Morphology, Mechanical Properties, and Biodegradability of Modified Thermoplastic Starch/PETG Blends with In Situ Generated Graft Copolymers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
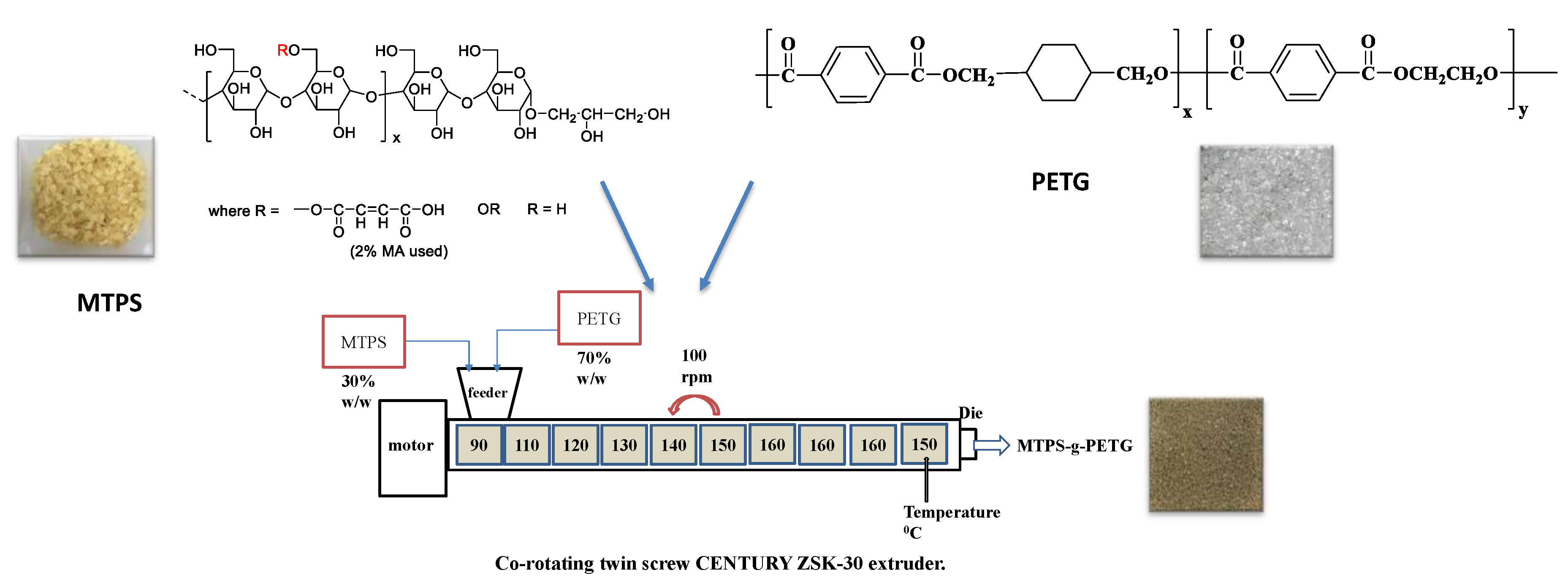
2.2. Preparation of MTPS–g–PETG Graft Copolymers
2.3. Characterization and Analysis
2.3.1. Soxhlet Extractions
2.3.2. Thermal Analysis
2.3.3. Tensile Testing
2.3.4. Impact Testing
2.3.5. Fourier Transform Infrared Spectroscopy
2.3.6. Scanning Electron Microscopy
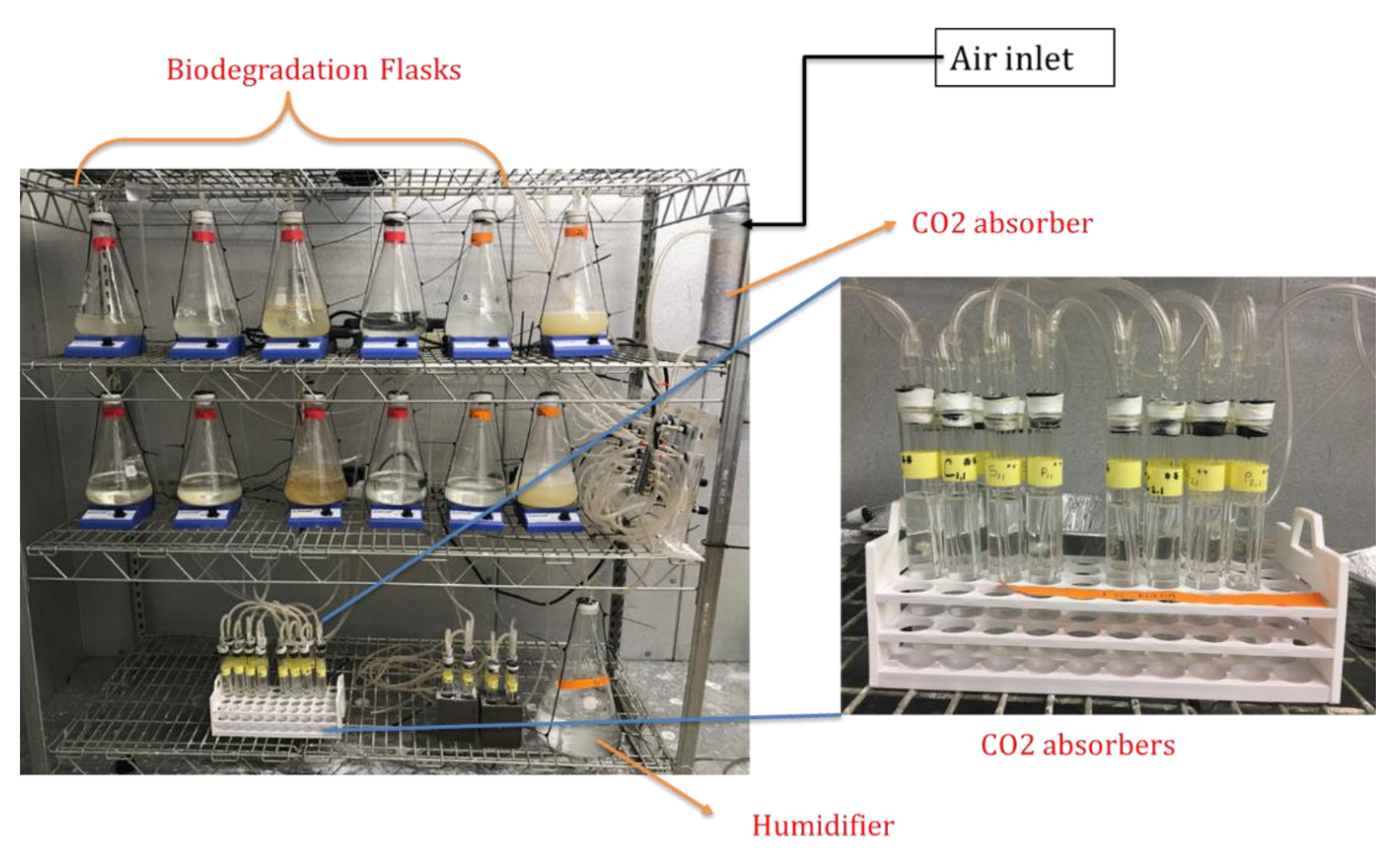
2.3.7. Aqueous Biodegradation
3. Results and Discussion
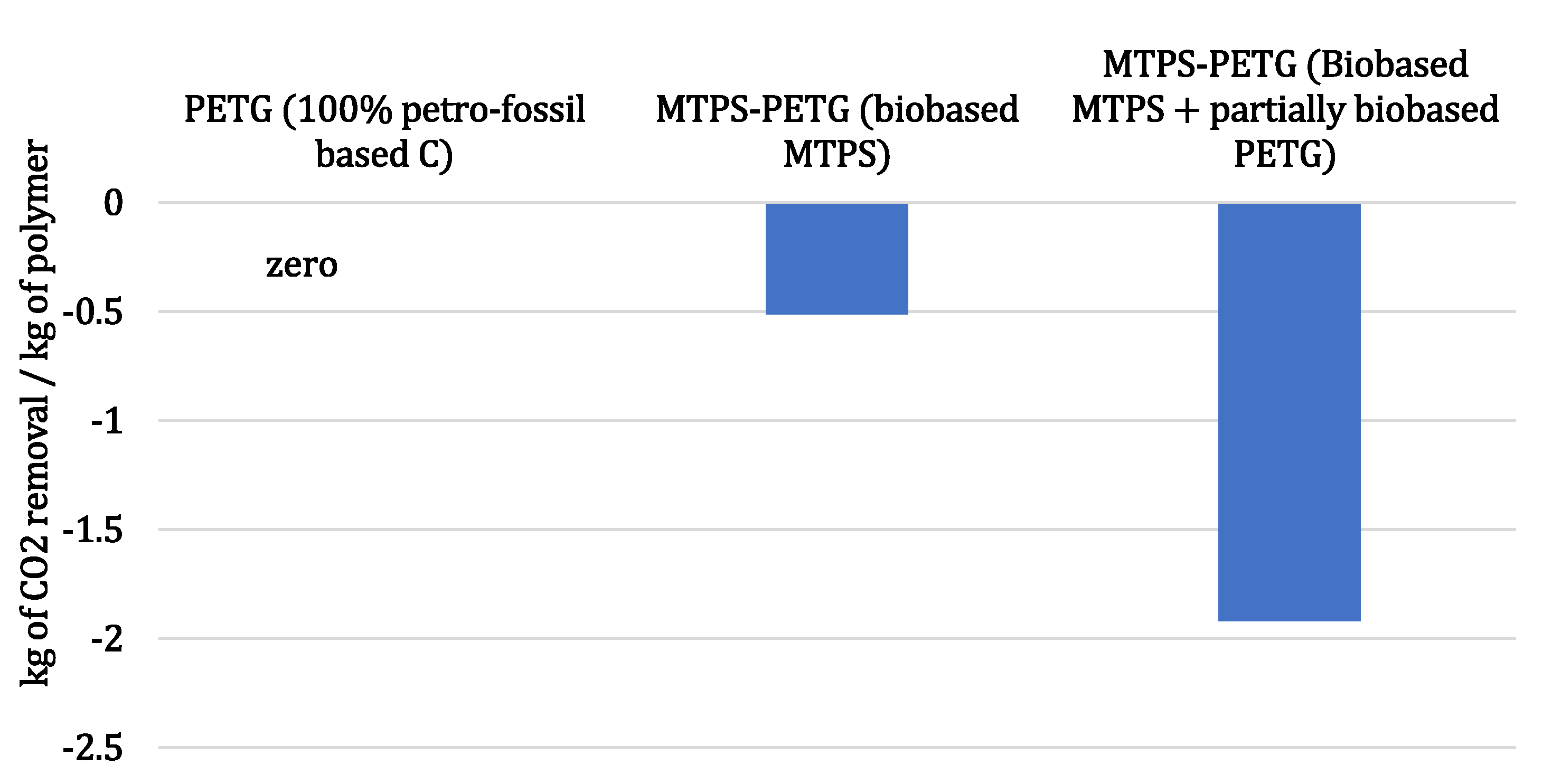
3.1. Biobased Value Proposition
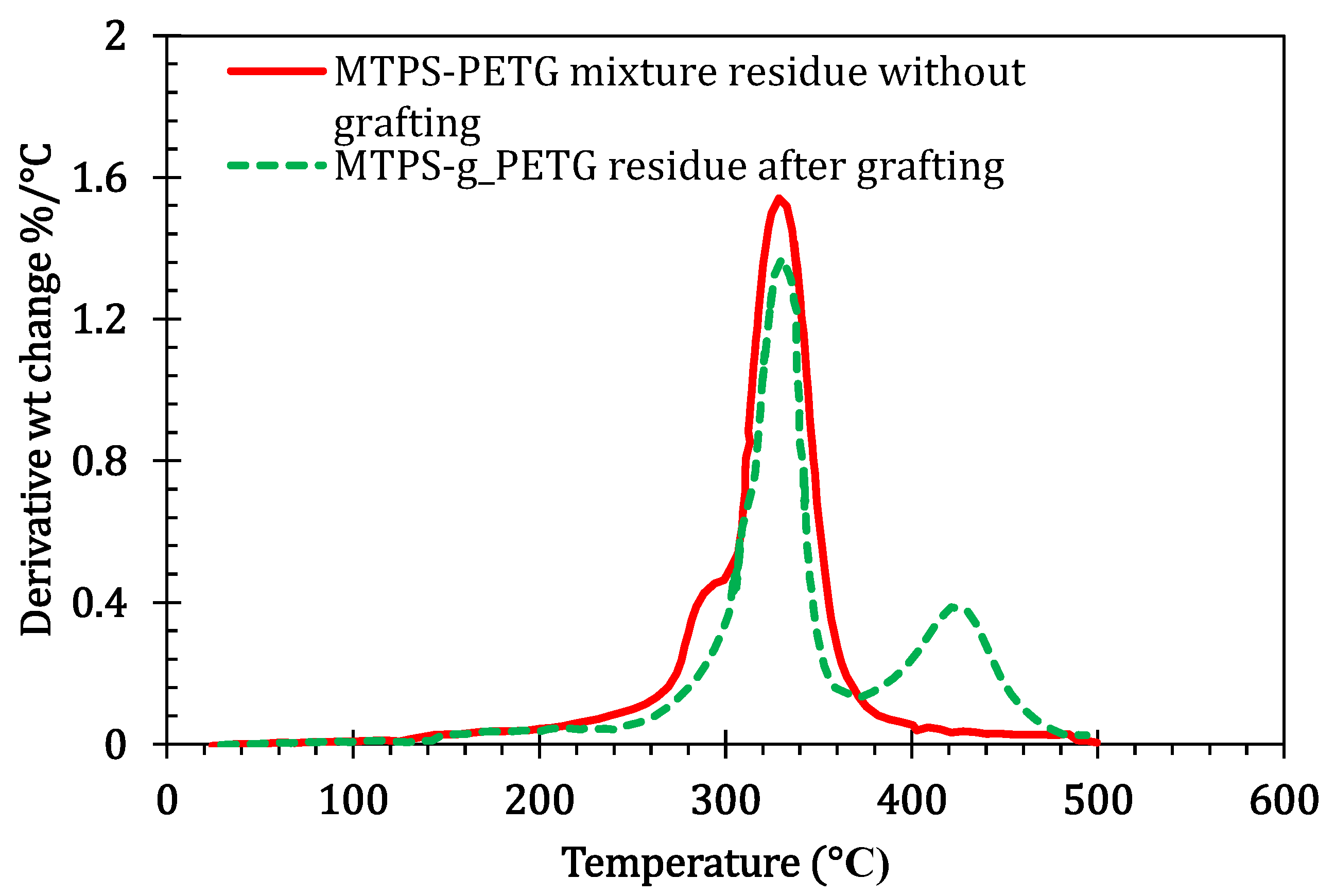
3.2. Soxhlet Extraction
3.3. FT-IR Analysis
3.4. Mechanical Properties
3.5. Phase Morphology
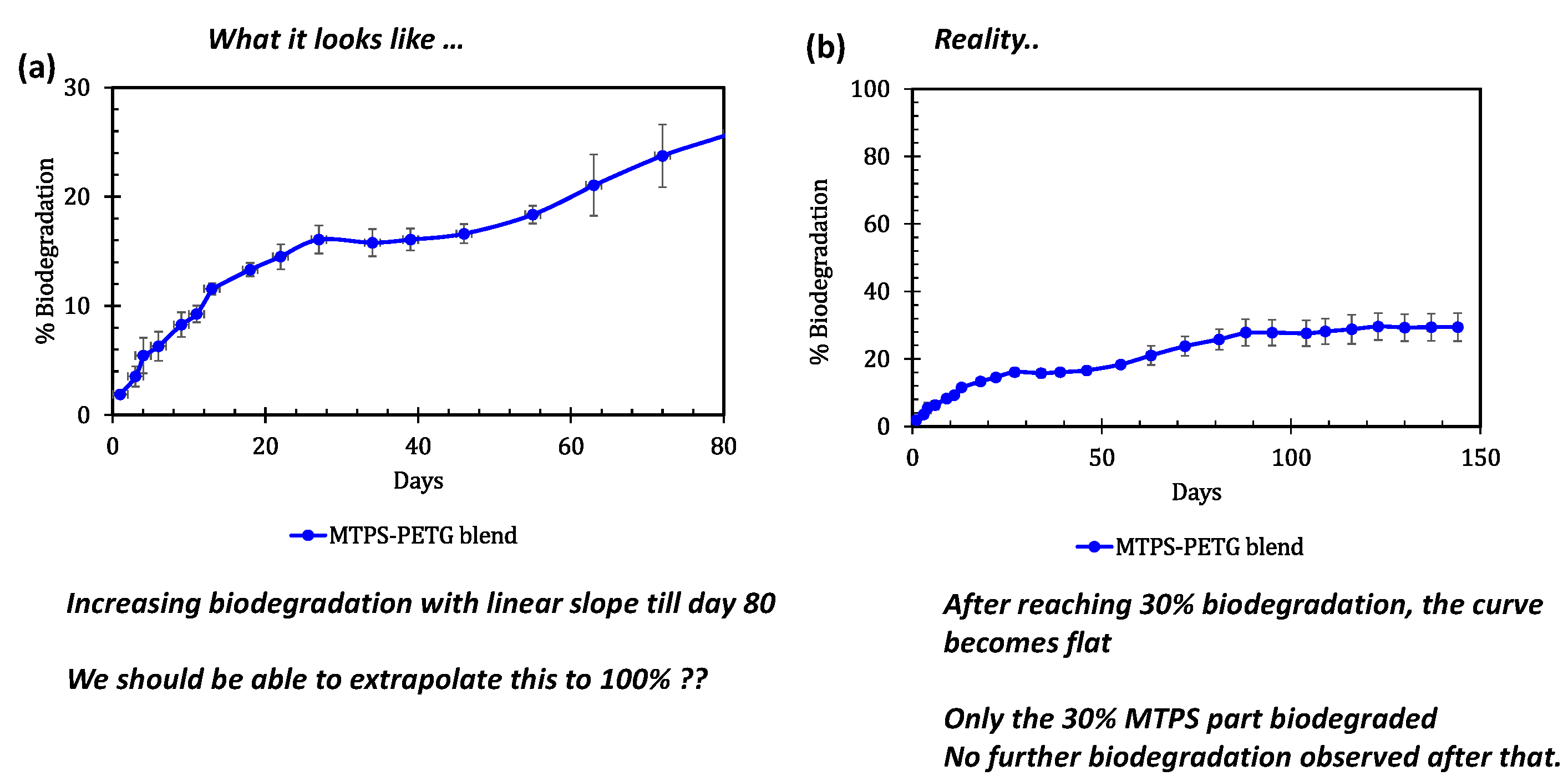
3.6. Biodegradability Testing
4. Conclusions and Future Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Narayan, R. Principles, Drivers, and Analysis of Biodegradable and Biobased Plastics. In Handbook of Biodegradable Polymers; Wiley: Hoboken, NJ, USA, 2020; pp. 455–473. [Google Scholar] [CrossRef]
- Ramani, N. Biobased and Biodegradable Polymer Materials: Rationale, Drivers, and Technology Exemplars. Degrad. Polym. Mater. 2005, 939, 282–306. [Google Scholar] [CrossRef]
- Law, K.L.; Narayan, R. Reducing environmental plastic pollution by designing polymer materials for managed end-of-life. Nat. Rev. Mater. 2021, 7, 104–116. [Google Scholar] [CrossRef]
- Narayan, R. Biodegradable and Biobased Plastics: An Overview. Soil Degrad. Bioplastics Sustain. Mod. Agric. 2017, 23–34. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Snowdon, M.R.; Misra, M.; Mohanty, A.K. Biobased Poly(ethylene terephthalate)/Poly(lactic acid) Blends Tailored with Epoxide Compatibilizers. ACS omega 2018, 3, 11759–11769. [Google Scholar] [CrossRef]
- Chen, T.; Jiang, G.; Li, G.; Wu, Z.; Zhang, J. Poly(ethylene glycol-co-1,4-cyclohexanedimethanol terephthalate) random copolymers: Effect of copolymer composition and microstructure on the thermal properties and crystallization behavior. RSC Adv. 2015, 5, 60570–60580. [Google Scholar] [CrossRef]
- Soleyman, E.; Aberoumand, M.; Soltanmohammadi, K.; Rahmatabadi, D.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Baghani, M. 4D printing of PET-G via FDM including tailormade excess third shape. Manuf. Lett. 2022, 33, 1–4. [Google Scholar] [CrossRef]
- Frenkel, D.; Ginsbury, E.; Sharabi, M. The Mechanics of Bioinspired Stiff-to-Compliant Multi-Material 3D-Printed Interfaces. Biomimetics 2022, 7, 170. [Google Scholar] [CrossRef]
- Soleyman, E.; Rahmatabadi, D.; Soltanmohammadi, K.; Aberoumand, M.; Ghasemi, I.; Abrinia, K.; Baniassadi, M.; Wang, K.; Baghani, M. Shape memory performance of PETG 4D printed parts under compression in cold, warm, and hot programming. Smart Mater. Struct. 2022, 31, 085002. [Google Scholar] [CrossRef]
- Bulatović, V.O.; Grgić, D.K.; Mandić, V.; Miloloža, M.; Dybal, J.; Gajdosova, V.; Slouf, M. Biodegradation of LDPE_TPS blends under controlled composting conditions. Polym. Bull. 2022, 1–27. [Google Scholar] [CrossRef]
- Romagnolli, C.M.N.; Leite, G.; Rodrigues, T.; Morelli, C.L. Blend of cassava starch and high-density polyethylene with green tea for food packaging. Polym. Renew. Resour. 2020, 11, 3–14. [Google Scholar] [CrossRef]
- Berruezo, M.; Ludueña, L.; Rodriguez, E.; Álvarez, V. Preparation and characterization of polystyrene/starch blends for packaging applications. J. Plast. Film. Sheet. 2014, 30, 141–161. [Google Scholar] [CrossRef]
- Pérezr, M.A.; Rivas, B.; Rodríguez-Llamazares, S. Polypropylene/starch blends: Study of thermal and morphological properties. J. Chil. Chem. Soc. 2013, 58, 1643–1646. [Google Scholar] [CrossRef]
- ISO 22526-2:2020; Plastics—Carbon and environmental footprint of biobased plastics—Part 2: Material carbon footprint, amount (mass) of CO2 removed from the air and incorporated into polymer molecule. International Organization for Standardization: Geneva, Switzerland, 2020. Available online: https://www.iso.org/standard/73390.html (accessed on 26 November 2022).
- Hablot, E.; Dewasthale, S.; Zhao, Y.; Zhiguan, Y.; Shi, X.; Graiver, D.; Narayan, R. Reactive extrusion of glycerylated starch and starch-polyester graft copolymers. Eur. Polym. J. 2013, 49, 873–881. [Google Scholar] [CrossRef]
- Nafchi, A.M.; Moradpour, M.; Saeidi, M.; Alias, A.K. Thermoplastic starches: Properties, challenges, and prospects. Starch/Staerke 2013, 65, 61–72. [Google Scholar] [CrossRef]
- Averous, L. Properties of thermoplastic blends: Starch–polycaprolactone. Polymer 2000, 41, 4157–4167. [Google Scholar] [CrossRef]
- Narayan, R. Starch-Polyester Biodegradable Graft Copolymers and a Method of Preparation Thereof. U.S. Patent 7,629,405 B2, 19 November 2009. [Google Scholar]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2019, 3, 8–18. [Google Scholar] [CrossRef]
- Raquez, J.M.; Nabar, Y.; Srinivasan, M.; Shin, B.; Narayan, R.; Dubois, P. Maleated thermoplastic starch by reactive extrusion. Carbohydr. Polym. 2008, 74, 159–169. [Google Scholar] [CrossRef]
- Kulkarni, A.; Narayan, R. Effects of Modified Thermoplastic Starch on Crystallization Kinetics and Barrier Properties of PLA. Polymers 2021, 13, 4125. [Google Scholar] [CrossRef]
- Humphreys, R. Thermoplastic Starch: A Renewable, Biodegradable Bioplastic. Polymer Innovation Blog. 4 March 2013. Available online: https://polymerinnovationblog.com/thermoplastic-starch-a-renewable-biodegradable-bioplastic/ (accessed on 1 March 2020).
- Shanks, R.; Kong, I. Thermoplastic Starch. In Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1991; Volume 302, pp. 627–637. [Google Scholar] [CrossRef]
- Aranda-García, F.J.; González-Núñez, R.; Jasso-Gastinel, C.; Mendizábal, E. Water Absorption and Thermomechanical Characterization of Extruded Starch/Poly(lactic acid)/Agave Bagasse Fiber Bioplastic Composites. Int. J. Polym. Sci. 2015, 2015, 343294. [Google Scholar] [CrossRef]
- Qiao, X.; Tang, Z.; Sun, K. Plasticization of corn starch by polyol mixtures. Carbohydr. Polym. 2011, 83, 659–664. [Google Scholar] [CrossRef]
- Raquez, J.M.; Nabar, Y.; Narayan, R.; Dubois, P. In Situ Compatibilization of Maleated Thermoplastic Starch/Polyester Melt-Blends by Reactive Extrusion. Polym. Eng. Sci. 2008, 48, 1747–1754. [Google Scholar] [CrossRef]
- Detyothin, S.; Selke, S.; Narayan, R.; Rubino, M.; Auras, R.A. Effects of molecular weight and grafted maleic anhydride of functionalized polylactic acid used in reactive compatibilized binary and ternary blends of polylactic acid and thermoplastic cassava starch. J. Appl. Polym. Sci. 2015, 132, 1–15. [Google Scholar] [CrossRef]
- Hwang, S.W.; Shim, J.; Selke, S.; Soto-Valdez, H.; Rubino, M.; Auras, R. Effect of Maleic-Anhydride Grafting on the Physical and Mechanical Properties of Poly(lactic acid)/Starch Blends. Macromol. Mater. Eng. 2013, 298, 624–633. [Google Scholar] [CrossRef]
- Clasen, S.H.; Müller, C.; Pires, A.T.N. Maleic Anhydride as a Compatibilizer and Plasticizer in TPS/PLA Blends. J. Braz. Chem. Soc. 2015, 26, 1583–1590. [Google Scholar] [CrossRef]
- Chen, L.; Qiu, X.; Deng, M.; Hong, Z.; Luo, R.; Chen, X.; Jing, X. The starch grafted poly(l-lactide) and the physical properties of its blending composites. Polymer 2005, 46, 5723–5729. [Google Scholar] [CrossRef]
- Najemi, L.; Jeanmaire, T.; Zerroukhi, A.; Raihane, M. Organic catalyst for ring opening polymerization of ε-caprolactone in bulk. Route to starch-graft-polycaprolactone. Starch–Stärke 2010, 62, 147–154. [Google Scholar] [CrossRef]
- Zerroukhi, A.; Jeanmaire, T.; Raveyre, C.; Ainser, A. Synthesis and characterization of hydrophobically modified starch by ring opening polymerization using imidazole as catalyst. Starch–Stärke 2012, 64, 613–620. [Google Scholar] [CrossRef]
- Giri, P.; Tambe, C.; Narayan, R. Greener Products: From Laboratory Fundamentals to Commercial Scale. In Biomass Extrusion and Reaction Technologies: Principles to Practices and Future Potential; American Chemical Society: Washington, DC, USA, 2018; Volume 1304, pp. 1–23. [Google Scholar] [CrossRef]
- Chandra, R.; Rustgi, R. Biodegradable polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Datta, D.; Halder, G. Enhancing degradability of plastic waste by dispersing starch into low density polyethylene matrix. Process Saf. Environ. Prot. 2018, 114, 143–152. [Google Scholar] [CrossRef]
- Zumstein, M.T.; Narayan, R.; Kohler, H.; McNeill, K.; Sander, M. Dos and Do Nots When Assessing the Biodegradation of Plastics. Environ. Sci. Technol. 2019, 53, 9967–9969. [Google Scholar] [CrossRef]
- Selke, S.; Auras, R.; Nguyen, T.; Aguirre, E.C.; Cheruvathur, R.; Liu, Y. Evaluation of biodegradation-promoting additives for plastics. Environ. Sci. Technol. 2015, 49, 3769–3777. [Google Scholar] [CrossRef]
- Nigam, M. Biobased Materials from Starch: Trans-Esterification Blends with Commercial Polymers—ProQuest. Michigan State University, East Lansing. 2018. Available online: https://www.proquest.com/openview/9eb6e906da75c40118d05a3ed23b6ed5/1.pdf?pq-origsite=gscholar&cbl=18750&diss=y (accessed on 5 November 2022).
- Giri, P. Design and Engineering of Value-Added Products from the Polylactide (PLA) Polymer—ProQuest. Michigan State University, East Lansing. 2019. Available online: https://www.proquest.com/openview/ec3fa3e96cf60e3701905b164704122c/1.pdf?pq-origsite=gscholar&cbl=18750&diss=y (accessed on 5 November 2022).
- ISO 14852:2018; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in an Aqueous Medium—Method by Analysis of Evolved Carbon Dioxide. International Standard: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/72051.html (accessed on 5 November 2022).
- Anellotech, Inc. Cost Competitive Bio-Sourced Chemicals and Fuels. Available online: https://anellotech.com/news-1 (accessed on 26 November 2022).
- López, O.V.; Ninago, M.D.; Lencina, M.M.S.; Ciolino, A.E.; Villar, M.A.; Andreucetti, N.A. Starch/Poly(ε-caprolactone) Graft Copolymers Synthetized by γ-Radiation and Their Application as Compatibilizer in Polymer Blends. J. Polym. Environ. 2019, 27, 2906–2914. [Google Scholar] [CrossRef]
- Amin, M.R.; Abu-Sharkh, B.; Al-Harthi, M. Effect of Starch Addition on the Properties of Low Density Polyethylene for Developing Environmentally Degradable Plastic Bags. J. Chem. Eng. 2012, 26, 38–40. [Google Scholar] [CrossRef]
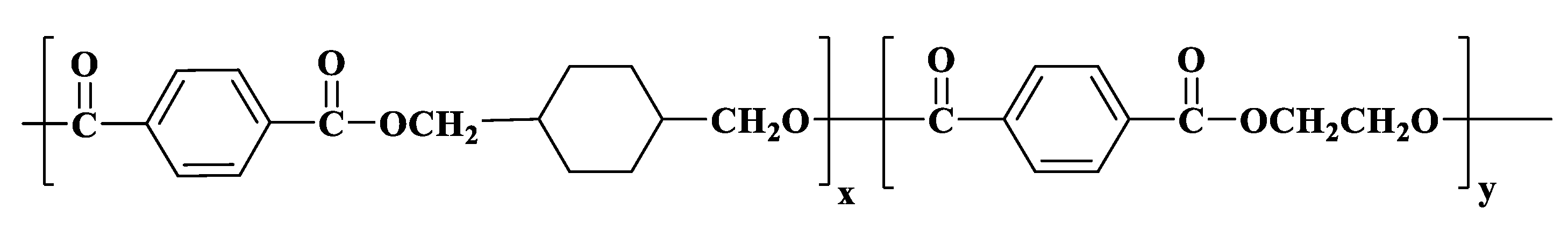


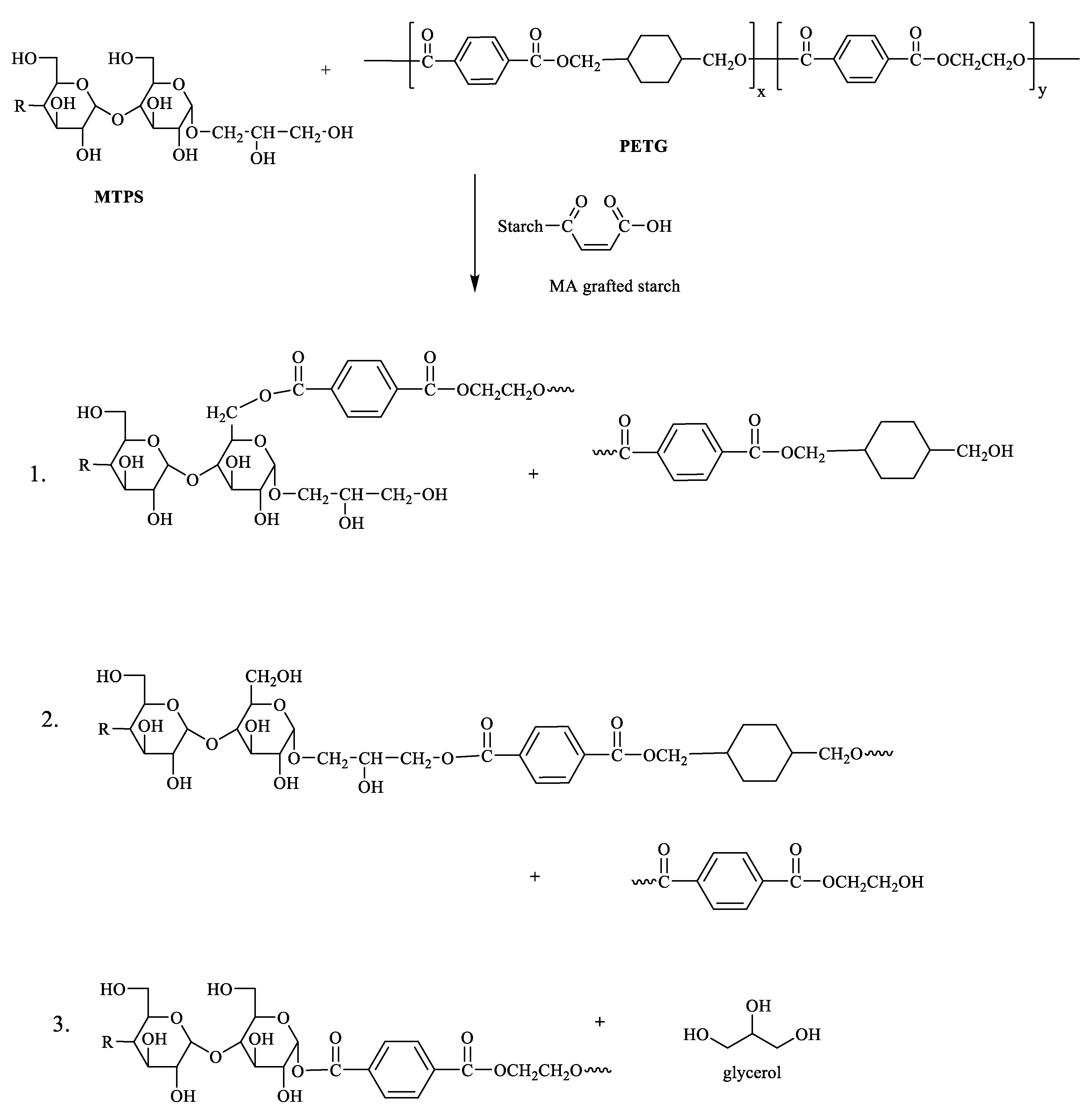
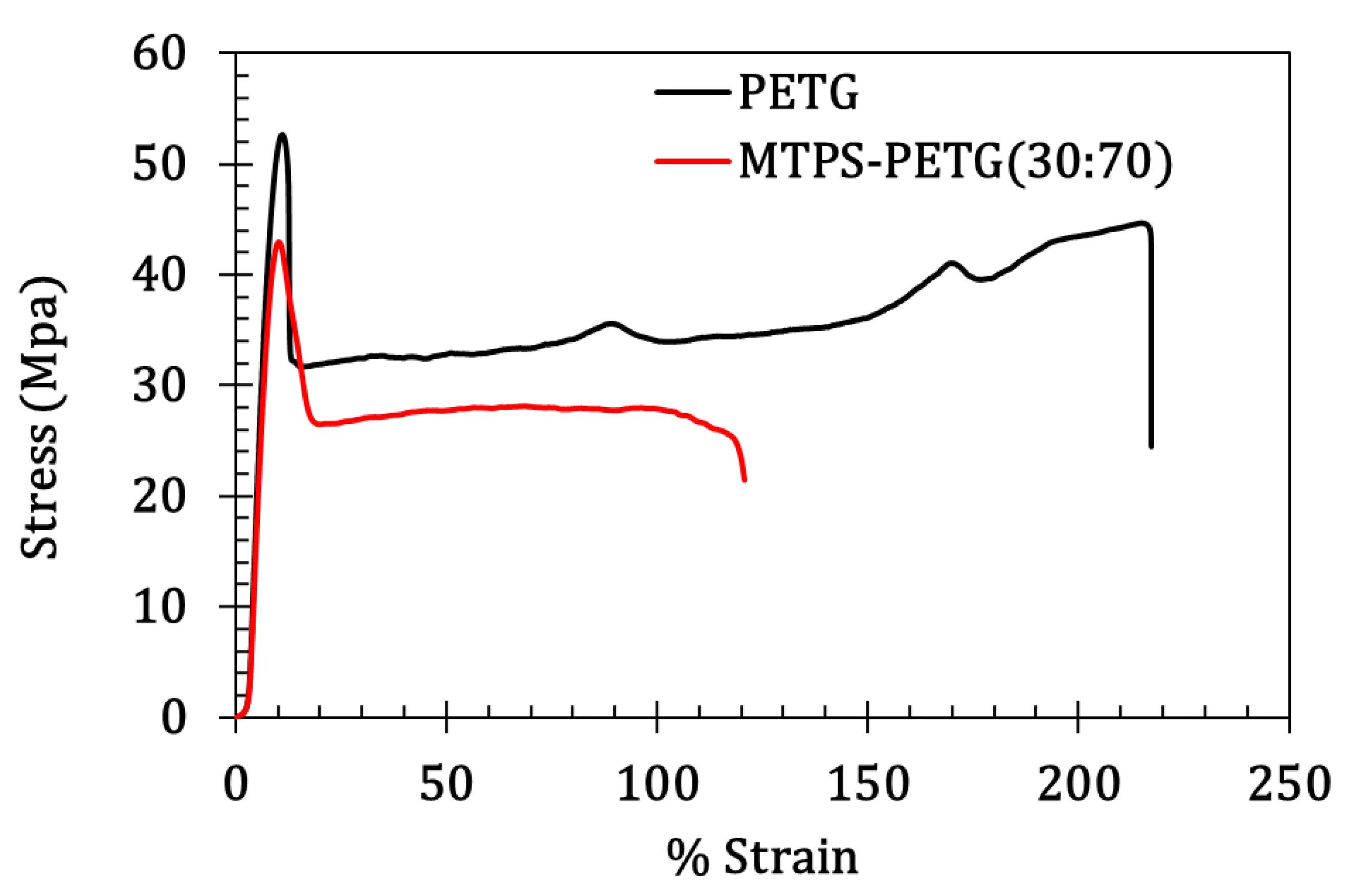


| Modulus (MPa) | Tensile Stress at Yield (MPa) | Tensile Strain at Yield (mm/mm) | Tensile Stress at Break (MPa) | Elongation at Break (%) | |
|---|---|---|---|---|---|
| PETG | 632.7 ± 45.7 | 53.8 ± 0.8 | 2.2 ± 0.1 | 41.9 ± 0.8 | 215 ± 10.6 |
| MTPS/PETG | 483.68 ± 29.3 | 44.5 ± 1.2 | 1.5 ± 0.1 | 22.9 ± 1.5 | 120.7 ± 15.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulkarni, A.; Narayan, R. Morphology, Mechanical Properties, and Biodegradability of Modified Thermoplastic Starch/PETG Blends with In Situ Generated Graft Copolymers. Sustainability 2023, 15, 2227. https://doi.org/10.3390/su15032227
Kulkarni A, Narayan R. Morphology, Mechanical Properties, and Biodegradability of Modified Thermoplastic Starch/PETG Blends with In Situ Generated Graft Copolymers. Sustainability. 2023; 15(3):2227. https://doi.org/10.3390/su15032227
Chicago/Turabian StyleKulkarni, Apoorva, and Ramani Narayan. 2023. "Morphology, Mechanical Properties, and Biodegradability of Modified Thermoplastic Starch/PETG Blends with In Situ Generated Graft Copolymers" Sustainability 15, no. 3: 2227. https://doi.org/10.3390/su15032227
APA StyleKulkarni, A., & Narayan, R. (2023). Morphology, Mechanical Properties, and Biodegradability of Modified Thermoplastic Starch/PETG Blends with In Situ Generated Graft Copolymers. Sustainability, 15(3), 2227. https://doi.org/10.3390/su15032227







