New Glass Ceramic Materials Obtained from Cathode Ray Tubes Glass Wastes and Fly Ash
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Dimensional Stability after Heat Treatment
3.2. Apparent Density, Apparent and Total Porosities
3.3. Phase Composition
3.4. Chemical Stability of the Samples
3.5. Mechanical Properties of the Glass Ceramics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhir, A.; Koshta, N.; Goyal, R.K.; Sakashita, M.; Almotairi, M. Behavioral reasoning theory (BRT) perspectives on E-waste recycling and management. J. Clean. Prod. 2021, 280, 124269. [Google Scholar] [CrossRef]
- Ahirwar, R.; Tripathi, A.K. E-waste management: A review of recycling process, environmental and occupational health hazards, and potential solutions. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100409. [Google Scholar] [CrossRef]
- Aboelmaged, M. E-waste recycling behaviour: An integration of recycling habits into the theory of planned behavior. J. Clean. Prod. 2021, 278, 124182. [Google Scholar] [CrossRef]
- Shevchenko, T.; Laitala, K.; Danko, Y. Understanding Consumer E-Waste Recycling Behavior: Introducing a New Economic Incentive to Increase the Collection Rates. Sustainability 2019, 11, 2656. [Google Scholar] [CrossRef]
- Kuehr, R. 1—Global e-waste initiatives. In Woodhead Publishing Series in Electronic and Optical Materials, Waste Electrical and Electronic Equipment (WEEE) Hand-Book; Goodship, V., Stevels, A., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 3–16. [Google Scholar]
- Wang, B.; Ren, C.; Dong, X.; Zhang, B.l.; Wang, Z. Determinants shaping willingness towards on-line recycling behaviour: An empirical study of household e-waste recycling in China. Resour. Conserv. Recycl. 2019, 143, 218–225. [Google Scholar] [CrossRef]
- Forti, V.; Balde, C.P.; Kuehr, R.; Bel, G. The Global E-Waste Monitor 2020: Quantities, Flows and the Circular Economy Potential; United Nations University/United Nations Institute for Training and Research, International Telecommunication Union, and International Solid Waste Association: Bonn, Germany, 2020. [Google Scholar]
- Shevchenko, T.; Saidani, M.; Danko, Y.; Golysheva, I.; Chovancová, J.; Vavrek, R. Towards a Smart E-Waste System Utilizing Supply Chain Participants and Interactive Online Maps. Recycling 2021, 6, 8. [Google Scholar] [CrossRef]
- Chi, X.; Wang, M.Y.L.; Reuter, M.A. E-waste collection channels and household recycling behaviors in Taizhou of China. J. Clean. Prod. 2014, 80, 87–95. [Google Scholar] [CrossRef]
- Baron, Y.; Maijala, A.; Lopez, V.; Thiebaud, E.; Haarman, A.; Kaartinen, H.; Herreras, L.; Hajosi, E.; Wuisan, L.; Winkler, J.; et al. The CEWASTE Assurance and Verification System for the Certi-fication of Waste Management Operators with CRM Focused Requirements. In Proceedings of the Electronic Goes Green, Berlin, Germany, 1 September 2020. [Google Scholar]
- Kurtuluş, R.; Kavas, T.; Kavaz, T.; Tekin, H.O.; Kurucu, Y. Synthesis and characterization of waste CRT glasses through physical, optical and structural properties: A comprehensive study on recycling. Optik 2021, 248, 168167. [Google Scholar] [CrossRef]
- Yao, Z.; Ling, T.K.; Sarker, P.K.; Su, W.; Liu, J.; Wu, W.; Tang, J. Recycling difficult-to-treat e-waste cathode-ray-tube glass as construction and building materials: A critical review. Renew. Sustain. Energy Rev. 2018, 81, 595–604. [Google Scholar] [CrossRef]
- Paunescu, L.; Dragoescu, M.F.; Axinte, S.M.; Cosmulescu, F. Nonconventional manufacture technique of cellular glass from recycled aluminosilicate glass-based waste. Mater. Sci. Eng. Int. J. 2021, 5, 11–16. [Google Scholar]
- Smolii, V.A.; Kosarev, A.S.; Yatsenko, E.A. Porophores for Cellular Glass Based on TPP Ash-Slag Materials. Glass Ceram. 2020, 77, 94–97. [Google Scholar] [CrossRef]
- Praxedes, F.M.; Teixeira, J.V.U.; Da Luz, P.T.S.; Fernandez, O.J.C.; Figueira, B.A.M.; de Oliveira Araújo, S.M.S. Use of industrial residues for production of cellular glasses of low environmental impact. Mater. Res. Express 2019, 6, 065513. [Google Scholar] [CrossRef]
- Yuan, H.; Wu, H.; Guan, J. Synthesis of foam glass-ceramic from CRT panel glass using one-step powder sintering. IOP Conf. Ser. Earth Environ. Sci. 2018, 186, 012020. [Google Scholar] [CrossRef]
- Xu, B.; Wang, F.; Yang, J.; Yang, B.; Zhao, J. Enhancing Pb Removal and Synthesizing Glass–Ceramics from Waste CRTs Funnel Glass by Red Mud. J. Sustain. Metall. 2020, 6, 367–374. [Google Scholar] [CrossRef]
- Zhang, Q.; He, F.; Shu, H.; Qiao, Y.; Mei, S.; Jin, M.; Xie, J. Preparation of high strength glass ceramic foams from waste cathode ray tube and germanium tailings. Constr. Build. Mater. 2016, 111, 105–110. [Google Scholar] [CrossRef]
- Lv, J.; Yang, H.; Jin, Z.; Zhao, M. Lead extraction and glass-ceramics synthesis from waste cathode ray tube funnel glass through cooperative smelting process with coal fly ash. Waste Manag. 2018, 76, 687–696. [Google Scholar] [CrossRef]
- Lu, X.; Yang, J.; Ning, X.A.; Shih, K.; Wang, F. Crystallization pathways in glass-ceramics by sintering cathode ray tube (CRT) glass with kaolin-based precursors. J. Eur. Ceram. Soc. 2018, 38, 5184–5191. [Google Scholar] [CrossRef]
- Revelo, R.J.; Menegazzo, A.P.; Ferreira, E.B. Cathode-Ray Tube panel glass replaces frit in transparent glazes for ceramic tiles. Ceram. Int. 2018, 44, 13790–13796. [Google Scholar] [CrossRef]
- Reben, M.; Kosmal, M.; Pałczyńska, N.; Pichniarczyk, P. Waste immobilization and environmental sustainability in glass-ceramics glazes development. E3S Web Conf. 2016, 10, 00071. [Google Scholar] [CrossRef]
- Mourou, C.; Martín-Morales, M.; Zamorano, M.; Ruiz, D.P. Light Reflectance Characterization of Waste Glass Coating for Tiles. Appl. Sci. 2022, 12, 1537. [Google Scholar] [CrossRef]
- Qi, Y.; Xiao, X.; Lu, Y.; Shu, J.; Wang, J.; Chen, M. Cathode ray tubes glass recycling: A review. Sci. Total Environ. 2019, 650, 2842–2849. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, E.; Esposito, L.; Rambaldi, E.; Tucci, A. Glass based stoneware’as a promising route for the recycling of waste glasses. Adv. Appl. Ceram. 2009, 108, 2–8. [Google Scholar] [CrossRef]
- Gopal, P.M.; Soorya Prakash, K. Wire electric discharge machining of silica rich E-waste CRT and BN reinforced hybrid magnesium MMC. Silicon 2019, 11, 1429–1440. [Google Scholar]
- Rana, V.; Kumar, H.; Kumar, A. Fabrication of hybrid metal matrix composites (HMMCs)—A review of comprehensive research studies. Mater. Today Proc. 2022, 56, 3102–3107. [Google Scholar] [CrossRef]
- Taurino, R.; Bondioli, F.; Messori, M. Use of different kinds of waste in the construction of new polymer composites: Review. Mater. Today Sustain. 2023, 21, 100298. [Google Scholar] [CrossRef]
- Popovici, A.; Corbu, O.; Popita, G.E.; Rosu, C.; Proorocu, M.; Sandu, A.V.; Abdullah, M.M.A.B. Modern mortars with electronic waste scraps (glass and plastic). Mater. Plast. 2015, 52, 588–592. [Google Scholar]
- Naganathan, S.; Silvadanan, S.; Chung, T.Y.; Nicolasselvam, M.F.; Thiruchelvam, S. Use of wastes in developing mortar—A review. Adv. Mater. Res. 2014, 935, 146–150. [Google Scholar] [CrossRef]
- Long, W.J.; Gu, Y.C.; Xing, F.; Khayat, K.H. Evaluation of the inhibiting effect of graphene oxide on lead leaching from waste cathode-ray tube glass incorporated in cement mortar. Cem. Concr. Compos. 2019, 104, 103337. [Google Scholar] [CrossRef]
- Luhar, S.; Luhar, I. Potential application of E-wastes in construction industry: A review. Constr. Build. Mater. 2019, 203, 222–240. [Google Scholar] [CrossRef]
- Liu, T.; Song, W.; Zou, D.; Li, L. Dynamic mechanical analysis of cement mortar prepared with recycled cathode ray tube (CRT) glass as fine aggregate. J. Clean. Prod. 2018, 174, 1436–1443. [Google Scholar] [CrossRef]
- Ling, T.C.; Poon, C.S.; Lam, W.S.; Chan, T.P.; Fung, K.K.L. Utilization of recycled cathode ray tubes glass in cement mortar for X-ray radiation-shielding applications. J. Hazard. Mater. 2012, 199, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Poon, C.S. Recycle of large amount cathode ray tube funnel glass sand to mortar with supplementary cementitious materials. Constr. Build. Mater. 2021, 308, 124953. [Google Scholar] [CrossRef]
- Zacco, A.; Borgese, L.; Gianoncelli, A.; Struis, R.P.; Depero, L.E.; Bontempi, E. Review of fly ash inertisation treatments and recycling. Environ. Chem. Lett. 2014, 12, 153–175. [Google Scholar] [CrossRef]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A comprehensive review on the applications of coal fly ash. Earth. Sc. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef]
- Kurda, R.; de Brito, J.; Silvestre, J.D. A comparative study of the mechanical and life cycle assessment of high-content fly ash and recycled aggregates concrete. J. Build. Eng. 2020, 29, 101173. [Google Scholar] [CrossRef]
- Tošić, N.; Marinković, S.; Pecić, N.; Ignjatović, I.; Dragaš, J. Long-term behaviour of reinforced beams made with natural or recycled aggregate concrete and high-volume fly ash concrete. Constr. Build. Mater. 2018, 176, 344–358. [Google Scholar] [CrossRef]
- Kurda, R.; de Brito, J.; Silvestre, J.D. Water absorption and electrical resistivity of concrete with recycled concrete aggregates and fly ash. Cem. Concr. Compos. 2019, 95, 169–182. [Google Scholar] [CrossRef]
- Kurda, R.; de Brito, J.; Silvestre, J.D. Combined economic and mechanical performance optimization of recycled aggregate concrete with high volume of fly ash. Appl. Sci. 2018, 8, 1189. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, N. Impact of fly ash incorporation in soil systems. Agric. Ecosyst. Environ. 2010, 136, 16–27. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Hooda, P.S.; Tsadilas, C.D. Opportunities and challenges in the use of coal fly ash for soil improvements—A review. J. Environ. Manag. 2014, 145, 249–267. [Google Scholar] [CrossRef]
- Sahu, G.; Bag, A.G.; Chatterjee, N.; Mukherjee, A.K. Potential use of fly ash in agriculture: A way to improve soil health. J. Pharmacogn. Phytochem. 2017, 6, 873–880. [Google Scholar]
- Hoy, M.; Horpibulsuk, S.; Arulrajah, A. Strength development of Recycled Asphalt Pavement—Fly ash geopolymer as a road construction material. Constr. Build. Mater. 2016, 117, 209–219. [Google Scholar] [CrossRef]
- Poltue, T.; Suddeepong, A.; Horpibulsuk, S.; Samingthong, W.; Arulrajah, A.; Rashid, A.S.A. Strength development of recycled concrete aggregate stabilized with fly ash-rice husk ash based geopolymer as pavement base material. Road Mater. Pavement Des. 2020, 21, 2344–2355. [Google Scholar] [CrossRef]
- Lu, Y.; Tian, A.; Zhang, J.; Tang, Y.; Shi, P.; Tang, Q.; Huang, Y. Physical and chemical properties, pretreatment, and recycling of municipal solid waste incineration fly ash and bottom ash for highway engineering: A literature review. Adv. Civ. Eng. 2020, 2020, 8886134. [Google Scholar] [CrossRef]
- Liu, J.; Dong, Y.; Dong, X.; Hampshire, S.; Zhu, L.; Zhu, Z.; Li, L. Feasible recycling of industrial waste coal fly ash for preparation of anorthite-cordierite based porous ceramic membrane supports with addition of dolomite. J. Eur. Ceram. Soc. 2016, 36, 1059–1071. [Google Scholar] [CrossRef]
- Yuan, Q.; Robert, D.; Mohajerani, A.; Tran, P.; Pramanik, B.K. Utilisation of waste-to-energy fly ash in ceramic tiles. Constr. Build. Mater. 2022, 347, 128475. [Google Scholar] [CrossRef]
- Jordán, M.M.; Montero, M.A.; Pardo-Fabregat, F. Technological behaviour and leaching tests in ceramic tile bodies obtained by recycling of copper slag and MSW fly ash wastes. J. Mater. Cycles. 2021, 23, 707–716. [Google Scholar] [CrossRef]
- Yuan, N.; Zhang, X.; Zhao, A.; Tan, K.; Cui, Y. High-alumina fly ash as sustainable aluminum sources for the in situ preparation of Al-based eco-MOFs. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128421. [Google Scholar] [CrossRef]
- Chen, T.; Yuan, N.; Wang, S.; Hao, X.; Zhang, X.; Wang, D.; Yang, X. The Effect of Bottom Ash Ball-Milling Time on Properties of Controlled Low-Strength Material Using Multi-Component Coal-Based Solid Wastes. Sustainability 2022, 14, 9949. [Google Scholar] [CrossRef]
- Cao, G.; Yang, L.; Yuan, G.; Hu, J.; Shao, G.; Yan, L. Chemical diversity of iron species and structure evolution during the oxidation of C14 Laves phase Zr(Fe,Nb)2 in subcritical environment. Corros. Sci. 2020, 162, 108218. [Google Scholar] [CrossRef]
- Cao, G.; Yun, Y.; Xu, H.; Yuan, G.; Hu, J.; Shao, G. A mechanism assessment for the anti-corrosion of zirconia coating under the condition of subcritical water corrosion. Corros. Sci. 2019, 152, 54–59. [Google Scholar] [CrossRef]
- Feng, N.; Chen, C.; Hu, J.; Shao, G.; Yuan, G.; Cao, G. Microstructural and mechanical evolution of amorphous Zr-Si with irradiation induced atomic reconfiguration and free volume variation. Surf. Interfaces 2022, 30, 101890. [Google Scholar] [CrossRef]
- Belviso, C. State-of-the-art applications of fly ash from coal and biomass: A focus on zeolite synthesis processes and issues. Prog. Energy Combust. Sci. 2018, 65, 109–135. [Google Scholar] [CrossRef]
- Panek, R.; Madej, J.; Bandura, L.; Słowik, G. Recycling of waste solution after hydrothermal conversion of fly ash on a semi-technical scale for zeolite synthesis. Materials 2021, 14, 1413. [Google Scholar] [CrossRef] [PubMed]
- Supelano, G.I.; Cuaspud, J.G.; Moreno-Aldana, L.C.; Ortiz, C.; Trujillo, C.A.; Palacio, C.A.; Parra Vargas, C.A.; Gómez, J.M. Synthesis of magnetic zeolites from recycled fly ash for adsorption of methylene blue. Fuel 2020, 263, 116800. [Google Scholar] [CrossRef]
- Ju, S.; Yoon, J.; Sung, D.; Pyo, S. Mechanical properties of coal ash particle-reinforced recycled plastic-based composites for sustainable railway sleepers. Polymers 2020, 12, 2287. [Google Scholar] [CrossRef] [PubMed]
- Nasir, N.H.M.; Usman, F.; Saggaf, A. Development of composite material from Recycled Polyethylene Terephthalate and fly ash: Four decades progress review. Curr. Res. Green Sustain. Chem. 2022, 5, 100280. [Google Scholar] [CrossRef]
- Anandhan, S. Recent trends in fly ash utilization in polymer composites. Int. J. Waste Resour. 2014, 4, 1000149. [Google Scholar]
- Long, W.J.; Zhang, X.; Xie, J.; Kou, S.; Luo, Q.; Wei, J.; Lin, C.; Feng, G.L. Recycling of waste cathode ray tube glass through fly ash-slag geopolymer mortar. Constr. Build. Mater. 2022, 322, 126454. [Google Scholar] [CrossRef]
- Liang, B.; Zhang, M.; Li, H.; Zhao, M.; Xu, P.; Deng, L. Preparation of ceramic foams from ceramic tile polishing waste and fly ash without added foaming agent. Ceram. Int. 2021, 47, 23338–23349. [Google Scholar] [CrossRef]
- Fan, Y.; Li, S.; Li, Y.; Liang, H.; Tang, M.; Huang, K.; Zhu, L. Recycling of municipal solid waste incineration fly ash in foam ceramic materials for exterior building walls. J. Build. Eng. 2021, 44, 103427. [Google Scholar] [CrossRef]
- Rabelo Monich, P.; Lucas, H.; Friedrich, B.; Bernardo, E. Recyclable Porous Glass-Ceramics from the Smelting of MSWI Bottom Ash. Ceramics 2021, 4, 1–11. [Google Scholar] [CrossRef]
- US EPA. Extraction Procedure Toxicity Test in: Stabilization/Solidification of CERCLA and RCRA Wastes, US EPA625/6-89/022; US EPA: Cincinnati, OH, USA, 1986.
- ASTM C1424-15; Standard Test Method for Monotonic Compressive Strength of Advanced Ceramics at Ambient Temperature Advanced Concrete Technology and its Structural Applications. ASTM International: West Conshohocken, PA, USA, 2019.
- Abubakar, M.; Muthuraja, A.; Rajak, D.K.; Ahmad, N.; Pruncu, C.I.; Lamberti, L.; Kumar, A. Influence of Firing Temperature on the Physical, Thermal and Microstructural Properties of Kankara Kaolin Clay: A Preliminary Investigation. Materials 2020, 13, 1872. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Valles, M.; Cuevas, D.; Alfonso, P.; Martínez, S. Thermal behaviour of ceramics obtained from the kaolinitic clays of Terra Alta, Catalonia, Spain. J. Therm. Anal. Calorim. 2022, 147, 5303–5312. [Google Scholar] [CrossRef]
- Valášková, M.; Blahůšková, V.; Vlček, J. Effects of Kaolin Additives in Fly Ash on Sintering and Properties of Mullite Ceramics. Minerals 2021, 11, 887. [Google Scholar] [CrossRef]
- Krstić, I.; Zec, S.; Lazarević, V.; Stanisavljević, M.; Golubović, T. Use of sintering to immobilize toxic metals present in galvanic sludge into a stabile glass-ceramic structure. Sci. Sinter. 2018, 50, 139–147. [Google Scholar] [CrossRef]
- ASTM C1272; Standard Specification for Heavy Vehicular Paving Brick. ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM C902; Standard Specification for Pedestrian and Light Traffic Paving Brick. ASTM International: West Conshohocken, PA, USA, 2014.
- Chopra, M.; Wanielista, M.; Mulligan, A.M. Compressive Strength of Pervious Concrete Pavements; Final Report; Stormwater Management Academy, University of Central Florida: Orlando, FL, USA, January 2007. [Google Scholar]
- Penteado, C.S.G.; de Carvalho, E.V.; Lintz, R.C.C. Reusing ceramic tile polishing waste in paving block manufacturing. J. Clean. Prod. 2015, 112, 514–520. [Google Scholar] [CrossRef]
- Rozenstrauha, I.; Sosins, G.; Petersone, L.; Krage, L.; Drille, M.; Filipenkov, V. Production of glass-ceramics from sewage sludge and waste glass. IOP Conf. Ser. Mater. Sci. Eng. 2011, 25, 012016. [Google Scholar] [CrossRef]
- Montaev, S.A.; Zharylgapov, S.M.; Montaeva, N.S.; Shakeshev, B.T. Research of possibility of producing ceramic paving stones by vibrocompression with the purpose of using them in the improvement of urban areas. IOP Conf. Ser. Mater. Sci. Eng. 2020, 775, 012118. [Google Scholar] [CrossRef]
- Prahara, E.; Meilani. Compressive Strength and Water Absorption of Pervious Concrete that Using the Fragments of Ceramics and Roof Tiles. EPJ Web Conf. 2014, 68, 00015. [Google Scholar] [CrossRef]
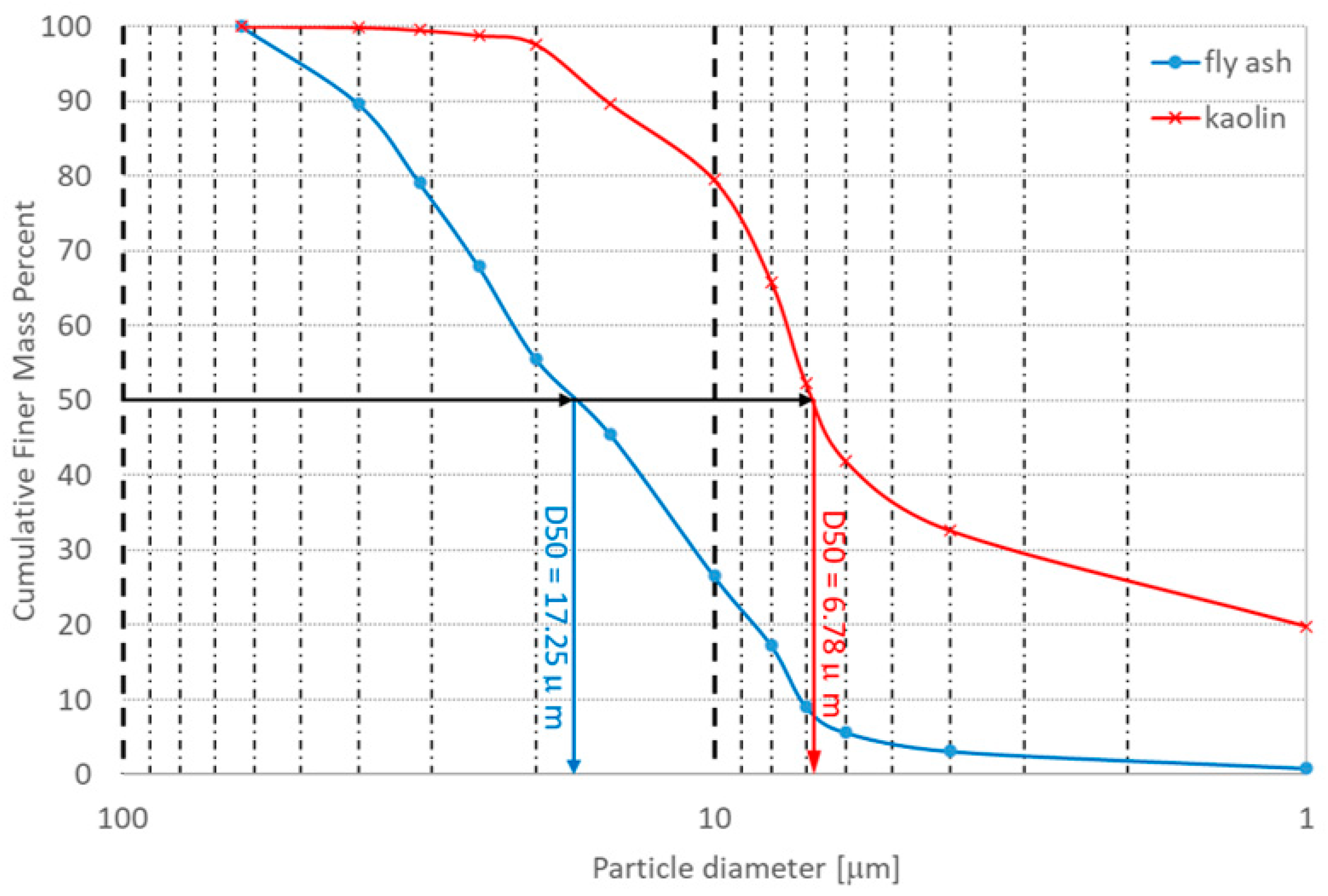








| Oxide | SiO2 | Na2O | K2O | CaO | MgO | Al2O3 | Fe2O3 | BaO | PbO | TiO2 | L.O.I |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CRT | 60.92 | 8.96 | 7.44 | 0.67 | 0.14 | 2.07 | 0.15 | 10.80 | 8.85 | - | - |
| Fly ash | 46.20 | 6.23 | 4.17 | 8.60 | 3.30 | 23.20 | 8.10 | 0.20 | |||
| Bojidar kaolin | 49.29 | 0.14 | 0.87 | 0.56 | 0.44 | 35.18 | 0.78 | - | - | 0.43 | 12.31 |
| Sample | Fly Ash | CRT Waste | Bojidar Kaolin | Fly Ash/CRT Ratio |
|---|---|---|---|---|
| 1 | 20.00 | 40.00 | 40.00 | 0.50 |
| 2 | 30.00 | 35.00 | 35.00 | 0.86 |
| 3 | 40.00 | 30.00 | 30.00 | 1.33 |
| 4 | 50.00 | 25.00 | 25.00 | 2.00 |
| 5 | 60.00 | 20.00 | 20.00 | 3.00 |
| Total Porosity (%) | Compression Strength (N·mm−2) | Reference |
|---|---|---|
| 3.0–29.0 | 4.68–15.08 | [74] |
| 12.1–13.5 | 43.90–62.40 | [75] |
| 8.0–24.0 | 52.67 | [76] |
| 18.6–20.40 | 25.67–31.26 | [77] |
| 15.0–20.0 | 3.50–27.50 | [78] |
| 9.2–36.7 | 1.42–11.83 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vancea, C.; Mosoarca, G.; Popa, S.; Dan, M.; Boran, S. New Glass Ceramic Materials Obtained from Cathode Ray Tubes Glass Wastes and Fly Ash. Sustainability 2023, 15, 3021. https://doi.org/10.3390/su15043021
Vancea C, Mosoarca G, Popa S, Dan M, Boran S. New Glass Ceramic Materials Obtained from Cathode Ray Tubes Glass Wastes and Fly Ash. Sustainability. 2023; 15(4):3021. https://doi.org/10.3390/su15043021
Chicago/Turabian StyleVancea, Cosmin, Giannin Mosoarca, Simona Popa, Mircea Dan, and Sorina Boran. 2023. "New Glass Ceramic Materials Obtained from Cathode Ray Tubes Glass Wastes and Fly Ash" Sustainability 15, no. 4: 3021. https://doi.org/10.3390/su15043021
APA StyleVancea, C., Mosoarca, G., Popa, S., Dan, M., & Boran, S. (2023). New Glass Ceramic Materials Obtained from Cathode Ray Tubes Glass Wastes and Fly Ash. Sustainability, 15(4), 3021. https://doi.org/10.3390/su15043021











