Local Food Systems under Global Influence: The Case of Food, Health and Environment in Five Socio-Ecosystems
Abstract
:1. Introduction
2. Analytical Approach
3. Impacts on Human Health: Nutritional and Dietary Transitions
4. Food Systems and Their Transformations
4.1. Regional Examples
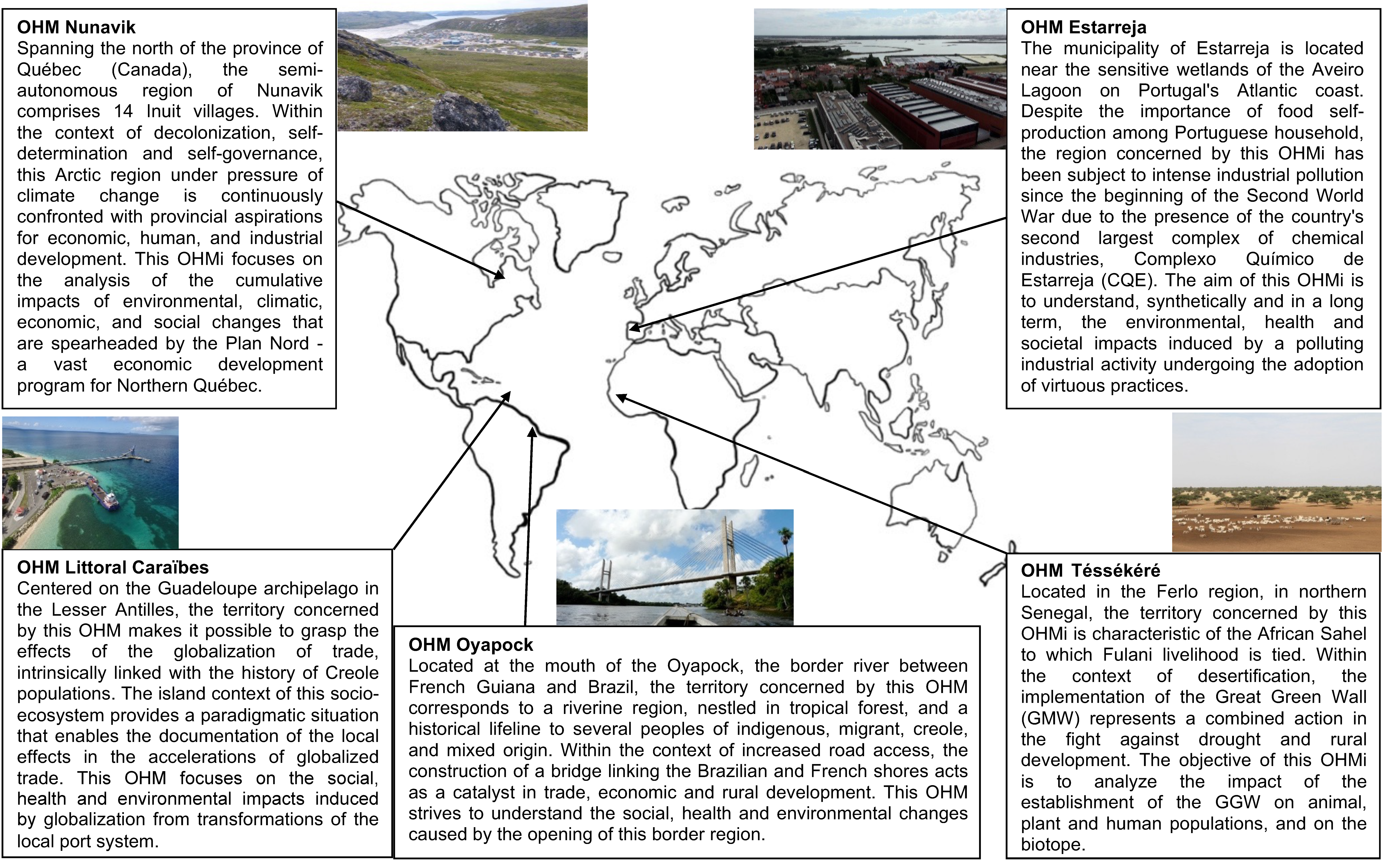
4.1.1. Nunavik, Canada
4.1.2. Ferlo, Senegal
4.1.3. Guadeloupe, France
4.1.4. French Guiana, France
4.1.5. Aveiro, Portugal
4.2. Food Profiles and Typologies
5. Health Outcomes of the Nutrition and Dietary Transitions in the Five OHMs’ Socio-Ecosystems
“There has been a big change in Saint-Georges since the 2000s; diabetes has doubled since the change in diet, the meals eaten now are not at all the meals eaten before because there was no freezing. To keep, it was only the things you could smoke, salt...”[84].
6. Conclusions: Interrogating the Relationship between Food Systems, the Environment and Health through the Lens of Populations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food: A Culinary History from Antiquity to the Present; Flandrin, J.L.; Montanari, M.; Sonnenfeld, A. (Eds.) Columbia University Press: New York, NY, USA, 1999. [Google Scholar]
- Monteiro, C.A.; Cannon, G.; Levy, R.; Moubarac, J.-C.; Jaime, P.; Paula Martins, A.; Canella, D.; Louzada, M.; Parra, D. NOVA. The Star Shines Bright. World Nutr. 2016, 7, 28–38. [Google Scholar]
- Benton, T.G. Using Scenario Analyses to Address the Future of Food. EFSA J. 2019, 17, e170703. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.-C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA Food Classification and the Trouble with Ultra-Processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, C.A.; Cannon, G.; Lawrence, M.; Da Costa Louzada, M.L.; Pereira Machado, P. Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System; FAO: Rome, Italy, 2019; ISBN 978-92-5-131701-3. [Google Scholar]
- Combris, P. 8.3. Les Transitions Nutritionnelles et Leurs Déterminants. In L’alimentation à Découvert; Esnouf, C., Fioramonti, J., Laurioux, B., Eds.; CNRS Éditions: Paris, France, 2015; pp. 277–278. [Google Scholar]
- Poulain, J.-P. Food in Transition: The Place of Food in the Theories of Transition. Sociol. Rev. 2021, 69, 702–724. [Google Scholar] [CrossRef]
- Wang, D.D.; Li, Y.; Afshin, A.; Springmann, M.; Mozaffarian, D.; Stampfer, M.J.; Hu, F.B.; Murray, C.J.L.; Willett, W.C. Global Improvement in Dietary Quality Could Lead to Substantial Reduction in Premature Death. J. Nutr. 2019, 149, 1065–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Obesity. Available online: https://www.who.int/news-room/facts-in-pictures/detail/6-facts-on-obesity (accessed on 8 June 2021).
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentham, J.; Di Cesare, M.; Bilano, V.; Bixby, H.; Zhou, B.; Stevens, G.A.; Riley, L.M.; Taddei, C.; Hajifathalian, K.; Lu, Y.; et al. Worldwide Trends in Body-Mass Index, Underweight, Overweight, and Obesity from 1975 to 2016: A Pooled Analysis of 2416 Population-Based Measurement Studies in 128·9 Million Children, Adolescents, and Adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide Trends in Hypertension Prevalence and Progress in Treatment and Control from 1990 to 2019: A Pooled Analysis of 1201 Population-Representative Studies with 104 Million Participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Whiteman, D.C.; Wilson, L.F. The Fractions of Cancer Attributable to Modifiable Factors: A Global Review. Cancer Epidemiol. 2016, 44, 203–221. [Google Scholar] [CrossRef]
- FAO. Sustainable Food Systems. Concept and Framework; FAO: Rome, Italy, 2018. [Google Scholar]
- Grenier, C. From the Geography of Globalization to Geographic Globalization. Ann. Georgr. 2019, 726, 58–80. [Google Scholar] [CrossRef]
- NRDC. Food Miles. How Far Your Food Travels Has Serious Consequences for Your Health and the Climate; Natural Resources Defense Council: New York, NY, USA, 2007. [Google Scholar]
- FAO. UN General Assembly Proclaims Decade of Action on Nutrition. Available online: https://www.fao.org/news/story/en/item/408970/icode/ (accessed on 1 January 2021).
- Dictionnaire Critique de La Mondialisation, 2nd ed.; Ghorra-Gobin, C. (Ed.) Armand Colin: Paris, France, 2012. [Google Scholar]
- Delisle, H. Transition Nutritionnelle. In Dictionnaire des Cultures Alimentaires; Poulain, J.-P., Ed.; PUF: Paris, France, 2018; pp. 1449–1453. [Google Scholar]
- Douglas, M. Les Structures Du Culinaire. Communications 1979, 31, 145–170. [Google Scholar] [CrossRef] [Green Version]
- Hubert, A. L’anthropologie Nutritionnelle: Aspects Socio-Culturels de l’alimentation. Cah. D’études Rech. Francoph. Santé 1991, 1, 165–168. [Google Scholar]
- Council of Canadian Academies. Aboriginal Food Security in Northern Canada: An Assessment of the State of Knowledge; The Expert Panel on the State of Knowledge of Food Security in Northern Canada, Council of Canadian Academies: Ottawa, ON, Canada, 2014. [Google Scholar]
- Giudice, F.; Caferra, R.; Morone, P. COVID-19, the Food System and the Circular Economy: Challenges and Opportunities. Sustainability 2020, 12, 7939. [Google Scholar] [CrossRef]
- Destoumieux-Garzón, D.; Mavingui, P.; Boetsch, G.; Boissier, J.; Darriet, F.; Duboz, P.; Fritsch, C.; Giraudoux, P.; Le Roux, F.; Morand, S.; et al. The One Health Concept: 10 Years Old and a Long Road Ahead. Front. Vet. Sci. 2018, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Chenorkian, R. Conception and Implementation of Interdisciplinarity in the Human-Environment Observatories (OHM, CNRS). Nat. Sci. Sociétés 2020, 28, 292–305. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and Future Köppen-Geiger Climate Classification Maps at 1-Km Resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [Green Version]
- Omran, A.R. The Epidemiologic Transition: A Theory of the Epidemiology of Population Change. Milbank Mem. Fund Q. 1971, 49, 509–538. [Google Scholar] [CrossRef]
- Notestein, F.W. Summary of the Demographic Background of Problems of Undeveloped Areas. Milbank Mem. Fund Q. 1948, 26, 249–255. [Google Scholar] [CrossRef]
- Notestein, F.W. Problems of Policy in Relation to Areas of Heavy Population Pressure. Milbank Mem. Fund Q. 1944, 22, 424–444. [Google Scholar] [CrossRef]
- Popkin, B.M. Nutritional Patterns and Transitions. Popul. Dev. Rev. 1993, 19, 138–157. [Google Scholar] [CrossRef]
- Drewnowski, A.; Popkin, B.M. The Nutrition Transition: New Trends in the Global Diet. Nutr. Rev. 1997, 55, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M. The Nutrition Transition in Low-Income Countries: An Emerging Crisis. Nutr. Rev. 1994, 52, 285–298. [Google Scholar] [CrossRef]
- Popkin, B.M. Nutrition in Transition: The Changing Global Nutrition Challenge. Asia Pac. J. Clin. Nutr. 2001, 10, S13–S18. [Google Scholar] [CrossRef]
- Maire, B.; Lioret, S.; Gartner, A.; Delpeuch, F. Nutritional Transition and Non-Communicable Diet-Related Chronic Diseases in Developing Countries. Cah. D’études Rech. Francoph. Santé 2002, 12, 45–55. [Google Scholar]
- Kuhnlein, H.V.; Soueida, R.; Receveur, O. Dietary Nutrient Profiles of Canadian Baffin Island Inuit Differ by Food Source, Season, and Age. J. Am. Diet. Assoc. 1996, 96, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Lemire, M.; Kwan, M.; Laouan-Sidi, A.E.; Muckle, G.; Pirkle, C.; Ayotte, P.; Dewailly, E. Local Country Food Sources of Methylmercury, Selenium and Omega-3 Fatty Acids in Nunavik, Northern Quebec. Sci. Total Environ. 2015, 509, 248–259. [Google Scholar] [CrossRef] [Green Version]
- Schuster, R.C.; Wein, E.E.; Dickson, C.; Chan, H.M. Importance of Traditional Foods for the Food Security of Two First Nations Communities in the Yukon, Canada. Int. J. Circumpolar Health 2011, 70, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Kuhnlein, H.V.; Receveur, O. Local Cultural Animal Food Contributes High Levels of Nutrients for Arctic Canadian Indigenous Adults and Children. J. Nutr. 2007, 137, 1110–1114. [Google Scholar] [CrossRef] [Green Version]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global Nutrition Transition and the Pandemic of Obesity in Developing Countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Shan, Z.; Rehm, C.D.; Rogers, G.; Ruan, M.; Wang, D.D.; Hu, F.B.; Mozaffarian, D.; Zhang, F.F.; Bhupathiraju, S.N. Trends in Dietary Carbohydrate, Protein, and Fat Intake and Diet Quality Among US Adults, 1999–2016. JAMA 2019, 322, 1178. [Google Scholar] [CrossRef] [Green Version]
- Kovalskys, I.; Fisberg, M.; Gómez, G.; Pareja, R.G.; Yépez García, M.C.; Cortés Sanabria, L.Y.; Herrera-Cuenca, M.; Rigotti, A.; Guajardo, V.; Zalcman Zimberg, I.; et al. Energy Intake and Food Sources of Eight Latin American Countries: Results from the Latin American Study of Nutrition and Health (ELANS). Public Health Nutr. 2018, 21, 2535–2547. [Google Scholar] [CrossRef] [Green Version]
- Saito, A.; Imai, S.; Htun, N.C.; Okada, E.; Yoshita, K.; Yoshiike, N.; Takimoto, H. The Trends in Total Energy, Macronutrients and Sodium Intake among Japanese: Findings from the 1995–2016 National Health and Nutrition Survey. Br. J. Nutr. 2018, 120, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H.; Zhang, B.; Popkin, B.M.; Du, S. Elevated Fat Intake Increases Body Weight and the Risk of Overweight and Obesity among Chinese Adults: 1991–2015 Trends. Nutrients 2020, 12, 3272. [Google Scholar] [CrossRef] [PubMed]
- Lipoeto, N.I.; Geok Lin, K.; Angeles-Agdeppa, I. Food Consumption Patterns and Nutrition Transition in South-East Asia. Public Health Nutr. 2013, 16, 1637–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galal, O.M. The Nutrition Transition in Egypt: Obesity, Undernutrition and the Food Consumption Context. Public Health Nutr. 2002, 5, 141–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wentzel-Viljoen, E.; Laubscher, R.; Vorster, H.H. Changes in Food Intake from 2005 to 2010 by a Cohort of Black Rural and Urban African Men and Women in the North West Province of South Africa: The PURE-NWP-SA Study. Public Health Nutr. 2018, 21, 2941–2958. [Google Scholar] [CrossRef] [PubMed]
- Vorster, H.H.; Kruger, A.; Margetts, B.M. The Nutrition Transition in Africa: Can It Be Steered into a More Positive Direction? Nutrients 2011, 3, 429–441. [Google Scholar] [CrossRef] [Green Version]
- Russell, C.; Baker, P.; Grimes, C.; Lindberg, R.; Lawrence, M.A. Global Trends in Added Sugars and Non-Nutritive Sweetener Use in the Packaged Food Supply: Drivers and Implications for Public Health. Public Health Nutr. 2022, 1–39. [Google Scholar] [CrossRef]
- Jensen, M.L.; Corvalán, C.; Reyes, M.; Popkin, B.M.; Taillie, L.S. Snacking Patterns among Chilean Children and Adolescents: Is There Potential for Improvement? Public Health Nutr. 2019, 22, 2803–2812. [Google Scholar] [CrossRef]
- Almoraie, N.M.; Saqaan, R.; Alharthi, R.; Alamoudi, A.; Badh, L.; Shatwan, I.M. Snacking Patterns throughout the Life Span: Potential Implications on Health. Nutr. Res. 2021, 91, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D. Snacking: A Cause for Concern. Physiol. Behav. 2018, 193, 279–283. [Google Scholar] [CrossRef]
- Taillie, L.S.; Afeiche, M.C.; Eldridge, A.L.; Popkin, B.M. Increased Snacking and Eating Occasions Are Associated with Higher Energy Intake among Mexican Children Aged 2–13 Years. J. Nutr. 2015, 145, 2570–2577. [Google Scholar] [CrossRef] [PubMed]
- Vandevijvere, S.; Jaacks, L.M.; Monteiro, C.A.; Moubarac, J.; Girling-Butcher, M.; Lee, A.C.; Pan, A.; Bentham, J.; Swinburn, B. Global Trends in Ultraprocessed Food and Drink Product Sales and Their Association with Adult Body Mass Index Trajectories. Obes. Rev. 2019, 20, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Kovic, Y.; Noel, J.K.; Ungemack, J.A.; Burleson, J.A. The Impact of Junk Food Marketing Regulations on Food Sales: An Ecological Study. Obes. Rev. 2018, 19, 761–769. [Google Scholar] [CrossRef]
- Popkin, B.M. Nutrition, Agriculture and the Global Food System in Low and Middle Income Countries. Food Policy 2014, 47, 91–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Du, S.; Su, C.; Zhang, B.; Wang, H.; Popkin, B.M. The Food Retail Revolution in China and Its Association with Diet and Health. Food Policy 2015, 55, 92–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuerrier, A.; Elders of Kangiqsualujjuaq. The Zoological Knowledge of the Inuit of Kangiqsualujjuaq, Nunavik; Avataq Cultural Institute: Inukjuak, QC, Canada, 2012. [Google Scholar]
- Rapinski, M.; Cuerrier, A.; Harris, C.S.; Elders of Ivujivik; Elders of Kangiqsujuaq; Lemire, M. Our Sea, Our Health; Avataq Cultural Institute: Westmount, QC, Canada, 2021. [Google Scholar]
- Cuerrier, A.; Elders of Kangiqsualujjuaq. The Botanical Knowledge of the Inuit of Kangiqsualujjuaq, Nunavik; Avataq Cultural Institute: Inukjuak, QC, Canada, 2011. [Google Scholar]
- Cuerrier, A.; Elders of Kangiqsujuaq. The Botanical Knowledge of the Inuit of Kangiqsujuaq; Avataq Cultural Institute: Inukjuak, QC, Canada, 2011. [Google Scholar]
- Cuerrier, A.; Elders of Umiujaq; Elders of Kuujjuarapik. The Botanical Knowledge of the Inuit of Umiujaq and Kuujjuarapik, Nunavik; Avataq Cultural Institute: Inukjuak, QC, Canada, 2011. [Google Scholar]
- Labrèche, Y. Terres Habitées, Interactions et Changement Au Temps de La. In Le Nord: Habitants et Mutations; Duhaime, G., Ed.; Les Presses de l’Université Laval (coll. «Atlas historique du Québec»): Québec, QC, Canada, 2001; pp. 7–22. ISBN 2763778046. [Google Scholar]
- Saladin d’Anglure, B. Les Inuits Du Nunavik. In Le Nord: Habitants et Mutations; Duhaime, G., Ed.; Les Presses de l’Université Laval (coll. «Atlas historique du Québec»): Québec, QC, Canada, 2001; pp. 85–102. [Google Scholar]
- Lamalice, A. Géographie Du Système Alimentaire Des Inuit Du Nunavik: Du Territoire Nourricier Au Supermarché. Ph.D. thesis, Université Montpellier et Université de Montréal, Montpellier, France, Montréal, QC, Canada, 2019. [Google Scholar]
- Lamalice, A.; Haillot, D.; Lamontagne, M.-A.; Herrmann, T.M.; Gibout, S.; Blangy, S.; Martin, J.-L.; Coxam, V.; Arsenault, J.; Munro, L.; et al. Building Food Security in the Canadian Arctic through the Development of Sustainable Community Greenhouses and Gardening. Écoscience 2018, 25, 325–341. [Google Scholar] [CrossRef]
- Lamalice, A.; Avard, E.; Coxam, V.; Herrmann, T.; Desbiens, C.; Wittrant, Y.; Blangy, S. Supporting Food Security in the Far North: Community Greenhouse Projects in Nunavik and Nunavut. Études/Inuit/Studies 2016, 40, 147–169. [Google Scholar] [CrossRef] [Green Version]
- Blanchet, C.; Rochette, L. Nutrition and Food Consumption among the Inuit of Nunavik. Nunavik Inuit Health Survey 2004, Qanuippitaa? How Are We? Institut National de Santé Publique du Québec (INSPQ) & Nunavik Regional Board of Health and Social Services (NRBHSS): Québec, QC, Canada, 2008. [Google Scholar]
- Rapinski, M.; Cuerrier, A.; Harris, C.; Ivujivik, E.; Kangiqsujuaq, E.; Lemire, M. Inuit Perception of Marine Organisms: From Folk Classification to Food Harvest. J. Ethnobiol. 2018, 38, 333–355. [Google Scholar] [CrossRef] [Green Version]
- Ka, A. Eating in Ferlo (Senegal). Anthropol. Sociétés 2020, 44, 241–258. [Google Scholar] [CrossRef]
- Macia, E.; Tibère, L.; Ka, A.; Seksik, P.; Faye, B.; Boëtsch, G.; Duboz, P. The Diet of the Fulani of Senegal; Comparison between Rural and Urban Contexts around Emblematic Elements. Anthropol. Food 2021, 1–18. [Google Scholar] [CrossRef]
- Ka, A.; Boëtsch, G.; Macia, E. The Feeding of the Fulani Pastoralists in Sahel. Cah. Nutr. Diététique 2020, 55, 47–52. [Google Scholar] [CrossRef]
- Ka, A. Manger à Widou Thiengoly (Nord-Sénégal). De l’abondance Remémorée à La Dépendance Au Marché. Ph.D. Thesis, Université Cheikh Anta Diop de Dakar, Dakar, Senegal, 2016. [Google Scholar]
- Bénéfice, E.; Chevassus-Agnès, S.; Barral, H. Nutritional Situation and Seasonal Variations for Pastoralist Populations of the Sahel (Senegalese Ferlo). Ecol. Food Nutr. 1984, 14, 229–247. [Google Scholar] [CrossRef]
- Foulquier, É. Transport Maritime Sous Régime de Froid. Mondialisation Des Circulations Des Marchandises Périssables. Le Déméter 2016. Econ. Strat. Agric. 2015, 22, 261–279. [Google Scholar]
- Food and Nutrition in the Overseas Departments and Regions; Méjean, C.; Debussche, X.; Martin-Prével, Y.; Réquillart, V.; Soler, L.-G.; Tibère, L. (Eds.) IRD Éditions (coll. «Expertise Collective»): Marseille, France, 2020. [Google Scholar]
- Colombet, Z.; Lamani, V.; Allès, B.; Terrieux, P.; Ducrot, A.; Drogué, S.; Méjean, C. The French West Indian Nutrition Transition Determinants. Cah. Nutr. Diététique 2022, 57, 37–58. [Google Scholar] [CrossRef]
- Weiss, J.; Duchêne, J.; Le Blond, S.; Guyader, O.; Demanèche, S.; Berthou, P.; Le Roy, E.; Leblond, E. Synthèse Des Pêcheries de Guadeloupe 2017. Ifremer-sih-2020.01; 2020. [Google Scholar]
- Stehlé, H. Les Associations Végétales de La Guadeloupe et Leur Intérêt Dans La Valorisation Rationnelle. Rev. Bot. Appliquée D’agriculture Colon. 1937, 17, 98–109. [Google Scholar] [CrossRef]
- Rasse, C.; Andrieu, N.; Diman, J.-L.; Fanchone, A.; Chia, E. Use of Agroecological Practices and Performances of Small Family Farming: The Case of Guadeloupe. Cah. Agric. 2018, 27, 55002. [Google Scholar] [CrossRef]
- Farani, M. Le Machinisme Agricole et Les Plantes à Tubercules. In Proceedings of the Caribbean Food Crops Society 31st Annual Meeting, Dover, Barbados, 10–14 July 1995; pp. 320–323. [Google Scholar]
- Guyot, H. Évolution de La Production et de La Technologie Fruitières En Guadeloupe. Fruits 1971, 26, 115–126. [Google Scholar]
- Davy, D.; Boudoux d’Hautefeuille, M.; Nicole, S.; Grenand, F. Du Manioc et Un Pont: Un Observatoire Hommes/Milieux Sur La Frontière Franco-Brésilienne. In Reformatações Fronteiriças no Platô das Guianas: (Re)territorialidades de Cooperações em Construção; Porto, J., Doff Sotta, E., Eds.; Publit Soluções Editoriais: Rio de Janeiro, Brazil, 2011; pp. 91–118. [Google Scholar]
- Vincent, N. De l’étranger Dans Le Quotidien... Évolutions et Adaptations de l’alimentation à Saint-Georges de l’Oyapock. Master 2 thesis, École des Hautes Études en Sciences Sociales, Toulouse, France,, 2013. [Google Scholar]
- Ouhoud-Renoux, F. Le Cas Palikur: Un Combat Pour Une Adaption à Des Contraintes Fortes. In Les Peuples des Forêts Tropicales Aujourd’hui, Vol. IV: Région Caraïbes; Grenand, P., Ed.; APFT: Bruxelles, Belgium, 2000; pp. 162–193. [Google Scholar]
- Grenand, P. Agriculture Sur Brûlis et Changements Culturels: Le Cas Des Indiens Wayapi et Palikur de Guyane. J. D’agriculture Tradit. Bot. Appliquée 1981, 28, 23–31. [Google Scholar] [CrossRef]
- Capiberibe, A.; Cristinoi, A.; Grenand, P. Un Peuple Arawak: Les Palikur. In Encyclopédies Palikur Wayana Wayãpi: Langue, Milieu et Histoire; Grenand, F., Ed.; Éditions du Comité des Travaux Historiques et Scientifiques & Presses Universitaires d’Orléans: Orléans, France, 2009; pp. 32–57. ISBN 9782735506927. [Google Scholar]
- Hansen, E.; Richard-Hansen, C. Faune de Guyane. Guide Des Principales Espèces Soumises à Réglementation; Éditions Roger Le Guen: L’Estéou, France, 2007. [Google Scholar]
- Rapinski, M. Ethnobiologie et Ethnomédecine Des Peuples Premiers d’Amérique (Cris d’Eeyou Istchee, Parikwene et Pekuakamilnuatsh): L’impact de l’alimentation et Des Médecines Locales Sur La Santé et Le Bien-Être Des Diabétiques. Ph.D. thesis, Université de Montréal et Université de Guyane, Montréal, QC, Canada, Cayenne, French Guiana, 2021. [Google Scholar]
- Berton, M.-È. Les Plantes Médicinales Chez Les Amérindiens Palikurs de St-Georges de l’Oyapock et Macouria (Guyane Française). Master thesis, Université de Paris VII, Paris, France, 1997. [Google Scholar]
- Iaparrá, M.; Martiniano, N.; Felício, J.A.; Orlando, A.; Green, H.; Green, D. Kinetihwa Kabayntiwa Ariku Parantunka Ariku Parikwaki. Comunique-Se Bem Na Lingua Palikur e Na Lingua Portuguesa; Sociedade Internacional de Linguística: Macapá, Brazil, 2017. [Google Scholar]
- de Granville, J.-J.; Gayot, M. Guide Palmiers de Guyane; ONF: Guyane, France, 2014. [Google Scholar]
- Grenand, P.; Moretti, C.; Jacquemin, H.; Prévost, M.-F. Pharmacopées Traditionnelles En Guyane: Créoles, Palikur, Wayapi.; IRD Éditions: Paris, France, 2004. [Google Scholar]
- Cabral-Pinto, M.M.S.; Inácio, M.; Neves, O.; Almeida, A.A.; Pinto, E.; Oliveiros, B.; Ferreira da Silva, E.A. Human Health Risk Assessment Due to Agricultural Activities and Crop Consumption in the Surroundings of an Industrial Area. Expo. Health 2020, 12, 629–640. [Google Scholar] [CrossRef]
- Inácio, M.; Neves, O.; Pereira, V.; Ferreira Da Silva, E.; Veiga, N. Heavy Metals Accumulation in Forage Plants and Crops Growing in Podzols of a Portuguese Industrial Site. Comun. Geológicas 2014, 101, 1019–1022. [Google Scholar]
- Inácio, M.; Neves, O.; Pereira, V.; Ferreira da Silva, E. Levels of Selected Potential Harmful Elements (PHEs) in Soils and Vegetables Used in Diet of the Population Living in the Surroundings of the Estarreja Chemical Complex (Portugal). Appl. Geochem. 2014, 44, 38–44. [Google Scholar] [CrossRef]
- Inácio, M.; Neves, O.; Pereira, V.; Silva, E. Concentration of As, Cu, Hg and Zn in Soil and Agricultural Products (Brassica Oleracea L., Lycopersicon Esculentum Mill and Zea Mays L.) in an Industrial Area in NW of Portugal. Rev. Ciências Agrárias 2013, 36, 229–237. [Google Scholar]
- Sousa, L.P.; Lillebø, A.I.; Soares, J.A.; Alves, F.L. The Management Story of Ria de Aveiro. In Coastal Lagoons in Europe: Integrated Water Resource Strategies; Lillebø, A.I., Stålnacke, P., Gooch, G.D., Eds.; IWA Publishing: London, UK, 2015; pp. 31–38. ISBN 9781780406299. [Google Scholar]
- Braga, H.O.; Azeiteiro, U.M.; Magalhães, L. A Case Study of Local Ecological Knowledge of Shellfishers about Edible Cockle (Cerastoderma Edule) in the Ria de Aveiro Lagoon, Western Iberia. J. Ethnobiol. Ethnomed. 2022, 18, 11. [Google Scholar] [CrossRef]
- Pereira, M.E.; Lillebø, A.I.; Pato, P.; Válega, M.; Coelho, J.P.; Lopes, C.B.; Rodrigues, S.; Cachada, A.; Otero, M.; Pardal, M.A.; et al. Mercury Pollution in Ria de Aveiro (Portugal): A Review of the System Assessment. Environ. Monit. Assess. 2009, 155, 39–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lillebø, A.I.; Ameixa, O.M.C.C.; Sousa, L.P.; Sousa, A.I.; Soares, J.A.; Dolbeth, M.; Alves, F.L. The Physio-Geographical Background and Ecology of Ria de Aveiro. In Coastal Lagoons in Europe: Integrated Water Resource StrategiesLagoons in Europe: Integrated Water Resource Strategies; Lillebø, A.I., Stålnacke, P., Gooch, G.D., Eds.; IWA Publishing: London, UK, 2015; pp. 21–29. ISBN 9781780406299. [Google Scholar]
- Cabral-Pinto, M.M.S.; Ordens, C.M.; Condesso de Melo, M.T.; Inácio, M.; Almeida, A.; Pinto, E.; Ferreira da Silva, E.A. An Inter-Disciplinary Approach to Evaluate Human Health Risks Due to Long-Term Exposure to Contaminated Groundwater Near a Chemical Complex. Expo. Health 2020, 12, 199–214. [Google Scholar] [CrossRef]
- Inácio, M.; Ferreira da Silva, E.; Perreira, V.; Guihard-Costa, A.-M.; Valente, S. Evaluation of Urinary Arsenic in Children and Their Mothers Living near an Industrial Complex (Estarreja, Portugal). In Proceedings of the GeoMed2011-4th International Conference on Medical Geology, Bari, Italy, 20–25 September 2011; Volume 408. [Google Scholar]
- Bento, S.; Gramaglia, C.; Fernandes, L.; Erdlenbruch, K.; Levasseur, P.; Condesso de Melo, M.T. Contaminação de Solos e Água Em Estarreja (Portugal): Que Efeitos Na Vida Dos Habitantes? CAPTAR 2021, 10, 11. [Google Scholar]
- Fumey, G. La Mondialisation de l’alimentation. Inf. Geogr. 2007, 71, 71–82. [Google Scholar] [CrossRef]
- Pitot, S.; Cornély, V. Typologie Des Comportements Alimentaires En Guadeloupe; Basse-Terre: Guadeloupe, France, 2012. [Google Scholar]
- Barrau, M.; Godard, E.; Suivant, C.; Ledrans, M. Common Base Methods for the Four Components of the Kannari Study on Health, Nutrition and Exposure to Chlordecone in the French West Indies, 2011–2018; Santé Publique France: Saint-Maurice, France, 2020. [Google Scholar]
- ORSAG. Présentation et Synthèse de l’enquête En Guadeloupe; Basse-Terre: Guadeloupe, France, 2012. [Google Scholar]
- Kuhnlein, H.V.; Receveur, O.; Soueida, R.; Egeland, G.M. Arctic Indigenous Peoples Experience the Nutrition Transition with Changing Dietary Patterns and Obesity. J. Nutr. 2004, 134, 1447–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egeland, G.M.; Johnson-Down, L.; Cao, Z.R.; Sheikh, N.; Weiler, H. Food Insecurity and Nutrition Transition Combine to Affect Nutrient Intakes in Canadian Arctic Communities. J. Nutr. 2011, 141, 1746–1753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagné, D.; Blanchet, R.; Lauzière, J.; Vaissière, É.; Vézina, C.; Ayotte, P.; Déry, S.; Turgeon O’Brien, H. Traditional Food Consumption Is Associated with Higher Nutrient Intakes in Inuit Children Attending Childcare Centres in Nunavik. Int. J. Circumpolar Health 2012, 71, 18401. [Google Scholar] [CrossRef] [PubMed]
- Proust, F.; Lucas, M.; Dewailly, E. Fatty Acid Profiles among the Inuit of Nunavik: Current Status and Temporal Change. Prostaglandins. Leukot. Essent. Fatty Acids 2014, 90, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Martin, T. Reflexive Modernity in Nunavik. Globe 2005, 8, 175–206. [Google Scholar] [CrossRef] [Green Version]
- Avard, E. Northern Greenhouses: An Alternative Local Food Provisioning Strategy for Nunavik. Ph.D. Thesis, Université Laval, Québec, QC, Canada, 2015. [Google Scholar]
- Pinto, C.M.T.M.Q. Atitudes e Perceções Dos Adolescentes Face à Alimentação: Estudo Exploratório Nos Agrupamentos de Escolas Do Município de Estarreja. Master thesis, Universidade Aberta, Lisbon, Portugal, 2013. [Google Scholar]
- Ribeiro, C.; Coelho, C. The Development of Agriculture and Industry in Estarreja (Portugal) and Its Relationship with the Environment: Documentary Information and Agent View. InterfacEHS Saúde Meio Ambient. Sustentabilidade 2015, 10, 42–52. [Google Scholar]
- Diallo, A.H. Marchés Hebdomadaires et Mutations Du Système de Production Pastoral Au Ferlo (Sahel Sénégalais). Ph.D. thesis, Université Cheikh Anta Diop de Dakar, Dakar, Senegal, 2020. [Google Scholar]
- Duboz, P.; Boëtsch, G.; Gueye, L.; Macia, E. Type 2 Diabetes in a Senegalese Rural Area. World J. Diabetes 2017, 8, 351–357. [Google Scholar] [CrossRef]
- Duboz, P.; Boëtsch, G.; Gueye, L.; Macia, E. Hypertension in the Ferlo (Northern Senegal): Prevalence, Awareness, Treatment and Control. Pan Afr. Med. J. 2016, 25, 177. [Google Scholar] [CrossRef]
- Sougou, N.; Boëtsch, G. Diet and Growth of Young Fulani Children in Widou Thiengoly (Ferlo, Sénégal). Bull. Mem. Soc. Anthropol. Paris 2016, 28, 145–154. [Google Scholar] [CrossRef]
- Brunone, L. The Influence of the Great Green Wall Development Project on Food in Widou Thiengoly (Ferlo, Senegal). Master Thesis, Université Toulous-Jean Jaurès, Toulouse, France, 2022. [Google Scholar]
- Scott, J.C. The Art of Not Being Governed: An Anarchist History of Upland Southeast Asia; Yale University Press: New Haven, CT, USA, 2009; ISBN 9780300152289. [Google Scholar]
- Dewailly, É.; Chateau-Degat, M.-L.; Ékoé, J.-M.; Ladouceur, R.; Rochette, L. Nunavik Inuit Health Survey 2004. Qanuippitaa? How Are We? Status of Cardiovascular Disease and Diabetes in Nunavik; Institut National de Santé Publique du Québec (INSPQ) & Nunavik Regional Board of Health and Social Services (NRBHSS): Québec, QC, Canada, 2007. [Google Scholar]
- Mongeau, L.; Audet, N.; Aubin, J.; Baraldi, R. L’Excès de Poids dans la Population Québecoise de 1987 à 2003; Institut National de Santé Publique du Québec (INSPQ): Québec, QC, Canada, 2005. [Google Scholar]
- Richard, J.-B.; Koivogui, A.; Carbunar, A.; Sasson, F.; Duplan, H.; Marrien, N.; Lacapère, F.; Pradines, N.; Beck, F. Premiers Résultat Du Baromètre Santé DOM 2014-Guyane; Santé Publique France: Saint-Maurice, France, 2015. [Google Scholar]
- Richard, J.-B.; Pitot, S.; Cornely, V.; Pradines, N.; Beck, F. Premiers Résultats Du Baromètre Santé DOM 2014-Guadeloupe; Santé Publique France: Saint-Maurice, France, 2015. [Google Scholar]
- Logframe. Diagnóstico Social Do Concelho de Estarreja 2019; Câmara Municipal de Estarreja: Estarreja, Portugal, 2019. [Google Scholar]
- Mandereau-Bruno, L.; Fosse-Edorh, S. Prévalence Du Diabète Traité Pharmacologiquement (Tous Types) En France En 2015. Disparités Territoriales et Socio-Économiques. Bull. Épidémiologique Hebd. 2017, 27–28, 586–591. [Google Scholar]
- Inamo, J.; Daigre, J.-L.; Boissin, J.-L.; Kangambega, P.; Larifla, L.; Chevallier, H.; Balkau, B.; Smadja, D.; Atallah, A. High Blood Pressure and Obesity. J. Hypertens. 2011, 29, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Glenisson, J. Un Tiers Des Guyanais Ont Retardé Ou Renoncé à Un Soin Médical En 2019. Insee Anal. Guyane 2021, 52, 1–4. [Google Scholar]
- Ndong, J.-R.; Romon, I.; Druet, C.; Prévot, L.; Hubert-Brierre, R.; Pascolini, E.; Thomasset, J.-P.; Cheungkin, R.; Bravo, A.; Chantry, M.; et al. Characteristics, Vascular Risk, Complications and Quality of Health Care in People with Diabetes in French Overseas Departments and Comparison with Metropolitan France: ENTRED 2007–2010, France. Bull. Épidémiologique Hebd. 2010, 42–43, 432–436. [Google Scholar]
- Guarmit, B.; Brousse, P.; Mosnier, E.; Martin, E.; Desrousseaux, G.; Fradin, S.; Verbanis, M.; Beauson, M.; Planes, L.; Lutin, Y.; et al. Bilan D’activité Des Centres Délocalisés de Prévention et de Soins (C.D.P.S.) de Guyane; Centre Hospitalier André Rosemon: Cayenne, French Guiana, 2017. [Google Scholar]
- Van Gastel, B.; Peiter, P.; Morel, V.; Roux, E.; Da Cruz Franco, V.; Mendes, A.P.; Coelho Eugenio, N.C.; Gomes, M. Frontières, Territoires de Santé et Réseaux de Soins. Accès Aux Soins, Prévention Des Maladies Vectorielles et Coopération Transfrontalière En Santé: Une Analyse Qualitative à La Frontière Brésilienne. In Maillages, Interfaces, Réseaux Transfrontaliers, de Nouveaux Enjeux Territoriaux de la Santé; Moullé, F., Reitel, B., Eds.; Presses Universitaires de Bordeaux: Pessac, France, 2021; pp. 111–127. [Google Scholar] [CrossRef]
- ORSAG. Surcharge Pondérale et Obésité Abdominale En Guadeloupe En 2013, KANNARI, Santé, Nutrition et Exposition Au Chlordécone Aux Antilles; Basse-Terre: Guadeloupe, France, 2016. [Google Scholar]
- Yacou, C.; Cornély, V. Données Sur Le Diabète en Guadeloupe; Observatoire Régional de la Santé de Guadeloupe: Baie-Mahault, Guadeloupe, 2015. [Google Scholar]
- ORSAG. Maladie Chronique: Diabète En Guadeloupe; Basse-Terre: Guadeloupe, France, 2021. [Google Scholar]
- DEAL; DAAF. Diagnostic Du Système Alimentaire Guadeloupéen-Rapport DIAG’Alim 2021; Direction de l’Environnement, de l’Aménagement et du Logement (DEAL) Guadeloupe: Basse-Terre, Guadeloupe, 2021. [Google Scholar]
- Dewailly, É.; Chateau-Degat, L.; Ékoé, J.-M.; Ladouceur, R.; Rochette, L. Enquête de Santé Auprès Des Inuits Du Nunavik 2004: État Des Maladies Cardiovasculaires et Du Diabète Au Nunavik; Institut National de Santé Publique du Québec (INSPQ) & Nunavik Regional Board of Health and Social Services (NRBHSS): Québec, QC, Canada, 2008. [Google Scholar]
- Duhaime, G.; Chabot, M.; Gaudreault, M. Food Consumption Patterns and Socioeconomic Factors among the Inuit of Nunavik. Ecol. Food Nutr. 2002, 41, 91–118. [Google Scholar] [CrossRef]
- Furgal, C.; Seguin, J. Climate Change, Health, and Vulnerability in Canadian Northern Aboriginal Communities. Environ. Health Perspect. 2006, 114, 1964–1970. [Google Scholar] [CrossRef] [Green Version]
- Huet, C.; Rosol, R.; Egeland, G.M. The Prevalence of Food Insecurity Is High and the Diet Quality Poor in Inuit Communities. J. Nutr. 2012, 142, 541–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couturier, C.; Lévesque, C. Les Contaminants et Leurs Impacts Sur La Santé Au Nunavik; Commission d’Enquête sur les Relations Entre les Autochtones et Certains Services Publics: Québec, QC, Canada, 2019. [Google Scholar]
- Carbone, S.; Del Buono, M.G.; Ozemek, C.; Lavie, C.J. Obesity, Risk of Diabetes and Role of Physical Activity, Exercise Training and Cardiorespiratory Fitness. Prog. Cardiovasc. Dis. 2019, 62, 327–333. [Google Scholar] [CrossRef] [PubMed]
- CCME. Canada Council of Ministers of the Environment (Updated 1999, 2001, 2011). Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 2011. [Google Scholar]
- Maury-Brachet, R.; Durrieu, G.; Dominique, Y.; Boudou, A. Mercury Distribution in Fish Organs and Food Regimes: Significant Relationships from Twelve Species Collected in French Guiana (Amazonian Basin). Sci. Total Environ. 2006, 368, 262–270. [Google Scholar] [CrossRef]
- Brousse, P.; Mosnier, E.; Guarmit, B.; Nacher, M.; Ville, M. Prise En Charge Des Populations Vivant En Forêt et Le Long Des Fleuves En Guyane. Actual. Doss. Santé Publique 2015, 91, 40–42. [Google Scholar]
- Maury-Brachet, R.; Gentes, S.; Dassié, E.P.; Feurtet-Mazel, A.; Vigouroux, R.; Laperche, V.; Gonzalez, P.; Hanquiez, V.; Mesmer-Dudons, N.; Durrieu, G.; et al. Mercury Contamination Levels in the Bioindicator Piscivorous Fish Hoplias Aïmara in French Guiana Rivers: Mapping for Risk Assessment. Environ. Sci. Pollut. Res. 2020, 27, 3624–3636. [Google Scholar] [CrossRef] [PubMed]
- Rimbaud, D.; Restrepo, M.; Louison, A.; Boukhari, R.; Ardillon, V.; Carles, G.; Lambert, V.; Jolivet, A. Blood Lead Levels and Risk Factors for Lead Exposure among Pregnant Women in Western French Guiana: The Role of Manioc Consumption. J. Toxicol. Environ. Health Part A 2017, 80, 382–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurice, L.; Barraza, F.; Blondet, I.; Ho-A-Chuck, M.; Tablon, J.; Brousse, P.; Demar, M.; Schreck, E. Childhood Lead Exposure of Amerindian Communities in French Guiana: An Isotopic Approach to Tracing Sources. Environ. Geochem. Health 2021. [Google Scholar] [CrossRef] [PubMed]
- Mosnier, E. Epidémiologie Des Maladies Infectieuses et Épidémiques En Milieu Isolé Amazonien. Ph.D. thesis, Université de Guyane, Cayenne, French Guiana, 2017. [Google Scholar]
- Devault, D.A.; Massat, F.; Lambourdière, J.; Maridakis, C.; Dupuy, L.; Péné-Annette, A.; Dolique, F. Micropollutant Content of Sargassum Drifted Ashore: Arsenic and Chlordecone Threat Assessment and Management Recommendations for the Caribbean. Environ. Sci. Pollut. Res. 2022, 29, 66315–66334. [Google Scholar] [CrossRef] [PubMed]
- Resiere, D.; Valentino, R.; Nevière, R.; Banydeen, R.; Gueye, P.; Florentin, J.; Cabié, A.; Lebrun, T.; Mégarbane, B.; Guerrier, G.; et al. Sargassum Seaweed on Caribbean Islands: An International Public Health Concern. Lancet 2018, 392, 2691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewailly, E.; Nantel, A.; Weber, J.-P.; Meyer, F. High Levels of PCBs in Breast Milk of Inuit Women from Arctic Quebec. Bull. Environ. Contam. Toxicol. 1989, 43, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Adamou, T.Y.; Riva, M.; Muckle, G.; Laouan Sidi, E.A.; Lemire, M.; Ayotte, P. Blood Mercury and Plasma Polychlorinated Biphenyls Concentrations in Pregnant Inuit Women from Nunavik: Temporal Trends, 1992–2017. Sci. Total Environ. 2020, 743, 140495. [Google Scholar] [CrossRef]
- Boucher, O.; Muckle, G.; Ayotte, P.; Dewailly, E.; Jacobson, S.W.; Jacobson, J.L. Altered Fine Motor Function at School Age in Inuit Children Exposed to PCBs, Methylmercury, and Lead. Environ. Int. 2016, 95, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Caron-Beaudoin, É.; Ayotte, P.; Blanchette, C.; Muckle, G.; Avard, E.; Ricard, S.; Lemire, M. Perfluoroalkyl Acids in Pregnant Women from Nunavik (Quebec, Canada): Trends in Exposure and Associations with Country Foods Consumption. Environ. Int. 2020, 145, 106169. [Google Scholar] [CrossRef]
- Couture, A.; Levesque, B.; Dewailly, É.; Muckle, G.; Déry, S.; Proulx, J.-F. Lead Exposure in Nunavik: From Research to Action. Int. J. Circumpolar Health 2012, 71, 18591. [Google Scholar] [CrossRef]
- Messier, V.; Lévesque, B.; Proulx, J.-F.; Rochette, L.; Libman, M.D.; Ward, B.J.; Serhir, B.; Couillard, M.; Ogden, N.H.; Dewailly, É.; et al. Seroprevalence of Toxoplasma Gondii Among Nunavik Inuit (Canada). Zoonoses Public Health 2009, 56, 188–197. [Google Scholar] [CrossRef]
- Bradette-Laplante, M.; Courtemanche, Y.; Desrochers-Couture, M.; Forget-Dubois, N.; Bélanger, R.E.; Ayotte, P.; Jacobson, J.L.; Jacobson, S.W.; Muckle, G. Food Insecurity and Psychological Distress in Inuit Adolescents of Nunavik. Public Health Nutr. 2020, 23, 2615–2625. [Google Scholar] [CrossRef]
- Lamoureux-Tremblay, V.; Muckle, G.; Maheu, F.; Jacobson, S.W.; Jacobson, J.L.; Ayotte, P.; Bélanger, R.E.; Saint-Amour, D. Risk Factors Associated with Developing Anxiety in Inuit Adolescents from Nunavik. Neurotoxicol. Teratol. 2020, 81, 106903. [Google Scholar] [CrossRef] [PubMed]
- Sadler, R.; Connell, D. Global Distillation in an Era of Climate Change. In Organic Pollutants Ten Years After the Stockholm Convention-Environmental and Analytical Update; Puzyn, T., Ed.; InTech: London, UK, 2012; pp. 191–216. ISBN 978-953-307-917-2. [Google Scholar]
- Ikonen, I.; Sotgiu, F.; Aydinli, A.; Verlegh, P.W.J. Consumer Effects of Front-of-Package Nutrition Labeling: An Interdisciplinary Meta-Analysis. J. Acad. Mark. Sci. 2020, 48, 360–383. [Google Scholar] [CrossRef]
- Biabiany, O.; Mandonnet, N.; Bolo, A.; Alexandre, G.; Chia, E. Family Farmers and Consumers in Guadeloupe Facing the COVID-19. Études Caribéennes 2021. [Google Scholar] [CrossRef]
- Malassis, L. Food Systems Analysis; University of California: Berkeley, CA, USA, 1983. [Google Scholar]
- Institute of Medicine. Improving Food Safety Through a One Health Approach: Workshop Summary; The National Academies Press: Washington, DC, USA, 2012; ISBN 978-0-309-25933-0. [Google Scholar]
| OHM | Social-Ecological Framework | Disrupting Event (Date) | Climate 1 |
|---|---|---|---|
| Téssékéré Senegal | Desertification of the Sahel region | Great Green Wall (2008) | Hot semi-arid (arid, steppe and hot; BSh) |
| Oyapock French Guiana, France | Opening of the Franco-Brazilian cross-border region by road access | Oyapock River Bridge (2017) | Tropical rainforest (Af) to tropical monsoon (Am) |
| Littoral Caraïbes Guadeloupe, France | Development of an urban port complex in an island context | Transformation and acceleration of maritime trade (from the 1970s) | Tropical rainforest (Af) |
| Nunavik Québec, Canada | Sedentarization, urbanization and economic development of Québec’s arctic region | Plan Nord, i.e., Plan North (2011) | Tundra (polar tundra; ET) to subarctic (cold, without dry season and cold summers; Dfc) |
| Estarreja Portugal | Environmental pollution linked to a complex of chemical industries (CQE) near the Aveiro Lagoon | Adoption of virtuous practices within the CQE (1990) | Warm-summer Mediterranean (temperate, dry and warm summers; Csb) |
| Factors of Influence | Examples |
|---|---|
| Purchasing power of individuals and households |
| Climate change, industrial pollution |
| Level of isolation and connectivity, proximity to markets |
| Supermarket chains, containerization, trade liberalization |
| Economic, geographical and industrial development, trade liberalization |
| Colonization, sedentarization, assimilation |
| Customs, practices, values, both shared and individual |
| Massification of crops, transformation and distribution of food products |
| Food contamination (e.g., pollutants), consumption of “good” and “bad” food |
| Illness | Nunavik (Canada) | French Guiana (France) | Guadeloupe (France) | Estarreja 1 (Portugal) | Téssékéré (Senegal) |
|---|---|---|---|---|---|
| Overweight (30 < BMI ≥ 25 kg/m2) | 29.8 (34) [123,124] | 34 (29) [125] | 31 (29) [126] | 6.0 (6.4) [127] | 13.2 (ND) [119] |
| Obesity (BMI ≥ 30 kg/m2) | 28.3 (15) [123,124] | 18 (12) [125] | 17 (12) [126] | 10.1 (8.0) [127] | 3.00 (ND) [119] |
| Diabetes | 4.8 (4.8) [123] | 8.08 (5) [128] | 9.12 (5) [128] | 8.3 (7.8) [127] | 4.2 (ND) [118] |
| Ratio (Overweight+Obesity): Diabetes | 12 (10) | 6.4 (8) | 5.3 (8) | 1.9 (1.8) | 3.9 (ND) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapinski, M.; Raymond, R.; Davy, D.; Herrmann, T.; Bedell, J.-P.; Ka, A.; Odonne, G.; Chanteloup, L.; Lopez, P.J.; Foulquier, É.; et al. Local Food Systems under Global Influence: The Case of Food, Health and Environment in Five Socio-Ecosystems. Sustainability 2023, 15, 2376. https://doi.org/10.3390/su15032376
Rapinski M, Raymond R, Davy D, Herrmann T, Bedell J-P, Ka A, Odonne G, Chanteloup L, Lopez PJ, Foulquier É, et al. Local Food Systems under Global Influence: The Case of Food, Health and Environment in Five Socio-Ecosystems. Sustainability. 2023; 15(3):2376. https://doi.org/10.3390/su15032376
Chicago/Turabian StyleRapinski, Michael, Richard Raymond, Damien Davy, Thora Herrmann, Jean-Philippe Bedell, Abdou Ka, Guillaume Odonne, Laine Chanteloup, Pascal Jean Lopez, Éric Foulquier, and et al. 2023. "Local Food Systems under Global Influence: The Case of Food, Health and Environment in Five Socio-Ecosystems" Sustainability 15, no. 3: 2376. https://doi.org/10.3390/su15032376
APA StyleRapinski, M., Raymond, R., Davy, D., Herrmann, T., Bedell, J.-P., Ka, A., Odonne, G., Chanteloup, L., Lopez, P. J., Foulquier, É., da Silva, E. F., El Deghel, N., Boëtsch, G., Coxam, V., Joliet, F., Guihard-Costa, A.-M., Tibère, L., Nazare, J.-A., & Duboz, P. (2023). Local Food Systems under Global Influence: The Case of Food, Health and Environment in Five Socio-Ecosystems. Sustainability, 15(3), 2376. https://doi.org/10.3390/su15032376







