The Study of Cu(II) Adsorption onto Synthetically Modified Geopolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Preparation of Adsorbent Samples
2.2. Cu(II) Syntetic Sollutions
2.3. Adsorption Experiments
2.4. Atomic Absorption Spectrometry
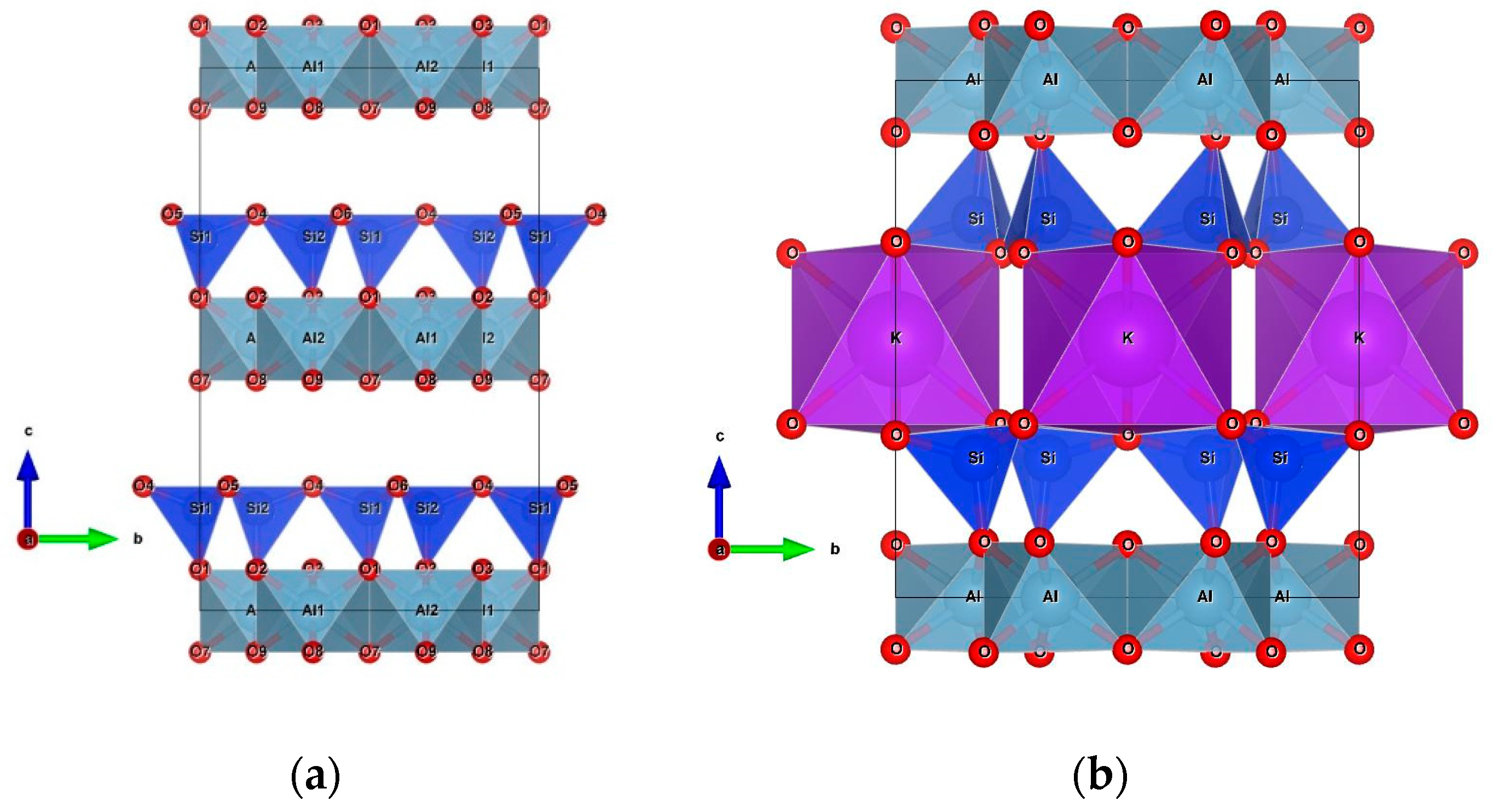
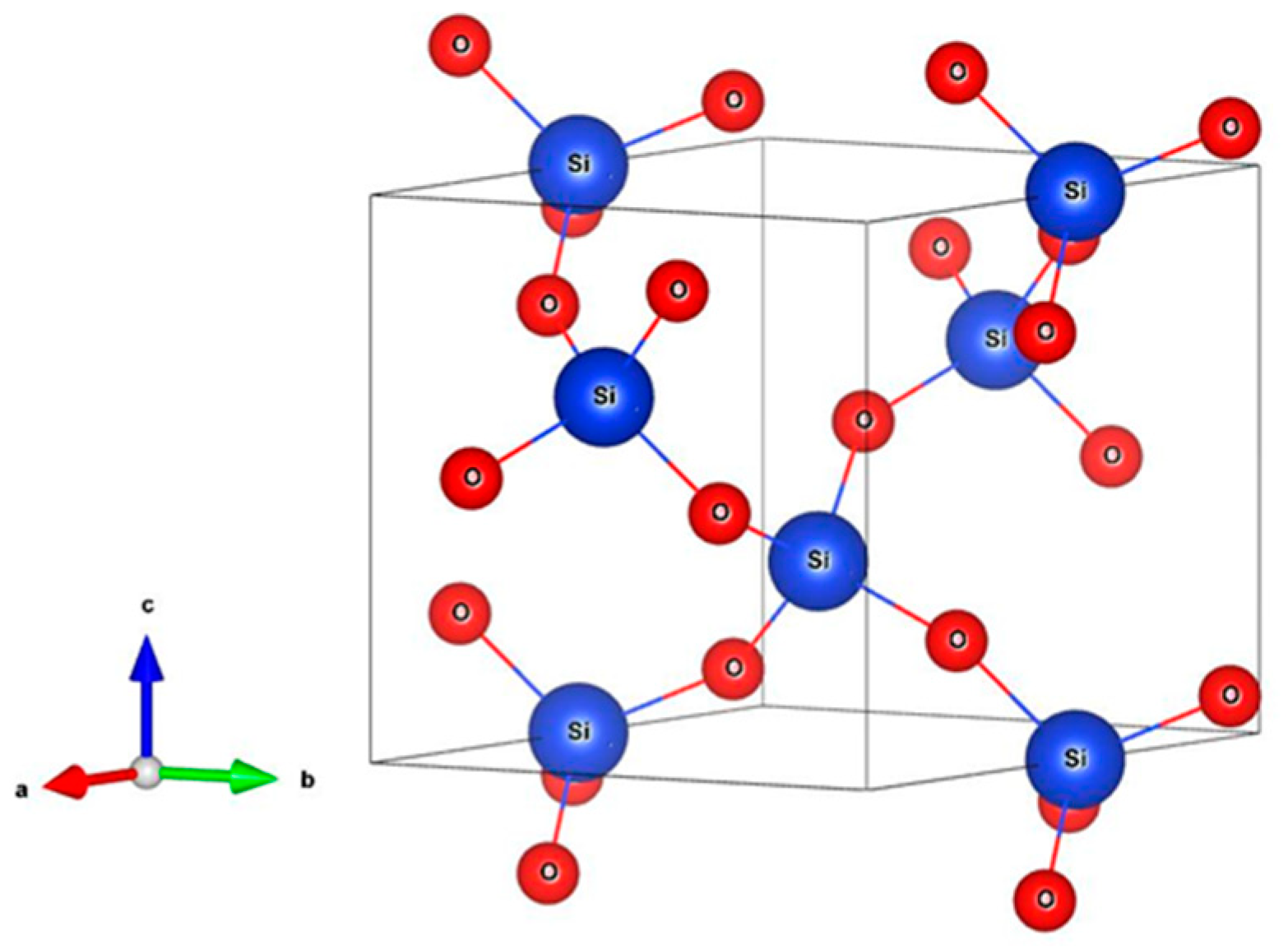
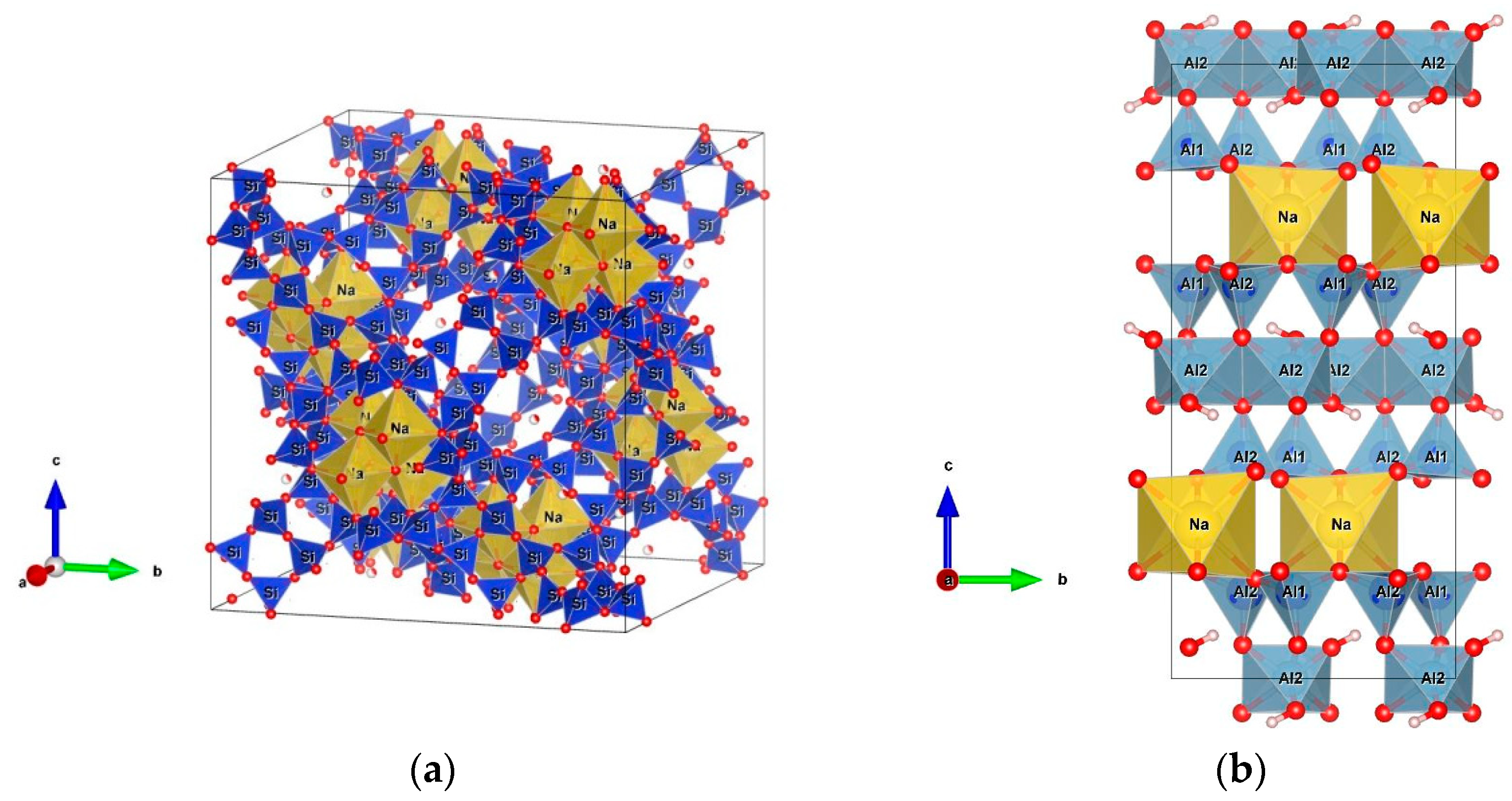
2.5. XRD Analysis
- PDF: 00-024-1047, Paragonite NaAl2(AlSi3)O10(OH)2
- PDF: 00-039-1380, Faujasite-NaNa2Al2Si4O12 · 8 H2O
- PDF: 01-078-1252, Quartz SiO2
- PDF: 01-089-0769, Zeolite X
- PDF: 00-043-0685, Illite KAl2(Si3Al)O10(OH)2
- PDF: 00-003-0052, Kaolinite Al2O3 · 2 SiO2 · 2 H2O
2.6. Diffuse Reflectance Infra Red Fourier Transform Spectroscopy (DRIFTS)
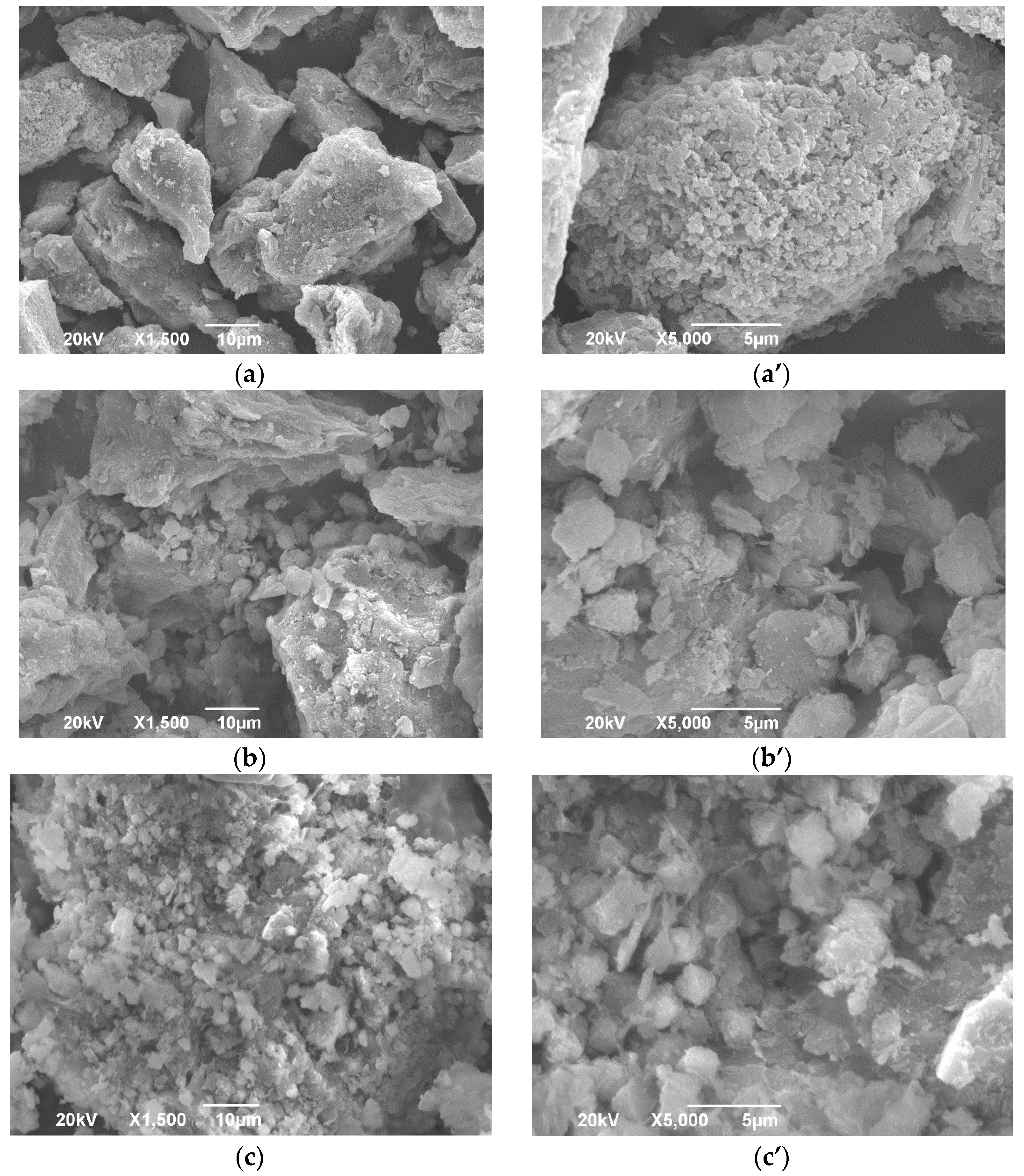
2.7. SEM Analysis
3. Results and Discussion
3.1. Characterization of the Adsorbents Samples
3.1.1. X-ray Diffraction
3.1.2. DRIFT Analysis
3.1.3. SEM Analysis
3.2. Adsorption Experiments
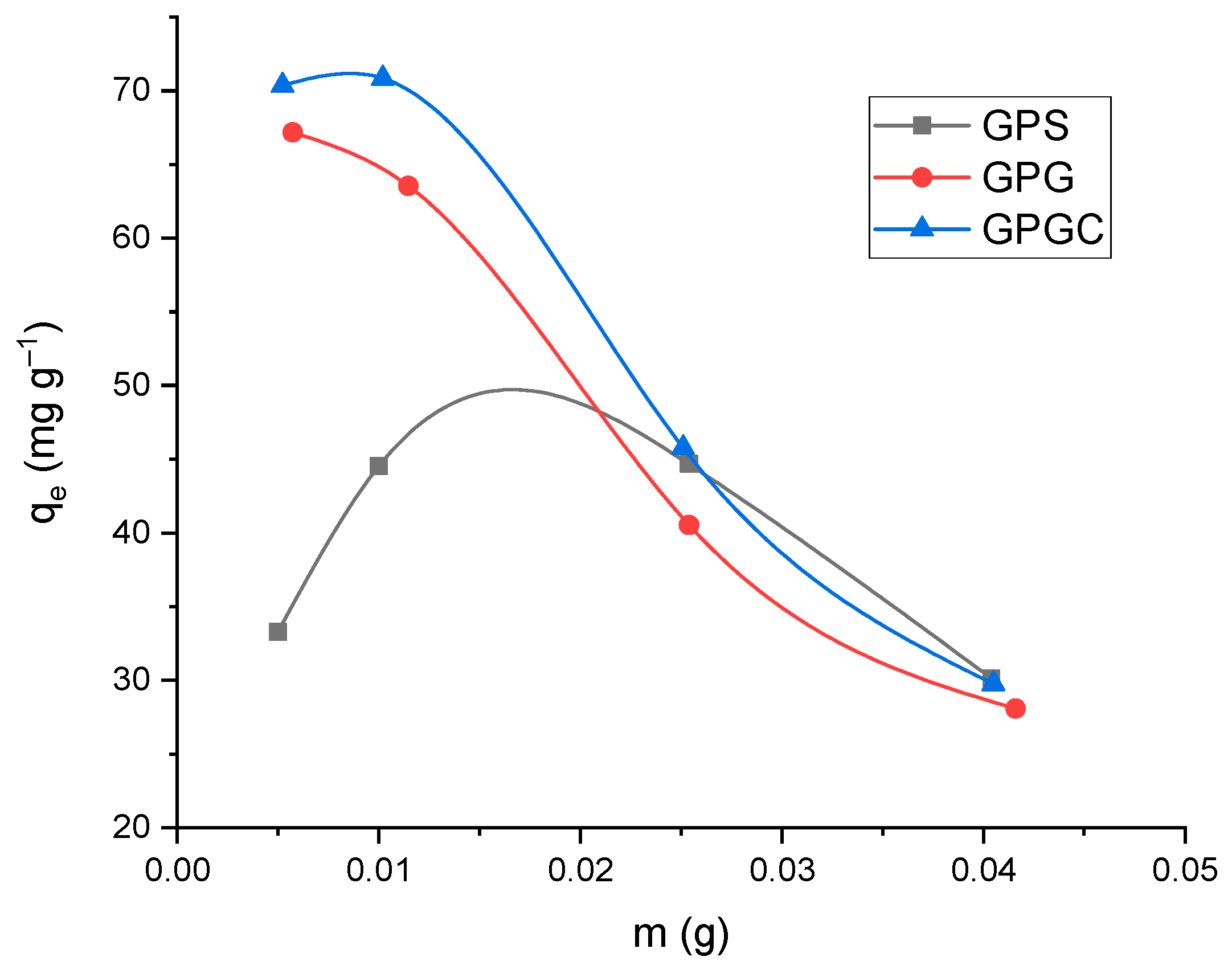
3.2.1. Effect of Adsorbent Dose
3.2.2. Effect of pH
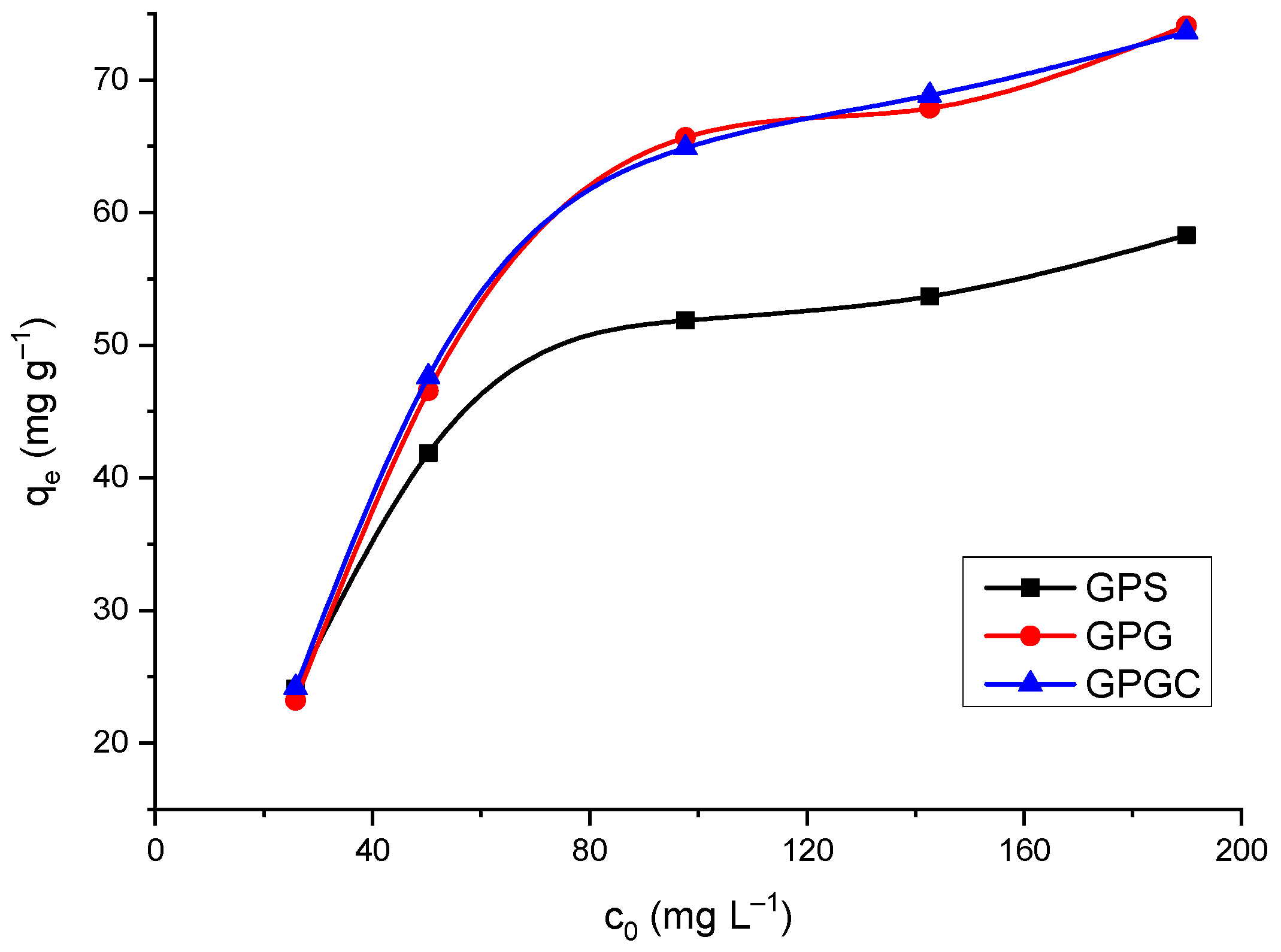
3.2.3. Effect of Initial Concentration Cu(II)
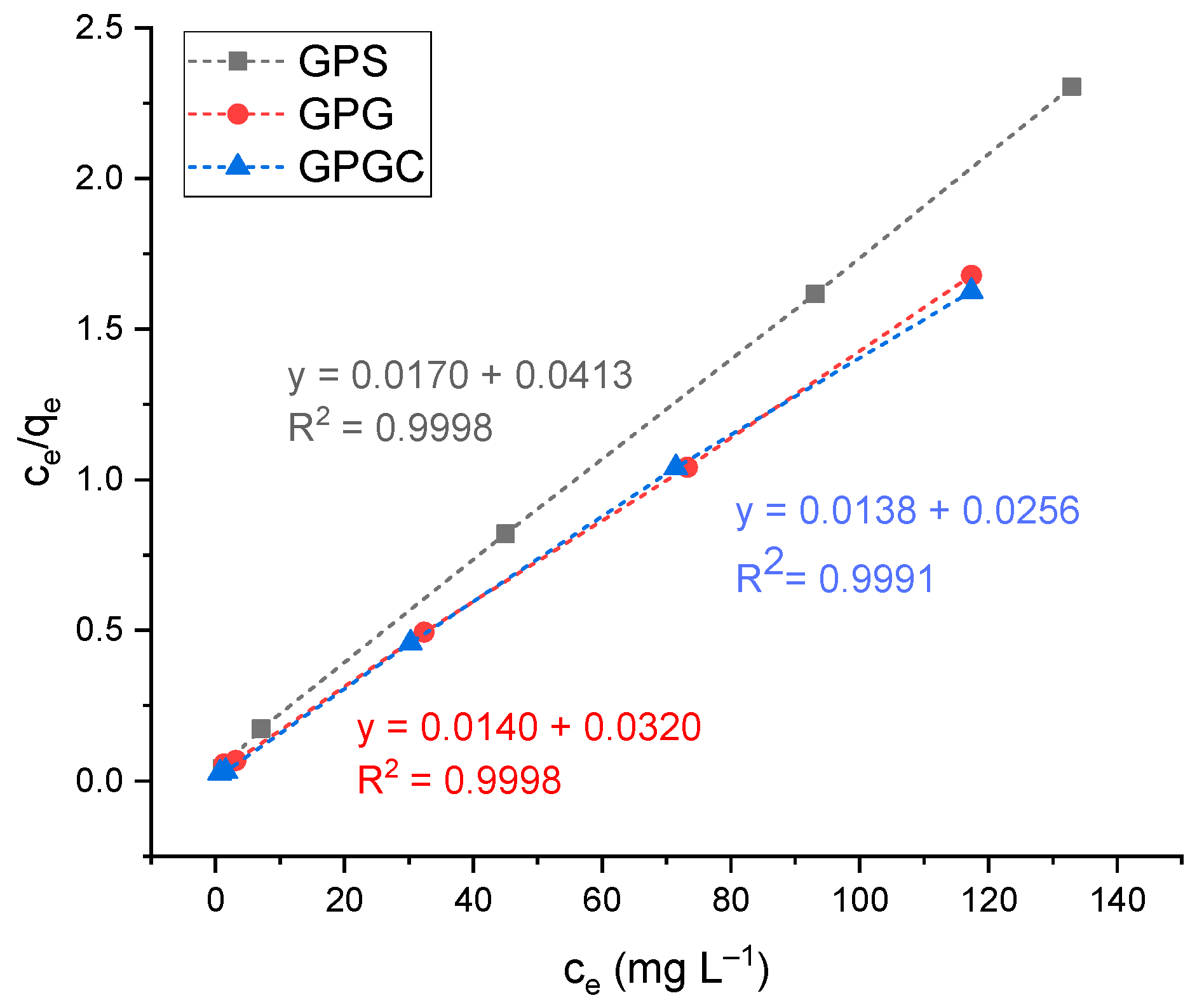
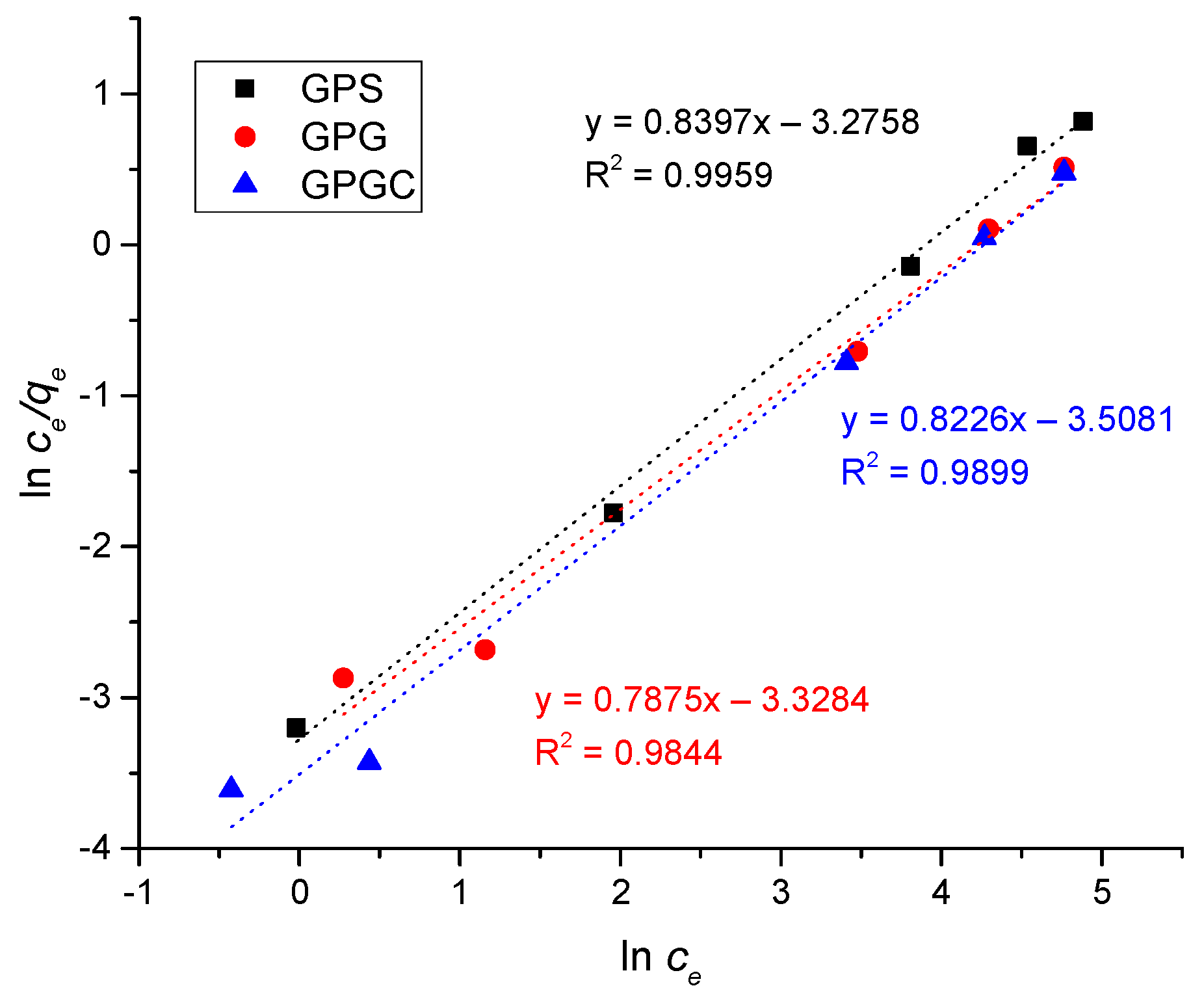
3.3. Adsorption Isotherms
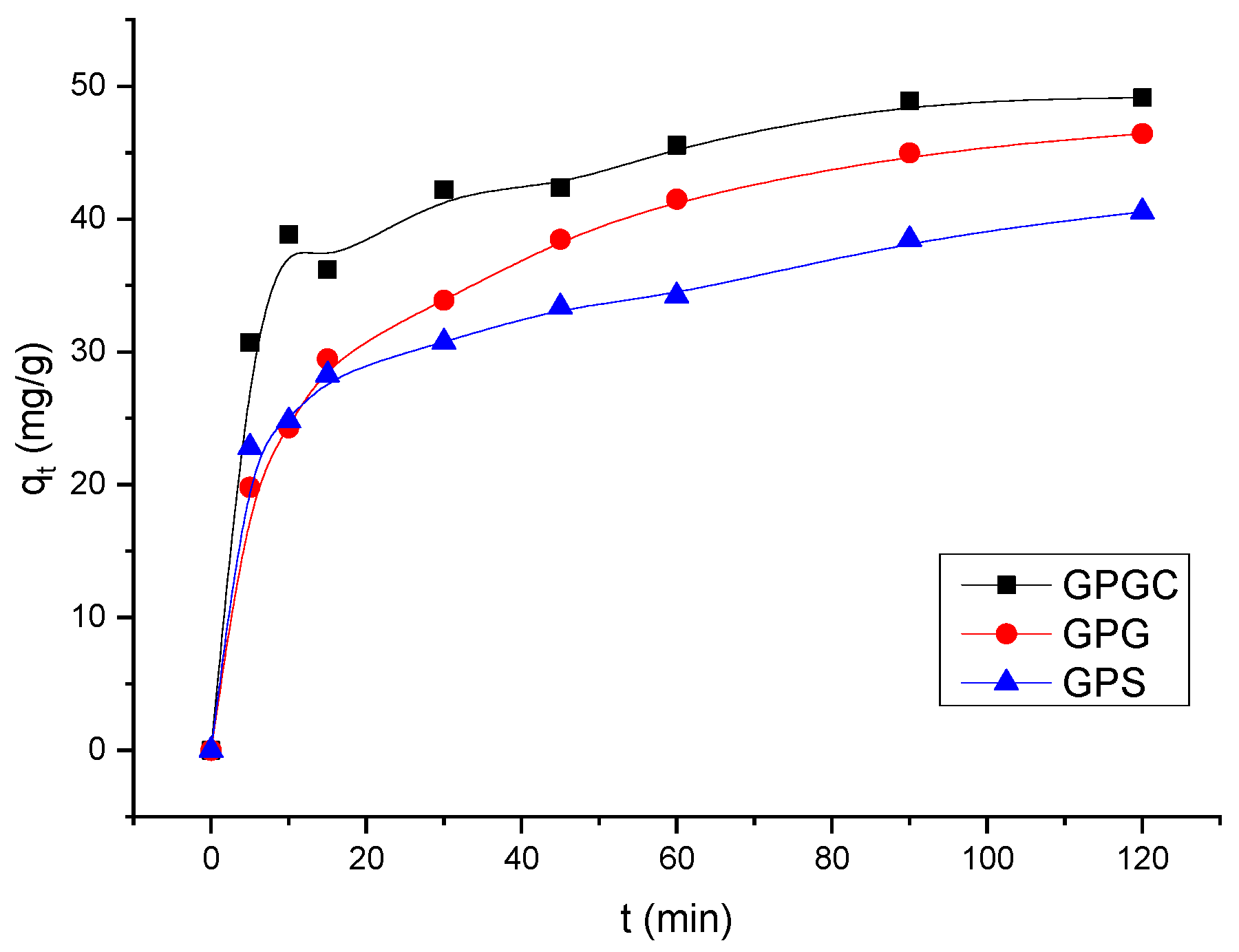
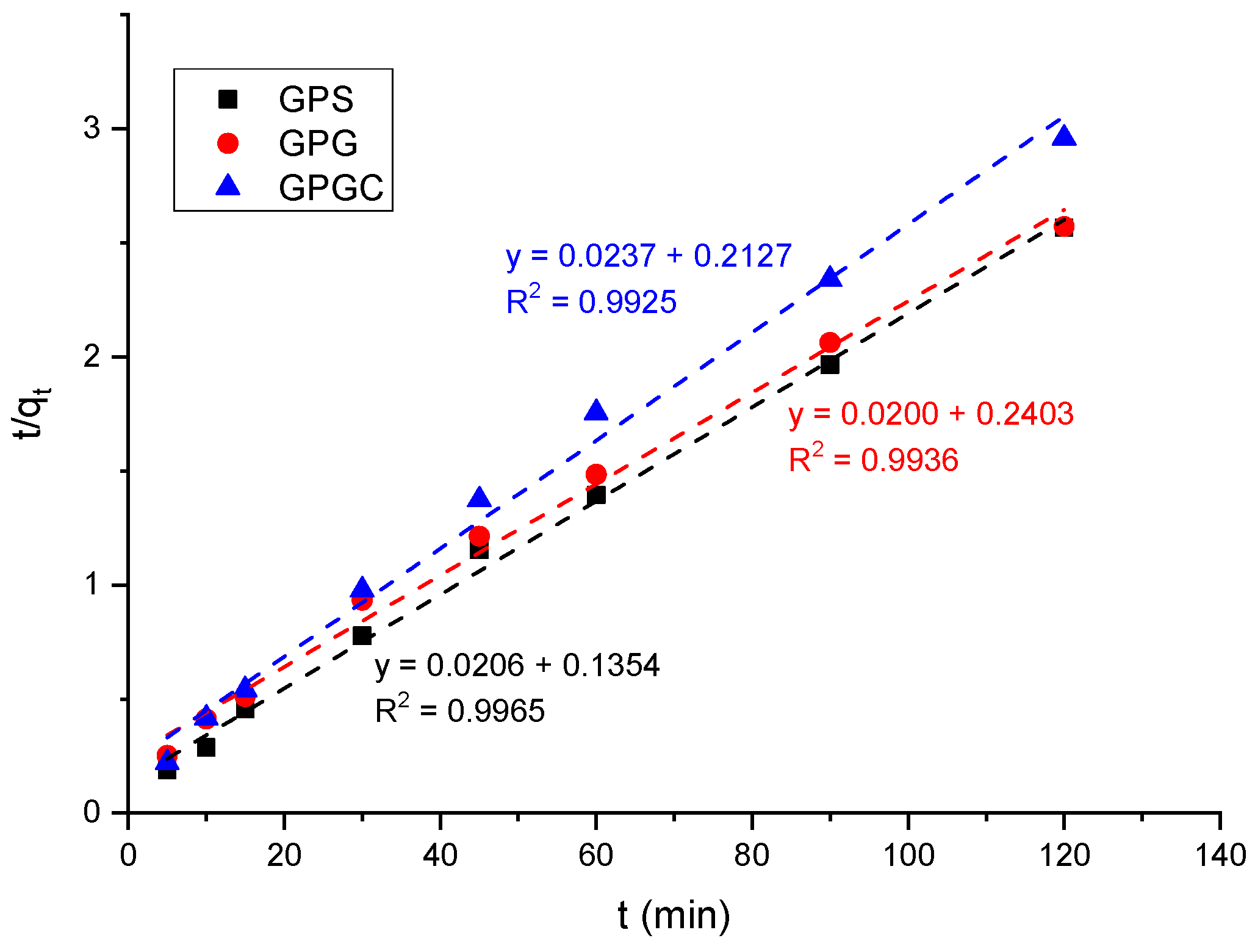
3.4. Kinetic Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tallentire, C.W.; Mackenzie, S.G.; Kyriazakis, I. Can Novel Ingredients Replace Soybeans and Reduce the Environmental Burdens of European Livestock Systems in the Future? J. Clean. Prod. 2018, 187, 338–347. [Google Scholar] [CrossRef]
- Gautam, R.K.; Sharma, S.K.; Mahiya, S.; Chattopadhyaya, M.C. CHAPTER 1. Contamination of Heavy Metals in Aquatic Media: Transport, Toxicity and Technologies for Remediation. In Heavy Metals in Water; Sharma, S., Ed.; Royal Society of Chemistry: Cambridge, UK, 2014; pp. 1–24. ISBN 978-1-84973-885-9. [Google Scholar]
- Siegel, F.R. Heavy Metals Mobility/Immobility in Environmental Media. In Environmental Geochemistry of Potentially Toxic Metals; Springer: Berlin/Heidelberg, Germany, 2002; pp. 45–59. ISBN 978-3-642-07554-4. [Google Scholar]
- Stern, B.R. Essentiality and Toxicity in Copper Health Risk Assessment: Overview, Update and Regulatory Considerations. J. Toxicol. Environ. Health A 2010, 73, 114–127. [Google Scholar] [CrossRef]
- Ashish, B.; Neeti, K.; Himanshu, K. Copper Toxicity: A Comprehensive Study. Res. J. Recent Sci. 2013, 2, 58–67. [Google Scholar]
- García-Rico, L.; Leyva-Perez, J.; Jara-Marini, M.E. Content and Daily Intake of Copper, Zinc, Lead, Cadmium, and Mercury from Dietary Supplements in Mexico. Food Chem. Toxicol. 2007, 45, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- de Romaña, D.L.; Olivares, M.; Uauy, R.; Araya, M. Risks and Benefits of Copper in Light of New Insights of Copper Homeostasis. J. Trace Elem. Med. Biol. 2011, 25, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hao, S.; Han, A.; Yang, Y.; Fang, G.; Liu, J.; Wang, S. Intracellular Fenton Reaction Based on Mitochondria-Targeted Copper(II)–Peptide Complex for Induced Apoptosis. J. Mater. Chem. B 2019, 7, 4008–4016. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Ma, Z.; Sun, M.; Li, M.; Zhang, Z.; Zhang, L.; Tang, Z.; Yao, Y.; Huang, B.; et al. Atomically Dispersed Cu Catalyst for Efficient Chemoselective Hydrogenation Reaction. Nano Lett. 2021, 21, 10284–10291. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.S.; Ananikov, V.P. Which Metals Are Green for Catalysis? Comparison of the Toxicities of Ni, Cu, Fe, Pd, Pt, Rh, and Au Salts. Angew. Chem. Int. Ed. 2016, 55, 12150–12162. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Q.; Soklun, H.; Qu, G.; Xia, T.; Guo, X.; Jia, H.; Zhu, L. A Green Strategy for Simultaneous Cu(II)-EDTA Decomplexation and Cu Precipitation from Water by Bicarbonate-Activated Hydrogen Peroxide/Chemical Precipitation. Chem. Eng. J. 2019, 370, 1298–1309. [Google Scholar] [CrossRef]
- Thomas, M.; Zdebik, D.; Białecka, B. Using Sodium Trithiocarbonate to Precipitate Heavy Metals from Industrial Wastewater—From the Laboratory to Industrial Scale. Pol. J. Environ. Stud. 2018, 27, 1753–1763. [Google Scholar] [CrossRef]
- Divrikli, U.; Kartal, A.; Soylak, M.; Elci, L. Preconcentration of Pb(II), Cr(III), Cu(II), Ni(II) and Cd(II) Ions in Environmental Samples by Membrane Filtration Prior to Their Flame Atomic Absorption Spectrometric Determinations. J. Hazard. Mater. 2007, 145, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Keane, M.A. The Removal of Copper and Nickel from Aqueous Solution Using Y Zeolite Ion Exchangers. Colloids Surf. Physicochem. Eng. Asp. 1998, 138, 11–20. [Google Scholar] [CrossRef]
- Dhiman, S.; Gupta, B. Partition Studies on Cobalt and Recycling of Valuable Metals from Waste Li-Ion Batteries via Solvent Extraction and Chemical Precipitation. J. Clean. Prod. 2019, 225, 820–832. [Google Scholar] [CrossRef]
- Xu, S.; Ng, J.; Du, A.J.; Liu, J.; Sun, D.D. Highly Efficient TiO2 Nanotube Photocatalyst for Simultaneous Hydrogen Production and Copper Removal from Water. Int. J. Hydrog. Energy 2011, 36, 6538–6545. [Google Scholar] [CrossRef]
- da Silva, J.R.P.; Merçon, F.; Guimarães Costa, C.M.; Radoman Benjo, D. Application of Reverse Osmosis Process Associated with EDTA Complexation for Nickel and Copper Removal from Wastewater. Desalin. Water Treat. 2016, 57, 19466–19474. [Google Scholar] [CrossRef]
- Tran, T.-K.; Chiu, K.-F.; Lin, C.-Y.; Leu, H.-J. Electrochemical Treatment of Wastewater: Selectivity of the Heavy Metals Removal Process. Int. J. Hydrog. Energy 2017, 42, 27741–27748. [Google Scholar] [CrossRef]
- Vo, K.A.; Xu, X.J.; Li, T.G.; Peng, R.H.; Liu, S.L.; Yue, X.L. Research on a New Electrochemical Method Combined with Chemical Coagulation in Removal of Lead, Zinc, and Copper from Wastewater. Desalin. Water Treat. 2016, 57, 15343–15352. [Google Scholar] [CrossRef]
- Veli, S.; Alyüz, B. Adsorption of Copper and Zinc from Aqueous Solutions by Using Natural Clay. J. Hazard. Mater. 2007, 149, 226–233. [Google Scholar] [CrossRef]
- Kucharski, P.; Białecka, B.; Thomas, M. Removal of Cadmium Ions from Polluted Waters Using Low-Cost Adsorbents: Process Optimization Study. Desalin. Water Treat. 2022, 256, 114–124. [Google Scholar] [CrossRef]
- Muya, F.N.; Sunday, C.E.; Baker, P.; Iwuoha, E. Environmental Remediation of Heavy Metal Ions from Aqueous Solution through Hydrogel Adsorption: A Critical Review. Water Sci. Technol. 2016, 73, 983–992. [Google Scholar] [CrossRef]
- Duricova, A.; Samesova, D. Distribution of the toxic metals in system water–sludge in the biological water treatment plant. Ecology and Environmental Protection. In Proceedings of the 14th International Multidisciplinary Scientific GeoConference SGEM, Albena, Bulgaria, 17–26 June 2014. [Google Scholar] [CrossRef]
- Yahya, Z.; Abdullah, M.M.A.B.; Hussin, K.; Ismail, K.N.; Razak, R.A.; Sandu, A.V. Effect of Solids-To-Liquids, Na2SiO3-To-NaOH and Curing Temperature on the Palm Oil Boiler Ash (Si + Ca) Geopolymerisation System. Materials 2015, 8, 2227–2242. [Google Scholar] [CrossRef]
- Gulicovski, J.; Nenadović, S.; Kljajević, L.; Mirković, M.; Nišavić, M.; Kragović, M.; Stojmenović, M. Geopolymer/CeO2 as Solid Electrolyte for IT-SOFC. Polymers 2020, 12, 248. [Google Scholar] [CrossRef] [PubMed]
- Ivanović, M.; Nenadović, S.; Pavlović, V.P.; Radović, I.; Kijevčanin, M.; Pavlović, V.B.; Kljajević, L. The Influence of Thermodynamic Parameters on Alkaline Activator of Geopolymers and Structure of Geopolymers. Maced. J. Chem. Chem. Eng. 2021, 40, 99. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers and Geopolymeric Materials. J. Therm. Anal. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Nenadović, S.; Gulicovski, J.; Mirković, M.; Kljajević, L.; Bošković, I.; Vukčević, M.; Nenadović, M. Structural, Mechanical and Chemical Properties of Low Content Carbon Geopolymer. Sustainability 2022, 14, 4885. [Google Scholar] [CrossRef]
- Yong, H.; Kamarudin, H.; Abdullah, M.M.A.B.; Musa, L.; Nizar, K.; Ming, L. Potential Application of Kaolin Without Calcine as Greener Concrete: A Review. Aust. J. Basic Appl. Sci. 2011, 5, 1026–1035. [Google Scholar]
- Al-Harahsheh, M.S.; Al Zboon, K.; Al-Makhadmeh, L.; Hararah, M.; Mahasneh, M. Fly Ash Based Geopolymer for Heavy Metal Removal: A Case Study on Copper Removal. J. Environ. Chem. Eng. 2015, 3, 1669–1677. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Zhu, Z.H. Solid-State Conversion of Fly Ash to Effective Adsorbents for Cu Removal from Wastewater. J. Hazard. Mater. 2007, 139, 254–259. [Google Scholar] [CrossRef]
- Jianguo, Z.; Provis, J.L.; Dingwu, F.; Deventer, J.S.J. van Geopolymers for Immobilization of Cr{sup 6+}, Cd{sup 2+}, and Pb{sup 2+}. J. Hazard. Mater. 2008, 157, 587–598. [Google Scholar] [CrossRef]
- Yousef, R.I.; El-Eswed, B.; Alshaaer, M.; Khalili, F.; Khoury, H. The Influence of Using Jordanian Natural Zeolite on the Adsorption, Physical, and Mechanical Properties of Geopolymers Products. J. Hazard. Mater. 2009, 165, 379–387. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Khan, M.I.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Siame, J.; Azizli, K.A. A Review on Geopolymers as Emerging Materials for the Adsorption of Heavy Metals and Dyes. J. Environ. Manag. 2018, 224, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Andrejkovičová, S.; Sudagar, A.; Rocha, J.; Patinha, C.; Hajjaji, W.; da Silva, E.F.; Velosa, A.; Rocha, F. The Effect of Natural Zeolite on Microstructure, Mechanical and Heavy Metals Adsorption Properties of Metakaolin Based Geopolymers. Appl. Clay Sci. 2016, 126, 141–152. [Google Scholar] [CrossRef]
- Su, Q.; Ye, Q.; Deng, L.; He, Y.; Cui, X. Prepared Self-Growth Supported Copper Catalyst by Recovering Cu (II) from Wastewater Using Geopolymer Microspheres. J. Clean. Prod. 2020, 272, 122571. [Google Scholar] [CrossRef]
- Ge, Y.; Cui, X.; Kong, Y.; Li, Z.; He, Y.; Zhou, Q. Porous Geopolymeric Spheres for Removal of Cu(II) from Aqueous Solution: Synthesis and Evaluation. J. Hazard. Mater. 2015, 283, 244–251. [Google Scholar] [CrossRef]
- Cheng, T.W.; Lee, M.L.; Ko, M.S.; Ueng, T.H.; Yang, S.F. The Heavy Metal Adsorption Characteristics on Metakaolin-Based Geopolymer. Appl. Clay Sci. 2012, 56, 90–96. [Google Scholar] [CrossRef]
- Mama, C.N.; Nwonu, D.C.; Akanno, C.C.; Chukwuemeka, O.E. Adsorption Capacity of Composite Bio-Modified Geopolymer for Multi-Component Heavy Metal System: Optimisation, Equilibrium and Kinetics Study. Environ. Monit. Assess. 2022, 194, 134. [Google Scholar] [CrossRef] [PubMed]
- Babić, B.M.; Milonjić, S.K.; Polovina, M.J.; Čupić, S.; Kaludjerović, B.V. Adsorption of Zinc, Cadmium and Mercury Ions from Aqueous Solutions on an Activated Carbon Cloth. Carbon 2002, 40, 1109–1115. [Google Scholar] [CrossRef]
- Haberhauer, G.; Gerzabek, M.H. Drift and Transmission FT-IR Spectroscopy of Forest Soils: An Approach to Determine Decomposition Processes of Forest Litter. Vib. Spectrosc. 1999, 19, 413–417. [Google Scholar] [CrossRef]
- Kljajević, L.; Melichová, Z.; Kisić, D.; Nenadovic, M.; Todorović, B.; Pavlovic, V.; Nenadović, S. The Influence of Alumino-Silicate Matrix Composition on Surface Hydrophobic Properties. Sci. Sinter. 2019, 51, 163–173. [Google Scholar] [CrossRef]
- Yan, K.; Guo, Y.; Fang, L.; Cui, L.; Cheng, F.; Li, T. Decomposition and Phase Transformation Mechanism of Kaolinite Calcined with Sodium Carbonate. Appl. Clay Sci. 2017, 147, 90–96. [Google Scholar] [CrossRef]
- Clay and Clay Minerals; Do Nascimento, G.M. (Ed.) IntechOpen: Rijeka, Croatia, 2021; ISBN 978-1-83969-563-6. [Google Scholar]
- Wan, Q.; Zhang, Y.; Zhang, R. Using Mechanical Activation of Quartz to Enhance the Compressive Strength of Metakaolin Based Geopolymers. Cem. Concr. Compos. 2020, 111, 103635. [Google Scholar] [CrossRef]
- Omerašević, M.; Lukić, M.; Savić-Biserčić, M.; Savić, A.; Matović, L.; Baščarević, Z.; Bučevac, D. Permanent Disposal of Cs Ions in the Form of Dense Pollucite Ceramics Having Low Thermal Expansion Coefficient. Nucl. Eng. Technol. 2020, 52, 115–122. [Google Scholar] [CrossRef]
- Alderton, D. Micas. In Encyclopedia of Geology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 326–333. ISBN 978-0-08-102909-1. [Google Scholar]
- Ríos, C.A.; Williams, C.D.; Fullen, M.A. Nucleation and Growth History of Zeolite LTA Synthesized from Kaolinite by Two Different Methods. Appl. Clay Sci. 2009, 42, 446–454. [Google Scholar] [CrossRef]
- Frost, R.L.; Johansson, U. Combination Bands in the Infrared Spectroscopy of Kaolins—A Drift Spectroscopic Study. Clays Clay Miner. 1998, 46, 466–477. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. The Geopolymerisation of Alumino-Silicate Minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Nenadović, S.; Ferone, C.; Nenadovic, M.; Cioffi, R.; Mirkovic, M.; Vukanac, I.; Kljajevic, L. Chemical, Physical and Radiological Evaluation of Raw Materials and Geopolymers for Building Applications. J. Radioanal. Nucl. Chem. 2020, 325, 435–445. [Google Scholar] [CrossRef]
- Maragkos, I.; Giannopoulou, I.P.; Panias, D. Synthesis of Ferronickel Slag-Based Geopolymers. Miner. Eng. 2009, 22, 196–203. [Google Scholar] [CrossRef]
- Ivanović, M.; Kljajević, L.; Gulicovski, J.; Petković, M.; Jankovic-Castvan, I.; Bucevac, D.; Nenadović, S. The Effect of the Concentration of Alkaline Activator and Aging Time on the Structure of Metakaolin Based Geopolymer. Sci. Sinter. 2020, 52, 219–229. [Google Scholar] [CrossRef]
- Salami, B.; Megat Johari, M.A.; Ahmad, Z.; Maslehuddin, M.; Adewumi, A. Impact of Al(OH)3 Addition to POFA on the Compressive Strength of POFA- Alkali-Activated Mortar. Constr. Build. Mater. 2018, 190, 65–82. [Google Scholar] [CrossRef]
- Kljajević, L.M.; Nenadović, S.S.; Nenadović, M.T.; Bundaleski, N.K.; Todorović, B.Ž.; Pavlović, V.B.; Rakočević, Z.L. Structural and Chemical Properties of Thermally Treated Geopolymer Samples. Ceram. Int. 2017, 43, 6700–6708. [Google Scholar] [CrossRef]
- Alahverdi, A.; Mehrpour, K.; Najafikani, E. Taftan Pozzolan-Based Geopolymer Cement. Int. J. Eng. Sci. Engl. 2008, 19, 1–5. [Google Scholar]
- Zaharaki, D.; Komnitsas, K.; Perdikatsis, V. Use of Analytical Techniques for Identification of Inorganic Polymer Gel Composition. J. Mater. Sci. 2010, 45, 2715–2724. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Wang, Y.; Yan, F. Preparation of Macroporous Ceramic from Metakaolinite-Based Geopolymer by Calcination. Ceram. Int. 2015, 41, 11177–11183. [Google Scholar] [CrossRef]
- Kosor, T.; Nakić-Alfirević, B.; Svilović, S. Geopolymer Depolymerization Index. Vib. Spectrosc. 2016, 86, 143–148. [Google Scholar] [CrossRef]
- Kljajević, L.M.; Melichova, Z.; Stojmenović, M.D.; Todorović, B.Z.; Pavlović, V.B.; Čitaković, N.M.; Nenadović, S.S. Structural and Electrical Properties of Geopolymer Materials Based on Different Precursors (Kaolin, Bentonite and Diatomite). Maced. J. Chem. Chem. Eng. 2019, 38, 283–292. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Xiang, Y.; Wang, P.; Zhang, J.; Zhang, F.; Wei, J.; Luo, L.; Lei, M.; Tang, L. Modification of Biochar Derived from Sawdust and Its Application in Removal of Tetracycline and Copper from Aqueous Solution: Adsorption Mechanism and Modelling. Bioresour. Technol. 2017, 245, 266–273. [Google Scholar] [CrossRef]
- Kim, D.-H.; Shin, M.-C.; Choi, H.-D.; Seo, C.-I.; Baek, K. Removal Mechanisms of Copper Using Steel-Making Slag: Adsorption and Precipitation. Desalination 2008, 223, 283–289. [Google Scholar] [CrossRef]
- Fornasiero, D.; Ralston, J. Effect of Surface Oxide/Hydroxide Products on the Collectorless Flotation of Copper-Activated Sphalerite. Int. J. Miner. Process. 2006, 78, 231–237. [Google Scholar] [CrossRef]
- Worrall, G.M.; Buswell, J.T.; English, C.A.; Hetherington, M.G.; Smith, G.D.W. A Study of the Precipitation of Copper Particles in a Ferrite Matrix. J. Nucl. Mater. 1987, 148, 107–114. [Google Scholar] [CrossRef]
- Mužek, M.N.; Svilović, S.; Ugrina, M.; Zelić, J. Removal of Copper and Cobalt Ions by Fly Ash-Based Geopolymer from Solutions-Equilibrium Study. Desalination Water Treat. 2016, 57, 10689–10699. [Google Scholar] [CrossRef]
- Yan, C.; Guo, L.; Ren, D.; Duan, P. Novel Composites Based on Geopolymer for Removal of Pb(II). Mater. Lett. 2019, 239, 192–195. [Google Scholar] [CrossRef]
- Luukkonen, T.; Sarkkinen, M.; Kemppainen, K.; Rämö, J.; Lassi, U. Metakaolin Geopolymer Characterization and Application for Ammonium Removal from Model Solutions and Landfill Leachate. Appl. Clay Sci. 2016, 119, 266–276. [Google Scholar] [CrossRef]
- Luukkonen, T.; Věžníková, K.; Tolonen, E.-T.; Runtti, H.; Yliniemi, J.; Hu, T.; Kemppainen, K.; Lassi, U. Removal of Ammonium from Municipal Wastewater with Powdered and Granulated Metakaolin Geopolymer. Environ. Technol. 2018, 39, 414–423. [Google Scholar] [CrossRef]
- Ge, Y.; Cui, X.; Liao, C.; Li, Z. Facile Fabrication of Green Geopolymer/Alginate Hybrid Spheres for Efficient Removal of Cu(II) in Water: Batch and Column Studies. Chem. Eng. J. 2017, 311, 126–134. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W.; Ren, D. Development of Fly Ash and Iron Ore Tailing Based Porous Geopolymer for Removal of Cu(II) from Wastewater. Ceram. Int. 2016, 42, 13507–13518. [Google Scholar] [CrossRef]
- Tunali Akar, S.; Çolo, H.; Sayin, F.; Kara, I.; Akar, T. Parametric Optimization of Cu(II) Removal Process by a Metakaolin-Based Geopolymer: Batch and Continuous Process Design. J. Clean. Prod. 2022, 366, 132819. [Google Scholar] [CrossRef]
- Singhal, A.; Gangwar, B.P.; Gayathry, J.M. CTAB Modified Large Surface Area Nanoporous Geopolymer with High Adsorption Capacity for Copper Ion Removal. Appl. Clay Sci. 2017, 150, 106–114. [Google Scholar] [CrossRef]
- Yadav, V.B.; Gadi, R.; Kalra, S. Clay Based Nanocomposites for Removal of Heavy Metals from Water: A Review. J. Environ. Manag. 2019, 232, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Boonamnuayvitaya, V.; Chaiya, C.; Tanthapanichakoon, W.; Jarudilokkul, S. Removal of Heavy Metals by Adsorbent Prepared from Pyrolyzed Coffee Residues and Clay. Sep. Purif. Technol. 2004, 35, 11–22. [Google Scholar] [CrossRef]







| Elements | wt, % | ||
|---|---|---|---|
| Sample: | GPS | GPG | GPGC |
| C | 17.59 | 18.33 | 19.80 |
| O | 45.75 | 45.13 | 44.53 |
| Na | 8.07 | 5.08 | 4.95 |
| Al | 8.20 | 9.78 | 9.59 |
| Si | 17.20 | 18.18 | 17.82 |
| K | 1.90 | 1.70 | 1.68 |
| Ca | 0.65 | 0.56 | 0.50 |
| Ti | 0.1 | 0.56 | 0.42 |
| Fe | 0.54 | 0.68 | 0.70 |
| Total: | 100.00 | 100.00 | 100.00 |
| Si/Al | 2.10 | 1.86 | 1.86 |
| Na/Al | 0.98 | 0.52 | 0.52 |
| Isotherm Model | Equation | |
|---|---|---|
| Langmuir | (4) | |
| Freundlich | (5) | |
| Redlich-Peterson | (6) | |
| Sips | (7) | |
| Parameters | GPS | GPG | GPGC |
|---|---|---|---|
| Langmuir | qm (mg g−1) = 58.48 ± 0.06 | qm (mg g−1) = 71.43 ± 0.02 | qm (mg g−1) = 72.46 ± 0.07 |
| b (L mg−1) = 0.3353 ± 0.0003 | b (L mg−1) = 0.4389 ± 0.0007 | b (L mg−1) = 0.5391 ± 0.0005 | |
| R2 = 0.9998 | R2 = 0.9998 | R2 = 0.9991 | |
| Freundlich | 1/n = 0.1592 ± 0.0079 | 1/n = 0.2109 ± 0.0099 | 1/n = 0.1773 ± 0.0087 |
| KF = 26.6502 ± 0.0033 | KF = 28.0543 ± 0.0039 | KF = 33.8870 ± 0.0042 | |
| R2 = 0.8887 | R2 = 0.8157 | R2 = 0.8202 | |
| Redlich-Peterson | β = 0.3053 ± 0.0012 | β = 0.3004 ± 0.0047 | β = 0.2851 ± 0.0021 |
| aR = 0.0378 ± 0.0002 | aR = 0.0359 ± 0.0002 | aR = 0.0300 ± 0.0003 | |
| KR = 0.2563 ± 0.0010 | KR = 0.2366 ± 0.0037 | KR = 0.2345 ± 0.0024 | |
| R2 = 0.9959 | R2 = 0.9844 | R2 = 0.9899 | |
| Sips | qmax (mg g−1) = 45.2489 ± 2.2624 | qmax (mg g−1) = 27.0270 ± 1.3514 | qmax (mg g−1) = 57.8035 ± 2.8902 |
| Ks (L mg−1) = 1.1510 ± 0.0576 | Ks (L mg−1) = 2.7407 ± 0.1370 | Ks (L mg−1) = 1.2815 ± 0.0641 | |
| nS = 6.7267 ± 0.3363 | nS = 5.1988 ± 0.2599 | nS = 7.6029 ± 0.3801 | |
| R2 = 0.9766 | R2 = 0.9709 | R2 = 0.9675 |
| Used Adsorbent Material | qm (mg g−1) | Author/’s |
|---|---|---|
| Sodium silicate based geopolymer | 48.78 | [39] |
| Green geopolymer/alginate hybrid spheres (GAS) | 62.50 | [70] |
| Geopolymer based on fly ash and iron ore tailing | 69.11 | [71] |
| Germany clay based geopolymer | 71.43 | In this study |
| Metakaolin-based geopolymer (MKG) | 86.60 | [72] |
| Geopolymer based on Serbian kaolinite | 58.48 | In this study |
| Beihai in Guangxi Province, China —based kaolin porous geopolymer | 52.63 | [38] |
| Cetyl-trimethylammonium bromide (CTAB)-based geopolymer | 40.00 | [73] |
| Unmodified kaolinite | 10.80 | [74] |
| Organic carbon based geopolymer prepared from coffee residue | 31.20 | [75] |
| Geopolymer based on Germany clay with addition of a carbon | 72.46 | In this study |
| Adsorbent | Concentration (mg L−1) | ||||
|---|---|---|---|---|---|
| 25 | 50 | 100 | 150 | 200 | |
| GPS | 0.1035 ± 0.0052 | 0.0560 ± 0.0028 | 0.0296 ± 0.0015 | 0.0205 ± 0.0010 | 0.0155 ± 0.0008 |
| GPG | 0.0810 ± 0.0041 | 0.0433 ± 0.0022 | 0.0228 ± 0.0011 | 0.0157 ± 0.0008 | 0.0119 ± 0.0006 |
| GPGC | 0.0670 ± 0.0034 | 0.0356 ± 0.0018 | 0.0186 ± 0.0009 | 0.0128 ± 0.0006 | 0.0097 ± 0.0005 |
| Pseudo-First Order | ||||
| Sample | Experimental qe (mg g−1) | k1 (L min−1) | Calculated qe (mg g−1) | R2 |
| GPS | 46.80 | 0.0071 ± 0.0004 | 18.81 ± 0.9405 | 0.1360 |
| GPG | 47.09 | 0.0069 ± 0.0003 | 25.54 ± 1.2770 | 0.1627 |
| GPGC | 50.52 | 0.0061 ± 0.0003 | 19.19 ± 0.9595 | 0.2039 |
| Pseudo-Second Order | ||||
| Sample | Experimental qe (mg g−1) | k2 (g mg−1 min−1) | Calculated qe (mg g−1) | R2 |
| GPS | 46.80 | 0.0026 ± 0.0002 | 42.19 ± 0.31 | 0.9965 |
| GPG | 47.09 | 0.0031 ± 0.0005 | 48.54 ± 0.17 | 0.9936 |
| GPGC | 50.52 | 0.0017 ± 0.0001 | 50.00 ± 0.32 | 0.9925 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šuránek, M.; Melichová, Z.; Mirković, M.M.; Ivanović, M.; Pavlović, V.B.; Kljajević, L.; Nenadović, S. The Study of Cu(II) Adsorption onto Synthetically Modified Geopolymers. Sustainability 2023, 15, 2869. https://doi.org/10.3390/su15042869
Šuránek M, Melichová Z, Mirković MM, Ivanović M, Pavlović VB, Kljajević L, Nenadović S. The Study of Cu(II) Adsorption onto Synthetically Modified Geopolymers. Sustainability. 2023; 15(4):2869. https://doi.org/10.3390/su15042869
Chicago/Turabian StyleŠuránek, Matej, Zuzana Melichová, Miljana M. Mirković, Marija Ivanović, Vladimir B. Pavlović, Ljiljana Kljajević, and Snežana Nenadović. 2023. "The Study of Cu(II) Adsorption onto Synthetically Modified Geopolymers" Sustainability 15, no. 4: 2869. https://doi.org/10.3390/su15042869
APA StyleŠuránek, M., Melichová, Z., Mirković, M. M., Ivanović, M., Pavlović, V. B., Kljajević, L., & Nenadović, S. (2023). The Study of Cu(II) Adsorption onto Synthetically Modified Geopolymers. Sustainability, 15(4), 2869. https://doi.org/10.3390/su15042869








