1. Introduction
With the expansion of urbanization and industrialization of human societies in the past few decades, many challenges have arisen, including global climate change [
1], environmental pollution, and the reduction of natural resources [
2]. Nowadays, the existence of serious and dangerous pollution in the environment has attracted a lot of attention [
3]. One of these environmental concerns is oil pollution in water [
4]. The presence of oily substances in water sources as potential pollutants can lead to damage to the aquatic ecosystem [
5] and cause environmental hazards for humans and aquatic organisms [
6]. The non-biodegradability [
7], high toxicity, and chemical stability of these oily substances are obvious obstacles that have added to the previous concerns [
8]. There are many strategies for modifying water pollution control, including decomposition [
9,
10], filtration [
11], ion exchange [
12], ionic liquid [
13], and membrane process [
14]; however, one of the most effective and cheapest methods of oil pollution treatment is the use of surface adsorption. During the last few decades, different high contact surface and high porosity materials have been used to remove environmental pollutants in the adsorption process, including bio-adsorbent material [
15], carbon actives, zeolites, mesoporous silicas, and Metal–Organic Frameworks (MOFs) [
16]. In recent years, MOFs have been commonly used to adsorb hazardous organic pollutants from wastewater owing to their high porosity and high specific area of the surface [
17], which are the most important parameters of adsorbent selection [
18].
The MOFs which are obtained by coordination bonds connecting to clusters/metal ions and organic ligands [
19] have attracted much attention owing to their high porosity [
20], high thermal [
21] and chemical stability [
22], and ability to adjust their structure and application [
23,
24,
25].
During thermal decomposition, MOFs can be transformed into porous carbons, metal-based compounds in the form of oxides/carbides/phosphides and metal sulfides [
26], which are important for adsorption [
27] and storage applications [
28]. The performance of these MOFs has already been studied in green projects such as the adsorption of carbon, the treatment of harmful gases [
29], and the removal of oil pollutants from water [
30]. Recently, the thermal conversion of MOFs into carbon-based materials and metal oxides in one-dimensional [
31] and two-dimensional morphologies [
32] have been well defined and have received much attention due to their special applications in the storage of gases [
33]. In general, controlling the chemical synthesis procedure, such as starting chemicals (metal precursors and organic ligands) [
34], synthesis conditions (temperature, pH value, concentration, etc.) [
35], and synthesis method (hydrothermal method, microwave radiation, etc.), can easily adjust the MOF’s structure and properties [
36].
In the process of surface adsorption, the most important factor in selecting an adsorbent is regeneration ability [
37]. Among the different materials, there is a particular preference for MOFs due to their surface modifiability [
38]. Among the improvements that can be made to surface interactions, electrostatic interactions are the most important [
39].
MOF applications in water purification processes can be categorized into two major classes: (i) the treatment of organic substances such as pesticides [
40], oil [
41], dyes [
42], and herbicides [
43,
44]; and (ii) the treatment of heavy metals [
45]. In 2015, Li et al. [
46] applied aluminum nitrate, aluminum oxide, alumina, and boehmite to synthesize MIL-53(Al) in order to perform dimethyl phthalate treatment. Moreover, Khan et al. [
47] studied the adsorption of diethyl phthalate (DEP) and phthalic acid (PA) by ZIF-8, UiO-66(Zr) and NH2-UiO-66(Zr) MOFs. In 2021, Xue et al. [
48] investigated the adsorption properties of porphyrinic Zr-MOF in the removal of water-soluble organic dyes.
Jing et al. [
49] studied a synthesized hydrophobic hierarchical MOF (HZIF-8) as an adsorbent for oil in water emulsion; it showed extraordinary performance in oil adsorption. In 2019, Gao et al. [
50] developed a strategy to synthesize the MOF-based materials and showed that synthesized MOFs contain an efficiently separate petroleum ether, toluene, chloroform, and n-hexane with high oil-in-water emulsion separation performance. The comparative study of MOFs based on the literature review was listed in
Table 1.
On the other hand, designing a process and correlating a phenomenon are the most important parts of a process [
26]. In fact, in order to better understand the process, mathematical modeling is a valuable tool that can be used to investigate operational parameters [
35]. By developing a strong process model, the effect of the process’s parameters on its efficiency can be correlated [
60]. Hitherto, considerable research has been carried out to investigate the equilibrium condition of the adsorption process; nevertheless, there is no comprehensive study to evaluate MOFs’ surface adsorption properties [
61]. Therefore, the adsorption mechanism and equilibrium capacity of MOFs have not been fully understood [
37]. Additionally, the mass transfer mechanism is still unknown and therefore it is not possible to predict the adsorption process using MOFs [
62].
In this study, to correlate the process of surface adsorption, different adsorption kinetic models have been considered for the treatment of oily wastewater on MOF adsorbents. In this way, the measured experimental data have been correlated by different kinetic models and the correlations compared with each other. Finally, the surface adsorption mechanism has been discussed carefully. Moreover, to study the mass transfer mechanism of oily pollutants in the surface adsorption process, different mass transfer models on porous adsorbents have been investigated; then, the obtained experimental data have been correlated using the mass transfer models, and finally the mass transfer mechanism of the adsorption process has been proposed.
2. Materials and Methods
The synthesis of MnFe
2O
4 was carried out using the co-precipitation method [
63], so that first 16.218 gm of FeCl
3·6H
2O was reacted with 5.937 gm of (MnCl)
2·4H
2O with a molar ratio of Fe:Mn = 2:1. In order to adjust the pH to the desired value, the NaOH solution (2M) was used. The solution was stirred at 85 ± 1 °C in an oven with nitrogen atmosphere. After changing the color of the solution, the precipitated MnFe
2O
4 was removed using a magnet. Then, the produced particles were washed twice using deionized water and dried using an oven at 95 °C for 12 h. Finally, the particles were heated to 560 ± 1 °C for 4 h in a furnace. The forming reaction of MnFe
2O
4 from MnCl
2 and FeCl
3 is as follows:
For the synthesis of the final MOF, the pre-synthesized MnFe
2O
4 substance was added to the synthesized UIO-66 compound. In order to synthesize the UIO-66 initially 0.636 gm of ZrCl
4 salt and 0.408 gm of dicarboxylic benzene (H
2BDC) with 0.21 gm of previously obtained MnFe
2O
4 were dissolved in 300 gm of DMF solvent by a stirrer for 24 h. For chemical activation, the obtained solution was washed twice using dimethyl and placed in chloroform for 3 days, and the synthesized crystals were placed in an oven at a temperature of 200 ± 1 °C for 24 h, as mentioned elsewhere [
64].
In order to obtain the adsorption experimental data, 35 mg of the obtained MOF was added to 150 mL of oil-in-water emulsion containing different initial concentrations of pollutant with continuous stirring in a dark place at different process conditions (temperature, pH, and adsorption time). The substances were analyzed using a UV spectrophotometer to measure the amount of residual pollutant in the sample [
64,
65].
The quality of the synthesized adsorbents in the treatment of oily wastewater was performed by producing a water–oil emulsion with a certain amount of gasoline. For the synthesis of the mentioned emulsion, first a certain amount of the pollutant was poured into double-distilled water and the desired emulsion was obtained using Tween80 as an emulsifier. The concentration of oil in the prepared feeds for different analysis was constant and was adjusted to 2000 mg/L (equivalent to 6400 ppm based on COD calculations). After adding Tween80 emulsifier to the system with a concentration of 100 mg/L, a high-speed homogenizer was applied to prepare an emulsion with high stability.
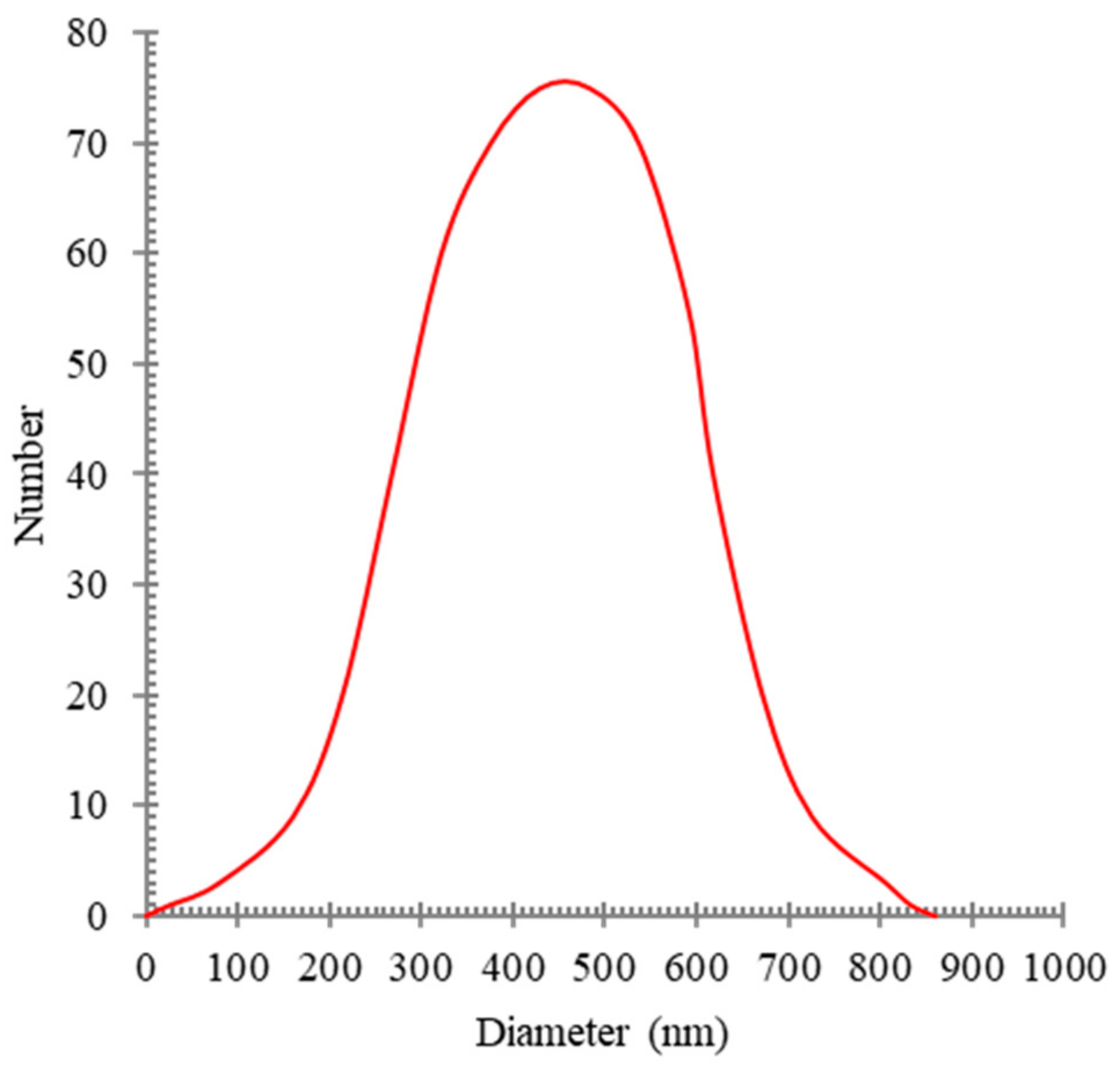
This homogenizing operation was performed for 30 min at 12,000 rpm. In order to measure the distribution of oil particle sizes in water, a DLS analysis (BeNano90) was performed. The synthesized oily wastewater particle sizes are shown in
Figure 1.
3. Adsorption Isotherm Models
The adsorption mechanism can be determined using isotherm models; in other words, the isotherms can be used to optimize the required adsorbent amount in a process. When the adsorbent is placed in contact with a pollutant, the concentration of the pollutant on the surface of the adsorbent increases until reaching thermodynamic equilibrium and then stabilizes at an equilibrium point, which is known as the adsorption isotherm and which is the primary basis for the design of adsorption systems. These equilibrium data can be applied to compare different adsorbents, as well as to design and optimize chemical processes [
66]. To investigate the behavior of adsorbents in pollutant removal different adsorption models can be used, such as Langmuir (Lg), Freundlich (Fl), and Temkin (Tk) isotherms [
67].
The Lg model was the first kinetically oriented theory presented for surface adsorption on a flat surface. In this theory, a continuous molecular mass transfer on the surface occurs so that the rate of accumulation of molecules on the surface remains equal to zero in the equilibrium state [
68].
One of the assumptions used in the Lg model is that the surface adsorption energy is constant and has the same value in all sites. In other words, the adsorbent surface is assumed to be homogeneous. According to this model, each adsorption site can adsorb one molecule, at most.
The linear equation of Lg isotherm model is presented in Equation (2), where
qm is the maximum adsorption capacity,
Ce is the concentration at equilibrium state,
qe is the equilibrium adsorbent concentration, and
Kl is the Lg constant that is related to the strength of molecule adsorption on the adsorbent surface. Therefore, the larger
Kl is, the more surface is covered with adsorbed molecules [
69]. As can be seen from Equation (2), a linear plot of
Ce/
qe versus
Ce can be used to check the Lg model validity in terms of experimental data reproduction.
To describe the equilibrium data, the Fl isotherm can also be applied; it is presented as Equation (3), where
n and
KF are temperature-dependent constants of the Fl isotherm. As it can be understood from Equation (3), by plotting ln
qe in terms of ln
Ce and calculating the intercept and the slope, the constants of the equation can be presented; consequently,
n and
KF can be calculated [
70].
KF represents the irreversibility of the process, and it is obvious that if
n = 1 the Fl equation will be linear, and that the larger the
n value the more the isotherm deviates from the linear state and will have a non-linear behavior [
71]. In this model, it is assumed that the surface is non-uniform in terms of energy distribution, and the sites with equal surface energy are placed next to each other. It is also assumed that each molecule is adsorbed on just one site [
68].
The linearized Tk isotherm is shown in Equation (4), where
R is the universal gas constant,
KT and
b are adjustable parameters, and
T is the temperature. As it can be seen, the constants of the equation can be presented by plotting
qe in terms of ln
Ce and calculating the intercept and the slope [
68].
4. Kinetics
To correlate the surface adsorption process, some kinetic models have been developed by different researchers and have been applied to correlate the experimentally obtained data [
72,
73,
74]. To investigate the kinetic adsorption mechanism, in this work, the pseudo-first-order (PFO), pseudo-second-order (PSO), and Elovich (ELO) models were applied. The pseudo-first-order kinetic model is based on physical adsorption; nevertheless, a chemical reaction is assumed in the pseudo-second order model [
75].
The equation of the PFO model is given in Equation (5) and the PSO model is presented in Equation (6), where
K1 and
K2 are the constant coefficients of the PFO and the PSO models, respectively [
76].
In kinetic process correlating, the oil adsorbent particles interact with the available active sites on the MOF adsorbent and form an unstable adsorbent–adsorbent complex, which subsequently becomes the final stable product [
77]. Equation (7) describes the reaction mechanism of the mentioned phenomena, where
M is the MOF adsorbent and
S is the solute.
The reaction rate for
MS*, which is an unstable intermediate complex, as well as the reversible reaction product,
MS, are presented in Equations (8) and (9), where
K1,
K2,
K3 and
K4 are the rate constants for the reversible reactions [
77].
The intermediate complex compound,
MS*, is an unstable molecule with a short lifetime which immediately turns into the
MS as product. Due to the presence of an intermediary complex with a short lifetime, it can be assumed that the
MS* concentration is negligible [
76]. Therefore, the rate of the intermediary complex concentration must be zero according to Equation (10) [
78]:
In surface adsorption, the initial concentration of the adsorbent (
S0) is equal to the total concentration of the active sites of the adsorbent (
S) which are free; therefore, the site of the reacted complex, and the sites of the adsorbent which are occupied, can be related as Equation (11) [
78].
As the occupied adsorbent sites do not become active adsorption sites without recovery and purification operations, the rate of the reverse reaction of
K4[
MS] is insignificant in the operation of surface adsorption; in other words, the rate of the reverse reaction is almost zero [
78]. Therefore, [
MS*] can be rewritten as Equation (12), where α = (
K2 + K3)/
K1.
Finally, the adsorption rate in the [
MS] form is rewritten as Equation (13).
For high concentrations of [
M] and low concentrations of [
MS], it can be found that [
MS] = 0 and α + [
M] = [
M]; Therefore, the adsorption rate equation can be simplified as follows [
78]:
One of the assumptions in the PSO model is that the surface adsorption is the rate-restricting step and the adsorbent is related to the active sites on the surface of the adsorbent which are free [
79]. Based on Equation (15), when the solution concentration is low, the PSO kinetic model is associated with the surface adsorption phenomenon, where
qt,
ks2, and
qe are the adsorption capacity at
t, the equilibrium rate constant, and the equilibrium adsorption capacity, respectively [
78].
The ELO model can also be applied for the chemical adsorption phenomenon. The ELO model is shown in Equation (16), where α is the initial rate of the process of the surface adsorption and
β is the constant of desorption [
80].
5. Mass Transfer
The mass transfer study, as one of the most important phenomena in the process of the surface adsorption, is the key parameter in a deep understanding of adsorption. The surface adsorption of oily pollutants on the adsorbent can be described as a three-step process [
81]:
- (1)
Liquid Film Diffusion (LF) or Extra-Particle Diffusion model; where oil particles diffuse from the boundary layer of liquid film to the adsorbent surface.
- (2)
Intra-Particle Diffusion model; where the diffusion of oil molecules occurs beneath the surface, inside the pores, or a combination of both.
- (3)
Surface Chemical Reaction; where oil molecules are adsorbed by electrostatic interaction and hydrogen bonding.
As the third step is a very fast one in the surface adsorption process, among these three, the first and second steps can control the adsorption rate. It should be noted that these three steps play a role in the reaction mechanism separately or in combination [
82].
As mentioned, in the LF (extra-particle diffusion) model, the oil molecules’ mass transfer from the liquid bulk to the adsorbent surface controls the rate; in the present study, due to the fact that increasing the stirring speed increases the solution agitation, and mixing between the adsorbent and the adsorbent in the discontinuous surface adsorption process, the thickness of the film boundary layer begins to decrease. As a result, the effect of extra-particle diffusion at high mixing rates can be ignored [
83].
In the intra-particle diffusion model, which is also known as the Weber and Morris (WM) model, the mass transfer of oil particles to the inner part of the synthesized adsorbent is determined using the intra-particle diffusion coefficient [
84]. The WM model is based on the second law of Fick’s mass transfer theory and is applied to investigate the mechanism of the surface mass transfer to the adsorbent pores. This model is expressed according to Equation (17) and is applied to measure the coefficient of diffusion inside the adsorbent pores [
85].
Usually, the diffusion of the particles inside the pores can control the adsorption process. In these situations, using the Bangham and Burt (BB) model can be useful [
77]. It should be noted that using the BB model can be applied whether the surface adsorption is rate-controlled by diffusion inside the pores or not. This model is presented in Equation (18), where
Ci is the initial concentration,
qt is the adsorption capacity at t,
m is the adsorbent mass, and
V is the solution volume. Moreover,
kb and
α are the BB equation constants [
86].
7. Conclusions
Hitherto, a considerable amount of research has been carried out to investigate the equilibrium condition of the adsorption process; nevertheless, there is no comprehensive study to evaluate the surface adsorption properties of MOFs. Therefore, the adsorption mechanism and equilibrium capacity of MOFs have not been fully understood. Moreover, the mass transfer mechanism is still unknown and therefore it is not possible to predict the process using MOFs. In this work, the UIO-66–MnFe2O4 MOF was synthesized as a novel high-performance metal–organic framework adsorbent, and its applications in oily wastewater treatment were studied. The study was performed in four phases, experimental, thermodynamic, kinetic, and mass transfer.
To correlate the process of surface adsorption, different adsorption kinetic models have been applied for the treatment of oil pollutant on MOF adsorbents. Finally, the surface adsorption mechanism has been discussed carefully. Moreover, the mass transfer mechanism of oily pollutants in the surface adsorption process has been investigated using different mass transfer models on porous adsorbents; then, the experimentally obtained data have been correlated with different models of mass transfer, and finally the mass transfer mechanism of the process has been presented. In this way the effects of temperature, amount of adsorbent, adsorption time, pH, and pollutant initial concentration have been studied in the treatment of oily wastewater in the experimental phase. This work is the first step in the analysis of mass transfer and the kinetics of the process of surface adsorption, which can be used to predict the properties of the adsorbent and the process of surface adsorption. In the future, with the expansion of mass transfer and kinetic models and the simultaneous investigation of these two phenomena, it is suggested that the possibility of predicting the adsorption process in other MOFs be investigated.