Enhancing Polyphenols and Tannins Concentration on Cotton Dyed with Red Tea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Tea Liquor Extract
2.3. Pre-Mordanting
2.4. Dyeing: Exhaustion Method
2.5. Polyphenols Characterization in Wastewater
2.6. Hydrolysable Tannin Characterisation
2.7. Condensed Tannin Characterisation
2.8. Colour Characterisation
2.9. Colour Fastness
2.10. Ultraviolet Protection Factor (UPF)
3. Results
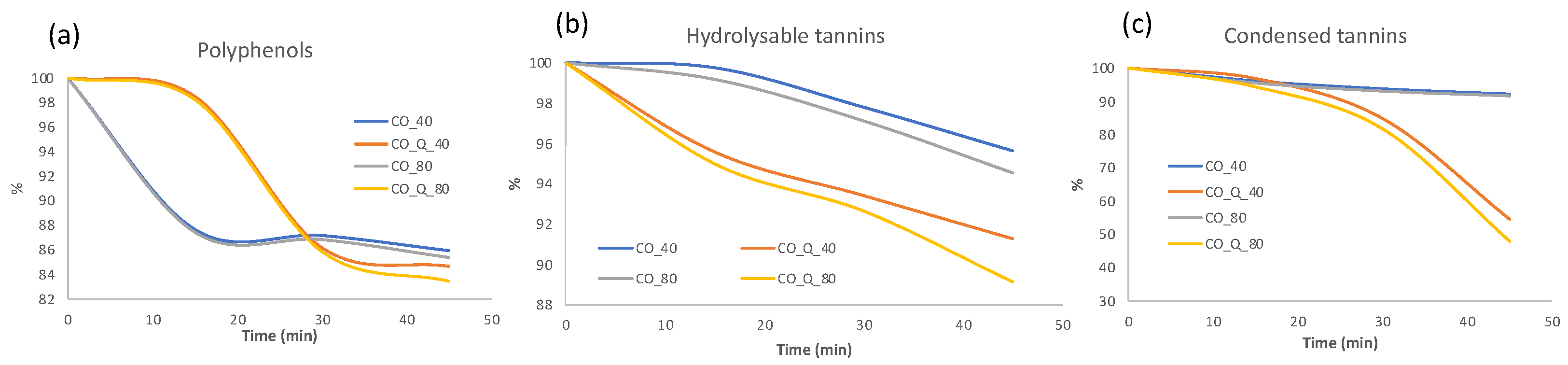
3.1. Phytocomponents Presence
3.2. Colour Characterization
3.3. Colour Fastness
3.4. Ultraviolet Protection Factor (UPF)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kadolph, S. Natural dyes: A traditional craft experiencing new attention. Delta Kappa Gamma Bull. 2008, 75, 14–17. [Google Scholar]
- Phan, K.; Van Den Broeck, E.; Speybroeck, V.; De Clerck, K.; Raes, K.; De Meester, S. The potential of anthocyanins from blueberries as a natural dye for cotton: A combined experimental and theoretical study. Dye. Pigment. 2020, 176, 108180. [Google Scholar] [CrossRef]
- Saxena, S.; Raja, A. Natural dyes: Sources, chemistry, application and sustainability issues. In Roadmap to Sustainable Textiles and Clothing; Springer: Berlin/Heidelberg, Germany, 2014; pp. 37–80. [Google Scholar]
- Shahid, M.; Mohammad, F. Recent advancements in natural dye applications: A review. J. Clean. Prod. 2013, 53, 310–331. [Google Scholar] [CrossRef]
- Vadood, M.; Haji, A. Application of ANN Weighted by Optimization Algorithms to Predict the Color Coordinates of Cellulosic Fabric in Dyeing with Binary Mix of Natural Dyes. Coatings 2022, 12, 1519. [Google Scholar] [CrossRef]
- Mohammad, F.; Shahid, I. Natural colorants in the presence of anchors so-called mordants as promising coloring and antimicrobial agents for textile materials. ACS Sustain. Chem. Eng. 2015, 3, 2361–2375. [Google Scholar]
- Haji, A.; Nasiriboroumand, M.; Qavamnia, S.S. Cotton dyeing and antibacterial finishing using agricultural waste by an eco-friendly process optimized by response surface methodology. Fibers Polym. 2018, 19, 2359–2364. [Google Scholar] [CrossRef]
- Toprak-Cavdur, T.; Uysal, S.; Gisbert-Paya, J. Dyeing Recycled Cotton Fibers Using Curcuma Longa and Pterocarpus Santalinus Natural Dyes and Bio-mordant Chitosan. J. Nat. Fibers 2022, 19, 13736–13752. [Google Scholar] [CrossRef]
- Bou-Belda, E.; Indrie, L.; Ilies, D.C.; Hodor, N.; Berdenov, Z.; Herman, G.; Caciora, T. Chitosan–a non-invasive approach for the preservation of historical textiles. Ind. Text. 2020, 71, 576–579. [Google Scholar] [CrossRef]
- Buyukakinci, Y.B.; Guzel, E.T.; Karadag, R. Organic cotton fabric dyed with dyer’s oak and barberry dye by microwave irradiation and conventional methods. Ind. Text. 2021, 72, 30–38. [Google Scholar] [CrossRef]
- Houshyar, S.; Amirshahi, H.S. Treatment of Cotton with Chitosan and Its Effect on Dyeability with Reactive Dyes. Iran. Polym. J. 2002, 11, 259–301. [Google Scholar]
- Yadav, M.; Singh, N.; Kaur, A.; Sahu, O. Antibacterial activity assessment of woolen fabric treated with natural dyes and chitosan. Agric. Nat. Resour. 2019, 53, 188–196. [Google Scholar]
- Butola, B.S. Effect of chitosan biological macromolecule on colorimetric analysis and radical scavenging activity of linen using pineapple peel extract biomolecules. Int. J. Biol. Macromol. 2019, 124, 708–715. [Google Scholar]
- Saini, S.; Gupta, A.; Singh, N.; Sheikh, J. Functionalization of linen fabric using layer by layer treatment with chitosan and green tea extract. J. Ind. Eng. Chem. 2020, 82, 138–143. [Google Scholar] [CrossRef]
- Haji, A. Dyeing of cotton fabric with natural dyes improved by mordants and plasma treatment. Prog. Color Color. Coat. 2019, 12, 191–201. [Google Scholar]
- Kampeerapappun, P.; Phattararittigul, T.; Jittrong, S.; Kullachod, D. Effect of chitosan and mordants on dyeability of cotton fabrics with Ruellia tuberosa Linn. Chiang Mai J. Sci. 2010, 38, 95–104. [Google Scholar]
- Hong, S.J.; Jeon, D.W.; Kim, J.J.; Choi, I.R. Effect of chitosan and mordant treatments on the color change of cotton and nylon fabrics dyed using rhusjara ica. Res. J. Costume Cult. 2005, 13, 380–390. [Google Scholar]
- Uzun, I. Kinetics of the adsorption of reactive dyes by chitosan. Dye. Pigment. 2006, 70, 76–83. [Google Scholar] [CrossRef]
- Sakkayawong, N.; Thiravetyan, P.; Nakbanpote, W. Adsorption mechanism of synthetic reactive dye wastewater by chitosan. J. Colloid Interface Sci. 2005, 286, 36–42. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Salehi, R.; Arami, M.; Bahrami, H. Dye removal from colored textile wastewater using chitosan in binary systems. Desalination 2011, 267, 64–72. [Google Scholar] [CrossRef]
- Sánchez-Duarte, R.G.; Sánchez-Machado, D.I.; López-Cervantes, J.; Correa-Murrieta, M.A. Adsorption of allura red dye by cross-linked chitosan from shrimp waste. Water Sci. Technol. 2012, 65, 618–623. [Google Scholar] [CrossRef]
- Kim, S.-H. Dyeing characteristics and UV protection property of green tea dyed cotton fabrics. Fibers Polym. 2006, 7, 255–261. [Google Scholar] [CrossRef]
- Bhuiyan, R.M.; Shaid, A.; Khan, M.A. Cationization of Cotton Fiber by Chitosan and Its Dyeing with Reactive Dye without Salt. Chem. Mater. Eng. 2014, 2, 96–100. [Google Scholar] [CrossRef]
- Vayalil, P.K.; Mittal, A.; Hara, Y.; Elmets, C.A.; Katiyar, S.K. Green tea polyphenols prevent ultraviolet light-induced oxidative damage and matrix metalloproteinases expression in mouse skin. J. Investig. Dermatol. 2004, 122, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Yoo, D.I. Use of chitosan to improve dyeability of DP-finished cotton (II). J. Appl. Polym. Sci. 1998, 67, 1515–1521. [Google Scholar] [CrossRef]
- Rippon, J.A. Improving the dye coverage of immature cotton fibres by treatment with chitosan. J. Soc. Dye. Colour. 1984, 100, 298–303. [Google Scholar] [CrossRef]
- Elmets, C.A.; Singh, D.; Tubesing, K.; Matsui, M.; Katiyar, S.; Mukhtar, H. Cutaneous photoprotection from ultraviolet injury by green tea polyphenols. J. Am. Acad. Dermatol. 2001, 44, 425–432. [Google Scholar] [CrossRef]
- Liu, X.D.; Nishi, N.; Tokura, S.; Sakairi, N. Chitosan coated cotton fiber: Preparation and physical properties. Carbohydr. Polym. 2001, 44, 233–238. [Google Scholar] [CrossRef]
- Xiong, X.; Sun, J.; Hu, D.; Xiao, C.; Wang, J.; Zhuo, Q.; Dai, L. Fabrication of polyvinyl alcohol hydrogels with excellent shape memory and ultraviolet-shielding behavior via the introduction of tea polyphenols. RSC Adv. 2020, 10, 35226–35234. [Google Scholar] [CrossRef]
- Bonet-Aracil, M.A.; Belda, B.E.; Diaz, P.; Sebastia, N. UV protection from cotton fabrics dyed with different tea extracts. Dye. Pigment. 2016, 134, 448–452. [Google Scholar] [CrossRef]
- Kanwar, J.; Taskeen, M.; Mohammad, I.; Huo, C.; Chan, T.H.; Dou, Q.P. Recent advances on tea polyphenols. Front. Biosci. (Elite Ed.) 2012, 4, 111–131. [Google Scholar] [CrossRef]
- Naima, R.; Oumam, M.; Hannache, H.; Sesbou, A.; Charrier, B.; Pizzi, A.; Charrier–El Bouhtoury, F. Comparison of the impact of different extraction methods on polyphenols yields and tannins extracted from Moroccan Acacia mollissima barks. Ind. Crops Prod. 2015, 70, 245–252. [Google Scholar] [CrossRef]
- Gironi, F.; Piemonte, V. Temperature and solvent effects on polyphenol extraction process from chestnut tree wood. Chem. Eng. Res. Des. 2011, 89, 857–862. [Google Scholar] [CrossRef]
- Lambrecht, L.; Gisbert-Payá, J.; Bou-Belda, E.; Bonet, M.Á. Optimization of tea extracts composition to dye cotton. Time and temperature influence. J. Appl. Res. Technol. Eng. 2020, 1, 3–7. [Google Scholar] [CrossRef]
- Hebeish, A.; El-Naggar, M.E.; Fouda, M.M.G.; Ramadan, M.A.; Al-Deyab, S.S.; El-Rafie, M.H. Highly effective antibacterial textiles containing green synthesized silver nanoparticles. Carbohydr.-Polym. 2015, 86, 936–940. [Google Scholar] [CrossRef]
- Sheshir, M.H. Textile Chemical Dictionarey; Wet Processing Technology: Allentown, PA, USA, 2014; p. 66. [Google Scholar]
- Singleton, V.; Rossi, J. Colorimetry of total phenolics with phospomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Bossu, C.F. Flow injection system for hydrolysable tannin determination. Microchem. J. 2006, 84, 88–92. [Google Scholar] [CrossRef]
- Schofield, P.; Mbugua, D.M.; Pell, A.N. Analysis of condensed tannins: A review. Anim. Feed. Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
- Scalbert, A.; Monties, B.; Janin, G. Tannins in wood: Comparative study on the phenolic acids identified and quantified in dry beans using HPLC as affected by different extraction and hydrolysis methods. Food Chem. 1989, 113, 1324–1329. [Google Scholar] [CrossRef]
- ISO 105-C06:2010; Colour Fastness to Commercial and Domestic Laundering. ISO: Geneva, Switzerland, 2010.
- Gabrijelčič, H.; Urbas, R.; Sluga, F.; Dimitrovski, K. Influence of fabric constructional parameters and thread colour on UV radiation protection. Fibres Text. East. Eur. 2009, 17, 46–54. [Google Scholar]
- AS/NZ 4399:1996; Sun Protecting Clothing. Evaluation and Characterization. Standards New Zealand: Wellington, New Zealand, 1996.
- Dawson, T.L.; Todd, J.C. Dye Diffusion-The Key to Efficient Coloration. J. Soc. Dye. Colour. 1979, 95, 417–426. [Google Scholar] [CrossRef]
- Deo, H.T.; Desai, B.K. Dyeing of cotton and jute with tea as a natural dye. Color. Technol. 1999, 115, 224–227. [Google Scholar] [CrossRef]
- Campos, J.; Diaz-Garcia, P.; Montava, I.; Bonet-Aracil, M.; Bou-Belda, E. Chitosan pretreatment for cotton dyeing with black tea. In IOP Conference Series-Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; p. 254. [Google Scholar]
- Mansour, R.; Ben Ali, H. Investigating the Use of Chitosan: Toward Improving the Dyeability of Cotton Fabrics Dyed with Roselle (Hibiscus sabdariffa L.). J. Nat. Fibers 2019, 18, 1007–1016. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Men, H.; Tong, J.; Zhou, J. Preparation and characterization of active films based on chitosan incorporated tea polyphenols. Food Hydrocoll. 2013, 32, 35–41. [Google Scholar] [CrossRef]
- Gao, H.X.; He, Z.; Sun, Q.; He, Q.; Zeng, W.C. A functional polysaccharide film forming by pectin, chitosan, and tea polyphenols. Carbohydr. Polym. 2019, 215, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Hou, X.; Ma, B.; Xu, H.; Yang, Y. Chitosan/gallnut tannins composite fiber with improved tensile, antibacterial and fluorescence properties. Carbohydr. Polym. 2019, 226, 115311. [Google Scholar] [CrossRef]



| Time (min) | Cotton 40 mL | Cotton + Chitosan 40 mL | Cotton 80 mL | Cotton + Chitosan 80 mL |
|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 |
| 15 | 20.15 | 15.68 | 18.75 | 11.76 |
| 30 | 35.76 | 39.44 | 38.94 | 40.89 |
| 45 | 49.78 | 52.42 | 50.46 | 54.32 |
| Reference | L | a | b | ∆L* | ∆a* | ∆b* | ∆E*ab |
|---|---|---|---|---|---|---|---|
| CO | 92.8158 | −0.3732 | 3.9564 | ||||
| CO_Q | 88.4979 | −0.4906 | 3.0713 | −4.3179 | −0.1174 | −0.8851 | 4.4092 |
| CO_40 | 82.5565 | 0.5826 | 10.8032 | −5.9414 | 1.0732 | 7.7319 | 9.8099 |
| CO_40_Q | 75.3886 | 2.9726 | 15.2524 | −13.1093 | 3.4631 | 12.1811 | 18.2271 |
| CO_80 | 78.5852 | 3.4205 | 13.6868 | −9.9127 | 3.9111 | 10.6156 | 15.0416 |
| CO_80_Q | 72.5898 | 3.7685 | 18.9044 | −15.9081 | 4.2591 | 15.8331 | 22.8451 |
| Reference | Discharge | Degradation | |
|---|---|---|---|
| Co | Wo | ||
| CO | 5 | 5 | 5 |
| CO_ Q | 5 | 5 | 5 |
| CO_40 | 4 | 4/5 | 3 |
| CO_40_Q | 4/5 | 5 | 4/5 |
| CO_80 | 4 | 4/5 | 2/3 |
| CO_80_Q | 4/5 | 5 | 4/5 |
| Sample | Dyed | Dyed + Washed | ||
|---|---|---|---|---|
| UPF 40 mL | UPF 80 mL | UPF 40 mL | UPF 80 mL | |
| Cotton | 1.31 | 1.31 | 1.45 | 1.45 |
| Cotton + chitosan | 1.37 | 1.37 | 1.51 | 1.51 |
| Cotton + red tea | 8.01 | 9.25 | 6.23 | 7.89 |
| Cotton + chitosan + red tea | 16.7 | 37.48 | 16.23 | 35.98 |
| UPF Range | Protection Category | Effective UVR Transmission (%) | Rating |
|---|---|---|---|
| 15–24 | Good | 6.7–4.2 | 15–20 |
| 25–39 | Very good | 4.1–2.6 | 25, 30, 35 |
| 50–50, 50+ | Excellent | ≤2.5 | 40, 45, 50, 50+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambrecht, L.; Capablanca, L.; Bou-Belda, E.; Montava, I.; Díaz-García, P.; Gisbert-Payá, J. Enhancing Polyphenols and Tannins Concentration on Cotton Dyed with Red Tea. Sustainability 2023, 15, 3062. https://doi.org/10.3390/su15043062
Lambrecht L, Capablanca L, Bou-Belda E, Montava I, Díaz-García P, Gisbert-Payá J. Enhancing Polyphenols and Tannins Concentration on Cotton Dyed with Red Tea. Sustainability. 2023; 15(4):3062. https://doi.org/10.3390/su15043062
Chicago/Turabian StyleLambrecht, Louise, Lucía Capablanca, Eva Bou-Belda, Ignacio Montava, Pablo Díaz-García, and Jaime Gisbert-Payá. 2023. "Enhancing Polyphenols and Tannins Concentration on Cotton Dyed with Red Tea" Sustainability 15, no. 4: 3062. https://doi.org/10.3390/su15043062
APA StyleLambrecht, L., Capablanca, L., Bou-Belda, E., Montava, I., Díaz-García, P., & Gisbert-Payá, J. (2023). Enhancing Polyphenols and Tannins Concentration on Cotton Dyed with Red Tea. Sustainability, 15(4), 3062. https://doi.org/10.3390/su15043062









