Microbial Enzyme Systems in the Production of Second Generation Bioethanol
Abstract
:1. Introduction
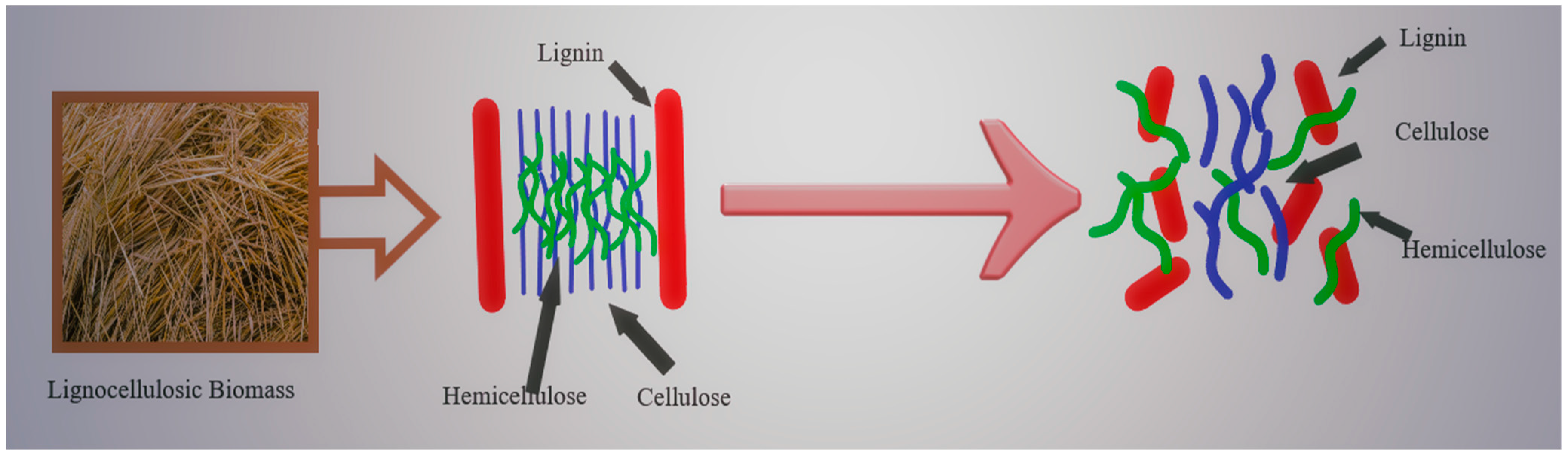
2. Composition of Agricultural and Agro-Industrial Waste Biomass, the Feedstocks for Second Generation Bioethanol
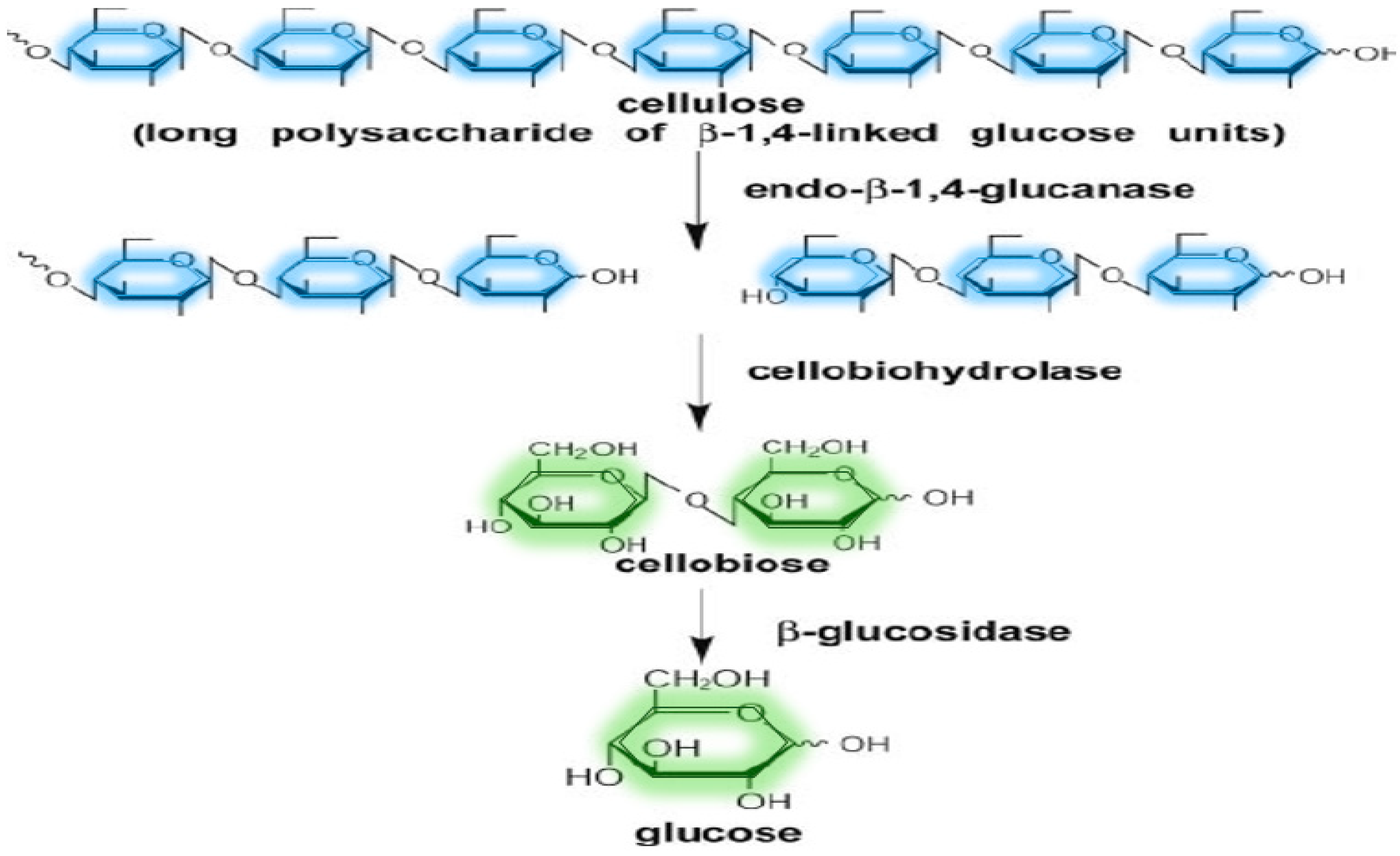
2.1. Cellulose (C6H10O5)n
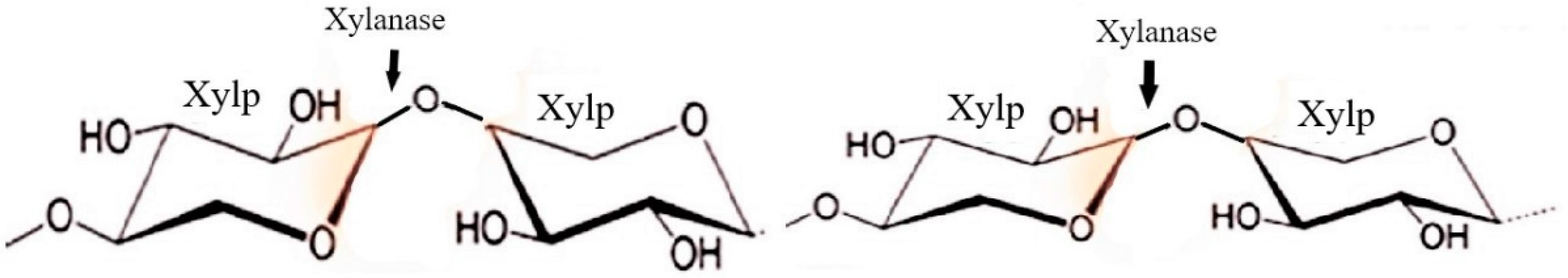
2.2. Hemicellulose (C5H8O4)n
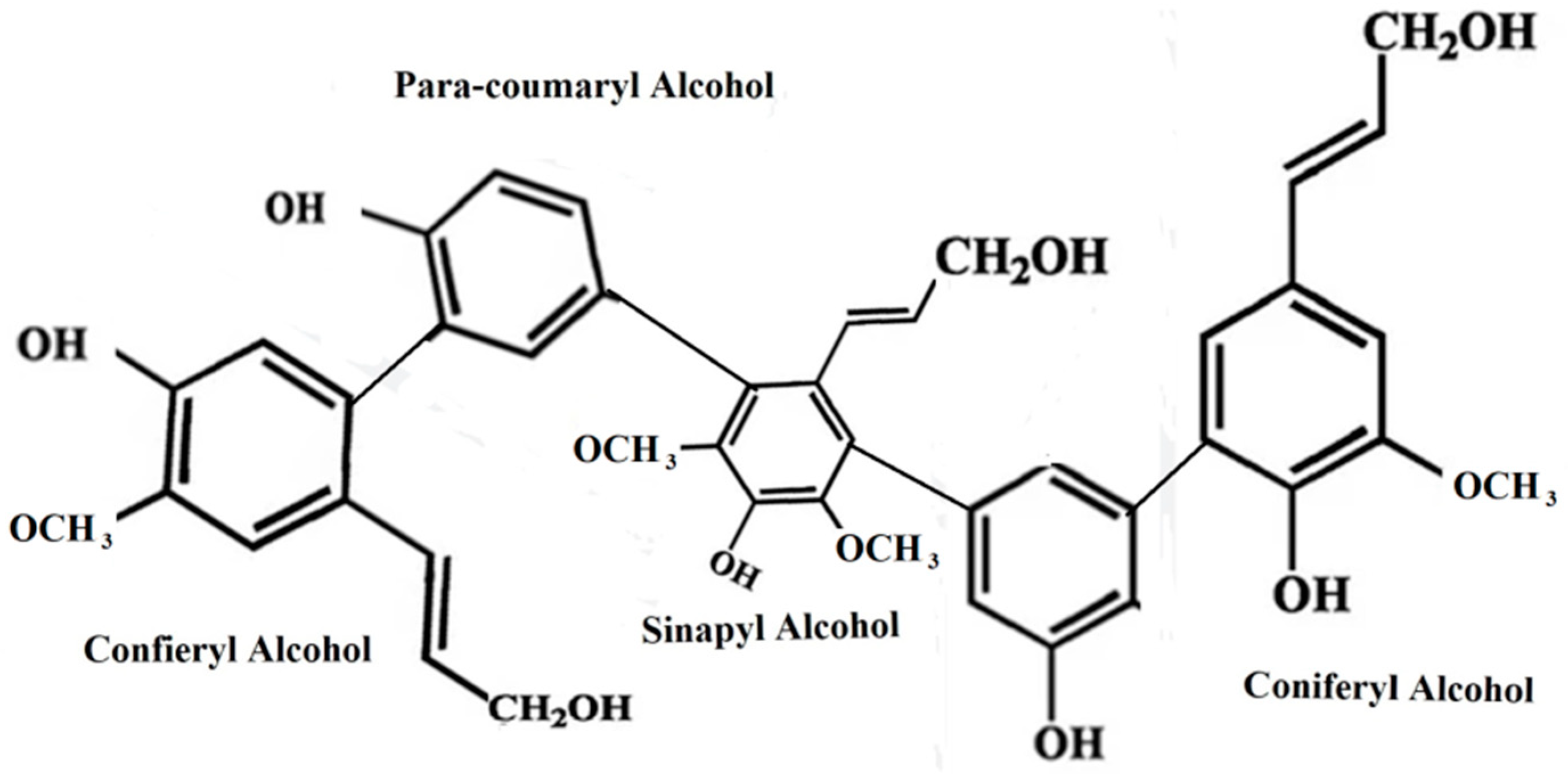
2.3. Lignin
2.4. Pectin
2.5. Starch
3. Conversion of Agricultural and Agro-Industrial Residues into Bioethanol
3.1. Pretreatment
3.1.1. Goal of Pretreatment
3.1.2. Factors Affecting the Choice of Pretreatment
3.1.3. Types of Pretreatments
Biological Pretreatment
3.2. Hydrolysis to Release Free Sugars for Fermentation into Ethanol
3.2.1. Acid Hydrolysis
3.2.2. Enzymatic Hydrolysis
Microbial Enzymes Involved in the Hydrolysis of Feedstocks for the Production of Second-Generation Bioethanol
Cellulases
Hemicellulases
Pectinases
Amylases
4. Production of Microbial Enzymes for Use in the Generation of Second-Generation Bioethanol
4.1. Solid-State Fermentation (SSF)
4.2. Liquid State Fermentation (Submerged and Surface)
5. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Bank. Urban Population (% of Total). Available online: http://data.worldbank.org/indicator/SP.URB.TOTL.IN.ZS. (accessed on 15 January 2019).
- Leahy, S. City Emits 60% More Carbon than Thought. National Geographic. 6 March 2018. Available online: https://www.nationalgeographic.com/news/2018/03/city-consumption-greenhouse-gases-carbon-c40-spd/ (accessed on 16 November 2022).
- Qiao, W.; Lu, H.; Zhou, G.; Azimi, M.; Yang, Q.; Tian, W. A hybrid algorithm for carbon dioxide emissions forecasting based on improved lion swarm optimizer. J. Clean. Prod. 2020, 244, 118612. [Google Scholar] [CrossRef]
- Hanaki, K.; Portugal-Pereira, J. The effect of biofuel production on greenhouse gas emission reductions. In Biofuels and Sustainability; Springer: Tokyo, Japan, 2018; pp. 53–71. [Google Scholar]
- Moukamnerd, C.; Kawahara, H.; Katakura, Y. Feasibility study of ethanol production from food wastes by consolidated continuous solid-state fermentation. J. Sustain. Bioenerg. Syst. 2013, 3, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Xue, J.; Yang, Y.; Lee, S.W. Thermal properties and combustion-related problems prediction of agricultural crop residues. Energies 2021, 14, 4619. [Google Scholar] [CrossRef]
- Subramaniam, Y.; Masron, T.A. The impact of economic globalization on biofuel in developing countries. Energy Convers. Manag. 2021, 1, 100064. [Google Scholar] [CrossRef]
- IEA. Bioenergy; IEA: Paris, France, 2022; Available online: https://www.iea.org/reports/bioenergy (accessed on 14 November 2022).
- Chugh, P.; Kaur, J.; Soni, R.; Sharma, A.; Soni, S.K. A low-cost process for efficient hydrolysis of deoiled rice bran and ethanol production using an inhouse produced multi-enzyme preparation from Aspergillus niger P-19. J. Mater. Cycles Waste Manag. 2022, 25, 359–375. [Google Scholar] [CrossRef]
- Kim, J.R.; Karthikeyan, K.G. Effects of severe pretreatment conditions and lignocellulose-derived furan byproducts on anaerobic digestion of dairy manure. Bioresour. Technol. 2021, 340, 125632. [Google Scholar] [CrossRef]
- Soni, S.K.; Dhull, N.P.; Soni, R.; Sharma, A. Microbiofuels: The Sustainable Energy Source for the Future. In Genomic, Proteomics, and Biotechnology; CRC Press: Boca Raton, FA, USA, 2022; pp. 357–380. [Google Scholar]
- Ziolkowska, J.R. Biofuels technologies: An overview of feedstocks, processes, and technologies. In Biofuels for a More Sustainable Future; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–19. [Google Scholar]
- Xie, Y.; Khoo, K.S.; Chew, K.W.; Devadas, V.V.; Phang, S.J.; Lim, H.R.; Show, P.L. Advancement of renewable energy technologies via artificial and microalgae photosynthesis. Bioresour. Technol. 2022, 363, 127830. [Google Scholar] [CrossRef]
- Arun, N.; Dalai, A.K. Environmental and socioeconomic impact assessment of biofuels from lignocellulosic biomass. In Lignocellulosic Biomass to Liquid Biofuels; Academic Press: Cambridge, MA, USA, 2020; pp. 283–299. [Google Scholar]
- Ahmed, J.O. The effect of biofuel crops cultivation on food prices stability and food security-A Review. Eurasian J. Biosci. 2020, 14, 613–621. [Google Scholar]
- Lima, D.R.S.; de Oliveira Paranhos, A.G.; Adarme, O.F.H.; Baêta, B.E.L.; Gurgel, L.V.A.; dos Santos, A.S.; de Aquino, S.F. Integrated production of second-generation ethanol and biogas from sugarcane bagasse pretreated with ozone. Biomass Convers. Biorefin. 2022, 12, 809–825. [Google Scholar] [CrossRef]
- Singh, A.; Prajapati, P.; Vyas, S.; Gaur, V.K.; Sindhu, R.; Binod, P.; Varjani, S. A comprehensive review of feed-stocks as sustainable substrates for next-generation biofuels. Bioenergy Res. 2022, 1–18. Available online: https://link.springer.com/article/10.1007/s12155-022-10440-2 (accessed on 21 November 2022).
- Machineni, L. Lignocellulosic biofuel production: Review of alternatives. Biomass Convers. Bioref. 2020, 10, 779–791. [Google Scholar] [CrossRef]
- Babu, S.; Rathore, S.S.; Singh, R.; Kumar, S.; Singh, V.K.; Yadav, S.K.; Wani, O.A. Exploring agricultural waste biomass for energy, food and feed production and pollution mitigation: A review. Bioresour. Technol. 2022, 360, 127566. [Google Scholar] [CrossRef]
- Mina, D.; Hadi, S.; Jalal, A. The incorporated environmental policies and regulations into bioenergy supply chain management: A literature review. Sci. Total Environ. 2022, 820, 153202. [Google Scholar]
- Santos, F.; Eichler, P.; de Queiroz, J.H.; Gomes, F. Production of second-generation ethanol from sugarcane. In Sugarcane Biorefinery, Technology and Perspectives; Academic Press: Cambridge, MA, USA, 2020; pp. 195–228. [Google Scholar]
- Oliva-Taravilla, A.; Moreno, A.D.; Demuez, M.; Ibarra, D.; Tomás-Pejó, E.; González-Fernández, C. Unraveling the effects of laccase treatment on enzymatic hydrolysis of steam-exploded wheat straw. Bioresour. Technol. 2015, 175, 209–215. [Google Scholar] [CrossRef]
- Khan, M.F.S.; Akbar, M.; Xu, Z.; Wang, H. A review on the role of pretreatment technologies in the hydrolysis of lignocellulosic biomass of corn stover. Biomass Bioenergy 2021, 155, 106276. [Google Scholar] [CrossRef]
- Verma, N.; Kumar, V. Impact of process parameters and plant polysaccharide hydrolysates in cellulase production by Trichoderma reesei and Neurospora crassa under wheat bran based solid state fermentation. Biotechnol. Rep. 2020, 25, e00416. [Google Scholar] [CrossRef]
- Partida-Sedas, G.; Montes-García, N.; Carvajal-Zarrabal, O.; López-Zamora, L.; Gómez-Rodríguez, J.; Aguilar-Uscanga, M.G. Optimization of hydrolysis process to obtain fermentable sugars from sweet sorghum bagasse using a Box–Behnken design. Sugar Tech. 2017, 19, 317–325. [Google Scholar] [CrossRef]
- Díaz-González, A.; Perez Luna, M.Y.; Ramírez Morales, E.; Saldaña-Trinidad, S.; Rojas Blanco, L.; de la Cruz-Arreola, S.; Robles-Ocampo, J.B. Assessment of the Pretreatments and Bioconversion of Lignocellulosic Biomass Recovered from the Husk of the Cocoa Pod. Energies 2022, 15, 3544. [Google Scholar] [CrossRef]
- Kou, L.; Song, Y.; Zhang, X.; Tan, T. Comparison of four types of energy grasses as lignocellulosic feedstock for the production of bio-ethanol. Bioresour. Technol. 2017, 241, 424–429. [Google Scholar] [CrossRef]
- Chugh, P.; Soni, R.; Soni, S.K. Deoiled rice bran: A substrate for co-production of a consortium of hydrolytic enzymes by Aspergillus niger P-19. Waste Biomass. Valor. 2016, 7, 513–525. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuel Bioprod, Bioref. 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Prasad, V.; Siddiqui, L.; Mishra, P.K.; Ekielski, A.; Talegaonkar, S. Recent advancements in lignin valorization and biomedical applications: A patent review. Recent Pat. Nanotechnol. 2022, 16, 107–127. [Google Scholar] [CrossRef]
- Picot-Allain, M.C.N.; Ramasawmy, B.; Emmambux, M.N. Extraction, characterisation, and application of pectin from tropical and sub-tropical fruits: A review. Food Rev. Int. 2022, 38, 282–312. [Google Scholar] [CrossRef]
- Gunaratne, A.; Corke, H. Starch, Analysis of Quality. Ref. Modul. Food Sci. 2016, 3, 202–212. [Google Scholar]
- Saini, J.K.; Kaur, A.; Mathur, A. Strategies to enhance enzymatic hydrolysis of lignocellulosic biomass for biorefinery applications: A review. Bioresour. Technol. 2022, 360, 127517. [Google Scholar] [CrossRef]
- Lay, C.H.; Dharmaraja, J.; Shobana, S.; Arvindnarayan, S.; Priya, R.K.; Saratlae, R.; Kumar, G. Lignocellulose biohydrogen towards net zero emission: A review on recent developments. Bioresour. Technol. 2022, 364, 128084. [Google Scholar] [CrossRef]
- Periyasamy, S.; Isabel, J.B.; Kavitha, S.; Karthik, V.; Mohamed, B.A.; Gizaw, D.G.; Aminabhavi, T.M. Recent Advances in Consolidated Bioprocessing for Conversion of Lignocellulosic Biomass into Bioethanol-A Review. Chem. Eng. J. 2022, 453, 139783. [Google Scholar] [CrossRef]
- Sharma, A.; Aggarwal, N.K. Pretreatment Strategies: Unlocking of Lignocellulosic Substrate. In Water Hyacinth: A Potential Lignocellulosic Biomass for Bioethanol; Springer: Berlin/Heidelberg, Germany, 2020; pp. 37–49. [Google Scholar]
- Meng, X.; Yoo, C.G.; Li, M.; Ragauskas, A.J. Physicochemical structural changes of cellulosic substrates during enzymatic saccharification. J. Appl. Biotechnl. Bioeng. 2016, 1, 87–94. [Google Scholar]
- Mahmood, H.; Moniruzzaman, M.; Iqbal, T.; Khan, M.J. Recent advances in the pretreatment of lignocellulosic biomass for biofuels and value-added products. Curr. Opin. Green Sustain. Chem. 2019, 20, 18–24. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.E.; Dale, B.E.; Elander, R.; Lee, Y.Y.; Holtzapple, M.T. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- García, V.; Päkkilä, J.; Ojamo, H.; Muurinen, E.; Keiski, R.L. Challenges in biobutanol production: How to improve the efficiency? Renew. Sustain. Energ. Rev. 2011, 15, 964–980. [Google Scholar] [CrossRef]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.V.T.; Vergaram, P.; Carbajo, J.M.; Villar, J.C.; dos Santos Rocha, J.M.; de Sousa, M.D.G.V. Bioconversion of pine stumps to ethanol: Pretreatment and simultaneous saccharification and fermentation. Holzforschung 2020, 74, 212–216. [Google Scholar] [CrossRef] [Green Version]
- Masran, R.; Zanirun, Z.; Bahrin, E.K.; Ibrahim, M.F.; Lai Yee, P.; Abd-Aziz, S. Harnessing the potential of ligninolytic enzymes for lignocellulosic biomass pretreatment. Appl. Microbiol. Biotechnol. 2016, 100, 5231–5246. [Google Scholar] [CrossRef] [PubMed]
- Gandam, P.K.; Chinta, M.L.; Pabbathi, N.P.P.; Baadhe, R.R.; Sharma, M.; Thakur, V.K.; Gupta, V.K. Second-generation bioethanol production from corncob–A comprehensive review on pretreatment and bioconversion strategies, including techno-economic and lifecycle perspective. Ind. Crops Prod. 2022, 186, 115245. [Google Scholar]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef]
- Periyasamy, S.; Karthik, V.; Senthil Kumar, P.; Isabel, J.B.; Temesgen, T.; Hunegnaw, B.M.; Vo, D.V.N. Chemical, physical and biological methods to convert lignocellulosic waste into value-added products. A review. Environ. Chem. Lett. 2022, 20, 1129–1152. [Google Scholar] [CrossRef]
- Aftab, M.N.; Iqbal, I.; Riaz, F.; Karadag, A.; Tabatabaei, M. Different Pretreatment Methods of Lignocellulosic Biomass for Use in Biofuel Production. In Biomass for Bioenergy-Recent Trends and Future Challenges; Intechopen: London, UK, 2019; pp. 15–92. [Google Scholar]
- Gu, B.J.; Dhumal, G.S.; Wolcott, M.P.; Ganjyal, G.M. Disruption of lignocellulosic biomass along the length of the screws with different screw elements in a twin-screw extruder. Bioresour. Technol. 2019, 275, 266–271. [Google Scholar] [CrossRef]
- Kumari, D.; Chahar, P.; Singh, R. Effect of ultrasonication on biogas and ethanol production from rice straw pretreated with petha waste water and dairy waste water. Int. J. Curr. Eng. Sci. Res. 2018, 5, 65–73. [Google Scholar]
- Puligundla, P.; Oh, S.E.; Mok, C. Microwave-assisted pretreatment technologies for the conversion of lignocellulosic biomass to sugars and ethanol: A review. Carbon Lett. 2016, 17, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mikulski, D.; Kłosowski, G.; Menka, A.; Koim-Puchowska, B. Microwave-assisted pretreatment of maize distillery stillage with the use of dilute sulfuric acid in the production of cellulosic ethanol. Bioresour. Technol. 2019, 278, 318–328. [Google Scholar] [CrossRef]
- Han, S.Y.; Park, C.W.; Endo, T.; Febrianto, F.; Kim, N.H.; Lee, S.H. Extrusion process to enhance the pretreatment effect of ionic liquid for improving enzymatic hydrolysis of lignocellulosic biomass. In Wood Science and Technology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–15. [Google Scholar]
- Meng, X.; Bhagia, S.; Wang, Y.; Zhou, Y.; Pu, Y.; Dunlap, J.R.; Shuai, L.; Ragauskas, A.J.; Yoo, C.G. Effects of the advanced organosolv pretreatment strategies on structural properties of woody biomass. Ind. Crops Prod. 2020, 146, 112144. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, G.; Wei, W.; Wang, J.; Fang, Z. A comparison of different preextraction methods followed by steam pretreatment of bamboo to improve the enzymatic digestibility and ethanol production. Energy 2020, 196, 117156. [Google Scholar] [CrossRef]
- Sarker, T.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K.; Meda, V.; Naik, S. Hydrothermal pretreatment technologies for lignocellulosic biomass: A review of steam explosion and subcritical water hydrolysis. Chemosphere 2021, 284, 131372. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Shen, F.; Yang, G.; Deng, S.; Long, L.; He, J.; Luo, L. Liquid hot water extraction followed by mechanical extrusion as a chemical-free pretreatment approach for cellulosic ethanol production from rigid hardwood. Fuel 2019, 252, 589–597. [Google Scholar] [CrossRef]
- Sanchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Curran, L.M.L.K.; Sale, K.L.; Simmons, B.A. Review of advances in the development of laccases for the valorization of lignin to enable the production of lignocellulosic biofuels and bioproducts. Biotechnol. Adv. 2021, 54, 107809. [Google Scholar] [CrossRef]
- Shi, J.; Chinn, M.S.; Sharma-Shivappa, R.R. Microbial pretreatment of cotton stalks by solid state cultivation of Phanerochaete chrysosporium. Bioresour Technol. 2008, 99, 6556–6564. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Verma, P. An overview of key pretreatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value added products. Biotech 2013, 5, 415–431. [Google Scholar] [CrossRef] [Green Version]
- Rabemanolontsoa, H.; Saka, S. Various pretreatments of lignocellulosics. Bioresour Technol. 2016, 199, 83–91. [Google Scholar] [CrossRef]
- Saha, B.C.; Qureshi, N.; Kennedy, G.J.; Cotta, M.A. Biological pretreatment of corn stover with white-rot fungus for improved enzymatic hydrolysis. Int. Biodeter. Biodegrad. 2016, 109, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Hafid, H.S.; Baharuddin, A.S.; Mokhtar, M.N.; Omar, F.N.; Mohammed, M.A.; Wakisaka, M. Enhanced laccase production for oil palm biomass delignification using biological pretreatment and its estimation at biorefinary scale. Biomass Bioenergy 2021, 144, 105904. [Google Scholar] [CrossRef]
- Ma, K.; Ruan, Z. Production of a lignocellulolytic enzyme system for simultaneous biodelignification and saccharification of corn stover employing co-culture of fungi. Bioresour Technol. 2015, 175, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Soni, R.; Kaur, J.; Soni, S.K. Unravelling the capability of Pyrenophora phaeocomes S-1 for the production of ligno-hemicellulolytic enzyme cocktail and simultaneous bio-delignification of rice straw for enhanced enzymatic saccharification. Bioresour. Technol. 2016, 222, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, Z.; Zhang, K.; Si, M.; Liu, M.; Chai, L. Bacteria-enhanced dilute acid pretreatment of lignocellulosic biomass. Bioresour. Technol. 2017, 245, 419425. [Google Scholar] [CrossRef]
- Liang, C.; Wang, Q.; Wang, W.; Lin, C.S.K.; Hu, Y.; Qi, W. Enhancement of an efficient enzyme cocktail from Penicillium consortium on biodegradation of pretreated poplar. Chem. Eng. J. 2023, 452, 139352. [Google Scholar] [CrossRef]
- Bhat, M.K. Cellulases and related enzymes in biotechnology. Biotechnol. Adv. 2000, 18, 355–383. [Google Scholar] [CrossRef] [PubMed]
- Sadaf, A.; Khare, S.K. Production of Sporotrichum thermophile xylanase by solid state fermentation utilizing deoiled Jatropha curcas seed cake and its application in xylooligosachharide synthesis. Bioresour. Technol. 2014, 153, 126–130. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Upadhyay, S.N.; Mishra, P.K.; Ramteke, P.W. Biofuels from Protein-Rich Lignocellulosic Biomass: New Approach. In Sustainable Approaches for Biofuels Production Technologies; Springer: Cham, Switherland, 2019; pp. 83–92. [Google Scholar]
- Janveja, C.; Rana, S.S.; Soni, S.K. Kitchen waste residues as potential renewable biomass resources for the production of multiple fungal carbohydrases and second generation bioethanol. J. Technol. Innov. Renew. Energy. 2013, 2, 186–200. [Google Scholar]
- Venkatanagaraju, E.; Bharathi, N.; Sindhuja, R.H.; Chowdhury, R.R.; Sreelekha, Y. Extraction and Purification of Pectin from Agro-Industrial Wastes. In Pectins-Extraction, Purification, Characterization and Applications; Intechopen: London, UK, 2019. [Google Scholar]
- Gupta, A.; Verma, J.P. Sustainable bio-ethanol production from agro-residues: A review. Renew. Sustain. Energ. Rev. 2015, 41, 550–567. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Z.; Li, X.; Liu, X.; Fan, J.; Clark, J.H.; Hu, C. The production of furfural directly from hemicellulose in lignocellulosic biomass: A review. Catal. Today 2019, 319, 14–24. [Google Scholar] [CrossRef]
- Peralta, A.G.; Venkatachalam, S.; Stone, S.C.; Pattathil, S. Xylan epitope profiling: An enhanced approach to study organ development-dependent changes in xylan structure, biosynthesis, and deposition in plant cell walls. Biotechnol. Biofuels 2017, 10, 245. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Li, X.; Zhao, J. Production and characteristics of a novel Xylose-and Alkali-tolerant GH 43 β-xylosidase from Penicillium oxalicum for promoting hemicellulose degradation. Sci. Rep. 2017, 7, 11600. [Google Scholar] [CrossRef] [Green Version]
- Dhawan, S.; Kaur, J. Microbial mannases: An overview of production and applications. Crit. Rev. Biotechnol. 2007, 27, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Bastawde, K.B. Xylan structure, microbial xylanases, and their mode of action. World J. Microb. Biotechnol. 1992, 8, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.R.S.; Filho, E.X.F. An overview of mannan structure and mannan-degrading enzyme systems. Appl. Microbiol. Biotechnol. 2008, 79, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Kabel, M.A.; Jurak, E.; Mäkelä, M.R.; De Vries, R.P. Occurrence and function of enzymes for lignocellulose degradation in commercial Agaricus bisporus cultivation. Appl. Microbiol. Biotechnol. 2017, 101, 4363–4369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondal, S.; Soren, J.P.; Mondal, J.; Rakshit, S.; Halder, S.K.; Mondal, K.C. Contemporaneous synthesis of multiple carbohydrate debranching enzymes from newly isolated Aspergillus fumigatus SKF-2 under solid state fermentation: A unique enzyme mixture for proficient saccharification of plant bioresources. Ind. Crops Prod. 2020, 150, 112409. [Google Scholar] [CrossRef]
- Zehra, M.; Syed, M.N.; Sohail, M. Banana Peels: A Promising Substrate for the Coproduction of Pectinase and Xylanase from Aspergillus fumigatus MS16. Pol. J. Microbiol. 2020, 69, 19–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olajuyigbe, F.M.; Fatokun, C.O.; Oni, O.I. Effective Substrate Loading for Saccharification of Corn Cob and Concurrent Production of Lignocellulolytic Enzymes by Fusarium oxysporum and Sporothrix carnis. Curr. Biotechnol. 2019, 8, 109–115. [Google Scholar] [CrossRef]
- Ezeilo, U.R.; Lee, C.T.; Huyop, F.; Zakaria, I.I.; Wahab, R.A. Raw oil palm frond leaves as cost-effective substrate for cellulase and xylanase productions by Trichoderma asperellum UC1 under solid-state fermentation. J. Environ. Manag. 2019, 243, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Ezeilo, U.R.; Wahab, R.A.; Mahat, N.A. Optimization studies on cellulase and xylanase production by Rhizopus oryzae UC2 using raw oil palm frond leaves as substrate under solid state fermentation. Renew. Energy 2019, 156, 1301–1312. [Google Scholar] [CrossRef]
- Cekmecelioglu, D.; Demirci, A. Production of Cellulase and Xylanase Enzymes Using Distillers Dried Grains with Solubles (DDGS) by Trichoderma reesei at Shake-Flask Scale and the Validation in the Benchtop Scale Bioreactor. Waste Biomass Valor. 2020, 11, 6575–6584. [Google Scholar] [CrossRef]
- Yan, S.; Xu, Y.; Yu, X.W. Rational engineering of xylanase hyper-producing system in Trichoderma reesei for efficient biomass degradation. Biotechnol. Biofuels. 2021, 14, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Blibech, M.; Farhat-Khemakhem, A.; Kriaa, M.; Aslouj, R.; Boukhris, I.; Alghamdi, O.A.; Chouayekh, H. Optimization of β-mannanase production by Bacillus subtilis US191 using economical agricultural substrates. Biotechnol. Prog. 2020, 36, 2989. [Google Scholar] [CrossRef]
- Yadav, A.; Ali, A.A.M.; Ingawale, M.; Raychaudhuri, S.; Gantayet, L.; Pandit, A. Enhanced co-production of pectinase, cellulase and xylanase enzymes from Bacillus subtilis ABDR01 upon ultrasonic irradiation. Proc. Biochem. 2020, 92, 197–203. [Google Scholar] [CrossRef]
- Khan, M.I.M.; Zafar, M.; Anwar, Z.; Imran, M. Effect of expression of additional catalytic domain on characteristics of Xylanase Z of Clostridium thermocellum. Biologia. 2019, 74, 1395–1403. [Google Scholar] [CrossRef]
- Hamann, P.R.; Gomes, T.C.; de MB Silva, L.; Noronha, E.F. Influence of lignin-derived phenolic compounds on the Clostridium thermocellum endo-β-1, 4-xylanase XynA. Proc. Biochem. 2020, 92, 1–9. [Google Scholar] [CrossRef]
- Sinjaroonsak, S.; Chaiyaso, T.; Aran, H. Optimization of Cellulase and Xylanase Productions by Streptomyces thermocoprophilus Strain TC13W Using Oil Palm Empty Fruit Bunch and Tuna Condensate as Substrates. Appl. Biochem. Biotechnol. 2019, 189, 76–86. [Google Scholar] [CrossRef]
- Pedrolli, D.B.; Monteiro, A.C.; Gomes, E.; Carmona, E.C. Pectin and pectinases: Production, characterization and industrial application of microbial pectinolytic enzymes. Open Biotechnol. J. 2009, 3, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Begum, G.; Munjam, S. Carbon and Nitrogen Sources Effect on Pectinase Synthesis by Aspergillus niger Under Submerged Fermentation. Biosci. Biotechnol. Res. Asia 2021, 18, 185–195. [Google Scholar] [CrossRef]
- Kumar, Y.S.; Varakumar, S.; Reddy, O.V. Production and optimization of polygalacturonase from mango (Mangifera indica L.) peel using Fusarium moniliforme in solid state fermentation. World J. Microbiol. Biotechnol. 2010, 26, 1973–1980. [Google Scholar] [CrossRef]
- Amin, F.; Mohsin, A.; Bhatti, H.N.; Bilal, M. Production, thermodynamic characterization, and fruit juice quality improvement characteristics of an Exo-polygalacturonase from Penicillium janczewskii. Biochim Biophy. Acta Proteins Proteom. 2020, 1868, 140379. [Google Scholar] [CrossRef]
- Siamphan, C.; Arnthong, J.; Tharad, S.; Zhang, F.; Yang, J.; Laothanachareon, T. Production of D-galacturonic acid from pomelo peel using the crude enzyme from recombinant Trichoderma reesei expressing a heterologous exopolygalacturonase gene. J. Clean. Prod. 2022, 331, 129958. [Google Scholar] [CrossRef]
- Zeni, J.; Cence, K.; Grando, C.E.; Tiggermann, L.; Colet, R.; Lerin, L.A.; Valduga, E. Screening of pectinase-producing microorganisms with polygalacturonase activity. Appl. Biochem. Biotechnol. 2011, 163, 383–392. [Google Scholar] [CrossRef]
- Saranraj, P.; Stella, D. Fungal amylase—A review. Int. J. Microbiol. Res. 2013, 4, 203–211. [Google Scholar]
- Adejuwon, A.O.; Tsygankova, V.A.; Alonge, O. Effect of cultivation conditions on activity of α-amylase from a tropical strain Aspergillus flavusLink. J. Microbiol. Biotechnol. Food Sci. 2021, 7, 571–575. [Google Scholar] [CrossRef]
- Bano, S.; Iqbal, S.; Siddiqui, K.; Abbasi, K. Purification and characterization of [beta]-galactosidase from Aspergillus fumigatus PCSIR-2013. Pak. J. Pharm. Sci. 2021, 34, 1333–1341. [Google Scholar]
- Bellaouchi, R.; Abouloifa, H.; Rokni, Y.; Hasnaoui, A.; Ghabbour, N.; Hakkou, A.; Bechchari, A.; Asehraou, A. Characterization and optimization of extracellular enzymes production by Aspergillus niger strains isolated from date by-products. J. Genet. Eng. Biotechnol. 2021, 19, 50. [Google Scholar] [CrossRef]
- Gopinath, S.C.; Anbu, P.; Arshad, M.M.; Lakshmipriya, T.; Voon, C.H.; Hashim, U.; Chinni, S.V. Biotechnological process in microbial amylase production. BioMed Res. Int. 2017, 2017, 1272193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohanram, S.; Amat, D.; Choudhary, J.; Arora, A.; Nain, L. Novel perspectives for evolving enzyme cocktails for lignocellulose hydrolysis in biorefineries. Sustain. Chem. Process. 2013, 1, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Sadh, P.K.; Chawla, P.; Bhandari, L.; Duhan, J.S. Bio-enrichment of functional properties of peanut oil cakes by solid state fermentation using Aspergillus oryzae. J. Food Meas. Character. 2017, 12, 622–633. [Google Scholar] [CrossRef]
- Leite, P.; Silva, C.; Salgado, J.M.; Belo, I. Simultaneous production of lignocellulolytic enzymes and extraction of antioxidant compounds by solid-state fermentation of agro-industrial wastes. Ind. Crops Prod. 2019, 137, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Marzo, C.; Díaz, A.B.; Caro, I.; Blandino, A. Valorization of agro-industrial wastes to produce hydrolytic enzymes by fungal solid-state fermentation. Waste Manag. Res. 2019, 37, 149–156. [Google Scholar] [CrossRef]
- Amande, T.; Adebayo-Tayo, B.; Ndubuisi-Nnaji, U.; Ado, B. Production and partial characterization of pectinases from mango peels by Aspergillus tamarii. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 59–62. [Google Scholar]
- Melnichuk, N.; Braia, M.J.; Anselmi, P.A.; Meini, M.R.; Romanini, D. Valorization of two agroindustrial wastes to produce alpha-amylase enzyme from Aspergillus oryzae by solid-state fermentation. Waste Manag. 2020, 106, 155–161. [Google Scholar] [CrossRef]
- Iqbal, H.M.N.; Ahmed, I.; Zia, M.A.; Irfan, M. Purification and characterization of the kinetic parameters of cellulase produced from wheat straw by Trichoderma viride under SSF and its detergent compatibility. Adv. Biosci. Biotechnol. 2011, 2, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Farinas, C.S. Developments in solid-state fermentation for the production of biomass-degrading enzymes for the bioenergy sector. Renew. Sustain. Energ. Rev. 2015, 52, 179–188. [Google Scholar] [CrossRef]
- Nene, S.N.; Joshi, K.S. A comparative study of production of hydrophobin like proteins (HYD-LPs) in submerged liquid and solid state fermentation from white rot fungus Pleurotus ostreatus. Biocatal. Agric. Biotechnol. 2020, 23, 101440. [Google Scholar]
- Barrios-González, J. Secondary Metabolites Production: Physiological Advantages in Solid-State Fermentation. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 257–283. [Google Scholar]
- El-Bakry, M.; Abraham, J.; Cerda, A.; Barrena, R.; Ponsa, S.; Gea, T.; Sánchez, A. From wastes to high value added products: Novel aspects of SSF in the production of enzymes. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1999–2042. [Google Scholar] [CrossRef] [Green Version]
- Rudakiya, D.M. Strategies to improve solid-state fermentation technology. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 155–180. [Google Scholar]
- Kaur, J.; Chugh, P.; Soni, R.; Soni, S.K. A low-cost approach for the generation of enhanced sugars and ethanol from rice straw using in-house produced cellulase-hemicellulase consortium from A. niger P-19. Bioresour. Technol. Rep. 2020, 11, 100469. [Google Scholar] [CrossRef]
- Soni, S.K.; Sharma, A.; Soni, R. Fungal cocktail of multiple hydrolytic enzymes and method of production thereof. Indian Patent 202213059023, 2022. Available online: https://ipindiaservices.gov.in/PatentSearch/PatentSearch/ViewApplicationStatus (accessed on 21 November 2022).
- Kalogeris, E.; Christakopoulos, P.; Katapodis, P.; Alexiou, A.; Vlachou, S.; Kekos, D.; Macris, B.J. Production and characterization of cellulolytic enzymes from the thermophilic fungus Thermoascus aurantiacus under solid state cultivation of agricultural wastes. Process Biochem. 2003, 38, 1099–1104. [Google Scholar] [CrossRef]
- Negi, S.; Banerjee, R. Optimization of extraction and purification of glucoamylase produced by A. awamori in solid state fermentation. Biotechnol. Bioprocess. Eng. 2009, 14, 60–66. [Google Scholar] [CrossRef]
- Prasoulas, G.; Gentikis, A.; Konti, A.; Kalantzi, S.; Kekos, D.; Mamma, D. Bioethanol Production from Food Waste Applying the Multienzyme System Produced On-Site by Fusarium oxysporum F3 and Mixed Microbial Cultures. Fermentation 2020, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Grover, A.; Maninder, A.; Sarao, L.K. Production of fungal amylase and cellulase enzyme via solid state fermentation using Aspergillus oryzae and Trichoderma reesei. Int. J. Adv. Res. Technol. 2013, 2, 108–124. [Google Scholar]
- Rana, S.S.; Janveja, C.; Soni, S.K. Brewer’s spent grain as a valuable substrate for low cost production of fungal cellulases by statistical modeling in solid state fermentation and generation of cellulosic ethanol. Int. J. Food. Ferment. Technol. 2013, 3, 41–55. [Google Scholar] [CrossRef]
- Kaur, P.S.; Kaur, S.; Kaur, H.; Sharma, A.; Raj, P.; Panwar, S. Solid substrate fermentation using agro industrial waste: New approach for amylase production by Bacillus licheniformis. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 712–717. [Google Scholar]
- Mahalakshmi, N.; Jayalakshmi, S. Amylase, cellulase and xylanase production from a novel bacterial isolate Achromobacter xylosoxidans isolated from marine environment. Int. J. Adv. Res. Biol. Sci. 2016, 3, 230–233. [Google Scholar]
- Marín, M.; Artola, A.; Sánchez, A. Optimization of down-stream for cellulases produced under solid-state fermentation of coffee husk. Waste Biomass Valor. 2019, 10, 2761–2772. [Google Scholar] [CrossRef] [Green Version]
- Marín, M.; Sánchez, A.; Artola, A. Production and recovery of cellulases through solid-state fermentation of selected lignocellulosic wastes. J. Clean. Prod. 2019, 209, 937–946. [Google Scholar] [CrossRef]
- Salomão, G.S.; Agnezi, J.C.; Paulino, L.B.; Hencker, L.B.; de Lira, T.S.; Tardioli, P.W.; Pinotti, L.M. Production of cellulases by solid state fermentation using natural and pretreated sugarcane bagasse with different fungi. Biocatal. Agric. Biotechnol. 2019, 17, 1–6. [Google Scholar] [CrossRef]
- Teles, A.S.; Chávez, D.W.; Oliveira, R.A.; Bon, E.P.; Terzi, S.C.; Souza, E.F.; Tonon, R.V. Use of grape pomace for the production of hydrolytic enzymes by solid-state fermentation and recovery of its bioactive compounds. Food Res. Int. 2019, 120, 441–448. [Google Scholar] [CrossRef]
- Almanaa, T.N.; Vijayaraghavan, P.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Alyahya, S.A. Solid state fermentation of amylase production from Bacillus subtilis D19 using agro-residues. J. King Saud. Univ. Sci. 2020, 32, 1555–1561. [Google Scholar] [CrossRef]
- Jovanović, M.; Vučurović, D.; Bajić, B.; Dodić, S.; Vlajkov, V.; Jevtić-Mučibabić, R. Optimization of the simultaneous production of cellulase and xylanase by submerged and solid-state fermentation of wheat chaff. J. Serb. Chem Soc. 2020, 85, 177–189. [Google Scholar] [CrossRef]
- Rodrigues, I.D.; Barreto, J.T.; Moutinho, B.L.; Oliveira, M.M.; da Silva, R.S.; Fernandes, M.F.; Fernandes, R.P.M. Production of xylanases by Bacillus sp. TC-DT13 in solid state fermentation using bran wheat. Prep. Biochem. Biotechnol. 2020, 50, 91–97. [Google Scholar]
- Khade, S.M.; Srivastava, S.K.; Kumar, K.; Sharma, K.; Goyal, A.; Tripathi, A.D. Optimization of clinical uricase production by Bacillus cereus under submerged fermentation, its purification and structure characterization. Process. Biochem. 2018, 75, 49–58. [Google Scholar] [CrossRef]
- Letti, L.A.; Vítola, F.M.; de Melo Pereira, G.V.; Karp, S.G.; Medeiros, A.B.; da Costa, E.S.; Soccol, C.R. Solid-State Fermentation for the Production of Mushrooms. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 285–318. [Google Scholar]
- Rodrigues, A.C.; Fontão, A.I.; Coelho, A.; Leal, M.; da Silva, F.A.; Wan, Y.; Dourado, F.; Gama, M. Response surface statistical optimization of bacterial nanocellulose fermentation in static culture using a low-cost medium. New Biotechnol. 2019, 49, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, S.; Chen, G.; Wu, R.; Cao, X.; Zeng, W.; Liang, Z. Non-sterile submerged fermentation of fibrinolytic enzyme by marine Bacillus subtilis harboring antibacterial activity with starvation strategy. Front. Microbiol. 2019, 10, 1025. [Google Scholar] [CrossRef] [PubMed]
- Darouneh, E.; Alavi, A.; Vosoughi, M.; Arjmand, M.; Seifkordi, A.; Rajabi, R. Citric acid production: Surface culture versus submerged culture. Afr. J. Microbiol. Res. 2009, 3, 541–545. [Google Scholar]
- Elegbede, J.A.; Lateef, A. Valorization of Corn-Cob by Fungal Isolates for Production of Xylanase in Submerged and Solid State Fermentation Media and Potential Biotechnological Applications. Waste Biomass Valor. 2018, 9, 1273. [Google Scholar] [CrossRef]
- Irfan, M.; Bakhtawar, J.; Shakir, H.A.; Khan, M.; Ali, S. Utilization of peanut shells as substrate for cellulase production in submerged fermentation through Box-Behnken Design. Int. J. Biol. Chem. 2020, 12, 28–39. [Google Scholar] [CrossRef]
- Fawole, O.B.; Odunfa, S.A. Some factors affecting production of pectic enzymes by Aspergillus niger. Int. Biodeterior. Biodegrad. 2003, 52, 223–227. [Google Scholar] [CrossRef]
- Krough, K.B.R.; Morkeberg, A.; Jorgensen, H.; Frisvad, J.C.; Olsson, L. Screening genus Penicillium for producers of cellulolytic and xylanolytic enzymes. Appl. Biochem. Biotechnol. 2004, 114, 389–401. [Google Scholar] [CrossRef]
- Elisashvili, V.; Penninckx, M.; Kachlishvili, E.; Asatiani, M.; Kvesitadze, G. Use of Pleurotus dryinus for lignocellulolytic enzymes production in submerged fermentation of mandarin peels and tree leaves. Enzym. Microb. Technol. 2006, 38, 998–1004. [Google Scholar] [CrossRef]
- Vidyalakshmi, R.; Paranthaman, R.; Indhumathi, J. Amylase production on submerged fermentation by Bacillus spp. World J. Chem. 2009, 4, 89–91. [Google Scholar]
- de Castro, A.M.; Pedro, K.C.N.R.; da Cruz, J.C.; Ferreira, M.C.; Leite, S.G.F.; Pereira, N. Trichoderma harzianum IOC-4038: A promising strain for the production of a cellulolytic complex with significant β-glucosidase activity from sugarcane bagasse cellulignin. Appl. Biochem. Biotechnol. 2010, 162, 2111–2122. [Google Scholar] [CrossRef]
- de Almeida, M.N.; Guimarães, V.M.; Bischoff, K.M.; Falkoski, D.L.; Pereira, O.L.; Gonçalves, D.S. Cellulases and hemicellulases from endophytic Acremonium species and its application on sugarcane bagasse hydrolysis. Appl. Biochem. Biotechnol. 2011, 165, 594–610. [Google Scholar] [CrossRef]
- Nagar, S.; Gupta, V.K.; Kumar, D.; Kumar, L.; Kuhad, R.C. Production and optimization of cellulase-free, alkali-stable xylanase by Bacillus pumilus SV-85S in submerged fermentation. J. Ind. Microbiol. Biotechnol. 2010, 37, 71–83. [Google Scholar] [CrossRef]
- Kavuthodi, B.; Sebastian, D. Biotechnological valorization of pineapple stem for pectinase production by Bacillus subtilis BKDS1: Media formulation and statistical optimization for submerged fermentation. Biocatal. Agricl. Biotechnol. 2018, 16, 715–722. [Google Scholar] [CrossRef]
- Ismail, S.A.; Khattab, O.K.H.; Nour, S.A.; Awad, G.E.; Abo-Elnasr, A.A.; Hashem, A.M. A Thermodynamic Study of Partially-Purified Penicillium humicola β-mannanase Produced by Statistical Optimization. Jordan J. Biol. Sci. 2019, 12, 209–217. [Google Scholar]
- Sharma, D.; Mahajan, R. Development of Methodology for Concurrent Maximum Production of Alkaline Xylanase–Pectinase Enzymes in Short Submerged Fermentation Cycle. Waste Biomass Valor. 2019, 11, 6065–6072. [Google Scholar] [CrossRef]
- Arekemase, M.O.; Omotosho, I.O.; Agbabiaka, T.O.; Ajide-Bamigboye, N.T.; Lawal, A.K.; Ahmed, T. Optimization of bacteria pectinolytic enzyme production using banana peel as substrate under submerged fermentation. Sci. World J. 2020, 15, 56–63. [Google Scholar]
- Kote, N.V.; Manjula, A.C.; Vishwanatha, T.; Aravind, G.P. Production, Partial Purification and Characterisation of α-Amylase from Aspergillus niger using Aqueous Two Phase System (ATPS). Res. J. Biotechnol. 2020, 15, 5. [Google Scholar]
- Liu, J.; Yang, J.; Wang, R.; Liu, L.; Zhang, Y.; Bao, H.M. Comparative characterization of extracellular enzymes secreted by Phanerochaete chrysosporium during solid-state and submerged fermentation. Int. J. Biol. Macromol. 2020, 152, 288–294. [Google Scholar] [CrossRef]
- Stephen, A.C.; Adeniyi, O.A.; Hadiza, J. Effect of optimization conditions on submerged fermentation of corn bran for the production of xylanase enzyme. World J. Adv. Res. Rev. 2020, 5, 19–25. [Google Scholar] [CrossRef]
- Thite, V.S.; Nerurkar, A.S.; Baxi, N.N. Optimization of concurrent production of xylanolytic and pectinolytic enzymes by Bacillus safensis M35 and Bacillus altitudinis J208 using agro-industrial biomass through Response Surface Methodology. Sci. Rep. 2020, 10, 3824. [Google Scholar] [CrossRef] [Green Version]
- Girio, F.M.; Fonseca, C.; Carvalheiro, F.; Duarte, C.L.; Marques, S.; Bogel-qukasik, R. Hemicelluloses for fuel ethanol: A review. Bioresour. Technol. 2010, 101, 4775–4800. [Google Scholar] [CrossRef]
- Melendez, J.R.; Mátyás, B.; Hena, S.; Lowy, D.A.; El Salous, A. Perspectives in the production of bioethanol: A review of sustainable methods, technologies, and bioprocesses. Renew. Sustain. Energy Rev. 2022, 160, 112260. [Google Scholar] [CrossRef]
- Sarkar, N.; Aikat, K. Aspergillus fumigatus NITDGPKA3 provides for increased cellulase production. Int. J. Chem. Eng. 2014, 5, 959845. [Google Scholar]
- Tayeh, A.H.; Naim, N.; Carlos, D.; Ahmed, T.; Hassan, A. Potential of bioethanol production from olive mill solid wastes. Bioresour. Technol. 2014, 152, 24–30. [Google Scholar] [CrossRef]
- Sarbishei, S.; Goshadrou, A.; Hatamipour, M.S. Mild sodium hydroxide pretreatment of tobacco product waste to enable efficient bioethanol production by separate hydrolysis and fermentation. Biomass Convers. Biorefin. 2021, 11, 2963–2973. [Google Scholar] [CrossRef]
- Roberto, I.C.; Castro, R.C.; Silva, J.P.; Mussatto, S.I. Ethanol Production from High Solid Loading of Rice Straw by Simultaneous Saccharification and Fermentation in a Non-Conventional Reactor. Energies 2020, 13, 2090. [Google Scholar] [CrossRef]
- Zhu, J.Q.; Zong, Q.J.; Li, W.C.; Chai, M.Z.; Xu, T.; Liu, H.; Fan, H.; Li, B.Z.; Yuan, Y.J. Temperature profiled simultaneous saccharification and co-fermentation of corn stover increases ethanol production at high solid loading. Energy Convers. Manag. 2020, 205, 112344. [Google Scholar] [CrossRef]
- Pandiyan, K.; Singh, A.; Singh, S.; Saxena, A.K.; Nain, L. Technological interventions for utilization of crop residues and weedy biomass for second generation bio-ethanol production. Renew. Energy 2019, 132, 723–741. [Google Scholar] [CrossRef]
- da Silva, F.L.; Dos Santos, D.A.; de Oliveira, C.A.; Magalhães, E.R.B.; Dos Santos, E.S. Evaluation of blend production of cellulases and xylanases Using pretreated and recycled carnauba straw. Appl. Biochem. Biotechnol. 2022, 194, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fu, X. Industrial technologies for bioethanol production from lignocellulosic biomass. Renew. Sustain. Energy Rev. 2016, 57, 468–478. [Google Scholar] [CrossRef]
- Bhatt, S.M.; Bal, J.S. Bioprocessing perspective in biorefineries. In Sustainable Approaches for Biofuels Production Technologies; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–23. [Google Scholar]





| Substrate | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Reference |
|---|---|---|---|---|
| Rice straw | 32–47 | 19–27 | 5–24 | [21] |
| Rice husk | 34.40 | 29.30 | 19.20 | [22] |
| Wheat straw | 35–45 | 20–30 | 8–15 | [21] |
| Corn straw | 42.60 | 21.30 | 8.20 | [21] |
| Corn cobs | 45.00 | 35.00 | 15.00 | [21] |
| Corn stover | 38.00 | 26.00 | 19.00 | [23] |
| Wheat bran | 25.30 | 14.60 | 3.20 | [24] |
| Sugarcane bagasse | 42.00 | 25–36 | 19–20 | [16] |
| Sweet sorghum | 48–49 | 20–26 | 19–20 | [25] |
| Coconut fiber | 36–43 | 0.15–0.25 | 41–45 | [21] |
| Cocoa pods husk | 35 | 10 | 14 | [26] |
| Soft wood | 40–44 | 25–29 | 25–31 | [21] |
| Banana fiber | 60–65 | 6–8 | 5–10 | [21] |
| Switch grass | 36–38 | 27 | 17–19 | [27] |
| De-oiled rice bran | 9.80 | 20.60 | 3.90 | [28] |
| Barley straw | 31–45 | 27–38 | 14–19 | [21] |
| Nature of Pretreatment | Method | Process | Impact | Reference |
|---|---|---|---|---|
| Mechanical or physical | Milling | Roll, ball, hammer, disk, and colloid milling | Decreases polymerization and crystalline structure of cellulose, increases specific surface area | [47] |
| Extrusion | Mixing, heating, and shearing of biomass | Alterations in the physical and chemical structure. Defibrillation and fiber shortening | [48] | |
| Pulse electric field | A sudden burst of high voltage between 5.0–20.0 kV/cm for nano to milliseconds | Disruption of the cell wall and electroporation | [49] | |
| Microwave | Irradiation with 2450 MHz microwaves (170–200 °C) | Alterations in the ultra-structure of cellulose, partially removes hemicelluloses and lignin | [50,51] | |
| Chemical | Acidic | Treatment with dilute HCl, H3PO4, HNO3, H2SO4, acetic acid, citric acid, oxalic acid, maleic acid, fumaric acid, etc | Hydrolysis of hemicellulose | [9] |
| Alkaline | Treatment with dilute NaOH, KOH, Ca(OH)2, NH4OH | Efficient removal of lignin | [9] | |
| Physicochemical | Wet Oxidation | Treatment with oxidative agents such as peracetic acid, sodium chlorite, KMnO4, and H2O2 at high temperatures | Higher lignin and hemicelluloses solubilization | [52] |
| Organosolv | Treatment with organic or aqueous–organic solvent systems with or without added catalysts in the temperature range of 100–250 °C | Hydrolysis of lignin and hemicellulose | [53] | |
| Ammonia Fibre Expansion treatment (AFEX) | Treatment with anhydrous or liquid ammonia at a temperature ranging from 90 to 100 °C followed by a successive lowering of pressure | Lignin removal | [54] | |
| Steam Explosion | Exposure to saturated steam under high pressure followed by a sudden lowering of pressure | Lignin removal and hemicellulose solubilization | [55] | |
| Liquid hot water | Use of high temperature of 170°–230 °C and pressure more than 5 MPa | Removal of hemicelluloses | [56] | |
| Biological | Enzymes or microorganisms | Acton of lignin-degrading enzymes such as peroxidases and laccases | Lignin degradation | [46,57] |
| Hydrolytic Enzyme | Classification | Mode of Action | Common Lignocellulosic Biomass | References |
|---|---|---|---|---|
| Cellulases | Endoglucanase or Endo-β-1,4-glucanase | Random hydrolysis of the interior glycosidic bonds in cellulolytic biomass | Wheat straw, rice straw, corn cobs, wheat bran, oat bran, Arundo donax, Populus tremuloides, deoiled rice bran, kitchen waste | [9,73,75,106] |
| Cellobiohydrolase or Exo-β-1,4-glucanase | Hydrolysis of beta-D-glucosidic linkages by releasing mainly cellobiose | |||
| Cellobiase or β-glucosidase (BG) | Cleavage of cellobiose | |||
| Hemicellulases | Endo-β-1,4-xylanase | Release of xylose from xylan by Endohydrolysis of (1 → 4)-beta-D-xylosidic linkages | Wheat bran, kitchen waste, Banana peels, Peanut oil cake, Brewer’s spent grain | [9,80,81,84,106,107,108] |
| Exo-β-1,4-xylanase or β-1,4-xylan xylohydrolase | Release monomeric xylose from the non-reducing end of xylan | |||
| β-1,4-xylosidase or Xylobiase | hydrolyzes disaccharides such as xylobiose and the higher xylooligosaccharides | |||
| Endo-β-1,4-mannanase | Randomly cleaving the mannan’s β-1,4-linkage internal links | |||
| Exo-β-mannosidase | Releases mannose sugar moieties by cleaving β-1,4-linked mannosides from the non-reducing ends of mannan | |||
| β-glucosidase | Hydrolyzes the 1,4-β-D-glucopyranose found at the non-reducing ends of the oligosaccharides | |||
| α-galactosidase | breaks down the α-1,6-linked D-galactopyranosyl side chains of the oligosaccharides | |||
| Acetyl mannan esterase | The debranching enzyme releases acetyl groups. | |||
| Pectinases | Protopectinases | Liberate soluble form polymerized pectin | Wheat bran, mango peel, banana peel, kitchen waste, Orange peels, exhausted sugar beet cassettes | [84,85,109,110] |
| Pectin Methyl Esterases | Deesterify the methyl group of pectin, releasing pectic acid and methanol | |||
| Pectin Acetyl Esterases | Hydrolysis of the acetyl esters found in pectin | |||
| Polymethylgalacturonases | Breaks α-1,4-glycosidic linkages in pectin | |||
| Polygalacturonases | Cleaves the polygalacturonic acid’s α-1,4-glycosidic linkages | |||
| Pectate Lyases | Release α-4,5-D-galacturonate from the glycosidic bonds in polygalacturonic acid | |||
| Pectin Lyases | randomly break the esterified pectin and create unsaturated methyloligogalacturonates. | |||
| Amylase | Endoamylases or α-amylase | Cleaves the α-1,4-bonds present in the inner regions of amylose and amylopectin | Rice bran, wheat bran, black gram bran, Soybean husk, flour mill waste | [101,105,111] |
| Exoamylase or β-amylase | Release limit dextrins and β-maltose | |||
| ƴ-amylase or Amyloglucosidase or Glucoamylase | Debranching enzyme releases glucose |
| Substrate | Microorganism | Enzymes | Major Breakthrough | References |
|---|---|---|---|---|
| Wheat straw, rice straw, corn cobs, wheat bran, oat bran, Arundo donax, Populus tremuloides | Thermoascus aurantiacus | Cellulases | Thermostable cellulolytic components production | [121] |
| Wheat bran | Aspergillus awamori Nakazawa (MTCC 6652) | Glucoamylase | Optimization of extraction and purification of glucoamylase | [122] |
| Wheat bran | Aspergillus niger NS-2 | Cellulases xylanase, mannanase, pectinase, amylases | Co-production of multiple enzymes for Bioethanol Production | [123] |
| Deoiled rice bran | Aspergillus niger, Aspergillus oryzae, Trichoderma reesei | Cellulase, amylase | Co-production of the thermostable multi-enzyme system for ethanol production | [9,124] |
| Kitchen waste | Aspergillus niger CJ-5 | Cellulases, xylanase, mannanase, pectinase, amylases | Co-production of multiple enzymes for Bioethanol Production from kitchen waste residues | [73] |
| Brewer’s spent grain | Fusarium oxysporum SS-25 | Cellulases | Production of cellulases for the production of ethanol from brewer’s spent grain | [125] |
| wheat straw, paddy straw, sugarcane waste, maize straw | Bacillus licheniformis | α-amylase | Production of amylase from the mixture of agricultural residue waste | [126] |
| Rice bran, wheat bran, black gram bran | Achromobacter xylosoxidans | Amylase, cellulase, xylanase | Co-production of multiple enzymes from various agro waste | [127] |
| Peanut oil cake | Aspergillus oryzae | Cellulase, xylanase, amylase | Enhancement in various functional properties during fermentation in addition to enzyme activities | [107] |
| Brewer’s spent grain | Aspergillus niger CECT2088 | Cellulase, xylanase | Simultaneous production of lignocellulolytic enzymes | [108] |
| Orange peel, apple pomace, and rice fiber | Compost from Municipal Solid Waste as inoculum | Cellulases | Development of a framework for a zero-waste enzyme production process | [128] |
| Coffee husk and wood chips | Compost from MSW as inoculum | Cellulases | Enhanced cellulase production | [129] |
| Orange peels and exhausted sugar beet cassettes | Aspergillus awamori 2B.361 U2/1 | Cellulase, xylanase, pectinase | Enhanced sugar production | [109] |
| Sugarcane bagasse | Penicillium sp., Rhizomucor sp., Trichoderma sp. | Cellulases | Use of sugarcane bagasse as an inducer for cellulase | [130] |
| Grape pomace with wheat bran | Aspergillus niger 3T5B8 | Cellulase, xylanase | Production of a cocktail of hydrolytic enzymes using Grape pomace with wheat bran | [131] |
| Wheat bran, banana peel, orange peel, rice bran, pine apple peel | Bacillus subtilis D19 | Amylase | Enhanced amylase production on various agro-waste residues | [132] |
| Mango peels | Aspergillus tamarii | Pectinase | Enhanced polygalacturonase and pectin lyase | [110] |
| Wheat chaff | Trichoderma reesei QM 9414 | Cellulases and xylanase | Simultaneous production of cellulase and xylanase | [133] |
| Rice straw | Aspergillus niger P-19 | Cellulases, hemicellulases | Enhanced sugars and ethanol from rice straw | [119] |
| Rice straw | Penicillium spp. | Cellulase | Potent cellulase cocktail production for lignocellulosic degradation | [69] |
| Soybean husk and flour mill waste | Aspergillus oryzae | Amylase | Production and purification of alpha-amylase | [111] |
| Wheat bran | Bacillus sp. TC-DT13 | Xylanase | Optimized production of extracellular xylanase | [134] |
| Wheat bran | Trichoderma reesei, Neurospora crassa | Cellulases | Optimization and standardization of various factors for cellulase production | [24] |
| Banana peels | Aspergillus fumigatus | Pectinase and xylanase | Coproduction of pectinase and xylanase | [84] |
| Kitchen waste | Aspergillus niger S-30 | Cellulases, Hemicellulases, Pectinases, Amylases | 19 hydrolytic enzymes from a single substrate and organism | [120] |
| Substrate | Microorganism | Enzymes | Major Breakthrough | References |
|---|---|---|---|---|
| Rice bran | Aspergillus niger | Pectinase | Enhanced Polygalacturonase and Pectinmethylesterase activity | [142] |
| Solka-Floc cellulose | Penicillium brasilianum IBT 20888 | Cellulases, xylanase | Coinduction of cellulolytic and xylanolytic | [143] |
| Mandarin peels and tree leaves | Pleurotus dryinus | Cellulases, xylanase, laccase, manganese peroxidase | Enhanced activity of cellulases, xylanase, laccase, manganese peroxidase | [144] |
| Starch | Bacillus sp. | Amylase | Optimization of enhanced amylase production | [145] |
| Partially delignified cellulignin | Trichoderma harzianum IOC-4038 | Cellulases | Simultaneous saccharification and fermentation process development using partially delignified cellulignin | [146] |
| Sugarcane bagasse, corn stover | Acremonium sp. | Cellulases, xylanase | Enhanced reducing sugar conversion | [147] |
| Wheat bran | Aspergillus tamarii MTCC5152 | Amylase | Production of a cellulase-free and alkali-stable xylanase | [148] |
| Corn cob | Aspergillus fumigatus SD5A | xylanase | Use of eight fungal strains in xylanase production | [140] |
| Pineapple stem | Bacillus subtilis BKDS1 | Pectinase | Economical production of the enzyme, pectinase using pineapple stem extract (PSE) medium | [149] |
| Coffee waste | Penicillium humicola | Mannanase | Statistical experimental designs to enhance the β-mannanase production | [150] |
| Wheat bran and citrus peel waste | Bacillus pumilus | Xylanase and pectinase | Maximum production of xylanase and pectinase in a short submerged fermentation cycle | [151] |
| Banana peels | Bacillus subtilis TYg4-3 and Bacillus amyloliquefaciens SW106 | Pectinase | Optimization of bacterial pectinsae | [152] |
| Coffee residue powder, date seeds powder, prickly pear seeds | Bacillus subtilis US191 | Mannanase | Statistical experimental designs to enhance the bacterial β-mannanase production | [90] |
| Peanut shells | Bacillus paralichniformis | Cellulases | Utilization of peanut shells for cellulase production through Box-Behnken Design | [141] |
| Wheat chaff | Trichoderma reesei QM 9414 | Cellulases and xylanase | Simultaneous production of cellulase and xylanase | [133] |
| Wheat bran, rice husk | Aspergillus niger | Amylase | Production and purification of amylase using an aqueous two-phase system | [153] |
| Corn stover | Phanerochaete chrysosporium PC2 | Cellulases and hemicellulases | Revealed the importance of carbohydrate-binding module in the hydrolysis process of lignocellulose | [154] |
| Corn bran | Aspergillus niger | Xylanase | Use of UV- rays for enhanced xylanase | [155] |
| Wheat bran and citrus peel waste | Bacillus safensis M35, Bacillus altitudinis J208 | Xylanase and pectinase | Concentration values for wheat bran and citrus peel substrates are to be amended in one single production medium for enhanced xylanase and pectinase | [156] |
| Banana peels | Aspergillus fumigatus | Pectinase and xylanase | Coproduction of pectinase and xylanase | [84] |
| Kitchen waste | Aspergillus niger S-30 | Cellulases, Hemicellulases, Pectinases, Amylases | 19 hydrolytic enzymes from a single substrate and organism | [120] |
| Fermentation Technology | Steps Involved | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| Separate hydrolysis and fermentation (SHF) |
| The conditions can be optimized separately for each step | End product inhibitionRequire separate reactors for each step High energy and time consumption | [161] |
| Simultaneous saccharification and fermentation (SSF) |
|
| Differences in the optimum condition for hydrolytic enzymes and fermenting microorganisms | [162] |
| Simultaneous Saccharification and Co-Fermentation (SSCF) |
|
| Differences in the optimum condition for hydrolytic enzymes and fermenting microorganisms | [163] |
| Consolidated Bio-Processing (CBP) | Pretreatment, enzyme production, Saccharification, and Fermentation |
| Differences in the optimal conditions for enzymes or microorganisms involved in the process | [164] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soni, S.K.; Sharma, A.; Soni, R. Microbial Enzyme Systems in the Production of Second Generation Bioethanol. Sustainability 2023, 15, 3590. https://doi.org/10.3390/su15043590
Soni SK, Sharma A, Soni R. Microbial Enzyme Systems in the Production of Second Generation Bioethanol. Sustainability. 2023; 15(4):3590. https://doi.org/10.3390/su15043590
Chicago/Turabian StyleSoni, Sanjeev Kumar, Apurav Sharma, and Raman Soni. 2023. "Microbial Enzyme Systems in the Production of Second Generation Bioethanol" Sustainability 15, no. 4: 3590. https://doi.org/10.3390/su15043590
APA StyleSoni, S. K., Sharma, A., & Soni, R. (2023). Microbial Enzyme Systems in the Production of Second Generation Bioethanol. Sustainability, 15(4), 3590. https://doi.org/10.3390/su15043590







