Facile Formulation of New Innovative Eco-Friendly Hybrid Protective Coating for Mild Steel in Acidic Media
Abstract
:1. Introduction
2. Experimental
2.1. Methods and Materials
2.2. Formulation, Characterization, and Application of Coating Samples
2.3. Corrosion Rate Determination of MS Samples in 1.0 M HCl
3. Results and Discussion
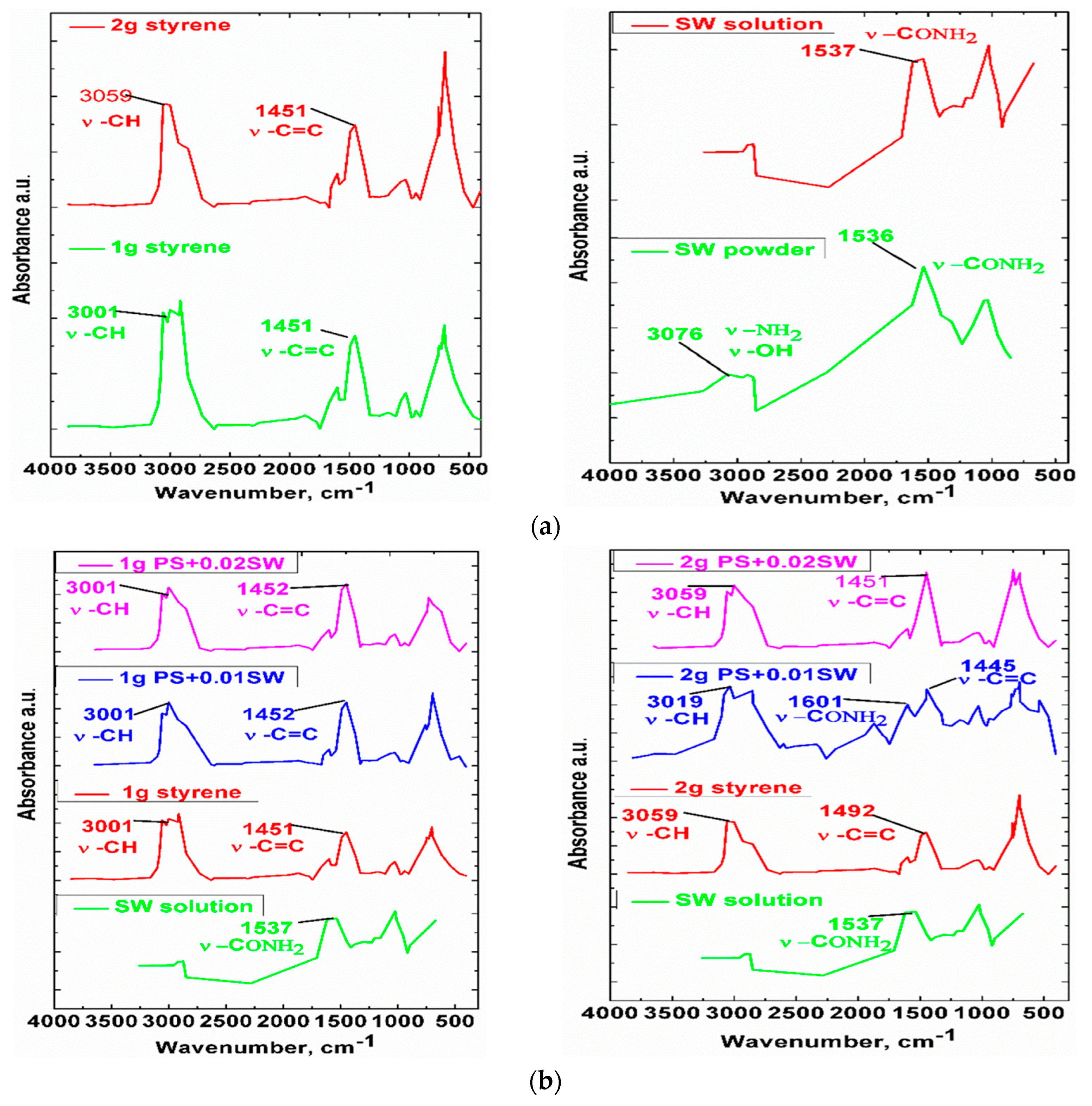
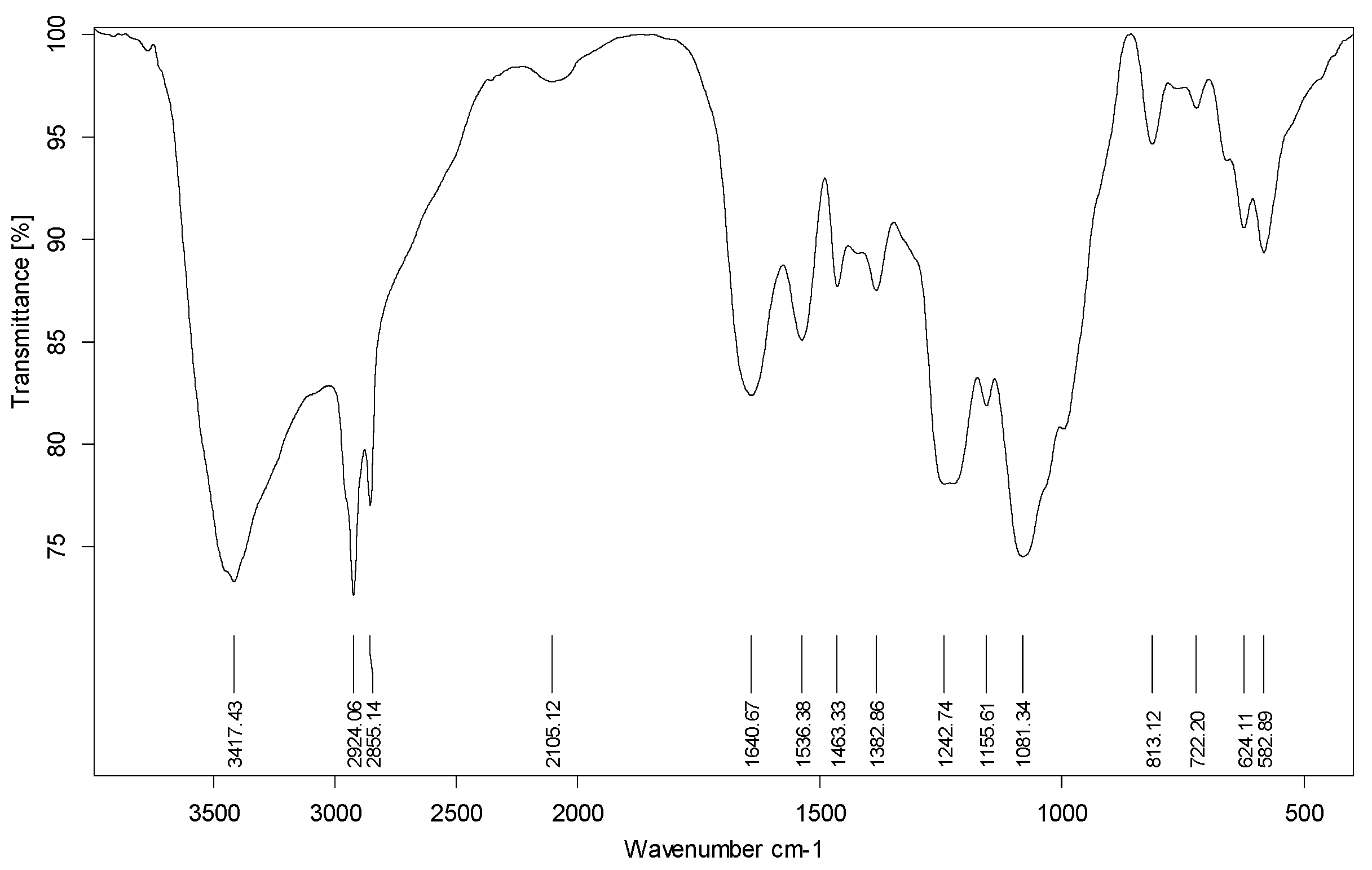
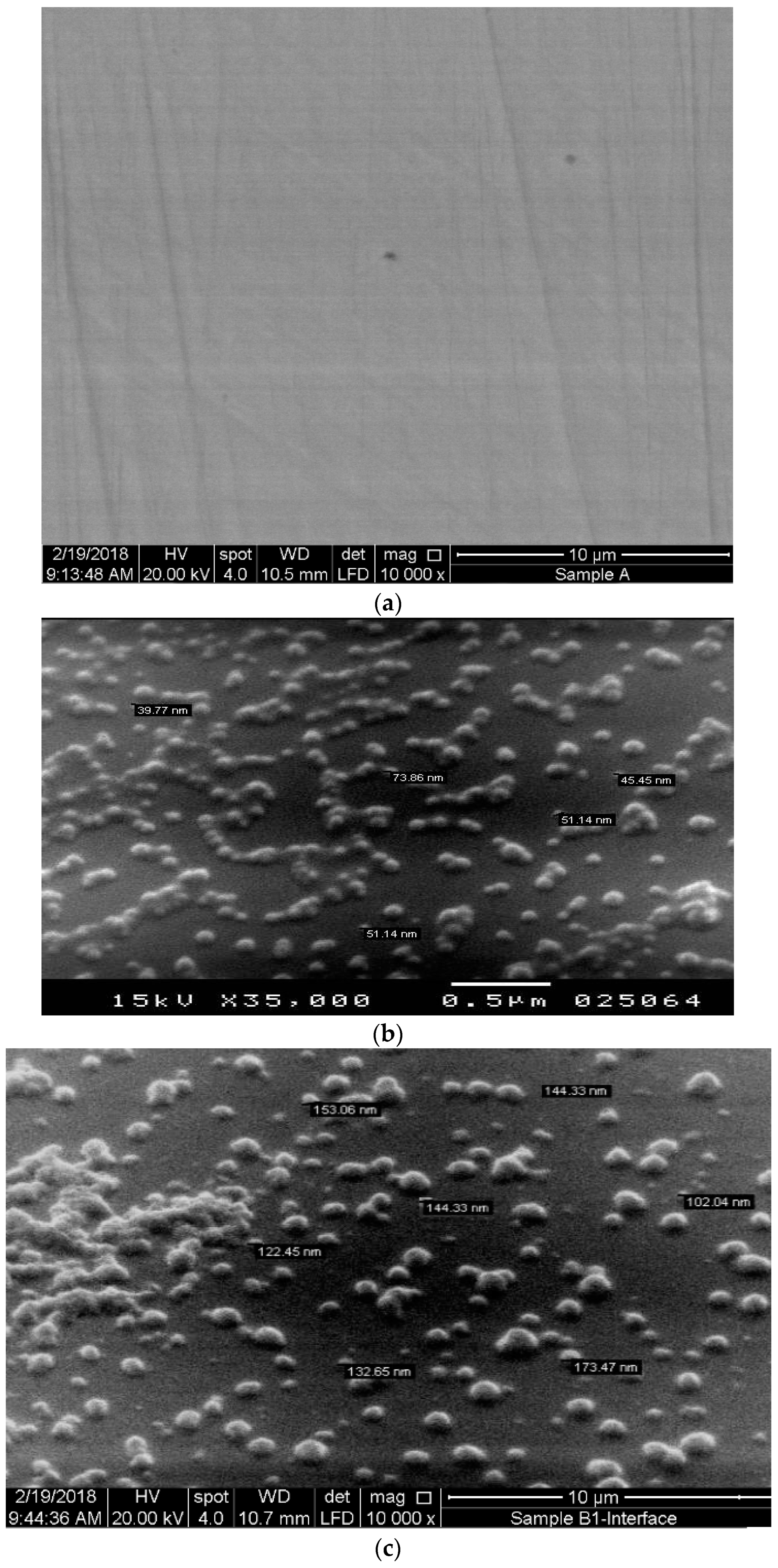
3.1. Characterization of SW–PS Coating Samples
3.2. Evaluation of SW–PS Coating for Mild Steel in 1.0 M HC1 at 30 °C
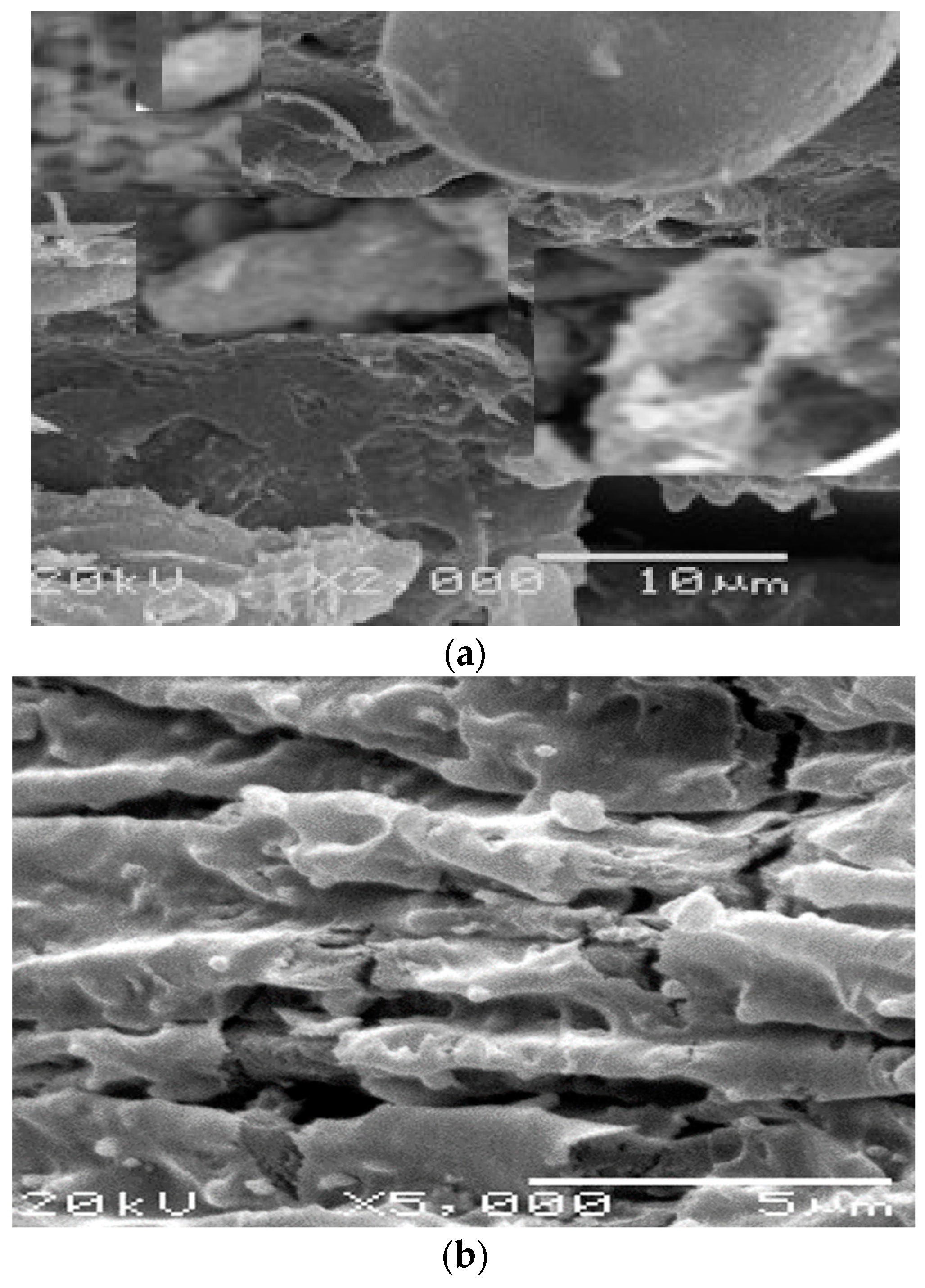
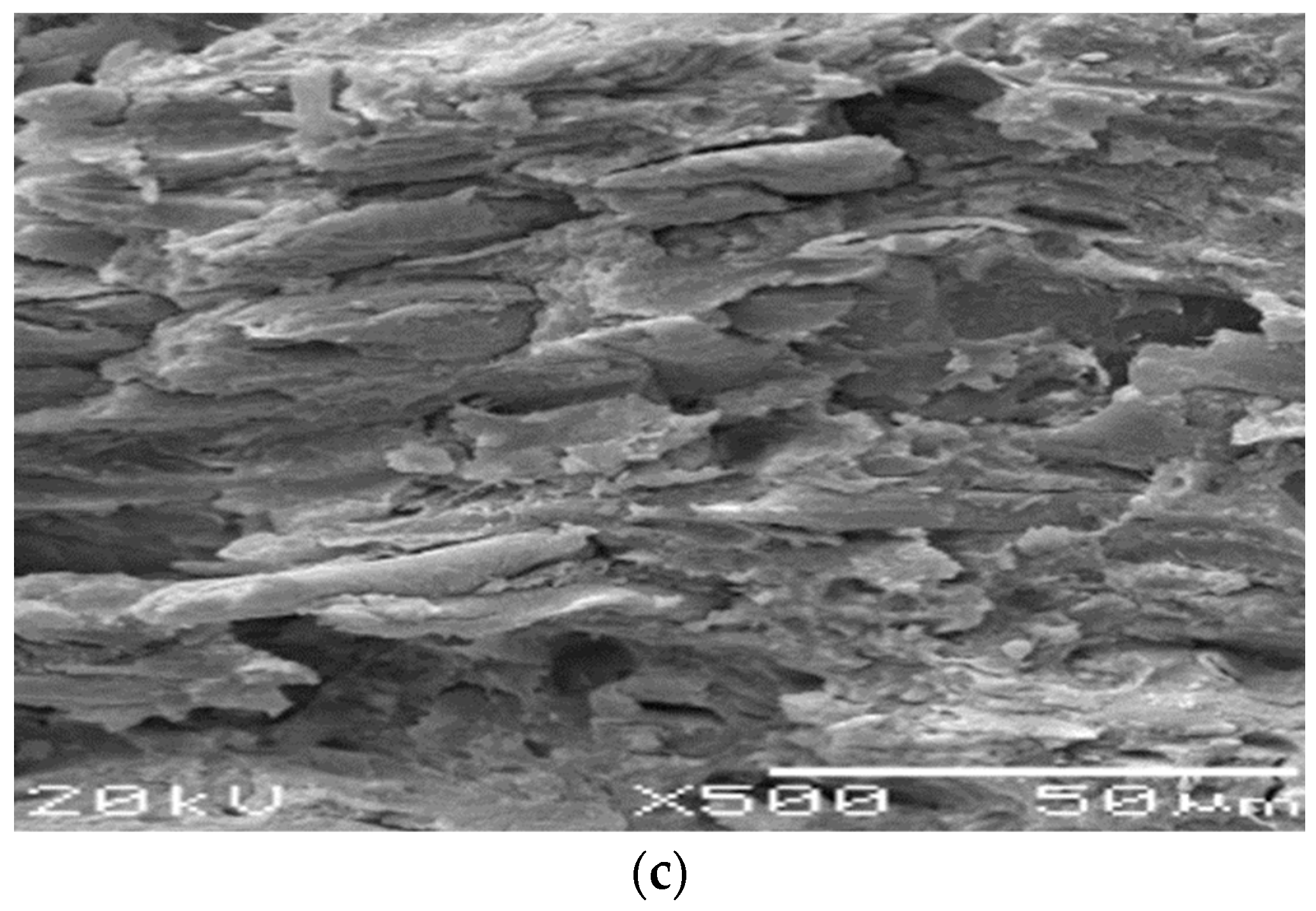
3.3. Surface Analysis of Mild Steel Samples
3.4. Evaluation of Physical Parameters of Coating Samples
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keshk, R.M.; Elgawad, G.E.A.; Sallam, E.R.; Alsubaie, M.S.; Fetouh, H.A. Synthesis and Characterization of Nicotinonitrile Derivatives as Efficient Corrosion Inhibitors for Acid Pickling of Brass Alloy in Nitric Acid. Chem. Sel. 2022, 7, e202202678. [Google Scholar] [CrossRef]
- Elbatouti, M.; Fetouh, H.A. Extraction of eco-friendly and biodegradable surfactant for inhibition of copper corrosion during acid pickling. Adsorpt. Sci. Technol. 2019, 37, 649–663. [Google Scholar] [CrossRef]
- Fetouh, H.A.; Hefnawy, A.; Attia, A.M.; Ali, E. Facile and low-cost green synthesis of eco-friendly chitosan-silver nanocomposite as novel and promising corrosion inhibitor for mild steel in chilled water circuits. J. Mol. Liq. 2020, 319, 114355. [Google Scholar] [CrossRef]
- Figueira, R.B. Hybrid sol–gel coatings for corrosion mitigation: A critical review. Polymers 2020, 12, 689. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Wang, Y.; Lu, Y. A review on fundamentals and strategy of epoxy-resin-based anticorrosive coating materials. Polym.-Plast. Technol. Mater. 2021, 60, 601–625. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Siva, T.; Ramadoss, A. Surface functionalized bioceramics coated on metallic implants for biomedical and anticorrosion performance—A review. J. Mater. Chem. B 2021, 9, 9433–9460. [Google Scholar] [CrossRef]
- Fetouh, H.A.; Abd-El-Nabey, B.A.; Goher, Y.M.; Karam, M.S. An electrochemical investigation in the anticorrosive properties of silver nanoparticles for the acidic corrosion of aluminium. J. Electrochem. 2018, 24, 89. [Google Scholar]
- Ghormade, V.; Pathan, E.K.; Deshpande, M.V. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017, 104, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Barghout, N.A.; El Nemr, A.; Abd-El-Nabey, B.A.; Fetouh, H.A.; Ragab, S.; Eddy, N.O. Use of orange peel extract as an inhibitor of stainless steel corrosion during acid washing in a multistage flash desalination plant. J. Appl. Electrochem. 2023, 53, 379–399. [Google Scholar] [CrossRef]
- Cottrell, A. An Introduction to Metallurgy; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Suryawanshi, N.; Jujjavarapu, S.E.; Ayothiraman, S. Marine shell industrial wastes—An abundant source of chitin and its derivatives: Constituents, pretreatment, fermentation, and pleiotropic applications-a revisit. Int. J. Environ. Sci. Technol. 2019, 16, 3877–3898. [Google Scholar] [CrossRef]
- Mathew, G.M.; Mathew, D.C.; Sukumaran, R.K.; Sindhu, R.; Huang, C.C.; Binod, P.; Sirohi, R.; Kim, S.H.; Pandey, A. Sustainable and eco-friendly strategies for shrimp shell valorization. Environ. Pollut. 2020, 267, 115656. [Google Scholar] [CrossRef]
- Wünsch, J.R. Polystyrene: Synthesis, Production and Applications; Rapra Technology LTD.: Shropshire, UK, 2000. [Google Scholar]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On coating techniques for surface protection: A review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Abd-El-Nabey, B.A.; Goher, Y.M.; Fetouh, H.A.; Karam, M.S. Anticorrosive properties of chitosan for the acid corrosion of aluminium. Port. Electrochim. Acta 2015, 33, 231–239. [Google Scholar] [CrossRef]
- Taha, A.A.; Shaban, S.M.; Fetouh, H.A.; Taha, S.T.; Sabet, V.M.; Kim, D.H. Synthesis and evaluation of nonionic surfactants based on dimethylaminoethylamine: Electrochemical investigation and theoretical modeling as inhibitors during electropolishing in-ortho-phosphoric acid. J. Mol. Liq. 2021, 328, 115421. [Google Scholar] [CrossRef]
- Omar, S.H.; Khalil, N.E.; EL-Ahwany, A.M.D.; El-Sayed, H.A.E.; Arafat, S.O.Y. Mitigation of Microbiologically Induced Corrosion (MIC) and Preventive Strategies. Res. Trends Microbiol. MedDocs 2021, 3, 1–11. [Google Scholar]
- Yu, Z.; Hu, J.; Meng, H. A review of recent developments in coating systems for hot-dip galvanized steel. Front. Mater. 2020, 7, 74. [Google Scholar] [CrossRef]
- Abd-El-Nabey, B.A.; Eldissouky, A.; Fetouh, H.A.; Mohamed, M.E. Role of anion in the electrochemical dissolution of copper and its inhibition by diethyl dithiocarbamate in neutral aqueous solutions. Phys. Chem. 2018, 8, 1. [Google Scholar]
- Fetouh, H.A.; Abdel-Fattah, T.M.; El-Tantawy, M.S. Novel plant extracts as green corrosion inhibitors for 7075-T6 aluminium alloy in an aqueous medium. Int. J. Electrochem. Sci. 2014, 9, 1565–1582. [Google Scholar]
- Choi, I.H.; Lee, J.H.; Chung, T.H. Polystyrene biodegradation using Zophobas morio. J. Entomol. Res. 2020, 44, 475–478. [Google Scholar] [CrossRef]
- Srinivasan, H.; Kanayairam, V.; Ravichandran, R. Chitin and chitosan preparation from shrimp shells Penaeus monodon and its human ovarian cancer cell line, PA-1. Int. J. Biol. Macromol. 2018, 107, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Izaki, K.; Yamamoto, K.; Kadokawa, J.I. Preparation of nanochitin/polystyrene composite particles by pickering emulsion polymerization using scaled-down chitin nanofibers. Coatings 2021, 11, 672. [Google Scholar] [CrossRef]
- Tanabe, K.; Izawa, H.; Ifuku, S. Preparation and recycling property of nanofiber-reinforced polystyrene molded product using the emulsion-forming ability of chitin nanofibers. Polym. J. 2022, 54, 615–621. [Google Scholar] [CrossRef]
- Yu, K.; Ji, X.; Yuan, T.; Cheng, Y.; Li, J.; Hu, X.; Liu, Z.; Zhou, X.; Fang, L. Robust Jumping Actuator with a Shrimp-Shell Architecture. Adv. Mater. 2021, 33, 2104558. [Google Scholar] [CrossRef] [PubMed]
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical extraction and modification of chitin and chitosan from shrimp shells. Eur. Polym. J. 2021, 159, 110709. [Google Scholar] [CrossRef]
- Yan, L.; Li, P.; Zhou, W.; Wang, Z.; Fan, X.; Chen, M.; Fang, Y.; Liu, H. Shrimp shell-inspired antifouling chitin nanofibrous membrane for efficient oil/water emulsion separation with in situ removal of heavy metal ions. ACS Sustain. Chem. Eng. 2018, 7, 2064–2072. [Google Scholar] [CrossRef]
- Moussian, B. Chitin: Structure, chemistry and biology. In Targeting Chitin-Containing Organisms; Yang, Q., Fukamizo, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 5–18. [Google Scholar]
- Saba, N.; Jawaid, M. A review on thermomechanical properties of polymers and fibers reinforced polymer composites. J. Ind. Eng. Chem. 2018, 67, 1–11. [Google Scholar] [CrossRef]
- Wendel, J.F.; Lenz Leite, M.; Furtat, P.; Tangermann-Gerk, K.; Schafföner, S.; Motz, G. New Coatings Systems with Improved Mechanical Properties by Combining Polymer Derived Ceramic and Physical Vapor Deposition Coating Methods. Adv. Mater. Interfaces 2022, 9, 2201654. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Z.; Cui, D.; Liu, X. Precisely Controlled Polymerization of Styrene and Conjugated Dienes by Group 3 Single-Site Catalysts. ChemCatChem 2018, 10, 42–61. [Google Scholar] [CrossRef]
- IkhtiarBakti, A.; Gareso, P.L. Characterization of active carbon prepared from coconuts shells using FTIR, XRD and SEM techniques. J. Ilm. Pendidik. Fis. Al-Biruni 2018, 7, 33–39. [Google Scholar]
- Thakkar, R.; Thakkar, R.; Pillai, A.; Ashour, E.A.; Repka, M.A. Systematic screening of pharmaceutical polymers for hot melt extrusion processing: A comprehensive review. Int. J. Pharm. 2020, 576, 118989. [Google Scholar] [CrossRef] [PubMed]

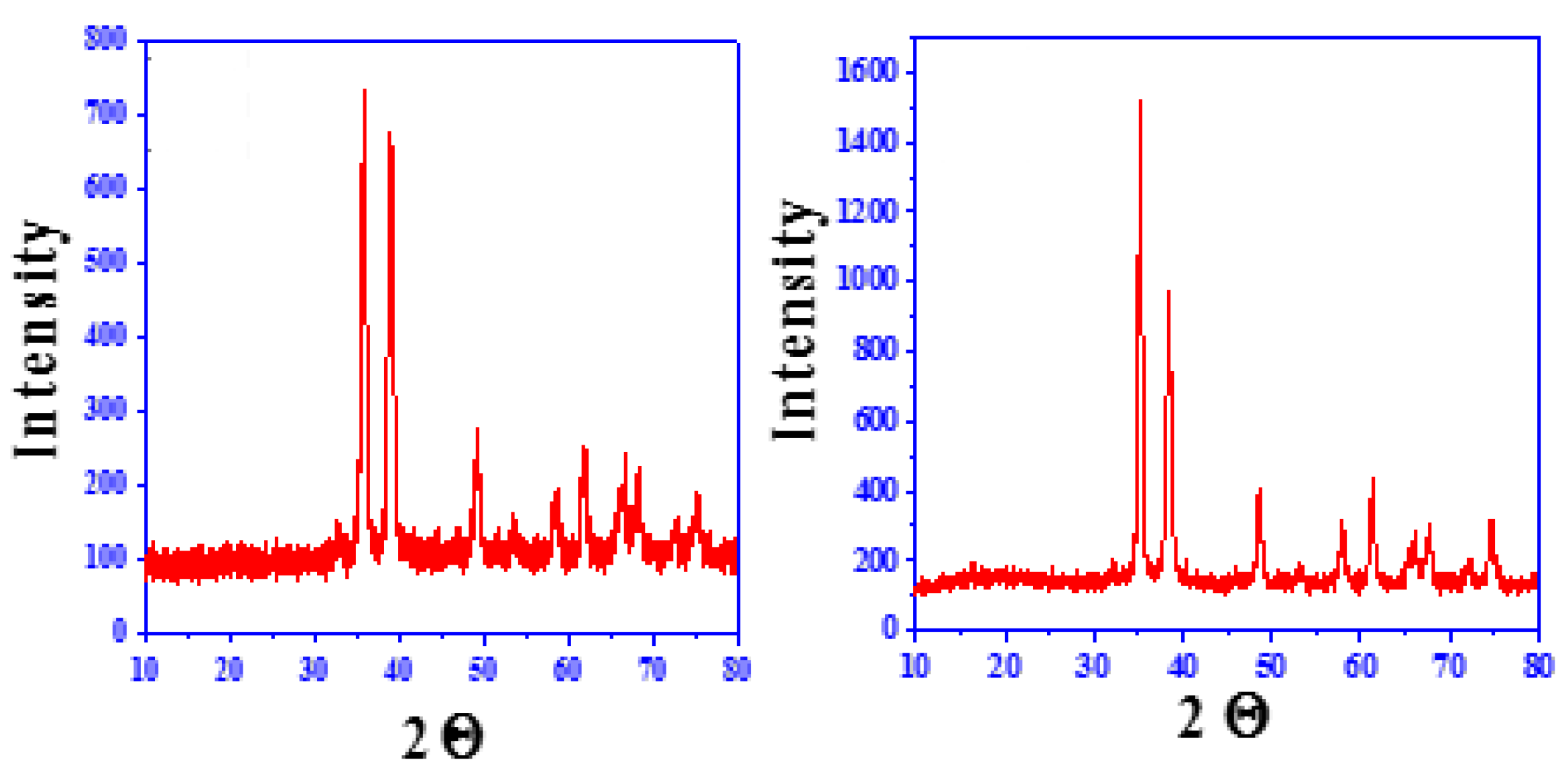
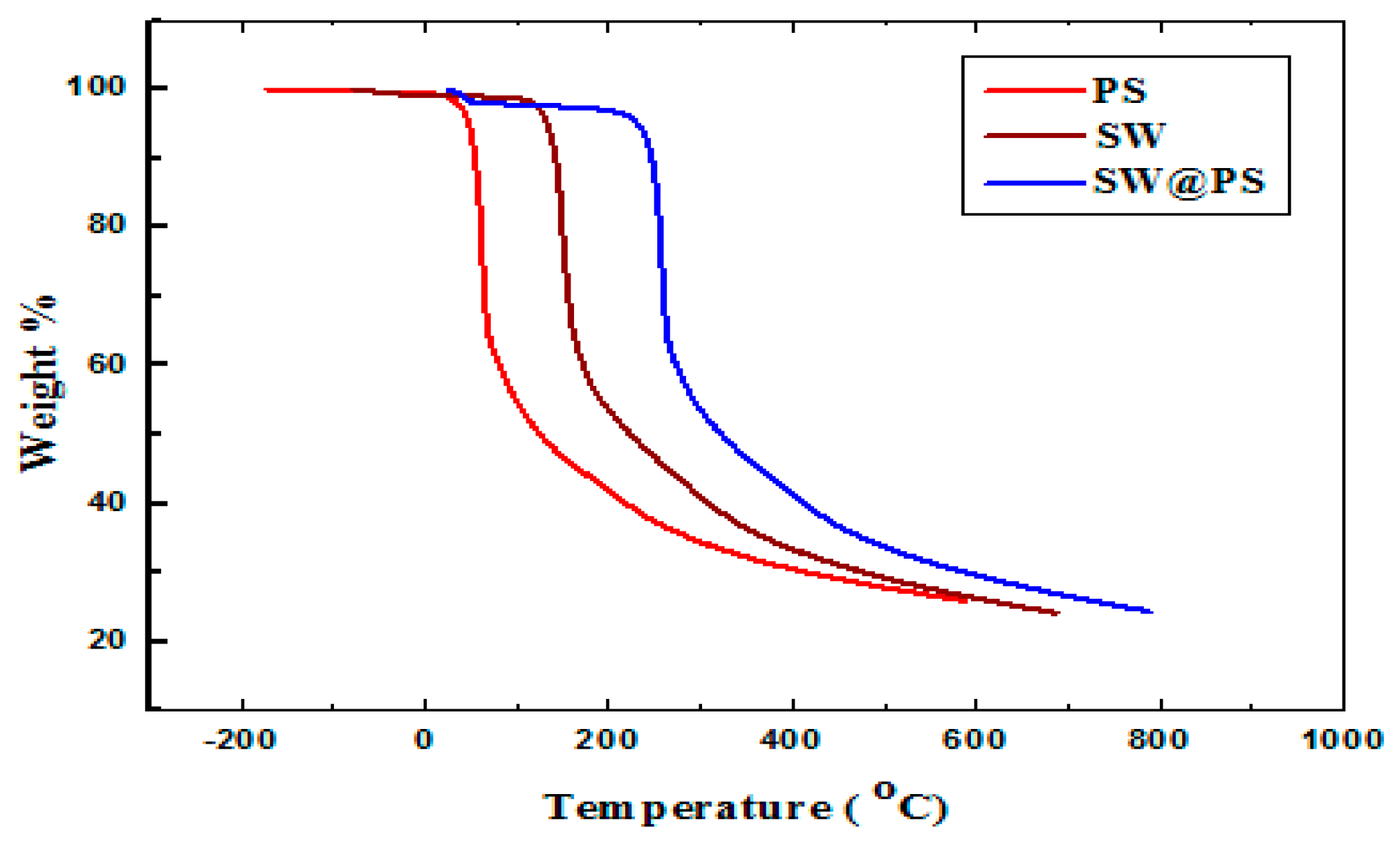


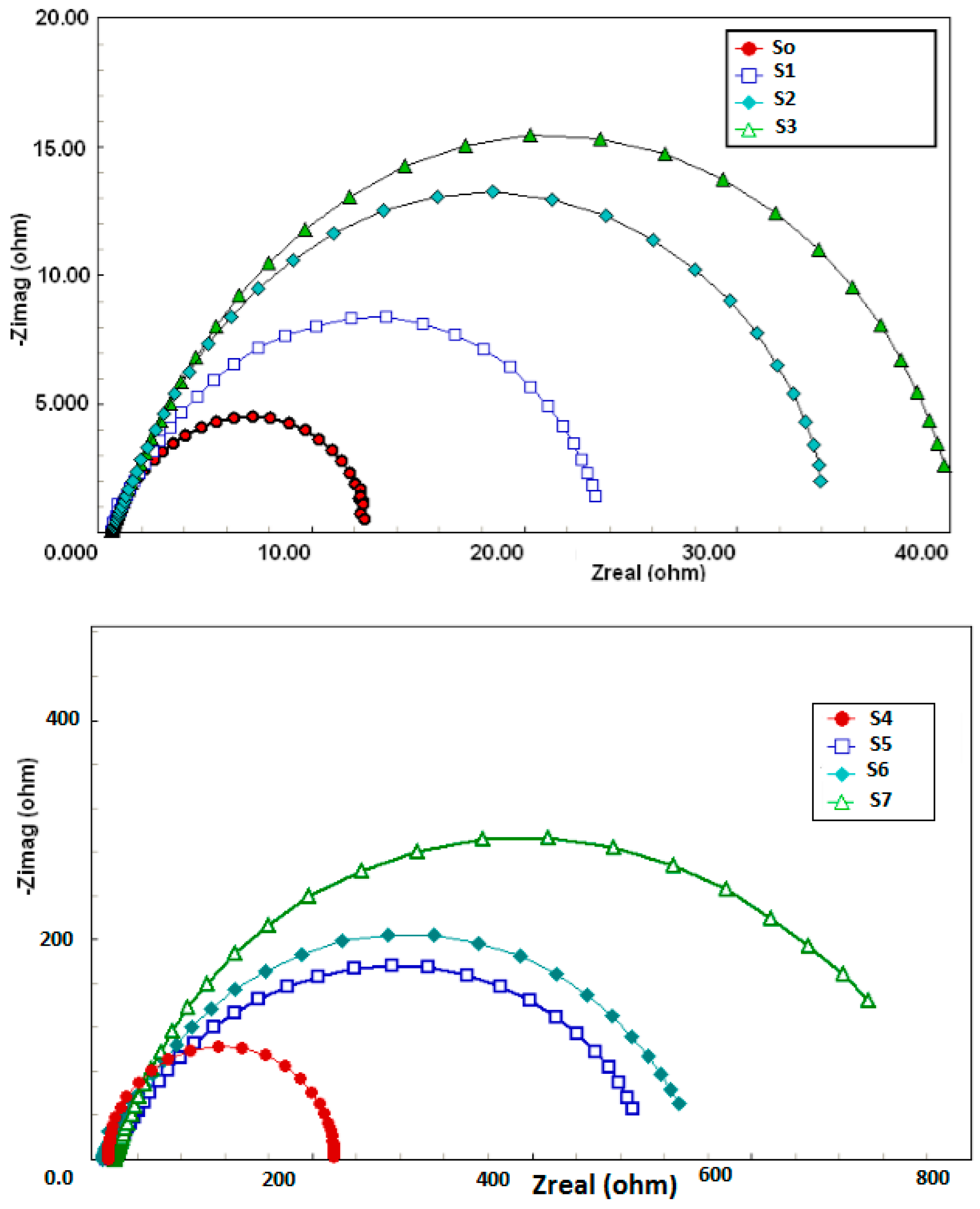
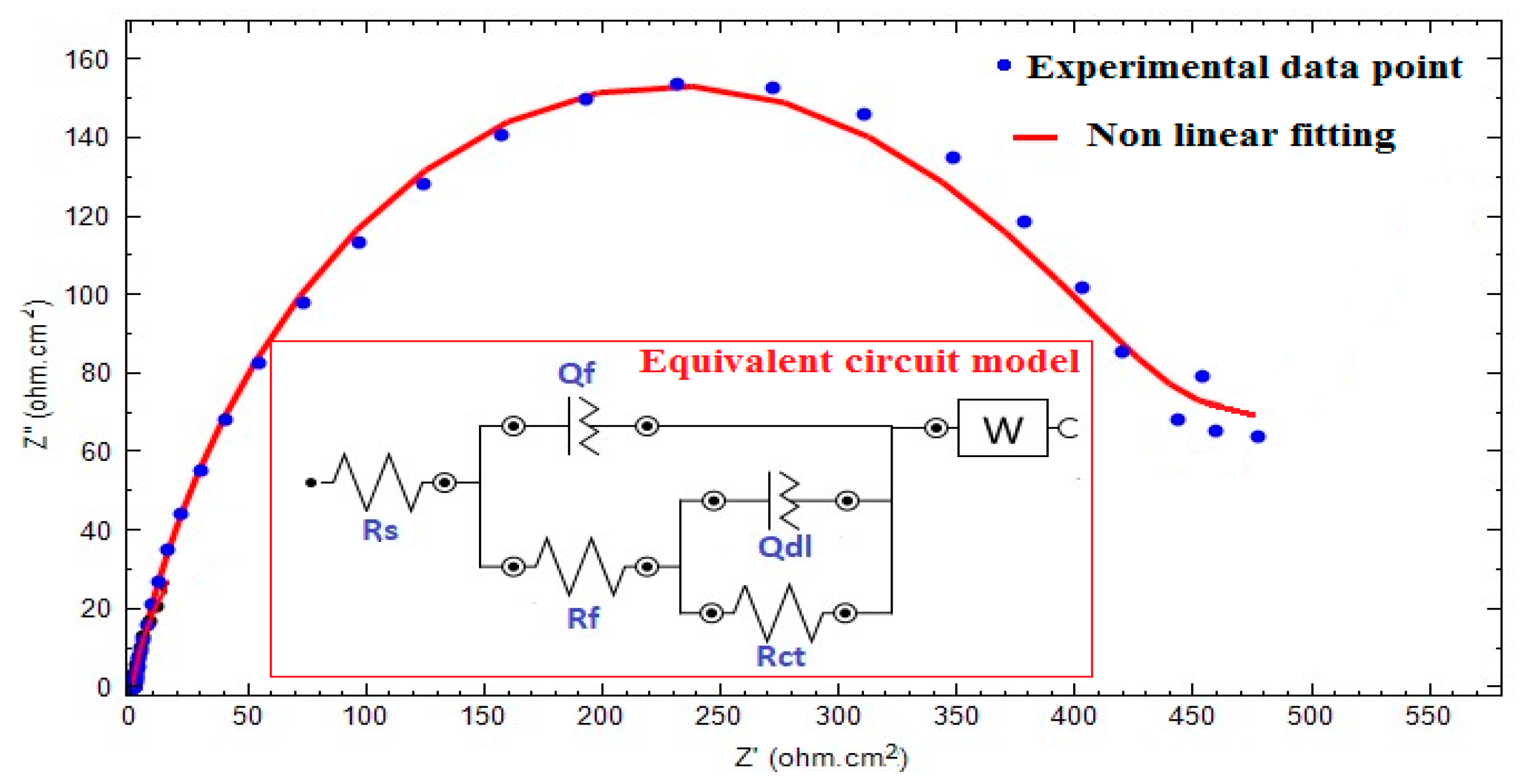
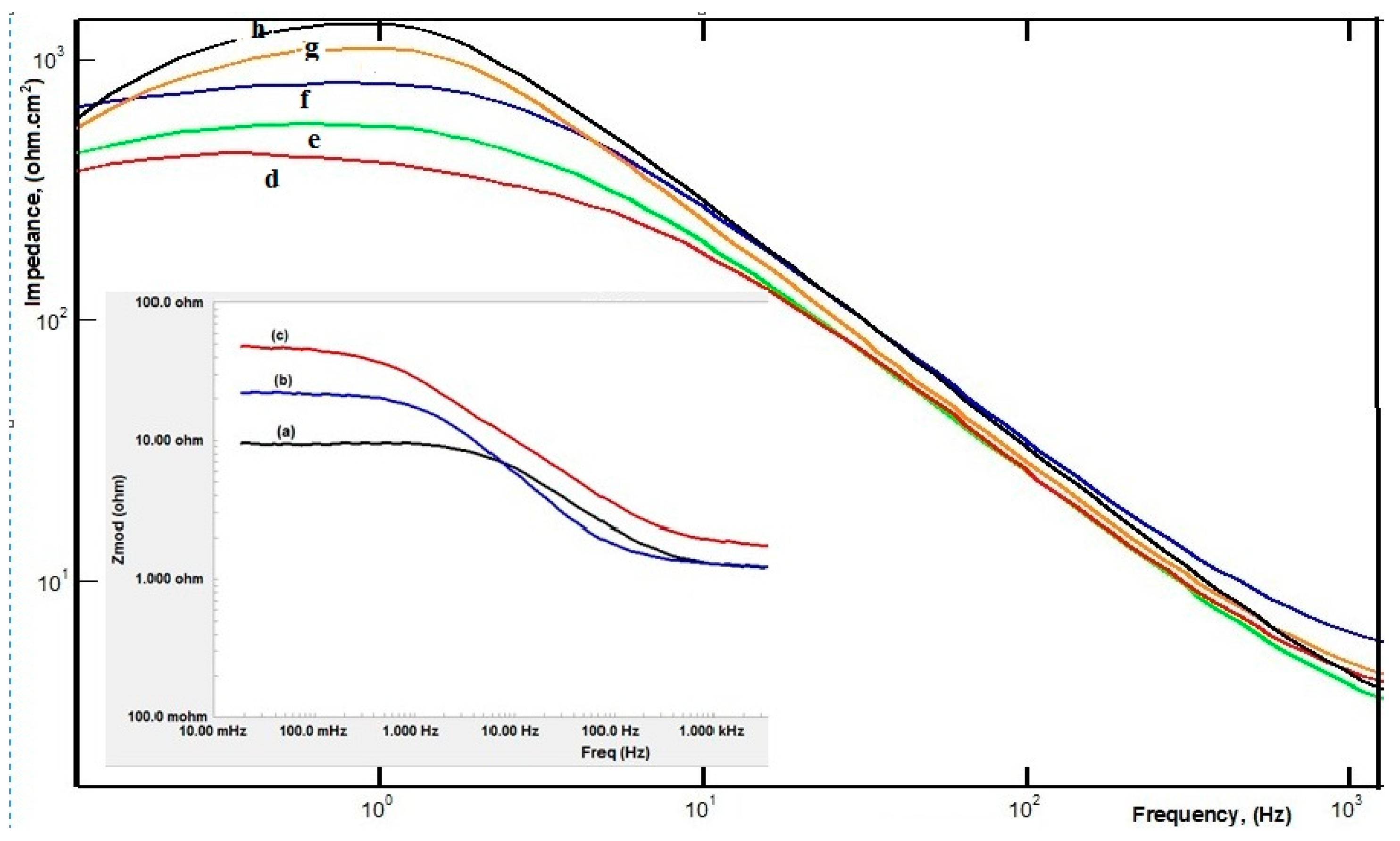
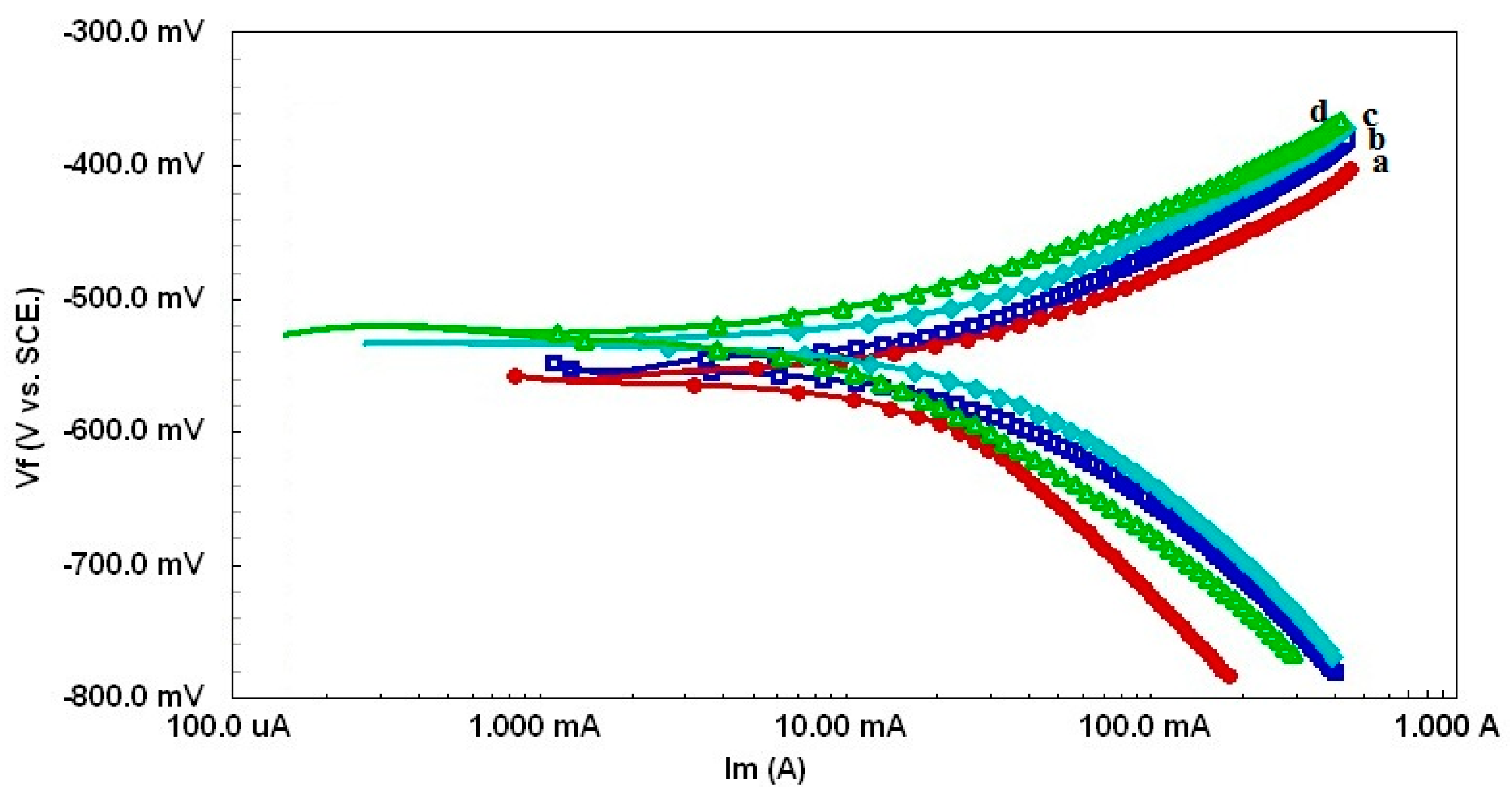


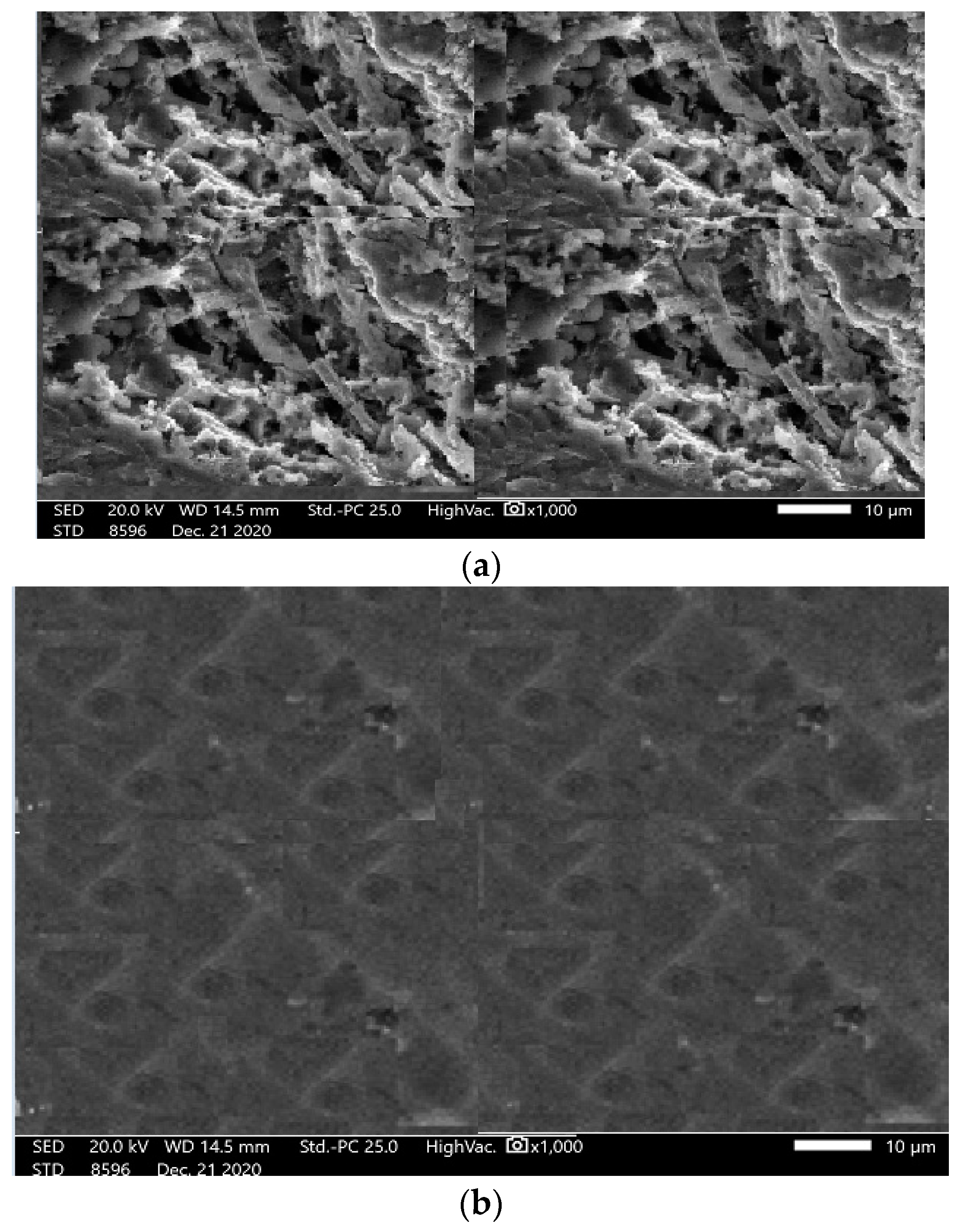
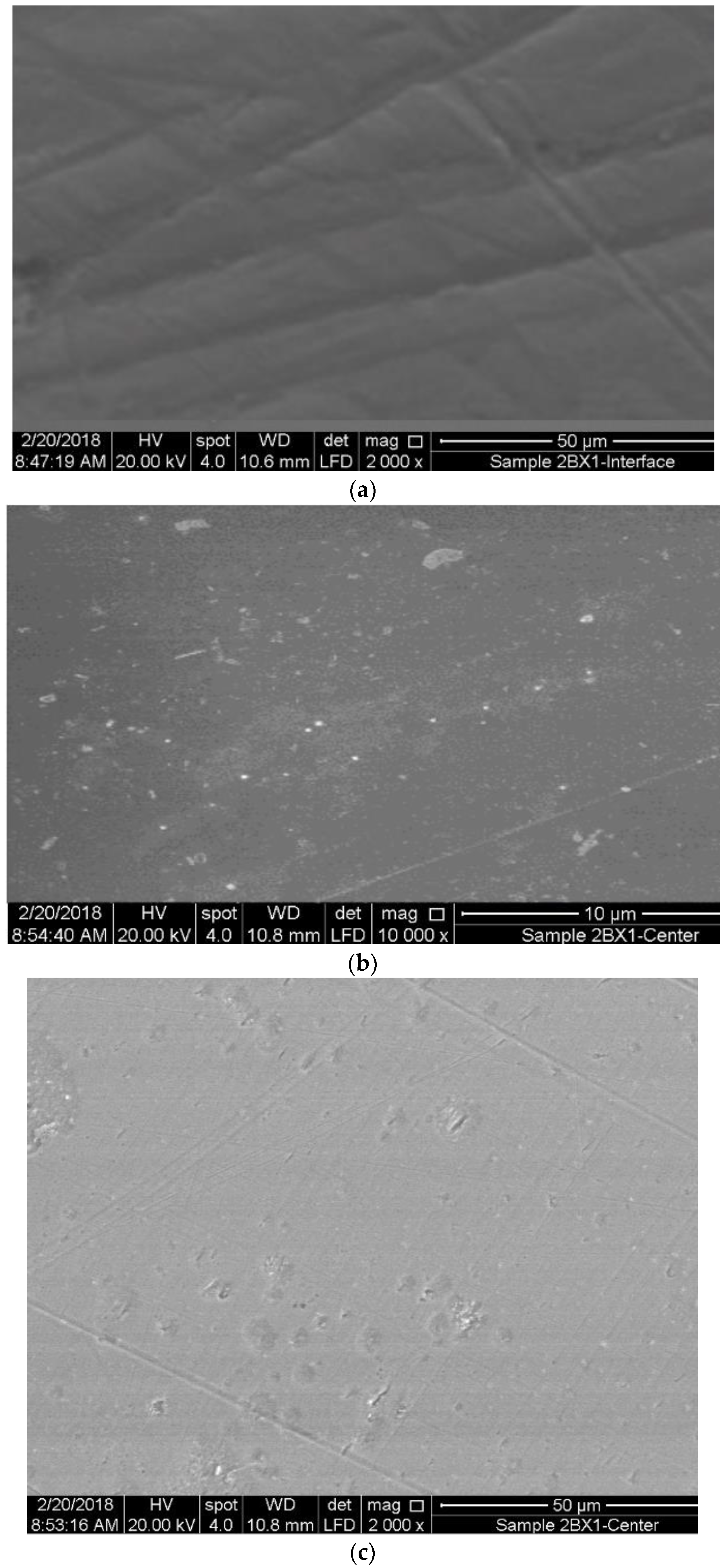

| Sample | RsOhm·cm−2 | nf | Rf | QdlµF·cm−2 | nEdl | Rct, Ohm·cm−2 | W, Ohm·cm−2 | %P |
|---|---|---|---|---|---|---|---|---|
| S0: bare metal | 2.1 | 0.9 | 5.3 | 1520 | 0.8 | 10 | 0.023 | - |
| S1: SW | 2.9 | 0.9 | 7.24 | 1430 | 0.7 | 13.4 | 0.020 | 25 |
| S2: 1.0 g PS | 4.3 | 0.9 | 7.94 | 1470 | 0.6 | 36.3 | 0.018 | 72 |
| S3: 2.0 g PS | 4.2 | 0.8 | 6.47 | 734 | 0.7 | 48.2 | 0.015 | 79 |
| S4: 1.0 g PS, 0.01 g SW | 3.1 | 0.8 | 8.54 | 625 | 0.6 | 250 | 0.013 | 96 |
| S5: 1.0 g PS, 0.02 g SW | 3.7 | 0.7 | 8.88 | 421 | 0.6 | 300 | 0.012 | 97 |
| S6: 2.0 g PS, 0.01 g SW | 3.6 | 0.8 | 8.84 | 330 | 0.7 | 520 | 0.010 | 98 |
| S7: 2.0 g PS, 0.02 g SW | 3.7 | 0.7 | 8.81 | 230 | 0.8 | 800 | 0.009 | 99 |
| wt.% (g) | −Ecorr (mV) | icorr (mA·cm−2) | βa | −βc | %P |
|---|---|---|---|---|---|
| mV·dec−1 | |||||
| S0: bare metal surface | 471 | 1.61 | 100 | 79 | - |
| S1: SW | 461 | 0.65 | 124 | 98 | 25 |
| S2: 1.0 g PS | 456 | 0.62 | 138 | 99 | 61 |
| S3: 2.0 g PS | 451 | 0.27 | 140 | 98 | 83 |
| S4: 1.0 g PS-0.01 g SW | 446 | 0.11 | 147 | 89 | 93 |
| S5: 1.0 g PS-0.02 g SW | 440 | 0.08 | 139 | 97 | 95 |
| S6: 2.0 g PS-0.01 g SW | 432 | 0.06 | 136 | 96 | 96 |
| S7: 2.0 g PS-0.02 g SW | 430 | 0.05 | 137 | 95 | 97 |
| Method | Sample No. | Parameter | |||
|---|---|---|---|---|---|
| 1 | 1 | 2 | 1 | wt.% PS | |
| ASTM | 0.02 | 0.01 | 0.02 | 0.01 | wt.% SW |
| ASTM D-938 | 67 | 63 | 65 | 63 | Congealing point, CP °C |
| ASTM D-36 | 101 | 78 | 85 | 79 | Softening point, SP °C |
| ASTM D-127 | 79 | 77 | 77 | 76 | Drop m.p., °C |
| ASTM-638 | 11 | 9 | 10 | 8 | Penetration at 25 °C, dmm |
| ASTM D-445 | 482 | 521 | 526 | 560 | Dynamic viscosity at 140 °C, cp |
| ASTM-638 | 846 | 813 | 839 | 809 | Tensile strength at break, 25 °C, psi |
| ASTM-D638 | 8.67 | 8.77 | 9.09 | 9.25 | %Elongation at break, 25 °C |
| - | 5B | 5B | 5B | 5B | Cross-cutter tester |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almufarij, R.S. Facile Formulation of New Innovative Eco-Friendly Hybrid Protective Coating for Mild Steel in Acidic Media. Sustainability 2023, 15, 2779. https://doi.org/10.3390/su15032779
Almufarij RS. Facile Formulation of New Innovative Eco-Friendly Hybrid Protective Coating for Mild Steel in Acidic Media. Sustainability. 2023; 15(3):2779. https://doi.org/10.3390/su15032779
Chicago/Turabian StyleAlmufarij, Rasmiah S. 2023. "Facile Formulation of New Innovative Eco-Friendly Hybrid Protective Coating for Mild Steel in Acidic Media" Sustainability 15, no. 3: 2779. https://doi.org/10.3390/su15032779
APA StyleAlmufarij, R. S. (2023). Facile Formulation of New Innovative Eco-Friendly Hybrid Protective Coating for Mild Steel in Acidic Media. Sustainability, 15(3), 2779. https://doi.org/10.3390/su15032779





