A New Method to Predict Final Products of Red Mud-Slag-Based Alkali-Activated Materials Using Complete Phase Analysis of Precursors
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Experimental Procedure
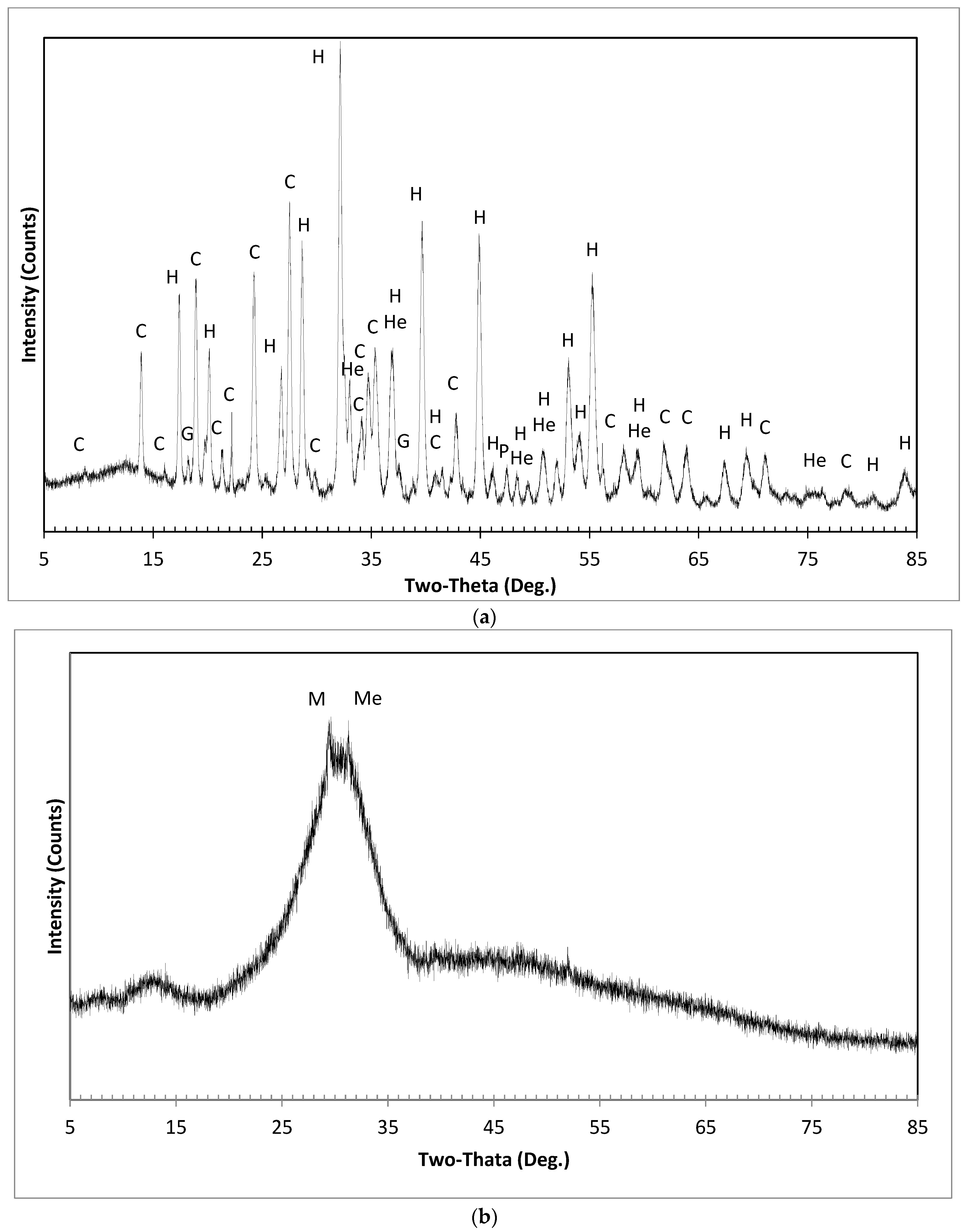
2.2.1. X-ray Diffraction
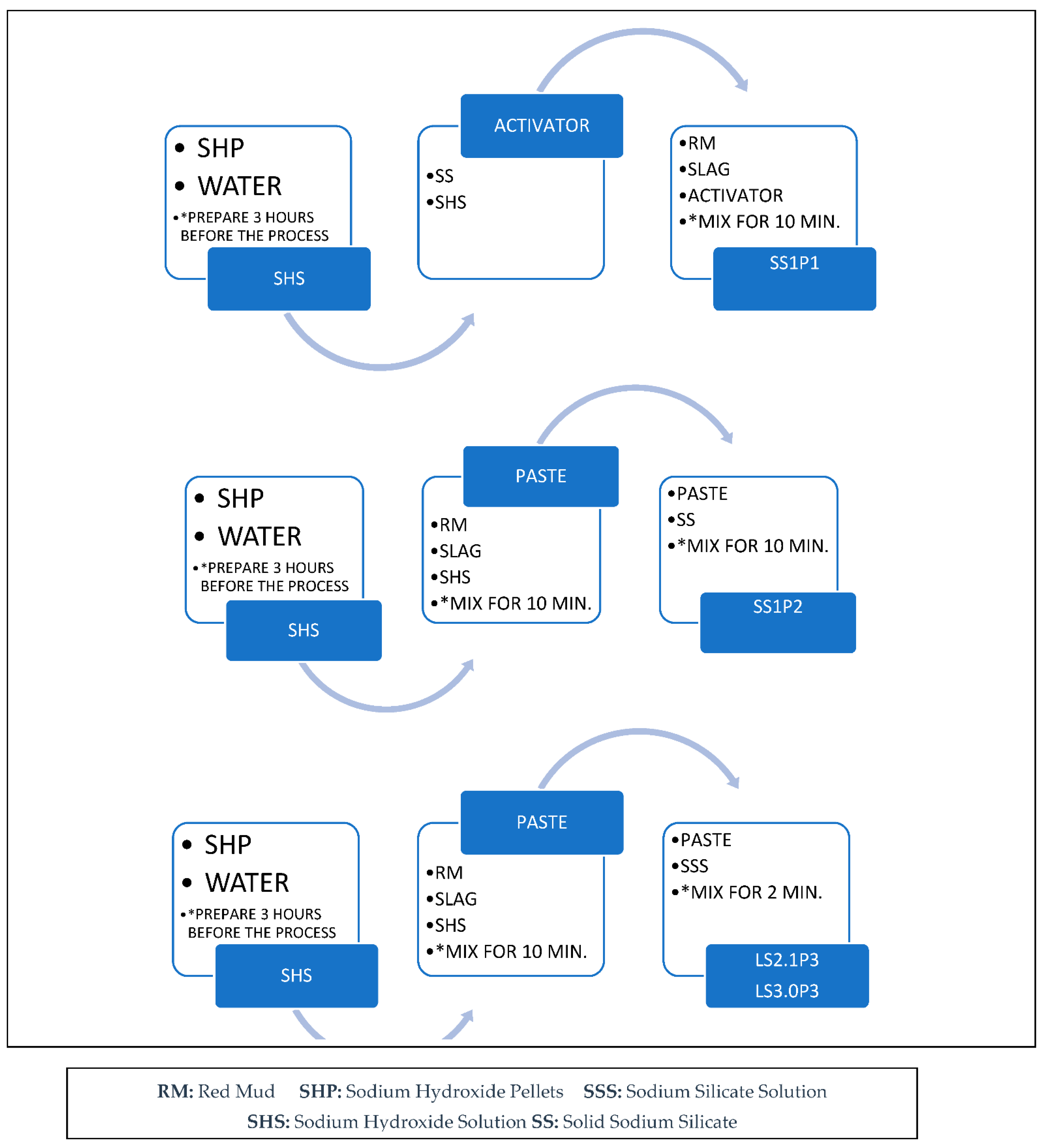
2.2.2. Mortar Sample Preparation and Mix Procedure
2.2.3. Paste Samples for XRD Analysis
3. Results and Discussion
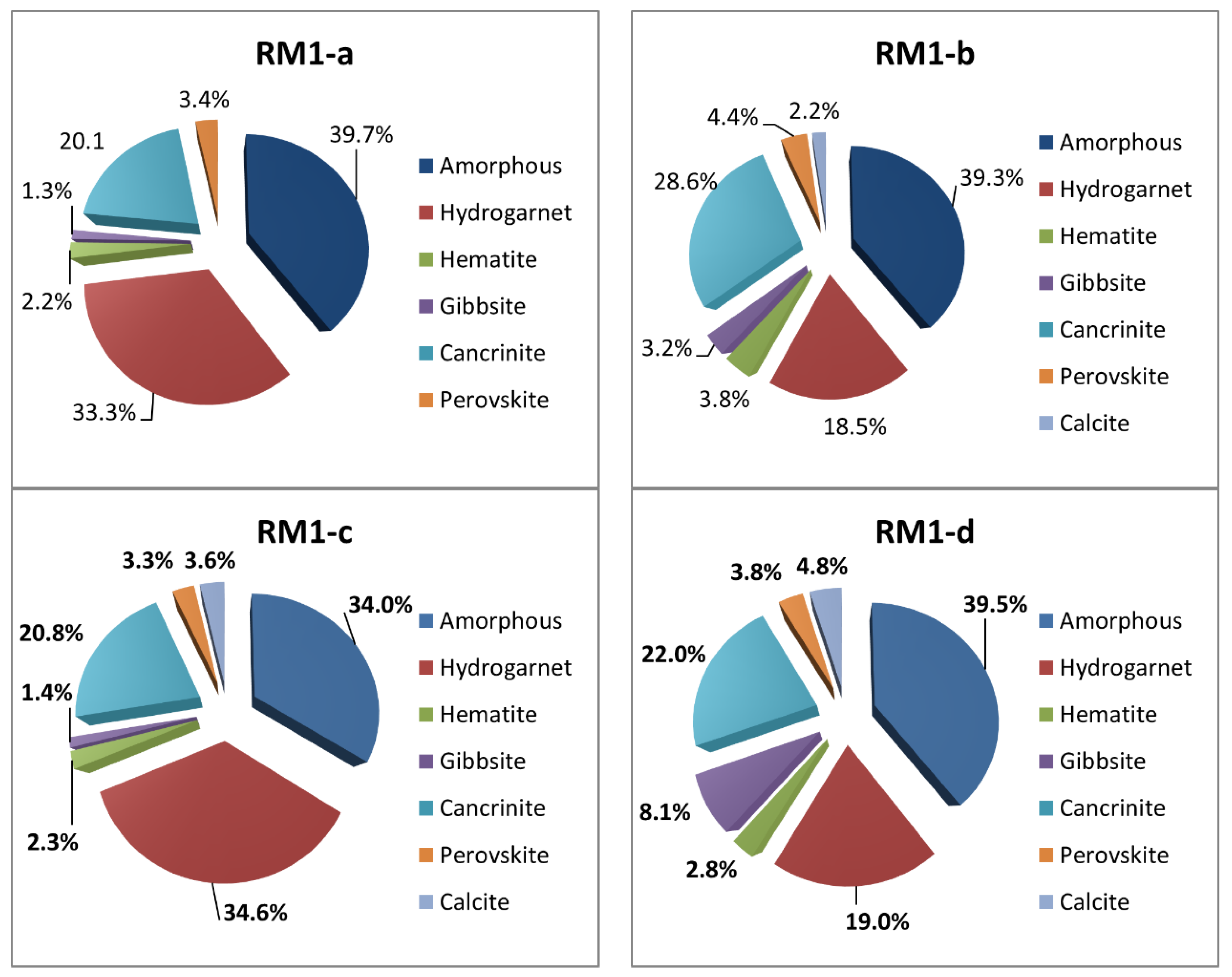
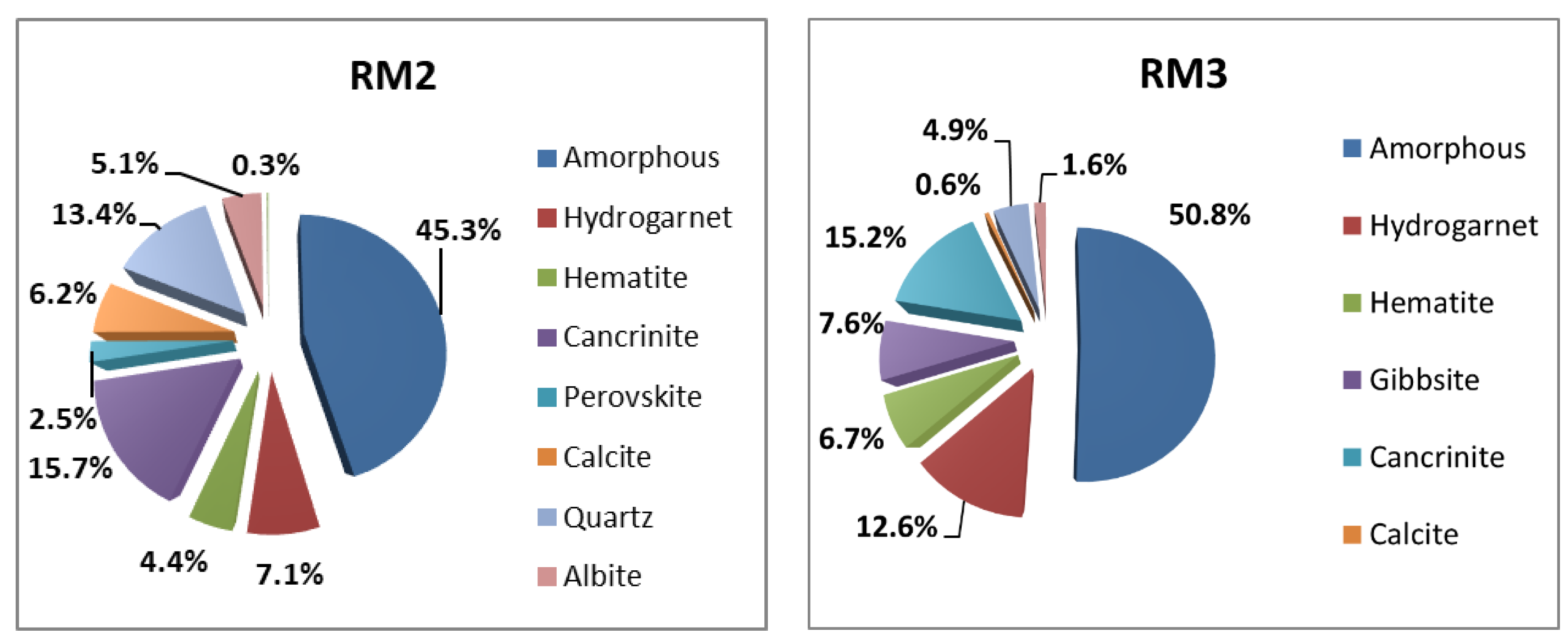
3.1. Quantitative Crystalline Phases Identification
3.2. Quantitative Vitreous Phase Identification
Reactive Alumina (Al2O3), Silica (SiO2), and Calcium Oxide (CaO)
3.3. Prediction of Final Products of RM-AAMs Based on Reactive Components
- Step 1: Based on the mix proportions of each sample, the total mass values of H2O, SiO2, Al2O3, CaO, and Na2O are computed. For example, the mass values of glassy silica in RM, slag, and sodium silicate (in solution or solid form) are accumulated for SiO2;
- Step 2: The quantities obtained in the previous step are then divided by the molar mass of each oxide or water.
- Dividing the results of Step 2 by the total molarity and then multiplying them by one hundred leads to the mole percentage of each oxide.
3.4. XRD Analysis of Final Products
4. Conclusions
- Although the XRF results present general ideas about the chemical compositions of precursors, AAMs should not be designed solely based on XRF analysis. A quantitative method could be employed to optimize the mix design of AAMs;
- A quantitative crystalline phase analysis of RM samples could identify various crystals along with mass percentages. Information about types and hardness of these crystals could help in understanding whether certain crystals may contribute in alkali-activation reactions (AARs). Increasing the concentrations of alkali solutions to dissolve the aforementioned crystals in the RM samples could unnecessarily increase material costs and adversely affect mechanical properties;
- Certain RM may contain hard crystals that can be considered micro-fillers and may enhance the mechanical properties of AAMs;
- A quantitative amorphous phase analysis of RM samples could be used to identify essential components for alkali-activation reactions, including the percentages of reactive calcium, silica, and alumina. Reasonably accurate estimations of the final products of AARs could be possible with the help of this information.
- A ternary diagram based on the total concentration of reactive CaO, SiO2, and Al2O3 can be created after a quantitative analysis of the crystalline and amorphous phases of the precursors for AAMs, such as RM and slag. This diagram could help predict the final products of alkali-activation;
- The new analysis technique for RM samples presented in this paper could successfully predict final product types, which is critical in optimizing the mix proportions of RM-slag-based AAMs;
- Depending on the amount of reactive alumina in RM samples, the formation of N-A-S-H and C (A)-S-H traces in the final products of AARs is possible. However, C-S-H was the major product of RM-slag-based AAMs for this study, which controls the mechanical properties of the AAM samples.
- Since the nanocrystals of C-S-H contribute to strength development, the Ca/Si ratio should be optimized in the design of AAMs based on RM, slag, and alkalis. Through the optimization process the amount of soluble silica, which would be available in sodium silicate solutions, is critical and should be considered in the calculation of Si.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaußen, F.M.; Friedrich, B. Phase Characterization and Thermochemical Simulation of (Landfilled) Bauxite Residue (“Red Mud”) in Different Alkaline Processes Optimized for Aluminum Recovery. Hydrometallurgy 2018, 176, 49–61. [Google Scholar] [CrossRef]
- Provis, J.; van Deventer, J. (Eds.) Alkali Activated Materials State-of-the-Art Report, RILEM TC 224-AAM, 1st ed.; Springer: Dordrecht, The Netherlands, 2014; ISBN 978-94-024-0278-0. [Google Scholar]
- Davidovits, J. Geopolymers-Inorganic Polymeric New Materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Shi, C.; Roy, D.; Krivenko, P. Alkali-Activated Cements and Concretes, 1st ed.; CRC Press: London, UK, 2003; ISBN 9781482266900. [Google Scholar]
- Buchwald, A.; Kaps, C.; Hohmann, M. Alkali-Activated Binder and Pozzolan Cement Binders-Compete Reaction or Two Side of the Story? In Proceedings of the 11th International Congress on the Chemistry of Cement (ICCC), Durban, South Africa, 11–16 May 2003; pp. 1238–1246. [Google Scholar]
- Li, Y.; Shen, L.; Mirmoghtadaei, R.; Ai, L. A Design of Experiment Approach to Study the Effects of Raw Material on the Performance of Geopolymer Concrete. Adv. Civ. Eng. Mater. 2017, 6, 20160007. [Google Scholar] [CrossRef]
- Wagh, A.; Douse, V. Silicate Bonded Unsintered Ceramics of Bayer Process Waste. J. Mater. Res. 1991, 6, 1094–1102. [Google Scholar] [CrossRef]
- Dimas, D.D.; Giannopoulou, I.P.; Panias, D. Utilization of Alumina Red Mud for Synthesis of Inorganic Polymeric Materials. Miner. Process. Extr. Metall. Rev. 2009, 30, 211–239. [Google Scholar] [CrossRef]
- Sun, W.; Feng, X.; Zhao, G. Effect of Distortion Degree on the Hydration of Red Mud Base Cementitious Material. J. Coal Sci. Eng. 2009, 15, 88–93. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S. Development of Paving Blocks from Synergistic Use of Red Mud and Fly Ash Using Geopolymerization. Constr. Build. Mater. 2013, 38, 865–871. [Google Scholar] [CrossRef]
- Pan, Z.; Cheng, L.; Lu, Y.; Yang, N. Hydration Products of Alkali-Activated Slag-Red Mud Cementitious Material. Cem. Concr. Res. 2002, 32, 357–362. [Google Scholar] [CrossRef]
- Krivenko, P.; Kovalchuk, O.; Pasko, A.; Croymans, T.; Hult, M.; Lutter, G.; Vandevenne, N.; Schreurs, S.; Schroeyers, W. Development of Alkali Activated Cements and Concrete Mixture Design with High Volumes of Red Mud. Constr. Build. Mater. 2017, 151, 819–826. [Google Scholar] [CrossRef]
- Li, C.; Zhang, N.; Zhang, J.; Song, S.; Zhang, Y. C-A-S-H Gel and Pore Structure Characteristics of Alkali-Activated Red Mud–Iron Tailings Cementitious Mortar. Materials 2022, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Yuan, S.; Straub, C.; Chen, W. Activation of Binary Binder Containing Fly Ash and Portland Cement Using Red Mud as Alkali Source and Its Application in Controlled Low-Strength Materials. J. Mater. Civ. Eng. 2020, 32, 04019356. [Google Scholar] [CrossRef]
- Song, S.; Zhang, N.; Yuan, J.; Zhang, Y. New Attempt to Produce Red Mud-Iron Tailing Based Alkali-Activated Mortar: Performance and Microstructural Characteristics. J. Build. Eng. 2021, 43, 103222. [Google Scholar] [CrossRef]
- X-ray Diffraction (XRD) and Scattering. Available online: https://www.bruker.com/products/x-ray-diffraction-and-elemental-analysis/x-ray-diffraction.html (accessed on 1 June 2020).
- Day, N. Crystallography Open Database. Available online: http://www.crystallography.net/cod/ (accessed on 1 June 2020).
- XRD Software-DIFFRAC.EVA. Available online: https://www.bruker.com/products/x-ray-diffraction-and-elemental-analysis/x-ray-diffraction/xrd-software/eva/overview.html (accessed on 1 June 2020).
- Mirmoghtadaei, R.; Shen, L. Design of Alkali-Activated Materials Based on Quantitative Microanalysis of Precursors and Reaction Kinetics. Ph.D. Thesis, University of Hawai’i at Manoa, Manoa, HI, USA, 2020. [Google Scholar]
- Coelho, A.A. TOPAS and TOPAS-Academic: An Optimization Program Integrating Computer Algebra and Crystallographic Objects Written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Li, X.; Snellings, R.; Scrivener, K.L. Quantification of Amorphous Siliceous Fly Ash in Hydrated Blended Cement Pastes by X-ray Powder Diffraction. J. Appl. Crystallogr. 2019, 52, 1358–1370. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; Provis, J.L.; van Deventer, J.S.J. Effect of Calcium Silicate Sources on Geopolymerisation. Cem. Concr. Res. 2008, 38, 554–564. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Selway, J.B.; Kato, A.; Matsubara, S.; Shimizu, M.; Grice, J.D.; Vajdak, J. Magnesiofoitite, (Mg2Al)Al6(Si6O18)(BO3)3(OH)4, a New Alkali-Deficient Tourmaline. Can. Mineral. 1999, 37, 1439–1443. [Google Scholar]
- Wikipedia Contributors Anatase. Available online: https://en.wikipedia.org/w/index.php?title=Anatase&oldid=920207787 (accessed on 1 June 2020).
- Koshy, N.; Dondrob, K.; Hu, L.; Wen, Q.; Meegoda, J.N. Mechanical Properties of Geopolymers Synthesized from Fly Ash and Red Mud under Ambient Conditions. Crystals 2019, 9, 572. [Google Scholar] [CrossRef]
- Gong, X.; Nie, Z.; Qian, M.; Liu, J.; Pederson, L.A.; Hobbs, D.T.; McDuffie, N.G. Gibbsite to Boehmite Transformation in Strongly Caustic and Nitrate Environments. Ind. Eng. Chem. Res. 2003, 42, 2163–2170. [Google Scholar] [CrossRef]
- Humad, A.M.; Provis, J.L.; Cwirzen, A. Alkali Activation of a High MgO GGBS-Fresh and Hardened Properties. Mag. Concr. Res. 2018, 70, 1256–1264. [Google Scholar] [CrossRef]
- Najafi Kani, E.; Allahverdi, A.; Provis, J.L. Efflorescence Control in Geopolymer Binders Based on Natural Pozzolan. Cem. Concr. Compos. 2012, 34, 25–33. [Google Scholar] [CrossRef]
- Škvára, F.; Kopecký, L.; Myšková, L.; Šmilauer, V.Í.T.; Alberovská, L.; Vinšová, L. Aluminosilicate Polymers-Influence of Elevated Temperatures, Efflorescence. Ceram.-Silik. 2009, 53, 276–282. [Google Scholar]
- Pacheco-Torgal, F.; Jalali, S. Influence of Sodium Carbonate Addition on the Thermal Reactivity of Tungsten Mine Waste Mud Based Binders. Constr. Build. Mater. 2010, 24, 56–60. [Google Scholar] [CrossRef]
- Lim, N.G.; Jeong, S.W.; Her, J.W.; Ann, K.Y. Properties of Cement-Free Concrete Cast by Finely Grained Nanoslag with the NaOH-Based Alkali Activator. Constr. Build. Mater. 2012, 35, 557–563. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Labrincha, J.; Leonelli, C.; Palomo, A.; Chindaprasit, P. Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier Science & Technology: Kent, UK, 2014; ISBN 9781782422884. [Google Scholar]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Resistance of Alkali-Activated Slag Concrete to Acid Attack. Cem. Concr. Res. 2003, 33, 1607–1611. [Google Scholar] [CrossRef]
- Garcia-lodeiro, I.; Palomo, A.; Fernández-jiménez, A.; Macphee, D.E. Compatibility Studies between N-A-S-H and C-A-S-H Gels. Study in the Ternary Diagram Na2O-CaO-Al2O3-SiO2-H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Grangeon, S.; Linard, Y.; Chiaberge, C. X-ray Diffraction: A Powerful Tool to Probe and Understand the Structure of Nanocrystalline Calcium Silicate Hydrates Research Papers. Struct. Sci. Cryst. Eng. Mater. 2013, B69, 465–473. [Google Scholar] [CrossRef]









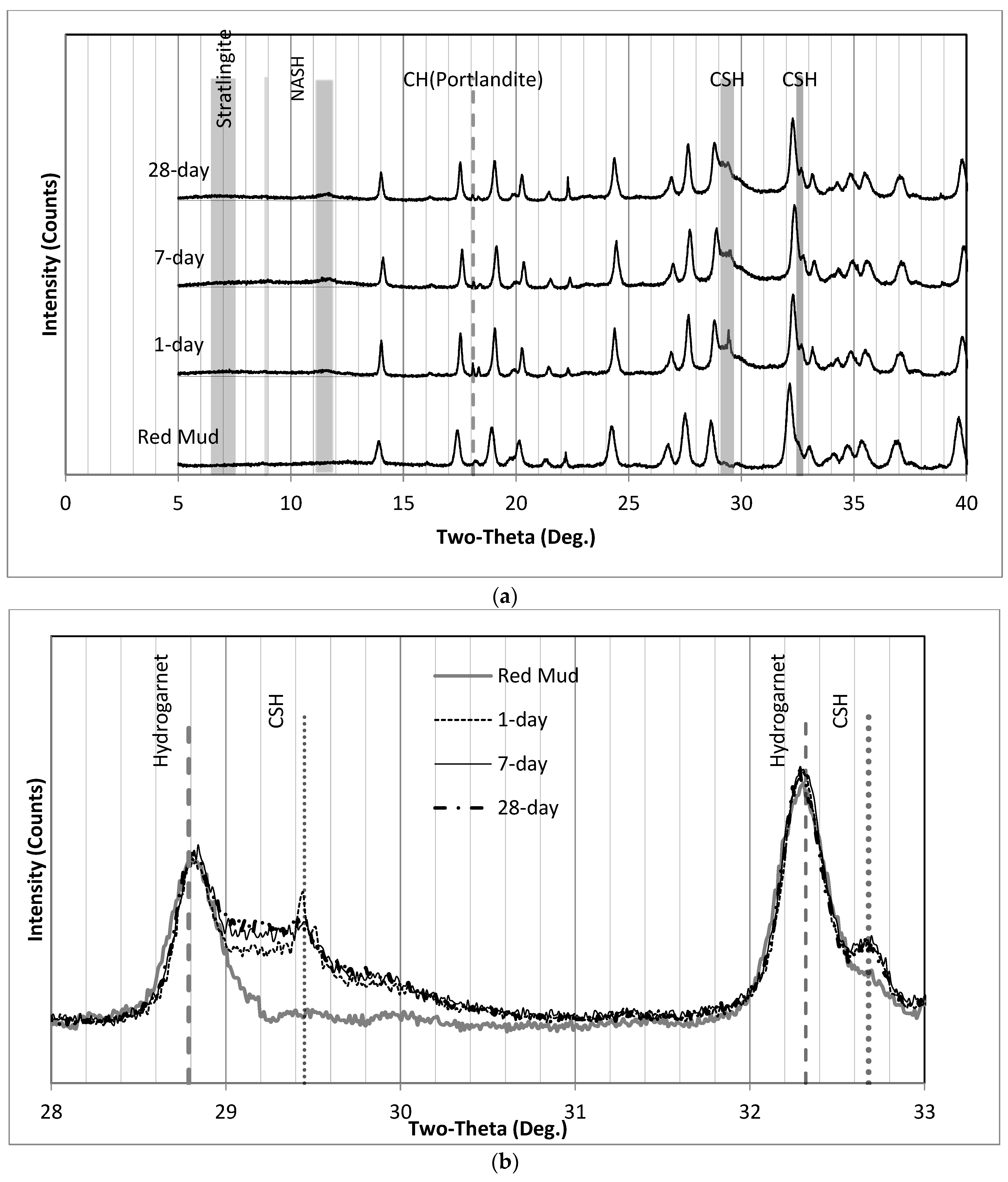

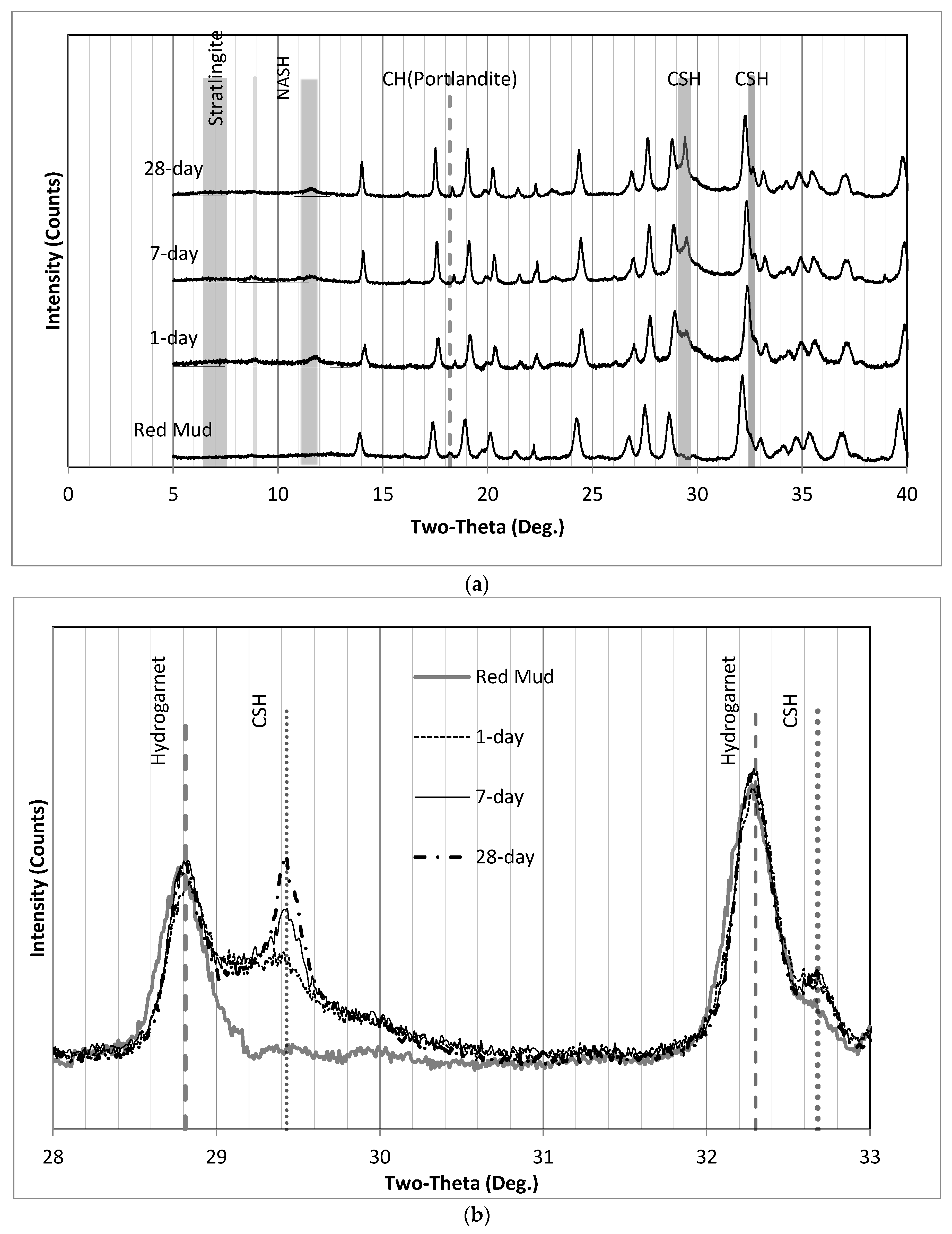
| Red Mud | Moisture Contents (%) |
|---|---|
| RM1-a | 30.67 |
| RM1-b | 37.87 |
| RM1-c | 29.94 |
| RM1-d | 2.37 |
| RM2 | 1.12 |
| RM3 | 34.86 |
| XRF Total Chemical Oxides (wt.%) | RM1-a | RM1-b | RM1-c | RM1-d | RM2 | RM3 | BFS |
|---|---|---|---|---|---|---|---|
| Al2O3 | 23.64 | 21.45 | 20.07 | 23.77 | 17.78 | 21.69 | 12.05 |
| SiO2 | 20.85 | 20.27 | 18.99 | 20.51 | 28.33 | 16.16 | 31.15 |
| CaO | 20.28 | 20.4 | 25.68 | 18.84 | 13.98 | 1.75 | 45.55 |
| Na2O | 9.32 | 7.05 | 6.59 | 8.24 | 6.73 | 12.97 | 0.31 |
| K2O | 1.48 | 1.99 | 1.67 | 2.09 | 1.62 | 0.00 | 0.58 |
| Fe2O3 | 5.58 | 9.94 | 7.89 | 6.93 | 17.29 | 31.58 | 0.49 |
| TiO2 | 4.42 | 5.69 | 4.84 | 3.94 | 3.31 | 6.08 | 0.53 |
| SO3 | 1.22 | 0.67 | 0.60 | 1.10 | 0.37 | 0.43 | 2.01 |
| MgO | 0.88 | 1.11 | 1.20 | 1.21 | 1.19 | 0.00 | 5.93 |
| P2O5 | 0.27 | 0.18 | 0.22 | 0.22 | 0.32 | 0.00 | 0.00 |
| Cl | 0.20 | 0.00 | 0.54 | 0.17 | 0.23 | 0.00 | 0.00 |
| ZrO2 | 0.15 | 0.19 | 0.17 | 0.13 | 0.00 | 0.22 | 0.03 |
| SrO | 0.11 | 0.00 | 0.14 | 0.11 | 0.14 | 0.00 | 0.09 |
| MnO | 0.00 | 0.00 | 0.00 | 0.00 | 0.13 | 0.00 | 0.11 |
| CuO | 0.00 | 0.00 | 0.00 | 0.00 | 0.15 | 0.00 | 0.00 |
| BaO | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 |
| LOI | 11.60 | 11.06 | 11.39 | 12.72 | 8.56 | 9.11 | 1.11 |
| Total w/LOI | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Row # | Mix Label | Si4+ Mole | OH− Mole | Water/ Solution | Solution/ Binder | Red Mud/ Precursor (wt.%) | BFS/ Precursor (wt.%) | Sand/ Precursor |
|---|---|---|---|---|---|---|---|---|
| 1 | RM1-aS40Ch1 | 0.91 | 2.30 | 0.84 | 0.37 | 60 | 40 | 1.00 |
| 2 | RM1-aS20Ch1 | 0.79 | 2.00 | 0.86 | 0.42 | 80 | 20 | |
| 3 | RM1-aS20Ch2 | 1.14 | 1.80 | 0.84 | 0.44 | 80 | 20 | |
| 4 | RM1-aS30Ch1 | 1.11 | 2.10 | 0.84 | 0.39 | 70 | 30 | |
| 5 | RM1-aS30Ch2-1 | 0.14 | 1.90 | 0.83 | 0.43 | 70 | 30 | |
| 6 | RM1-aS40Ch2-1 | 0.17 | 2.40 | 0.79 | 0.37 | 60 | 40 | |
| 7 | RM1-aS30Ch2-2 | 0.18 | 2.70 | 0.78 | 0.37 | 70 | 30 | |
| 8 | RM1-aS30Ch2-3 | 0.26 | 2.40 | 0.74 | 0.4 | 70 | 30 | |
| 9 | RM1-aS30Ch2-4 | 0.34 | 2.30 | 0.71 | 0.42 | 70 | 30 | |
| 10 | RM1-aS30Ch2-5 | 0.28 | 2.60 | 0.73 | 0.58 | 70 | 30 | |
| 11 | RM1-aS30Ch2-6 | 0.25 | 2.40 | 0.75 | 0.6 | 70 | 30 | |
| 12 | RM1-aS30Ch3 | 1.11 | 2.10 | 0.84 | 0.39 | 70 | 30 | |
| 13 | RM1-bS20Ch1 | 0.43 | 1.80 | 0.89 | 0.46 | 80 | 20 | |
| 14 | RM1-bS30Ch2 | 0.99 | 1.90 | 0.85 | 0.43 | 70 | 30 | |
| 15 | RM1-cS20Ch1 | 1.46 | 1.60 | 0.82 | 0.49 | 80 | 20 | |
| 16 | RM1-cS30Ch2 | 1.12 | 2.10 | 0.84 | 0.39 | 70 | 30 | |
| 17 | RM1-dS30Ch1 | 1.05 | 2.00 | 0.84 | 0.35 | 70 | 30 | |
| 18 | RM2S40Ch1 | 1.28 | 2.40 | 0.82 | 0.31 | 60 | 40 | |
| 19 | RM2S25Ch1 | 1.16 | 2.20 | 0.83 | 0.33 | 75 | 25 | |
| 20 | RM3S40Ch1 | 0.62 | 2.10 | 0.87 | 0.33 | 60 | 40 | |
| 21 | RM3S20Ch2 | 0.61 | 1.50 | 0.89 | 0.4 | 80 | 20 |
| Mix Label | Si4+ Mole | OH− Mole | Water/ Solution | Solution/ Binder | Red Mud/ Precursor (wt.%) | BFS/ Precursor (wt.%) | Sodium Silicate Type | Mix Proc. |
|---|---|---|---|---|---|---|---|---|
| SS1P1 | 1.11 | 2.11 | 0.84 | 0.39 | 70 | 30 | Solid | P1 |
| SS1P2 | P2 | |||||||
| LS2.1P3 | Solution (Si/Na = 2.1) | P3 | ||||||
| LS3.0P3 | Solution (Si/Na = 3.0) |
| RM Components | Al2O3 | SiO2 | CaO | Na2O | K2O | Fe2O3 | TiO2 | SO3 | MgO | |
|---|---|---|---|---|---|---|---|---|---|---|
| RM1-a | XRF | 23.6 | 20.9 | 20.3 | 9.3 | 1.5 | 5.5 | 4.5 | 1.2 | 0.9 |
| Crystalline, % by mass | 18.8 | 7.0 | 15.5 | 4.7 | 0.0 | 2.2 | 2.1 | 0.0 | 0.0 | |
| Amorphous, % by mass | 4.8 | 13.9 | 4.8 | 4.6 | 1.5 | 3.3 | 2.4 | 1.2 | 0.9 | |
| RM1-b | XRF | 21.5 | 20.3 | 20.4 | 7.1 | 2.0 | 9.9 | 5.7 | 0.7 | 1.1 |
| Crystalline, % by mass | 18.5 | 10.1 | 8.7 | 7.0 | 0.0 | 3.7 | 0.0 | 0.0 | 0.0 | |
| Amorphous, % by mass | 3.0 | 10.2 | 11.7 | 0.1 | 2.0 | 6.2 | 5.7 | 0.7 | 1.1 | |
| RM1-c | XRF | 20.1 | 19.0 | 25.7 | 6.6 | 1.7 | 7.9 | 4.8 | 0.6 | 1.2 |
| Crystalline, % by mass | 19.6 | 10.3 | 14.0 | 5.5 | 0.0 | 2.8 | 2.0 | 0.0 | 0.0 | |
| Amorphous, % by mass | 0.5 | 8.7 | 11.7 | 1.1 | 1.7 | 5.1 | 2.8 | 0.6 | 1.2 | |
| RM1-d | XRF | 23.8 | 20.5 | 18.8 | 8.3 | 2.1 | 6.9 | 4.0 | 1.1 | 1.2 |
| Crystalline, % by mass | 20.1 | 7.6 | 11.9 | 5.2 | 0.0 | 2.8 | 2.2 | 0.0 | 0.0 | |
| Amorphous, % by mass | 3.7 | 12.9 | 6.9 | 3.1 | 2.1 | 4.1 | 1.8 | 1.1 | 1.2 | |
| RM2 | XRF | 17.8 | 28.3 | 14.0 | 6.8 | 1.6 | 17.3 | 3.4 | 0.4 | 1.2 |
| Crystalline, % by mass | 8.4 | 23.0 | 8.1 | 3.8 | 0.0 | 4.4 | 1.5 | 0.0 | 0.0 | |
| Amorphous, % by mass | 9.4 | 5.3 | 5.9 | 3.0 | 1.6 | 12.9 | 1.9 | 0.4 | 1.2 | |
| RM3 | XRF | 21.7 | 16.2 | 1.8 | 13.0 | 0.0 | 31.6 | 6.1 | 0.4 | 0.0 |
| Crystalline, % by mass | 15.5 | 12.1 | 0.4 | 7.0 | 0.0 | 7.2 | 1.7 | 0.0 | 0.0 | |
| Amorphous, % by mass | 6.2 | 4.1 | 1.4 | 6.0 | 0.0 | 24.4 | 4.4 | 0.4 | 0.0 | |
| Row Number | Mix Label | n(H2O) (mol%) | n(SiO2) (mol%) | n(Al2O3) (mol%) | n(CaO) (mol%) | n(Na2O) (mol%) | CaO/SiO2 | Al2O3/SiO2 |
|---|---|---|---|---|---|---|---|---|
| 1 | RM1S40Ch1 | 70.95 | 10.84 | 2.05 | 11.14 | 5.02 | 1.03 | 0.19 |
| 2 | RM1S20Ch1 | 78.73 | 8.39 | 1.49 | 6.26 | 5.13 | 0.75 | 0.18 |
| 3 | RM1S20Ch2 | 78.98 | 8.55 | 1.39 | 5.82 | 5.27 | 0.68 | 0.16 |
| 4 | RM1S30Ch1 | 74.12 | 9.72 | 1.78 | 8.71 | 5.68 | 0.9 | 0.18 |
| 5 | RM1S30Ch2-1 | 75.47 | 9.21 | 1.69 | 8.28 | 5.36 | 0.9 | 0.18 |
| 6 | RM1S40Ch2-1 | 71.58 | 10.38 | 1.96 | 10.62 | 5.47 | 1.02 | 0.19 |
| 7 | RM1S30Ch2-2 | 72.81 | 10.08 | 1.85 | 9.06 | 6.2 | 0.9 | 0.18 |
| 8 | RM1S30Ch2-3 | 73.73 | 9.92 | 1.68 | 8.26 | 6.41 | 0.83 | 0.17 |
| 9 | RM1S30Ch2-4 | 74.58 | 9.76 | 1.52 | 7.44 | 6.71 | 0.76 | 0.16 |
| 10 | RM1S30Ch2-5 | 76.93 | 8.32 | 1.17 | 5.75 | 7.83 | 0.69 | 0.14 |
| 11 | RM1S30Ch2-6 | 78.87 | 7.62 | 1.07 | 5.26 | 7.17 | 0.69 | 0.14 |
| 12 | RM1S30Ch3 | 74.17 | 9.65 | 1.78 | 8.72 | 5.68 | 0.9 | 0.18 |
| 13 | RM2S20Ch1 | 82.64 | 5.84 | 1.02 | 7.18 | 3.33 | 1.23 | 0.17 |
| 14 | RM2S30Ch2 | 75.36 | 8.84 | 1.33 | 9.32 | 5.16 | 1.05 | 0.15 |
| 15 | RM3S20Ch1 | 80.86 | 7.19 | 0.62 | 6.77 | 4.56 | 0.94 | 0.09 |
| 16 | RM3S30Ch2 | 74.55 | 8.6 | 1.15 | 10.8 | 4.9 | 1.26 | 0.13 |
| 17 | RM4S30Ch1 | 73.35 | 10.17 | 1.72 | 9.47 | 5.28 | 0.93 | 0.17 |
| 18 | RM5S40Ch1 | 68.04 | 10.08 | 3.36 | 12.68 | 5.83 | 1.26 | 0.33 |
| 19 | RM5S25Ch1 | 73.87 | 7.88 | 3.2 | 9.08 | 5.96 | 1.15 | 0.41 |
| 20 | RM6S40Ch1 | 70.89 | 9.41 | 2.58 | 12.18 | 4.93 | 1.29 | 0.27 |
| 21 | RM6S20Ch2 | 81.86 | 5.7 | 1.86 | 5.83 | 4.75 | 1.02 | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirmoghtadaei, R.; Shen, L.; Hargraves, J. A New Method to Predict Final Products of Red Mud-Slag-Based Alkali-Activated Materials Using Complete Phase Analysis of Precursors. Sustainability 2023, 15, 3473. https://doi.org/10.3390/su15043473
Mirmoghtadaei R, Shen L, Hargraves J. A New Method to Predict Final Products of Red Mud-Slag-Based Alkali-Activated Materials Using Complete Phase Analysis of Precursors. Sustainability. 2023; 15(4):3473. https://doi.org/10.3390/su15043473
Chicago/Turabian StyleMirmoghtadaei, Reza, Lin Shen, and Jonathan Hargraves. 2023. "A New Method to Predict Final Products of Red Mud-Slag-Based Alkali-Activated Materials Using Complete Phase Analysis of Precursors" Sustainability 15, no. 4: 3473. https://doi.org/10.3390/su15043473
APA StyleMirmoghtadaei, R., Shen, L., & Hargraves, J. (2023). A New Method to Predict Final Products of Red Mud-Slag-Based Alkali-Activated Materials Using Complete Phase Analysis of Precursors. Sustainability, 15(4), 3473. https://doi.org/10.3390/su15043473








