Author Contributions
Conceptualisation, S.S. and J.L.; methodology, L.P.; formal analysis, S.V.-T.; investigation, S.V.-T., S.S. and J.L.; resources, S.S. and J.L.; data curation, L.P. and S.V.-T.; writing—original draft preparation, L.P., S.S., J.M.J. and S.V.-T.; writing—review and editing, J.L.; supervision, S.S. and J.L.; project administration, S.S. and J.L.; funding acquisition, S.S. and J.L. All authors have read and agreed to the published version of the manuscript.
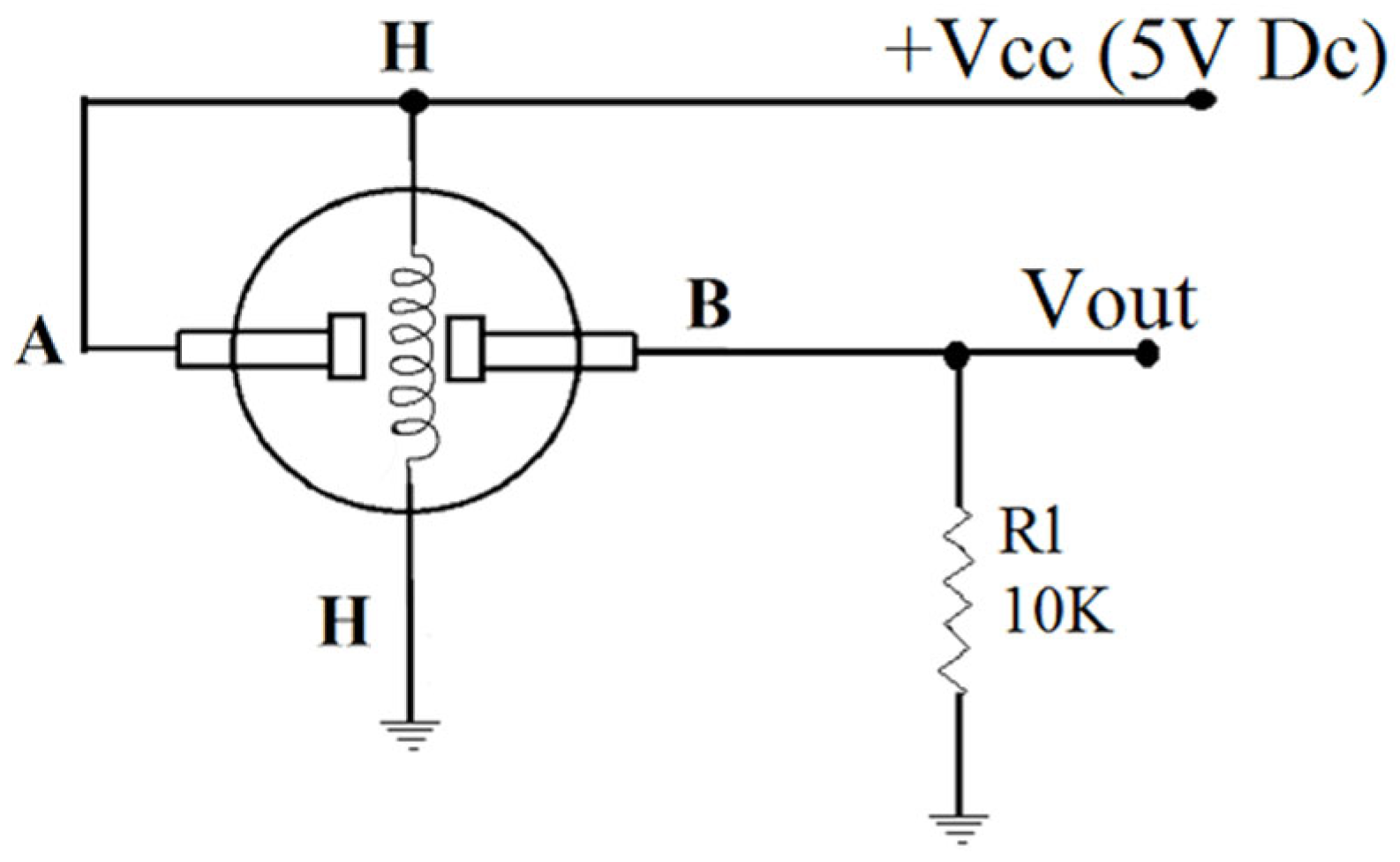
Figure 1.
MQ-XX Gas sensor connection diagram.
Figure 1.
MQ-XX Gas sensor connection diagram.
Figure 2.
Measuring device. (a) Connection diagram of our gas monitoring node. (b) Prototype developed during preliminary tests. (c) Detailed view of measuring chamber.
Figure 2.
Measuring device. (a) Connection diagram of our gas monitoring node. (b) Prototype developed during preliminary tests. (c) Detailed view of measuring chamber.
Figure 3.
Sample of essential oil of Cistus ladanifer.
Figure 3.
Sample of essential oil of Cistus ladanifer.
Figure 4.
Measures of MQ-XX sensors for Cistus ladanifer essential oil for 11 h. The axes represent the sensor response for each one of the chemical compounds, with no units. (A–F) display each of the responses to the different sensors. (A) shows the response to compound 1, (B) shows the response to compound 2, (C) shows the response to compound 3, (D) shows the response to compound 4, (E) shows the response to compound 5, and (F) shows the response to compound 6, 8 and 9.
Figure 4.
Measures of MQ-XX sensors for Cistus ladanifer essential oil for 11 h. The axes represent the sensor response for each one of the chemical compounds, with no units. (A–F) display each of the responses to the different sensors. (A) shows the response to compound 1, (B) shows the response to compound 2, (C) shows the response to compound 3, (D) shows the response to compound 4, (E) shows the response to compound 5, and (F) shows the response to compound 6, 8 and 9.
Figure 5.
Measures of MQ-XX sensors for Pinus pinaster essential oil for 11 h. The axes represent the sensor response for each one of the chemical compounds, with no units. (A–F) display each of the responses to the different sensors. (A) shows the response to compound 1, (B) shows the response to compound 2, (C) shows the response to compound 3, (D) shows the response to compound 4, (E) shows the response to compound 5, and (F) shows the response to compound 6, 8 and 9.
Figure 5.
Measures of MQ-XX sensors for Pinus pinaster essential oil for 11 h. The axes represent the sensor response for each one of the chemical compounds, with no units. (A–F) display each of the responses to the different sensors. (A) shows the response to compound 1, (B) shows the response to compound 2, (C) shows the response to compound 3, (D) shows the response to compound 4, (E) shows the response to compound 5, and (F) shows the response to compound 6, 8 and 9.
Figure 6.
Measures of MQ-XX sensors for Cistus ladanifer adulterated essential oil for 11 h. The axes represent the sensor response for each one of the chemical compounds, with no units. (A–F) display each of the responses to the different sensors. (A) shows the response to compound 1, (B) shows the response to compound 2, (C) shows the response to compound 3, (D) shows the response to compound 4, (E) shows the response to compound 5, and (F) shows the response to compound 6, 8 and 9.
Figure 6.
Measures of MQ-XX sensors for Cistus ladanifer adulterated essential oil for 11 h. The axes represent the sensor response for each one of the chemical compounds, with no units. (A–F) display each of the responses to the different sensors. (A) shows the response to compound 1, (B) shows the response to compound 2, (C) shows the response to compound 3, (D) shows the response to compound 4, (E) shows the response to compound 5, and (F) shows the response to compound 6, 8 and 9.
Figure 7.
Measures of MQ-XX sensors after removing the Cistus ladanifer essential oil for 11 h. The axes represent the sensor response for each one of the chemical compounds, with no units. (A–F) display each of the responses to the different sensors. (A) shows the response to compound 1, (B) shows the response to compound 2, (C) shows the response to compound 3, (D) shows the response to compound 4, (E) shows the response to compound 5, and (F) shows the response to compound 6, 8 and 9.
Figure 7.
Measures of MQ-XX sensors after removing the Cistus ladanifer essential oil for 11 h. The axes represent the sensor response for each one of the chemical compounds, with no units. (A–F) display each of the responses to the different sensors. (A) shows the response to compound 1, (B) shows the response to compound 2, (C) shows the response to compound 3, (D) shows the response to compound 4, (E) shows the response to compound 5, and (F) shows the response to compound 6, 8 and 9.
Figure 8.
Measures of MQ-XX sensors after removing the Pinus pinaster essential oil for 11 h. The axes represent the sensor response for each one of the chemical compounds, with no units. (A–F) display each of the responses to the different sensors. (A) shows the response to compound 1, (B) shows the response to compound 2, (C) shows the response to compound 3, (D) shows the response to compound 4, (E) shows the response to compound 5, and (F) shows the response to compound 6, 8 and 9.
Figure 8.
Measures of MQ-XX sensors after removing the Pinus pinaster essential oil for 11 h. The axes represent the sensor response for each one of the chemical compounds, with no units. (A–F) display each of the responses to the different sensors. (A) shows the response to compound 1, (B) shows the response to compound 2, (C) shows the response to compound 3, (D) shows the response to compound 4, (E) shows the response to compound 5, and (F) shows the response to compound 6, 8 and 9.
Figure 9.
Correlation coefficients of the multivariate analysis with the different data of the previous subsection. The X indicates that the correlation is not significant.
Figure 9.
Correlation coefficients of the multivariate analysis with the different data of the previous subsection. The X indicates that the correlation is not significant.
Figure 10.
Dendrogram with the data from the previous subsection (a) with all the data and (b) with the data of the initial 5 h.
Figure 10.
Dendrogram with the data from the previous subsection (a) with all the data and (b) with the data of the initial 5 h.
Figure 11.
Correlation coefficients of the multivariate analysis with the selected data. The X value indicates that the correlation is not significant.
Figure 11.
Correlation coefficients of the multivariate analysis with the selected data. The X value indicates that the correlation is not significant.
Figure 12.
Dendrograms with the selected data: (a) with all the data and (b) only with the data of the initial 5 h.
Figure 12.
Dendrograms with the selected data: (a) with all the data and (b) only with the data of the initial 5 h.
Figure 13.
Two-dimensional classification diagram of the ANN for the MQ3-3 and MQ8-1 sensors.
Figure 13.
Two-dimensional classification diagram of the ANN for the MQ3-3 and MQ8-1 sensors.
Table 1.
Existing MQ-based sensors and their sensitivities.
Table 1.
Existing MQ-based sensors and their sensitivities.
| Sensor | Sensitive to |
|---|
| MQ135 [26] | Air Quality (CO, CO2, Ammonia, Benzene, Alcohol, smoke) |
| MQ-131 [27] | Ozone |
| MQ136 [28] | Hydrogen Sulphide gas |
| MQ-137 [29] | Ammonia, NH3, Ethanol, CO |
| MQ-138 [30] | Benzene, Toluene, Alcohol, Acetone, Propane, Formaldehyde gas, Hydrogen |
| MQ214 [31] | Methane, Natural gas |
| MQ2 [32] | Methane, Butane, LPG, smoke |
| MQ3 [33] | Alcohol, Ethanol, smoke |
| MQ4 [34] | Methane, CNG Gas |
| MQ5 [35] | Natural gas, LPG |
| MQ6 [36] | LPG, butane gas |
| MQ7 [37] | Carbon Monoxide |
| MQ8 [38] | Hydrogen Gas |
| MQ-9 [39] | Carbon Monoxide, LPG, CH4 |
Table 2.
Used MQ sensors and their sensibility to the different compounds.
Table 2.
Used MQ sensors and their sensibility to the different compounds.
| Sensor | Sensible to Compound |
|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|
| MQ2 | x | x | x | x | | x | | x | |
| MQ3 | | x | x | | | | x | | |
| MQ4 | | x | x | x | x | | | | x |
| MQ5 | x | x | x | | x | | | | |
| MQ6 | x | x | x | x | x | | | | |
| MQ7 | x | x | x | x | x | | | | |
| MQ8 | x | x | x | x | x | | | | |
| MQ135 | | | x | x | | | x | | x |
Table 3.
Description of the digits of the sample ID of
Figure 10.
Table 3.
Description of the digits of the sample ID of
Figure 10.
| First Digit of the Sample ID | Description |
|---|
| 1 | Essential oil of Cistus ladanifer |
| 2 | Adulterated essential oil of Cistus ladanifer |
| 3 | Essential oil of Pinus pinaster |
| 4 | Empty chamber after Cistus ladanifer measurement |
| 5 | Empty chamber after Pinus pinaster measurement |
Table 4.
Confusion matrix when all data are used in the ANN.
Table 4.
Confusion matrix when all data are used in the ANN.
| Current Sample | Assigned Sample |
|---|
| 1 | 2 | 3 | 4 | 5 |
|---|
| 1 | 100% | 0% | 0% | 0% | 0% |
| 2 | 0% | 100% | 0% | 0% | 0% |
| 3 | 0% | 0% | 100% | 0% | 0% |
| 4 | 0% | 0% | 0% | 100% | 0% |
| 5 | 0% | 0% | 0% | 0% | 100% |
Table 5.
Summary of ANOVA results for different sensors and predefined equations for each compound.
Table 5.
Summary of ANOVA results for different sensors and predefined equations for each compound.
| Sensor | F-Value | p-Value | Selected | Sensor | F-Value | p-Value | Selected |
|---|
| MQ2-1 | 3.39 | 0.0156 | | MQ5-5 | 116.65 | 0.000 | |
| MQ2-2 | 24.98 | 0.000 | | MQ6-1 | 23.94 | 0.000 | |
| MQ2-3 | 17.44 | 0.000 | | MQ6-2 | 32.52 | 0.000 | x |
| MQ2-4 | 20.56 | 0.000 | | MQ6-3 | 5.01 | 0.0018 | |
| MQ2-6 | 25.77 | 0.000 | x | MQ6-4 | 14.88 | 0.000 | |
| MQ3-2 | 99.12 | 0.000 | | MQ6-5 | 31.35 | 0.000 | |
| MQ3-3 | 112.2 | 0.000 | x | MQ7-1 | 114.94 | 0.000 | |
| MQ3-7 | 3.09 | 0.0236 | | MQ7-2 | 766.27 | 0.000 | x |
| MQ4-2 | 12.36 | 0.000 | | MQ7-3 | 120.26 | 0.000 | |
| MQ4-3 | 17.65 | 0.000 | | MQ7-4 | 159.39 | 0.000 | |
| MQ4-4 | 33.39 | 0.000 | | MQ7-5 | 1.13 | 0.3532 | |
| MQ4-5 | 45.87 | 0.000 | | MQ8-1 | 247.82 | 0.000 | x |
| MQ4-9 | 62.04 | 0.000 | x | MQ8-2 | 110.46 | 0.000 | |
| MQ5-1 | 119.92 | 0.000 | x | MQ8-3 | 24.92 | 0.000 | |
| MQ5-2 | 25 | 0.000 | | MQ8-4 | 170.72 | 0.000 | |
| MQ5-3 | 104.87 | 0.000 | | MQ8-5 | 34.53 | 0.000 | |
| MQ4 | 108.98 | 0.000 | | | | | |
Table 6.
Summation of correctly classified cases for ANN with the combination of the selected sensors.
Table 6.
Summation of correctly classified cases for ANN with the combination of the selected sensors.
| Combination | MQ2-6 | MQ3-3 | MQ4-9 | MQ5-1 | MQ6-2 | MQ7-2 | MQ8-1 |
|---|
| MQ2-6 | x | | | | | | |
| MQ3-3 | 89.09 | x | | | | | |
| MQ4-9 | 92.73 | 96.36 | x | | | | |
| MQ5-1 | 85.45 | 85.45 | 90.91 | x | | | |
| MQ6-2 | 85.46 | 90.91 | 87.27 | 87.27 | x | | |
| MQ7-2 | 89.09 | 94.55 | 92.73 | 94.55 | 98.18 | x | |
| MQ8-1 | 98.18 | 100 | 96.36 | 96.36 | 92.73 | 89.09 | x |
Table 7.
Comparison of attained accuracy in other published papers and our case.
Table 7.
Comparison of attained accuracy in other published papers and our case.
| Ref. | Main Use | Tested Products | Included Sensors | Selected Sensors | Classification Method | Classification Accuracy (%) |
|---|
| [22] | Fraudulent products | Ghee and vanaspati | MQ2, MQ3, MQ4, MQ5, MQ6, MQ7, MQ8, MQ9, and MQ135 | MQ2, MQ3, MQ6, MQ7, MQ8 | DFA | 90.9 |
| [20] | Fraudulent products | Tomato paste with starch/pumpkin/potato | TGS2600. TGS2620, MQ3, TGS880, and TGS2610 | TGS2610 and MQ3 | PCA and FDA | 79.1 to 92.2 |
| [26] | Fraudulent products | Beef meat with pork meat | MQ2, MQ4, MQ6, MQ-9, MQ135, MQ136, MQ137, and MQ138 | All | Deep learning and PCA | 99.97 |
| [21] | Fraudulent products | Edible oils | MQ3, MQ9, MQ135, MQ136, TGS813, TGS822, TGS2602, and TGS2620 | All | CA, PCA, DFA, and ANN, among others | 97.3 |
| [23] | Quantify alcohol | Beer and wine | MQ2, MQ3, MQ4 and MQ135, | MQ3 | Several AI-based | 93.39; 86.78, 80.16, and 88.43 |
| [24] | Differentiate products | Tea | TGS800, TGS813, TGS822, TGS826, TGS832, TGS2600, TGS2610, TGS2620, MQ3, MQ5, MQ6, MQ8, MQK2, 2M00, 2M012 | MQK2, MQ8 (second option MQK2, MQ3, MQ8 | Correlation analyses and CA | 93.84, 94.35 (98.87 with 10 sensors) |
| [25] | Differentiate products | Coffee | MQ2, MQ7, MQ135, MQ-137 | All | Correlation analyses | - |
| [27] | Differentiate products | Essential oils | MQ3, MQ4, MQ8, MQ-9, MQ136, MQ136, TGS813, TGS822 | All | PCA and DFA | 100 |
| Our | Fraudulent products | Essential oils | MQ2, MQ3, MQ4, MQ5, MQ6, MQ7, MQ8 | MQ3 and MQ8 | CA and ANN | 100 |