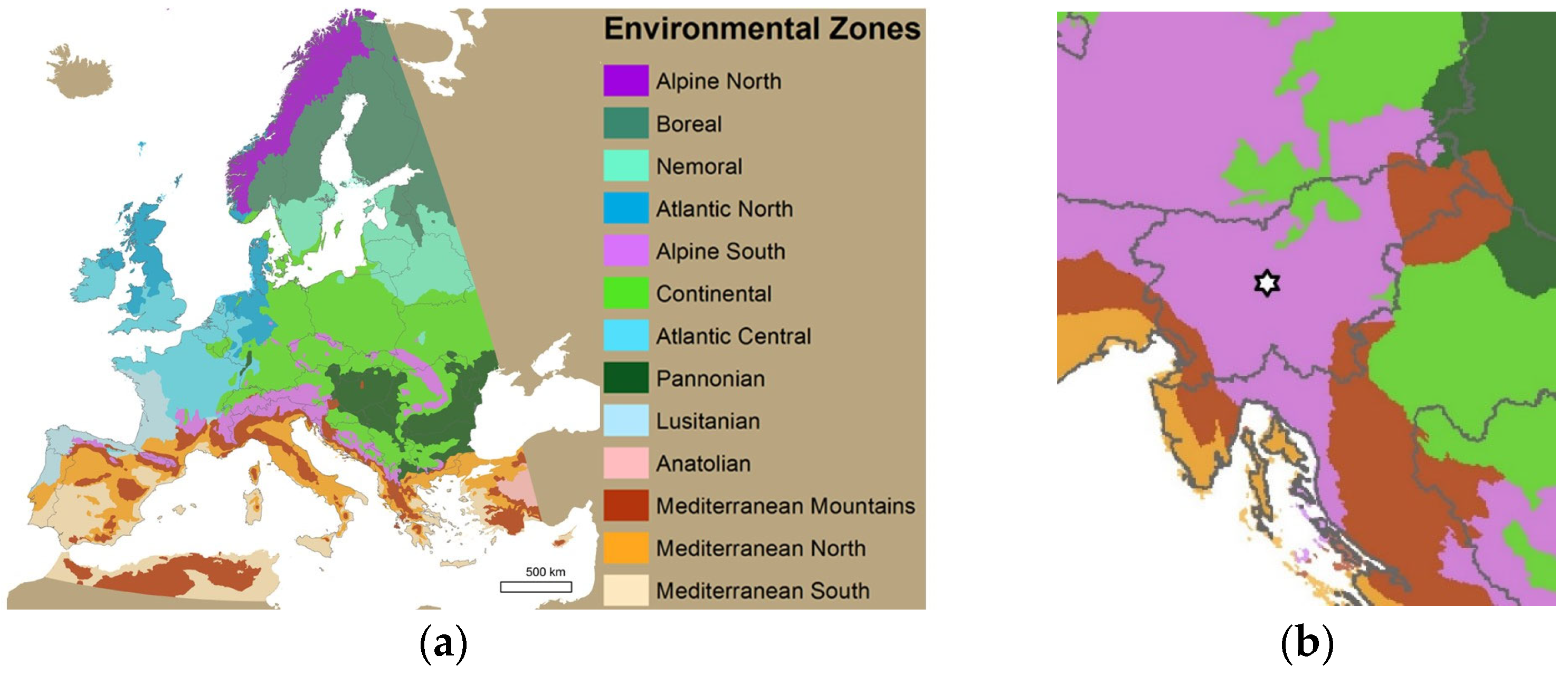
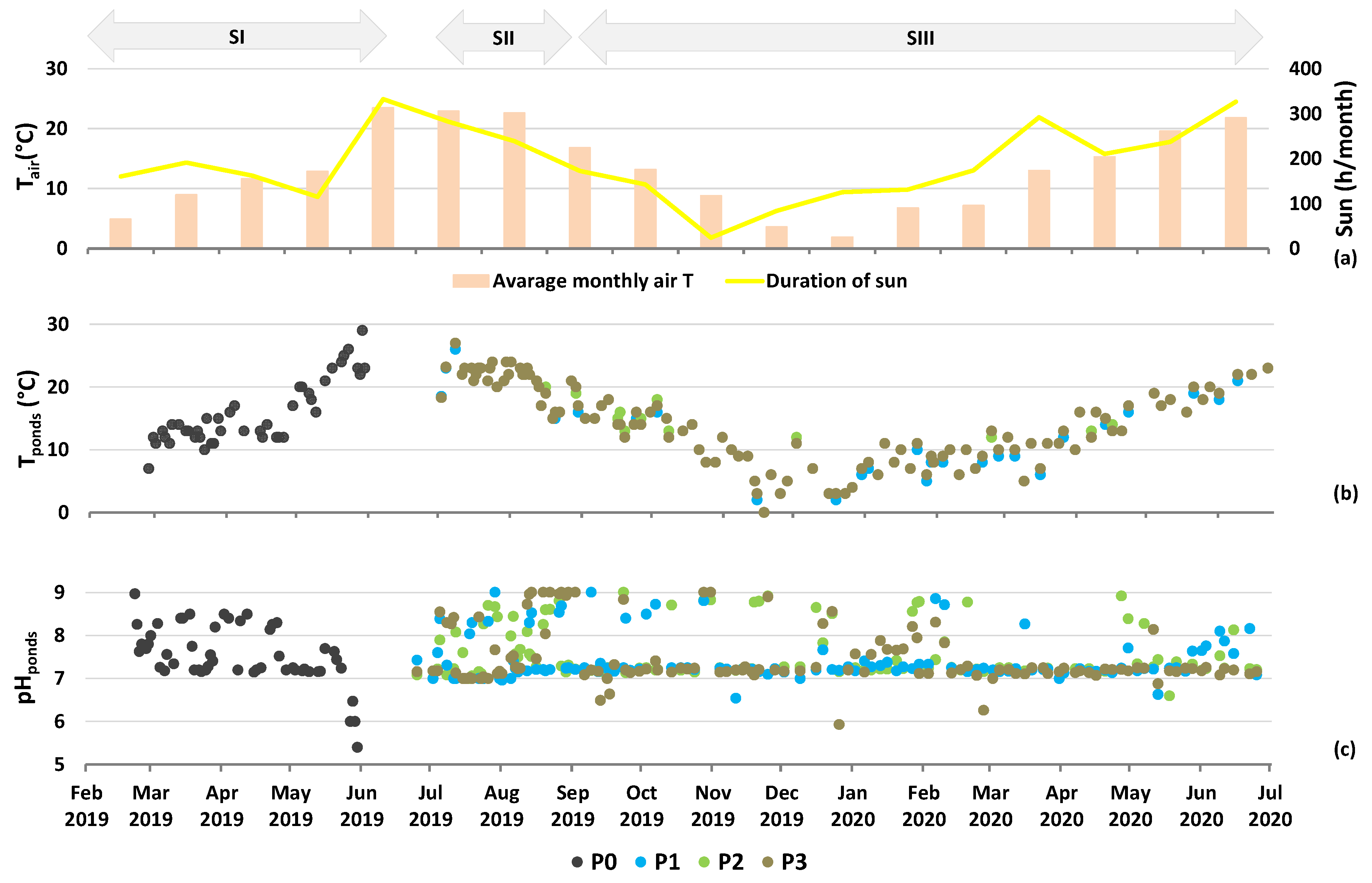
2.4. Climate and Pond Conditions
2.5. Sampling and Analysis
2.6. Experiment Stages
- Learning/Design Stage (SI): initial monitoring from February 2019 to July 2019 to establish testing conditions. The algae were fed by untreated digestate, increasing from 2 L day−1 in February 2019 to 27 L day−1 in June 2019 (corresponding to from 0.42 up to 4.38 g total daily nitrogen input, TNin m−2 day−1, resulting in EC levels between 1900 µS cm−1 in March 2019 and max. 5800 µS cm−1 in June 2019). Inoculum OD680 at the beginning of SI was 0.57.
- Development/Testing Stage (SII): three ponds were exposed to varying digestate treatments and EC levels in July 2019. P1 had low nutrition (EC < 1500 µS cm−1), P2 had medium nutrition, presumably the most optimal (EC 1500–2500 µS cm−1), and P3 had high nutrition (EC > 2500 µS cm−1). These EC levels corresponded to <1, 0.5–3.0 and >2 g total daily nitrogen input (TNin m−2 day−1) in P1, P2, and P3, respectively. Inoculum OD680 at the beginning of SII was 1.06, 1.48, and 1.21 in P1, P2, and P3, respectively. This stage concluded in late August 2019 due to potential culture collapse.
- Calibration/Verification Stage (SIII): started in September 2019 and lasted until July 2020 and included the adjustment of culturing and harvesting regimes to ensure sustainable cultivation of microalgae. The conditions were as follows: average EC 712 µS cm−1, 938 µS cm−1, 1316 µS cm−1; average TNin m−2 day−1 0.3, 0.6, and 1.0 g; and inoculum OD680 at the beginning of SIII was 1.06, 1.48, and 1.21 in P1, P2, and P3, respectively.

2.7. Productivity Calculations
2.8. Statistical Analysis
3. Results
3.1. Factors Influencing Microalgae Production
3.2. Dynamics of Physio-Chemical and Biological Parameters during the Experiment
3.3. Dynamics of Biological Parameters in Ponds: DFI, OD, and Species Composition
3.4. Biomass Productivity, Seasonal Biomass Yield, and Nitrogen Utilization Efficiency
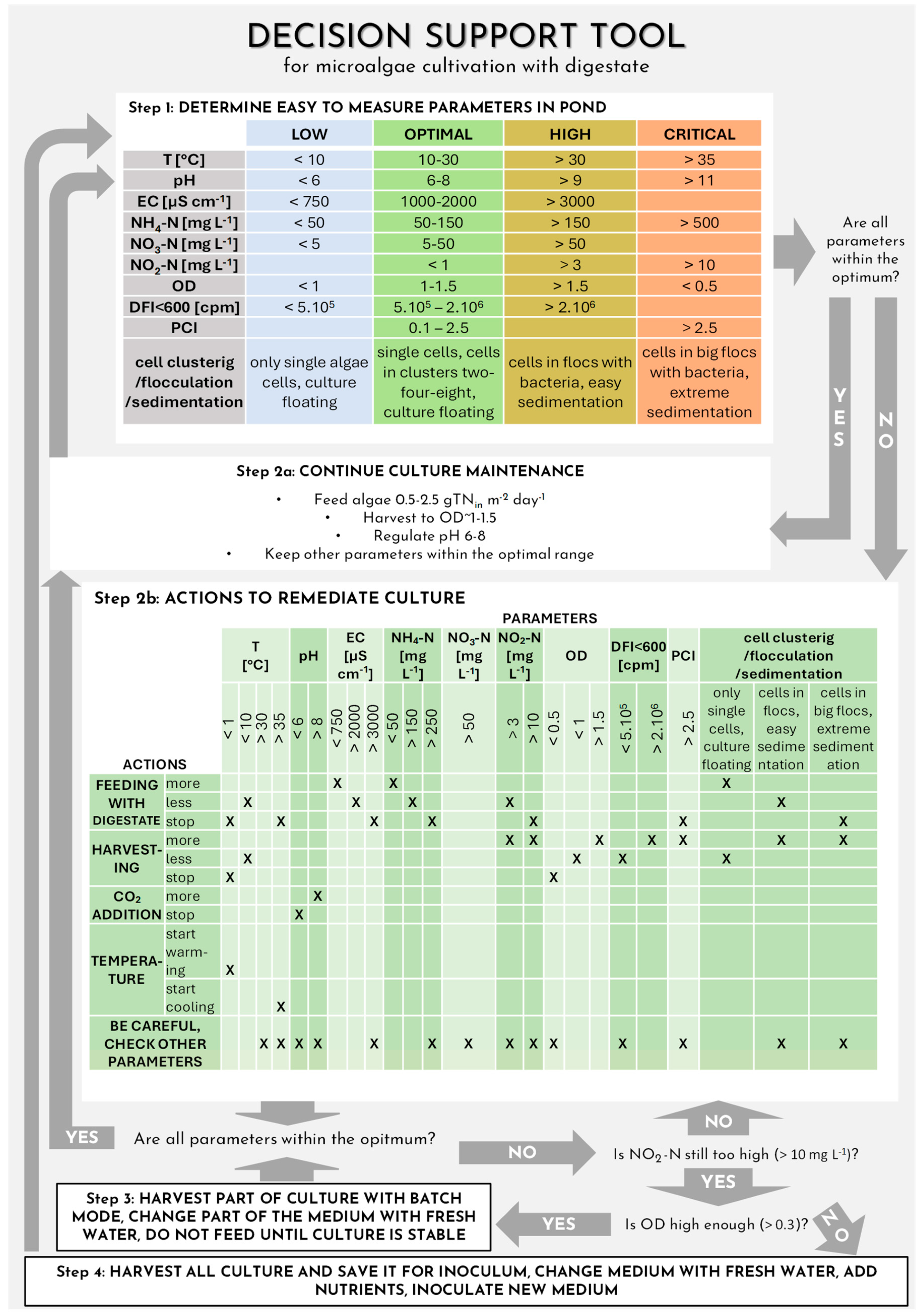
3.5. Decision Support Tool Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Microalgae Species 1 and Bacteria and/or Flocs Present in the Community | |||||
|---|---|---|---|---|---|
| Stage | Season | P0 | P1 | P2 | P3 |
| SI | spring | S. dimorphus, S. quadricauda, S. obliquus separate single cells mode in case of S. dimorphus | / | / | / |
| summer | S. dimorphus, S. quadricauda, S. obliquus some flocs, some bacteria | / | / | / | |
| SII | summer | / | S. dimorphus, S. quadricauda mostly single cells mode, some floc seen at the end of summer | S. dimorphus, S. quadricauda, single cells mode, flocs at the end of summer | S. dimorphus, S. quadricauda, Dictyosphaerium sp. whole cells at the beginning and then single cells mode; other species present in higher numbers |
| SIII | fall | / | S. dimorphus, S. quadricauda,diatoms, Dictyosphaerium sp. dominant algae in whole cells mode, at the end flocs with bacteria seen | S. dimorphus, S. quadricauda, cyanobacteria dominant algae in whole cells mode, at the end flocs with bacteria seen | S. dimorphus, S. quadricauda, cyanobacteria, Dictyospaherium sp., diatoms, Scenedesmus sp. whole cells mode, flocs of bacteria seen |
| winter | / | S. dimorphus, S. quadricauda, diatoms, Scenedesmus sp., cyanobacteria whole and single cells mode of S. dimorphus, some flocs | S. dimorphus, S. quadricauda, Scenedesmus sp. whole and single cells od S. dimorphus, some rotifera presnet | S. dimorphus., S. quadricauda, Scenedesmus sp. start: single cells mode, flocs, end: single cells and whole, flocs, some cyanobacteria, and grazers present (vorticella) | |
| spring | / | S. dimorphus, S. quadricauda, Scenedesmus sp. S. dimorphus mostly in single cell mode, at the end also whole cells; flocs, bacteria present, grazers | S. dimorphus, S. quadricauda, Scenedesmus sp. S. dimorphus in single cell mode mostly, flocs with bacteria present | S. dimorphus, S. quadricauda, Scenedesmus sp., Dictyosphaerium sp. more flocs than other ponds, bacteria and cyanobacteria, whole cells of S. dimorphus, grazers (rotifera) | |
| summer | / | S. dimorphus, S. quadricauda, Scenedesmus sp. mostly whole cell mode, flocs presnet, bacteria, grazers | S. dimorphus, S. quadricauda, Scenedesmus sp. whole cell mode, more of bacteria seen (form flocs as well), flocs present, thread-like cyanobacteria in higher numbers | S. dimorphus, S. quadricauda, Scenedesmus sp., Dictyosphaerium sp. bigger flocs, bacteria in flocs (round and thred-like), whole cells mode, Dictyospaherium sp. in big colonies; more S. quadricauda than S. dimorphus at the end of the period | |
References
- De Carvalho, J.C.; Molina-Aulestia, D.T.; Martinez-Burgos, W.J.; Karp, S.G.; Manzoki, M.C.; Medeiros, A.B.P.; Rodrigues, C.; Scapini, T.; Vandenberghe, L.P.D.S.; Vieira, S.; et al. Agro-Industrial Wastewaters for Algal Biomass Production, Bio-Based Products, and Biofuels in a Circular Bioeconomy. Fermentation 2022, 8, 728. [Google Scholar] [CrossRef]
- Haider, M.N.; Liu, C.-G.; Tabish, T.A.; Balakrishnan, D.; Show, P.-L.; Qattan, S.Y.A.; Gull, M.; Mehmood, M.A. Resource Recovery of the Wastewater-Derived Nutrients into Algal Biomass Followed by Its Cascading Processing to Multiple Products in a Circular Bioeconomy Paradigm. Fermentation 2022, 8, 650. [Google Scholar] [CrossRef]
- Casagli, F.; Beline, F.; Ficara, E.; Bernard, O. Optimizing Resource Recovery from Wastewater with Algae-Bacteria Membrane Reactors. Chem. Eng. J. 2023, 451, 138488. [Google Scholar] [CrossRef]
- Masojídek, J.; Gómez-Serrano, C.; Ranglová, K.; Cicchi, B.; Encinas Bogeat, Á.; Câmara Manoel, J.A.; Sanches Zurano, A.; Silva Benavides, A.M.; Barceló-Villalobos, M.; Robles Carnero, V.A.; et al. Photosynthesis Monitoring in Microalgae Cultures Grown on Municipal Wastewater as a Nutrient Source in Large-Scale Outdoor Bioreactors. Biology 2022, 11, 1380. [Google Scholar] [CrossRef] [PubMed]
- Srimongkol, P.; Sangtanoo, P.; Songserm, P.; Watsuntorn, W.; Karnchanatat, A. Microalgae-Based Wastewater Treatment for Developing Economic and Environmental Sustainability: Current Status and Future Prospects. Front. Bioeng. Biotechnol. 2022, 10, 904046. [Google Scholar] [CrossRef]
- Malik, S.; Shahid, A.; Haider, M.N.; Amin, M.; Betenbaugh, M.J.; Mehmood, M.A.; Haq, M.A.U.; Syafiuddin, A.; Boopathy, R. Prospects of Multiproduct Algal Biorefineries Involving Cascading Processing of the Biomass Employing a Zero-Waste Approach. Curr. Pollut. Rep. 2022, 8, 147–158. [Google Scholar] [CrossRef]
- Morillas-España, A.; Lafarga, T.; Sánchez-Zurano, A.; Acién-Fernández, F.G.; González-López, C. Microalgae Based Wastewater Treatment Coupled to the Production of High Value Agricultural Products: Current Needs and Challenges. Chemosphere 2022, 291, 132968. [Google Scholar] [CrossRef]
- La Bella, E.; Baglieri, A.; Fragalà, F.; Puglisi, I. Multipurpose Agricultural Reuse of Microalgae Biomasses Employed for the Treatment of Urban Wastewater. Agronomy 2022, 12, 234. [Google Scholar] [CrossRef]
- Alobwede, E.; Cotton, A.; Leake, J.R.; Pandhal, J. The Fate and Distribution of Microalgal Nitrogen When Applied as an Agricultural Soil Fertiliser and Its Effect on Soil Microbial Communities. Phycology 2022, 2, 297–318. [Google Scholar] [CrossRef]
- Osorio-Reyes, J.G.; Valenzuela-Amaro, H.M.; Pizaña-Aranda, J.J.P.; Ramírez-Gamboa, D.; Meléndez-Sánchez, E.R.; López-Arellanes, M.E.; Castañeda-Antonio, M.D.; Coronado-Apodaca, K.G.; Gomes Araújo, R.; Sosa-Hernández, J.E.; et al. Microalgae-Based Biotechnology as Alternative Biofertilizers for Soil Enhancement and Carbon Footprint Reduction: Advantages and Implications. Mar. Drugs 2023, 21, 93. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Wood, E.E.; Llewellyn, C.A. Algae Biostimulants: A Critical Look at Microalgal Biostimulants for Sustainable Agricultural Practices. Biotechnol. Adv. 2021, 49, 107754. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, D.; Gołaszewski, J.; Białowiec, A. The Pyrolysis and Gasification of Digestate from Agricultural Biogas Plant/Piroliza i Gazyfikacja Pofermentu z Biogazowni Rolniczych. Arch. Environ. Prot. 2015, 41, 70–75. [Google Scholar] [CrossRef]
- Rehl, T.; Müller, J. Life Cycle Assessment of Biogas Digestate Processing Technologies. Resour. Conserv. Recycl. 2011, 56, 92–104. [Google Scholar] [CrossRef]
- Baral, K.R.; Jégo, G.; Amon, B.; Bol, R.; Chantigny, M.H.; Olesen, J.E.; Petersen, S.O. Greenhouse Gas Emissions during Storage of Manure and Digestates: Key Role of Methane for Prediction and Mitigation. Agric. Syst. 2018, 166, 26–35. [Google Scholar] [CrossRef]
- Häfner, F.; Hartung, J.; Möller, K. Digestate Composition Affecting N Fertiliser Value and C Mineralisation. Waste Biomass Valor. 2022, 13, 3445–3462. [Google Scholar] [CrossRef]
- Kovačević, D.; Manojlović, M.; Čabilovski, R.; Ilić, Z.S.; Petković, K.; Štrbac, M.; Vijuk, M. Digestate and Manure Use in Kohlrabi Production: Impact on Plant-Available Nutrients and Heavy Metals in Soil, Yield, and Mineral Composition. Agronomy 2022, 12, 871. [Google Scholar] [CrossRef]
- Stiles, W.A.V.; Styles, D.; Chapman, S.P.; Esteves, S.; Bywater, A.; Melville, L.; Silkina, A.; Lupatsch, I.; Fuentes Grünewald, C.; Lovitt, R.; et al. Using Microalgae in the Circular Economy to Valorise Anaerobic Digestate: Challenges and Opportunities. Bioresour. Technol. 2018, 267, 732–742. [Google Scholar] [CrossRef]
- Tawfik, A.; Eraky, M.; Alhajeri, N.S.; Osman, A.I.; Rooney, D.W. Cultivation of Microalgae on Liquid Anaerobic Digestate for Depollution, Biofuels and Cosmetics: A Review. Environ. Chem. Lett. 2022, 20, 3631–3656. [Google Scholar] [CrossRef]
- Sobolewska, E.; Borowski, S.; Nowicka-Krawczyk, P.; Banach, K. Treatment of Liquid Digestate by Green Algal Isolates from Artificial Eutrophic Pond. Molecules 2022, 27, 6856. [Google Scholar] [CrossRef]
- Akkaya, E.; Can-Güven, E. Efficient Use of Liquid Digestate in Microalgae Cultivation for High Biomass Production and Nutrient Recovery. Water Supply 2022, 22, 7085–7095. [Google Scholar] [CrossRef]
- Kisielewska, M.; Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Quattrocelli, P.; Bordiean, A. Effects of Liquid Digestate Treatment on Sustainable Microalgae Biomass Production. Bioenerg. Res. 2022, 15, 357–370. [Google Scholar] [CrossRef]
- Algae Bioenergy Siting, Commercial Deployment and Development Analysis. Available online: https://energy.ec.europa.eu/publications/algae-bioenergy-siting-commercial-deployment-and-development-analysis_en (accessed on 4 October 2023).
- Pechsiri, J.S.; Thomas, J.-B.E.; Bahraoui, N.E.; Fernandez, F.G.A.; Chaouki, J.; Chidami, S.; Tinoco, R.R.; Martin, J.P.; Gomez, C.; Combe, M.; et al. Comparative Life Cycle Assessment of Conventional and Novel Microalgae Production Systems and Environmental Impact Mitigation in Urban-Industrial Symbiosis. Sci. Total Environ. 2023, 854, 158445. [Google Scholar] [CrossRef] [PubMed]
- Hermadi, I.; Setiadianto, I.R.; Al Zahran, D.F.; Simbolon, M.N.; Saefurahman, G.; Wibawa, D.S.; Arkeman, Y. Development of Smart Algae Pond System for Microalgae Biomass Production. IOP Conf. Ser. Earth Environ. Sci. 2021, 749, 012068. [Google Scholar] [CrossRef]
- Elisabeth, B.; Rayen, F.; Behnam, T. Microalgae Culture Quality Indicators: A Review. Crit. Rev. Biotechnol. 2021, 41, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Cafiero, A.; Giannino, F.; Mazzoleni, S.; Diano, M.M. A Monitoring, Modeling and Decision Support System (DSS) for a Microalgae Production Plant Based on Internet of Things Structure. Procedia Comput. Sci. 2017, 113, 519–524. [Google Scholar] [CrossRef]
- Metzger, M.J.; Bunce, R.G.H.; Jongman, R.H.G.; Mücher, C.A.; Watkins, J.W. A Climatic Stratification of the Environment of Europe: A Climatic Stratification of the European Environment. Glob. Ecol. Biogeogr. 2005, 14, 549–563. [Google Scholar] [CrossRef]
- Metzger, M.J. The Environmental Stratification of Europe, [dataset]; University of Edinburgh: Edinburgh, UK, 2018. [Google Scholar] [CrossRef]
- Home|Algaebiogas Project. Available online: https://algaebiogas.eu/ (accessed on 6 October 2023).
- Kützing, F.T. Synopsis Diatomearum Oder Versuch Einer Systematischen Zusammenstellung Der Diatomeen. Linnaea 1833, 8, 529–620. [Google Scholar] [CrossRef]
- Brébisson, L.A.; Godey, L.L. Algues Des Environs de Falaise, Décrites et Dessinées Par MM. de Brébisson et Godey. Mémoires de la Société Académique des Sciences, Artes et Belles-Lettres de Falaise. 1835, pp. 1–62, 256–269. Available online: https://www.algaebase.org/search/bibliography/detail/?biblio_id=38611 (accessed on 28 November 2023).
- Meteo.Si—Uradna Vremenska Napoved Za Slovenijo—Državna Meteorološka Služba RS—Arhiv Meritev. Available online: https://meteo.arso.gov.si/met/sl/archive/ (accessed on 4 October 2023).
- Bridgewater, L.L.; Baird, R.B.; Eaton, A.D.; Rice, E.W.; American Public Health Association; American Water Works Association; Water Environment Federation (Eds.) Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017; ISBN 978-0-87553-287-5. [Google Scholar]
- ISO 14255:1998; Determination of Nitrate Nitrogen, Ammonium Nitrogen and Total Soluble Nitrogen in Air-Dry Soils Using Calcium Chloride Solution as Extractant. ISO: Geneva, Switzerland, 1998.
- Fast and Accurate Gallery Discrete Industrial Analyzers—Automated Nutrient Analysis and Water Quality Monitoring. Available online: https://assets.thermofisher.com/TFS-Assets/CMD/brochures/eb-d17523-da-gallery-nutrient-water-ebd17523-en.pdf (accessed on 28 November 2023).
- Hotos, G.N.; Avramidou, D.; Bekiari, V. Calibration Curves of Culture Density Assessed by Spectrophotometer for Three Microalgae (Nephroselmis sp., Amphidinium carterae and Phormidium sp.). Eur. J. Biol. Biotechnol. 2020; 6, 1–7. [Google Scholar] [CrossRef]
- Schneckenburger, H.; Schmidt, W. Time-Resolved Chlorophyll Fluorescence of Spruce Needles after Different Light Exposure. J. Plant Physiol. 1996, 148, 593–598. [Google Scholar] [CrossRef]
- Monti, M.; Zrimec, A.; Beran, A.; Zrimec, M.B.; Drinovec, L.; Kosi, G.; Tamberlich, F. Delayed Luminescence of Prorocentrum Minimum under Controlled Conditions. Harmful Algae 2005, 4, 643–650. [Google Scholar] [CrossRef]
- Berden-Zrimec, M.; Drinovec, L.; Zrimec, A. Delayed Fluorescence. In Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications; Suggett, D.J., Prášil, O., Borowitzka, M.A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 293–309. ISBN 978-90-481-9267-0. [Google Scholar]
- Berden-Zrimec, M.; Drinovec, L.; Molinari, I.; Zrimec, A.; Umani, S.F.; Monti, M. Delayed Fluorescence as a Measure of Nutrient Limitation in Dunaliella Tertiolecta. J. Photochem. Photobiol. B Biol. 2008, 92, 13–18. [Google Scholar] [CrossRef]
- ISO 10694:1995; Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis). ISO: Geneva, Switzerland, 1995.
- Mining Laboratory Services. Available online: https://www.bvna.com/other-markets/mining-laboratory-services (accessed on 4 October 2023).
- Marazzi, F.; Bellucci, M.; Fornaroli, R.; Bani, A.; Ficara, E.; Mezzanotte, V. Lab-scale Testing of Operation Parameters for Algae Based Treatment of Piggery Wastewater. J. Chem. Technol. Biotechnol. 2020, 95, 967–974. [Google Scholar] [CrossRef]
- Dobermann, A. Fertilizer Best. Management Practices: General. Principles, Strategy for Their Adoption and Voluntary Initiatives vs Regulations: Papers Presented at the IFA International Workshop on Fertilizer Best. Management Practices, 7–9 March 2007, Brussels, Belgium; International Fertilizer Industry Association: Paris, France, 2007; ISBN 978-2-9523139-2-6. [Google Scholar]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 4 October 2023).
- Fox, J. The R Commander: A Basic-Statistics Graphical User Interface to R. J. Stat. Soft. 2005, 14, 1–42. [Google Scholar] [CrossRef]
- Vigani, M.; Parisi, C.; Rodríguez-Cerezo, E.; Barbosa, M.J.; Sijtsma, L.; Ploeg, M.; Enzing, C. Food and Feed Products from Micro-Algae: Market Opportunities and Challenges for the EU. Trends Food Sci. Technol. 2015, 42, 81–92. [Google Scholar] [CrossRef]
- Slegers, P.M.; Lösing, M.B.; Wijffels, R.H.; Van Straten, G.; Van Boxtel, A.J.B. Scenario Evaluation of Open Pond Microalgae Production. Algal Res. 2013, 2, 358–368. [Google Scholar] [CrossRef]
- Kenny, P.; Flynn, K.J. Physiology Limits Commercially Viable Photoautotrophic Production of Microalgal Biofuels. J. Appl. Phycol. 2017, 29, 2713–2727. [Google Scholar] [CrossRef] [PubMed]
- Pizzera, A.; Scaglione, D.; Bellucci, M.; Marazzi, F.; Mezzanotte, V.; Parati, K.; Ficara, E. Digestate Treatment with Algae-Bacteria Consortia: A Field Pilot-Scale Experimentation in a Sub-Optimal Climate Area. Bioresour. Technol. 2019, 274, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Xia, A.; Murphy, J.D. Microalgal Cultivation in Treating Liquid Digestate from Biogas Systems. Trends Biotechnol. 2016, 34, 264–275. [Google Scholar] [CrossRef]
- Kebede-Westhead, E.; Pizarro, C.; Mulbry, W.W. Treatment of Swine Manure Effluent Using Freshwater Algae: Production, Nutrient Recovery, and Elemental Composition of Algal Biomass at Four Effluent Loading Rates. J. Appl. Phycol. 2006, 18, 41–46. [Google Scholar] [CrossRef]
- Esteves, A.F.; Soares, S.M.; Salgado, E.M.; Boaventura, R.A.R.; Pires, J.C.M. Microalgal Growth in Aquaculture Effluent: Coupling Biomass Valorisation with Nutrients Removal. Appl. Sci. 2022, 12, 12608. [Google Scholar] [CrossRef]
- Lavrič, L. Obdelava Anaerobnega Digestata z Mikroalgami in Termofilna Proizvodnja Bioplina Iz Mikroalgne Biomase. Ph.D. Thesis, Univerza v Ljubljani, Biotehniška Fakulteta, Ljubljana, Slovenia, 2019. [Google Scholar]
- Biasiolo, M.; Ballarin, M.; Tassinato, G.; Stoppato, A.; Cavinato, C. Semi-Continuous Chlorella Vulgaris Cultivation Using Anaerobic Digestate Liquid Fraction Pre-Treated by Ultrasonic Cavitation to Improve Carbon Dioxide Solubilization. Chem. Eng. Trans. 2022, 92, 151–156. [Google Scholar] [CrossRef]
- Wágner, D.S.; Cazzaniga, C.; Steidl, M.; Dechesne, A.; Valverde-Pérez, B.; Plósz, B.G. Optimal Influent N-to-P Ratio for Stable Microalgal Cultivation in Water Treatment and Nutrient Recovery. Chemosphere 2021, 262, 127939. [Google Scholar] [CrossRef] [PubMed]
- Ali Al Meselmani, M. Nutrient Solution for Hydroponics. In Recent Research and Advances in Soilless Culture; Turan, M., Argin, S., Yildirim, E., Güneş, A., Eds.; IntechOpen: London, UK, 2023; ISBN 978-1-80355-168-5. [Google Scholar]
- Ji, M.-K.; Abou-Shanab, R.A.I.; Hwang, J.-H.; Timmes, T.C.; Kim, H.-C.; Oh, Y.-K.; Jeon, B.-H. Removal of Nitrogen and Phosphorus from Piggery Wastewater Effluent Using the Green Microalga Scenedesmus Obliquus. J. Environ. Eng. 2013, 139, 1198–1205. [Google Scholar] [CrossRef]
- Salama, E.-S.; Kurade, M.B.; Abou-Shanab, R.A.I.; El-Dalatony, M.M.; Yang, I.-S.; Min, B.; Jeon, B.-H. Recent Progress in Microalgal Biomass Production Coupled with Wastewater Treatment for Biofuel Generation. Renew. Sustain. Energy Rev. 2017, 79, 1189–1211. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, S.M. Effect of the N/P Ratio on Biomass Productivity and Nutrient Removal from Municipal Wastewater. Bioprocess Biosyst. Eng. 2015, 38, 761–766. [Google Scholar] [CrossRef]
- Xing, D.; Li, X.; Wang, Y.; Deng, S.; Jin, C.; Zhao, Y.; Guo, L. The Comprehensive Impact of Phosphorus Sources on Microalgae Biochemical Metabolism and Phosphorus Transformation. J. Water Process Eng. 2023, 51, 103477. [Google Scholar] [CrossRef]
- Jiang, Y.; Pu, X.; Zheng, D.; Zhu, T.; Wang, S.; Deng, L.; Wang, W. Cultivation of Lipid-Producing Microalgae in Struvite-Precipitated Liquid Digestate for Biodiesel Production. Biotechnol. Biofuels 2018, 11, 101. [Google Scholar] [CrossRef]
- Fernandes, F.; Silkina, A.; Gayo-Peláez, J.I.; Kapoore, R.V.; De La Broise, D.; Llewellyn, C.A. Microalgae Cultivation on Nutrient Rich Digestate: The Importance of Strain and Digestate Tailoring under PH Control. Appl. Sci. 2022, 12, 5429. [Google Scholar] [CrossRef]
- Moheimani, N.R.; Borowitzka, M.A. Increased CO2 and the Effect of pH on Growth and Calcification of Pleurochrysis Carterae and Emiliania Huxleyi (Haptophyta) in Semicontinuous Cultures. Appl. Microbiol. Biotechnol. 2011, 90, 1399–1407. [Google Scholar] [CrossRef]
- Al-Mallahi, J.; Ishii, K. Attempts to Alleviate Inhibitory Factors of Anaerobic Digestate for Enhanced Microalgae Cultivation and Nutrients Removal: A Review. J. Environ. Manag. 2022, 304, 114266. [Google Scholar] [CrossRef]
- Posadas, E.; Alcántara, C.; García-Encina, P.A.; Gouveia, L.; Guieysse, B.; Norvill, Z.; Acién, F.G.; Markou, G.; Congestri, R.; Koreiviene, J.; et al. Microalgae Cultivation in Wastewater. In Microalgae-Based Biofuels and Bioproducts; Woodhead Publishing Series in Energy; Woodhead Publishing: Cambridge, UK, 2017; pp. 67–91. ISBN 978-0-08-101023-5. [Google Scholar]
- Singh, S.P.; Singh, P. Effect of Temperature and Light on the Growth of Algae Species: A Review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Gao, B.; Hong, J.; Chen, J.; Zhang, H.; Hu, R.; Zhang, C. The Growth, Lipid Accumulation and Adaptation Mechanism in Response to Variation of Temperature and Nitrogen Supply in Psychrotrophic Filamentous Microalga Xanthonema Hormidioides (Xanthophyceae). Biotechnol. Biofuels 2023, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Cabello, J.; Toledo-Cervantes, A.; Sánchez, L.; Revah, S.; Morales, M. Effect of the Temperature, pH and Irradiance on the Photosynthetic Activity by Scenedesmus Obtusiusculus under Nitrogen Replete and Deplete Conditions. Bioresour. Technol. 2015, 181, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.-B.; Zhang, Y.-L.; Yang, L.-B.; Chu, H.-Q.; Guo, J. Outdoor Cultures of Chlorella Pyrenoidosa in the Effluent of Anaerobically Digested Activated Sludge: The Effects of pH and Free Ammonia. Bioresour. Technol. 2016, 200, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Difusa, A.; Talukdar, J.; Kalita, M.C.; Mohanty, K.; Goud, V.V. Effect of Light Intensity and pH Condition on the Growth, Biomass and Lipid Content of Microalgae Scenedesmus Species. Biofuels 2015, 6, 37–44. [Google Scholar] [CrossRef]
- Goldman, J.C.; Azov, Y.; Riley, C.B.; Dennett, M.R. The Effect of pH in Intensive Microalgal Cultures. I. Biomass Regulation. J. Exp. Mar. Biol. Ecol. 1982, 57, 1–13. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Moheimani, N.R. (Eds.) Algae for Biofuels and Energy; Springer: Dordrecht, The Netherlands, 2013; ISBN 978-94-007-5478-2. [Google Scholar]
- Azov, Y.; Goldman, J.C. Free Ammonia Inhibition of Algal Photosynthesis in Intensive Cultures. Appl. Environ. Microbiol. 1982, 43, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-J.; Jho, E.H.; Jang, H.-S.; Hwang, S.-J. Inhibition Effects of Free Ammonia (FA) on the Rates of Growth, Photosynthesis and Respiration of Chlorella Vulgaris. KSCE J. Civ. Eng. 2022, 26, 2567–2574. [Google Scholar] [CrossRef]
- Singh, H.; Bruce, D. Electrical Conductivity and pH Guide for Hydroponics. Oklahoma Cooperative Extension Fact Sheets. 2017. Available online: https://extension.okstate.edu/fact-sheets/electrical-conductivity-and-ph-guide-for-hydroponics.html (accessed on 4 October 2023).
- Moya, C.; Oyanedel, E.; Verdugo, G.; Flores, M.F.; Urrestarazu, M.; Álvaro, J.E. Increased Electrical Conductivity in Nutrient Solution Management Enhances Dietary and Organoleptic Qualities in Soilless Culture Tomato. Horts 2017, 52, 868–872. [Google Scholar] [CrossRef]
- Rehman, M.; Kesharvani, S.; Dwivedi, G.; Gidwani Suneja, K. Impact of Cultivation Conditions on Microalgae Biomass Productivity and Lipid Content. Mater. Today Proc. 2022, 56, 282–290. [Google Scholar] [CrossRef]
- Zafar, A.M.; Javed, M.A.; Aly Hassan, A. Unprecedented Biodesalination Rates–Shortcomings of Electrical Conductivity Measurements in Determining Salt Removal by Algae and Cyanobacteria. J. Environ. Manag. 2022, 302, 113947. [Google Scholar] [CrossRef]
- Figler, A.; B-Béres, V.; Dobronoki, D.; Márton, K.; Nagy, S.A.; Bácsi, I. Salt Tolerance and Desalination Abilities of Nine Common Green Microalgae Isolates. Water 2019, 11, 2527. [Google Scholar] [CrossRef]
- Sforza, E.; Al Emara, M.K.; Sharif, A.; Bertucco, A. Exploitation of Urban Landfill Leachate as Nutrient Source for Microalgal Biomass Production. Chem. Eng. Trans. 2015, 43, 373–378. [Google Scholar] [CrossRef]
- Barreiro-Vescovo, S.; Barbera, E.; Bertucco, A.; Sforza, E. Integration of Microalgae Cultivation in a Biogas Production Process from Organic Municipal Solid Waste: From Laboratory to Pilot Scale. ChemEngineering 2020, 4, 25. [Google Scholar] [CrossRef]
- Lopes, A.P.; Santos, F.M.; Silva, T.F.C.V.; Vilar, V.J.P.; Pires, J.C.M. Outdoor Cultivation of the Microalga Chlorella Vulgaris in a New Photobioreactor Configuration: The Effect of Ultraviolet and Visible Radiation. Energies 2020, 13, 1962. [Google Scholar] [CrossRef]
- Acién, F.G.; Gómez-Serrano, C.; Morales-Amaral, M.M.; Fernández-Sevilla, J.M.; Molina-Grima, E. Wastewater Treatment Using Microalgae: How Realistic a Contribution Might It Be to Significant Urban Wastewater Treatment? Appl. Microbiol. Biotechnol. 2016, 100, 9013–9022. [Google Scholar] [CrossRef]
- Mishra, N.; Mishra, P.; Gupta, E.; Singh, P. Synergistic effects of nitrogen deprivation and high irradiance to enhance biomass and lipid production in nannochloropsis. J. Microb. Biotech. Food Sci. 2023, 12, e3632. [Google Scholar] [CrossRef]
- Zhang, B.; Ogden, K. Nitrogen Balances and Impacts on the Algae Cultivation-Extraction-Digestion-Cultivation Process. Algal Res. 2019, 39, 101434. [Google Scholar] [CrossRef]
- Gupta, S.; Pawar, S.B.; Pandey, R.A. Current Practices and Challenges in Using Microalgae for Treatment of Nutrient Rich Wastewater from Agro-Based Industries. Sci. Total Environ. 2019, 687, 1107–1126. [Google Scholar] [CrossRef]
- Aparicio, S.; Robles, Á.; Ferrer, J.; Seco, A.; Borrás Falomir, L. Assessing and Modeling Nitrite Inhibition in Microalgae-Bacteria Consortia for Wastewater Treatment by Means of Photo-Respirometric and Chlorophyll Fluorescence Techniques. Sci. Total Environ. 2022, 808, 152128. [Google Scholar] [CrossRef]
- González-Camejo, J.; Montero, P.; Aparicio, S.; Ruano, M.V.; Borrás, L.; Seco, A.; Barat, R. Nitrite Inhibition of Microalgae Induced by the Competition between Microalgae and Nitrifying Bacteria. Water Res. 2020, 172, 115499. [Google Scholar] [CrossRef]
- Ge, S.; Wang, S.; Yang, X.; Qiu, S.; Li, B.; Peng, Y. Detection of Nitrifiers and Evaluation of Partial Nitrification for Wastewater Treatment: A Review. Chemosphere 2015, 140, 85–98. [Google Scholar] [CrossRef] [PubMed]
- González-Camejo, J.; Aparicio, S.; Pachés, M.; Borrás, L.; Seco, A. Comprehensive Assessment of the Microalgae-Nitrifying Bacteria Competition in Microalgae-Based Wastewater Treatment Systems: Relevant Factors, Evaluation Methods and Control Strategies. Algal Res. 2022, 61, 102563. [Google Scholar] [CrossRef]
- González-Camejo, J.; Aparicio, S.; Ruano, M.V.; Borrás, L.; Barat, R.; Ferrer, J. Effect of Ambient Temperature Variations on an Indigenous Microalgae-Nitrifying Bacteria Culture Dominated by Chlorella. Bioresour. Technol. 2019, 290, 121788. [Google Scholar] [CrossRef] [PubMed]
- Vergara, C.; Muñoz, R.; Campos, J.L.; Seeger, M.; Jeison, D. Influence of Light Intensity on Bacterial Nitrifying Activity in Algal-Bacterial Photobioreactors and Its Implications for Microalgae-Based Wastewater Treatment. Int. Biodeterior. Biodegrad. 2016, 114, 116–121. [Google Scholar] [CrossRef]
- Luo, Y.; Le-Clech, P.; Henderson, R.K. Assessment of Membrane Photobioreactor (MPBR) Performance Parameters and Operating Conditions. Water Res. 2018, 138, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Beardall, J.; Young, E.; Roberts, S. Approaches for Determining Phytoplankton Nutrient Limitation: Aquat. Sci. 2001, 63, 44–69. [Google Scholar] [CrossRef]
- Berden-Zrimec, M.; Drinovec, L.; Zrimec, A.; Tišler, T. Delayed Fluorescence in Algal Growth Inhibition Tests. Open Life Sci. 2007, 2, 169–181. [Google Scholar] [CrossRef]
- Praveen, P.; Guo, Y.; Kang, H.; Lefebvre, C.; Loh, K.-C. Enhancing Microalgae Cultivation in Anaerobic Digestate through Nitrification. Chem. Eng. J. 2018, 354, 905–912. [Google Scholar] [CrossRef]
- Kumar, N.; Banerjee, C.; Negi, S.; Shukla, P. Microalgae Harvesting Techniques: Updates and Recent Technological Interventions. Crit. Rev. Biotechnol. 2023, 43, 342–368. [Google Scholar] [CrossRef]
- Zhang, T.-Y.; Yu, Y.; Wu, Y.-H.; Hu, H.-Y. Inhibitory Effects of Soluble Algae Products (SAP) Released by Scenedesmus Sp. LX1 on Its Growth and Lipid Production. Bioresour. Technol. 2013, 146, 643–648. [Google Scholar] [CrossRef]
- Park, J.B.K.; Craggs, R.J.; Shilton, A.N. Recycling Algae to Improve Species Control and Harvest Efficiency from a High Rate Algal Pond. Water Res. 2011, 45, 6637–6649. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Lo, E.; Legall, N.; Philippidis, G.P. A Critical Review of Growth Media Recycling to Enhance the Economics and Sustainability of Algae Cultivation. Energies 2023, 16, 5378. [Google Scholar] [CrossRef]
- Wu, M.; Du, M.; Wu, G.; Lu, F.; Li, J.; Lei, A.; Zhu, H.; Hu, Z.; Wang, J. Water Reuse and Growth Inhibition Mechanisms for Cultivation of Microalga Euglena Gracilis. Biotechnol. Biofuels 2021, 14, 132. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Loftus, S.; Sha, J.; Wang, W.; Park, M.S.; Zhang, X.; Johnson, Z.I.; Hu, Q. Water Reuse for Sustainable Microalgae Cultivation: Current Knowledge and Future Directions. Resour. Conserv. Recycl. 2020, 161, 104975. [Google Scholar] [CrossRef]
- Pulgarin, A.; Kapeller, A.G.; Tarik, M.; Egloff, S.; Mariotto, M.; Ludwig, C.; Refardt, D. Cultivation of Microalgae at High-Density with Pretreated Liquid Digestate as a Nitrogen Source: Fate of Nitrogen and Improvements on Growth Limitations. J. Clean. Prod. 2021, 324, 129238. [Google Scholar] [CrossRef]
- Marcilhac, C.; Sialve, B.; Pourcher, A.-M.; Ziebal, C.; Bernet, N.; Béline, F. Digestate Color and Light Intensity Affect Nutrient Removal and Competition Phenomena in a Microalgal-Bacterial Ecosystem. Water Res. 2014, 64, 278–287. [Google Scholar] [CrossRef]
- Zeshan; Visvanathan, C. Evaluation of Anaerobic Digestate for Greenhouse Gas Emissions at Various Stages of Its Management. Int. Biodeterior. Biodegrad. 2014, 95, 167–175. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Singh, R.P.; Suman, R. Enhancing Smart Farming through the Applications of Agriculture 4.0 Technologies. Int. J. Intell. Netw. 2022, 3, 150–164. [Google Scholar] [CrossRef]





| Digestate Batches | ||||||
|---|---|---|---|---|---|---|
| Parameter | Unit | D1 | D2 | D3 | D4 | D5 |
| pH | - | 7.75 ± 0.15 | 8.02 ± 0.26 | 7.75 ± 0.10 | 8.16 ± 0.52 | 8.24 ± 0.09 |
| EC | µS cm−1 | 13,026 ± 355 | 13,118 ± 300 | 11,995 ± 204 | 8770 ± 1432 | 11,310 ± 289 |
| NO3-N | g kg−1 d.m.−1 | 0.33 ± 0.03 | 0.08 ± 0.04 | 0.06 ± 0.05 | 0.03 ± 0.02 | 0.002 ± 0.001 |
| NH4-N | g kg−1 d.m.−1 | 140 ± 2 | 571 ± 45 | 119 ± 28 | 15 ± 5 | 68 ± 4 |
| TS | g L−1 | 8.13 ± 0.12 | 4.98 ± 0.34 | 5.08 ± 0.80 | 5.88 ± 0.60 | 4.02 ± 0.42 |
| OM | g kg−1 d.m.−1 | n.d. | 390 ± 19 | 330 ± 31 | 580 ± 31 | 360 ± 51 |
| TC | g kg−1 d.m.−1 | 347 ± 72 | 407 ± 60 | 293 ± 55 | 326 ± 43 | 364 ± 17 |
| TN | g kg−1 d.m.−1 | 150 ± 14 | 229 ± 17 * | 155 ± 33 | 141 ± 25 | 213 ± 15 |
| P | g kg−1 d.m.−1 | 8.65 | 3 | 4 | 4.05 | 3.98 |
| K | g kg−1 d.m.−1 | 80.8 | >100 | >100 | >100 | >100 |
| S | g kg−1 d.m.−1 | 11 | n.d. | 18.8 | 5.3 | 13.4 |
| Ca | mg kg−1 d.m.−1 | 17,800 | 13,684 | 23,700 | 8500 | 24,800 |
| Mg | mg kg−1 d.m.−1 | 7090 | 11,670 | 11,290 | 10,210 | 12,410 |
| Na | mg kg−1 d.m.−1 | >55,000 | >55,000 | >55,000 | >55,000 | >55,000 |
| Fe | mg kg−1 d.m.−1 | 2710 | 216 | 400 | 530 | 800 |
| B | mg kg−1 d.m.−1 | 62 | 98 | 86 | 101 | 100 |
| Mn | mg kg−1 d.m.−1 | 47 | 11 | 30 | 10 | 23 |
| Zn | mg kg d.m.−1 | 63.1 | 15.4 | 10.1 | 18.5 | 21.3 |
| Cu | mg kg−1 d.m.−1 | 15.38 | <0.7 | 0.05 | 2.32 | 4.37 |
| Ni | mg kg−1 d.m.−1 | 12.4 | 10.9 | 8.6 | 18.4 | 8.1 |
| Cr | mg kg−1 d.m.−1 | 4.7 | 1.72 | 2 | 2.7 | 4.6 |
| Hg | mg kg−1 d.m.−1 | 0.041 | n.d. | 0.005 | 0.029 | 0.014 |
| Pb | mg kg−1 d.m.−1 | 3.42 | <3.5 | 0.19 | 0.63 | 1.18 |
| Cd | mg kg−1 d.m.−1 | 0.1 | <0.7 | 0.02 | <0.01 | 0.01 |
| C/N | ratio | 2.3 | 1.8 | 1.9 | 2.3 | 1.7 |
| N/P | ratio | 17 | 79 | 39 | 35 | 54 |
| TAN/TN | ratio | 93% | 250% ** | 77% | 11% | 32% |
| Average Input of Digestate (L m−3 day−1) | Average Nitrogen Supply Rate (g Nin m−2 day−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stage | Months | Nutrient Source | P0 | P1 | P2 | P3 | P0 | P1 | P2 | P3 |
| SI | March–June | D1 | 8.9 ± 5.4 | 2.26 ± 1.3 | ||||||
| SII | July–September | D2 | 2.8 ± 2.1 | 8.6 ± 5.9 | 11.5 ± 7.0 | 0.79 ± 0.52 | 1.94 ± 1.29 | 2.60 ± 1.61 | ||
| SIII | October–December | D3 | 2.6 ± 1.1 | 6.1 ± 5.5 | 8.8 ± 5.2 | 0.35 ± 0.20 | 0.68 ± 0.50 | 1.28 ± 0.94 | ||
| January–May | D4 | 1.2 ± 0.8 | 2.3 ± 1.4 | 3.9 ± 2.8 | 0.17 ± 0.08 | 0.34 ± 0.16 | 0.56 ± 0.32 | |||
| May–July | D5 | 1.5 ± 1.0 | 3.7 ± 2.5 | 5.8 ± 4.5 | 0.23 ± 0.16 | 0.46 ± 0.32 | 0.72 ± 0.48 | |||
| Stage | Season | Average BY (gMh m−2 day−1) | Relative BY (P2 = 100%) | NUE (gMh gNin−1) | Relative NUE (P2 = 100%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P1 | P2 | P3 | P1 | P2 | P3 | P1 | P2 | P3 | ||
| SII | summer | 4.8 a | 13.1 b | 10.5 ab | 38% | 100% | 86% | 11.4 a | 6.9 a | 4.7 a | 164% | 100% | 68% |
| SIII | fall | 9.1 a | 9.9 a | 10.9 a | 91% | 100% | 112% | 22.3 a | 13.9 a | 11.0 a | 160% | 100% | 79% |
| winter | 2.6 a | 2.7 a | 3.1 a | 96% | 100% | 117% | 28.5 a | 15.4 a | 11.6 a | 185% | 100% | 76% | |
| spring | 7.0 a | 9.8 a | 8.3 a | 72% | 100% | 85% | 48.1 b | 27.5 ab | 16.8 a | 175% | 100% | 61% | |
| summer | 7.8 a | 11.1 b | 10.4 b | 70% | 100% | 94% | 44.8 a | 28.9 a | 24.9 a | 155% | 100% | 86% | |
| Average ± stdev | 6.1 ± 2.6 | 9.0 ± 3.9 | 8.6 ± 3.4 | 73% ± 23% | 100% | 99% ± 15% | 31.0 ± 6.9 | 18.5 ± 4.2 | 13.8 ± 3.4 | 168% ± 5% | 100% | 74% ± 4% | |
| Stage | Pond | pH | Average Amount of Digestate (L pond−1 day−1) | TNin (g m−2 day−1) | EC in Pond (µS cm−1) | NH4-N in Pond (mg L−1) | NO3-N in Pond (mg L−1) | NO2-N in Pond (mg L−1) | OD680 | DFI ˂ 600 (cpm) |
|---|---|---|---|---|---|---|---|---|---|---|
| SI | P0 | 7.20 | 10–15 | 1.6–2.4 | 2000 | 100 | ˂20 | ˂3 | 0.7–1.3 | 0.5–3.5 M |
| SII | P1 | 7.20 | 5–10 | ˂1 | ˂1500 | 25–50 | ˂5 | ˂3 | 0.7–0.9 | 0.5–1 M |
| P2 | 7.20 | 10–15 | 0.5–3.0 | 1500–2500 | 50–150 | ˂20 | ˂3 | 0.9–1.1 | 1–2 M | |
| P3 | 7.20 | 15–30 | >2 | >2500 | 150–300 | ˂50 | ˂3 | 1.1–1.3 | 2–3.5 M |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resman, L.; Berden Zrimec, M.; Žitko, V.; Lazar, B.; Reinhardt, R.; Cerar, A.; Mihelič, R. Microalgae Production on Biogas Digestate in Sub-Alpine Region of Europe—Development of Simple Management Decision Support Tool. Sustainability 2023, 15, 16948. https://doi.org/10.3390/su152416948
Resman L, Berden Zrimec M, Žitko V, Lazar B, Reinhardt R, Cerar A, Mihelič R. Microalgae Production on Biogas Digestate in Sub-Alpine Region of Europe—Development of Simple Management Decision Support Tool. Sustainability. 2023; 15(24):16948. https://doi.org/10.3390/su152416948
Chicago/Turabian StyleResman, Lara, Maja Berden Zrimec, Vid Žitko, Borut Lazar, Robert Reinhardt, Ana Cerar, and Rok Mihelič. 2023. "Microalgae Production on Biogas Digestate in Sub-Alpine Region of Europe—Development of Simple Management Decision Support Tool" Sustainability 15, no. 24: 16948. https://doi.org/10.3390/su152416948






