The Influence of CO2 Injection into Manure as a Pretreatment Method for Increased Biogas Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock Characteristics and Pretreatment
2.2. Anaerobic Digestion Process
3. Results and Discussion
3.1. CO2 Pretreatment Influence on Feedstock pH and ORP
3.2. Biogas, Methane Yields and Energy Value
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Anaerobic digestion |
| CH4 | Methane |
| ChM | Chicken manure |
| CO2 | Carbon dioxide |
| CoM | Cow manure |
| H2S | Hydrogen sulfide |
| HCO3− | Carbonic acid |
| N | Nitrogen |
| NH3 | Ammonia |
| O2 | Oxygen |
| OLR | Organic loading rate |
| ORP | Oxidation-reduction potential |
| P | Phosphorus |
| PM | Pig manure |
| RM | Raw manure |
| TC | Total carbon |
| TS | Total solids |
| VFA | Volatile fatty acids |
| VS | Volatile solids |
References
- Dalkilic, K.; Ugurlu, A. Enhancement of Biogas Production from Cattle Manure in a Combined Microbial Electrolysis Cell and Anaerobic Digestion System at Short Hydraulic Retention Time. Res. Sq. 2022; preprint. [Google Scholar] [CrossRef]
- Dong, L.; Cao, G.; Guo, X.; Liu, T.; Wu, J.; Ren, N. Efficient Biogas Production from Cattle Manure in a Plug Flow Reactor: A Large-Scale Long-Term Study. Bioresour. Technol. 2019, 278, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Teixeira Franco, R.; Buffière, P.; Bayard, R. Cattle Manure for Biogas Production. Does Ensiling and Wheat Straw Addition Enhance Preservation of Biomass and Methane Potential? Biofuels 2020, 11, 671–682. [Google Scholar] [CrossRef] [Green Version]
- Vanegas, M.; Romani, F.; Jiménez, M. Pilot-Scale Anaerobic Digestion of Pig Manure with Thermal Pretreatment: Stability Monitoring to Improve the Potential for Obtaining Methane. Processes 2022, 10, 1602. [Google Scholar] [CrossRef]
- Ye, J.; Li, D.; Sun, Y.; Wang, G.; Yuan, Z.; Zhen, F.; Wang, Y. Improved Biogas Production from Rice Straw by Co-Digestion with Kitchen Waste and Pig Manure. Waste Manag. 2013, 33, 2653–2658. [Google Scholar] [CrossRef]
- Buivydas, E.; Navickas, K.; Venslauskas, K.; Žalys, B.; Župerka, V.; Rubežius, M. Biogas Production Enhancement through Chicken Manure Co-Digestion with Pig Fat. Appl. Sci. 2022, 12, 4652. [Google Scholar] [CrossRef]
- Yin, D.M.; Qiao, W.; Negri, C.; Adani, F.; Fan, R.; Dong, R.J. Enhancing Hyper-Thermophilic Hydrolysis Pre-Treatment of Chicken Manure for Biogas Production by in-Situ Gas Phase Ammonia Stripping. Bioresour. Technol. 2019, 287, 121470. [Google Scholar] [CrossRef]
- Venslauskas, K.; Navickas, K.; Rubežius, M.; Tilvikienė, V.; Supronienė, S.; Doyeni, M.O.; Barčauskaitė, K.; Bakšinskaitė, A.; Bunevičienė, K. Environmental Impact Assessment of Sustainable Pig Farm via Management of Nutrient and Co-Product Flows in the Farm. Agronomy 2022, 12, 760. [Google Scholar] [CrossRef]
- Dar, R.A.; Parmar, M.; Dar, E.A.; Sani, R.K.; Phutela, U.G. Biomethanation of Agricultural Residues: Potential, Limitations and Possible Solutions. Renew. Sustain. Energy Rev. 2021, 135, 110217. [Google Scholar] [CrossRef]
- Esteves, E.M.M.; Herrera, A.M.N.; Esteves, V.P.P.; Morgado, C.d.R.V. Life Cycle Assessment of Manure Biogas Production: A Review. J. Clean. Prod. 2019, 219, 411–423. [Google Scholar] [CrossRef]
- Roy, R.; Rahman, M.S.; Raynie, D.E. Recent Advances of Greener Pretreatment Technologies of Lignocellulose. Curr. Res. Green Sustain. Chem. 2020, 3, 100035. [Google Scholar] [CrossRef]
- Han, G.; Shin, S.G.; Cho, K.; Lee, J.; Kim, W.; Hwang, S. Temporal Variation in Bacterial and Methanogenic Communities of Three Full-Scale Anaerobic Digesters Treating Swine Wastewater. Environ. Sci. Pollut. Res. 2019, 26, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Lukehurst, P.; Frost, T.A.; Seadi, C.T.A.; Braun, A.R.; Soares, B.G.; Mc Farlan, C.A. Utilization of Digestate from Biogas Plants as Biofertilizer; IEA Bioenergy: Esbjerg, Denmark, 2010. [Google Scholar]
- Fernando, W.A.R.N.; Xia, K.; Rice, C.W. Sorption and Desorption of Ammonium from Liquid Swine Waste in Soils. Soil Sci. Soc. Am. J. 2005, 69, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Walsh, J.J.; Jones, D.L.; Edwards-Jones, G.; Williams, A.P. Replacing Inorganic Fertilizer with Anaerobic Digestate May Maintain Agricultural Productivity at Less Environmental Cost. J. Plant Nutr. Soil Sci. 2012, 175, 840–845. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Yamakawa, C.K.; van der Maas, L.; Dragone, G. New Trends in Bioprocesses for Lignocellulosic Biomass and CO2 Utilization. Renew. Sustain. Energy Rev. 2021, 152, 111620. [Google Scholar] [CrossRef]
- Zhao, M.J.; Xu, Q.Q.; Li, G.M.; Zhang, Q.Z.; Zhou, D.; Yin, J.Z.; Zhan, H.S. Pretreatment of Agricultural Residues by Supercritical CO2 at 50–80 °C to Enhance Enzymatic Hydrolysis. J. Energy Chem. 2019, 31, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Gu, T. Pretreatment of Lignocellulosic Biomass Using Supercritical Carbon Dioxide as a Green Solvent. In Green Biomass Pretreatment for Biofuels Production; Springer: Berlin/Heidelberg, Germany, 2013; pp. 107–125. [Google Scholar]
- Rubežius, M.; Venslauskas, K.; Navickas, K.; Bleizgys, R. Influence of Aerobic Pretreatment of Poultry Manure on the Biogas Production Process. Processes 2020, 8, 1109. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of Lignocellulosic Materials as Substrates for Fermentation Processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef] [Green Version]
- Andriani, D.; Wresta, A.; Atmaja, T.D.; Saepudin, A. A Review on Optimization Production and Upgrading Biogas through CO2 Removal Using Various Techniques. Appl. Biochem. Biotechnol. 2014, 172, 1909–1928. [Google Scholar] [CrossRef]
- Wang, R.; Yue, J.; Jiang, J.; Li, J.; Zhao, J.; Xia, H.; Wang, K.; Xu, J. Hydrothermal CO2-Assisted Pre-treatment of Wheat Straw for Hemicellulose Degradation Followed with Enzymatic Hydrolysis for Glucose Production. Waste Biomass Valorization 2021, 12, 1483–1492. [Google Scholar] [CrossRef]
- Park, C.; Lee, J. Recent Achievements in CO2 Assisted and CO2 Catalyzed Biomass Conversion Reactions. Green Chem. 2020, 22, 2628–2642. [Google Scholar] [CrossRef]
- Fu, S.; Angelidaki, I.; Zhang, Y. In Situ Biogas Upgrading by CO2-to-CH4 Bioconversion. Trends Biotechnol. 2021, 39, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Muntau, M.; Lebuhn, M.; Polag, D.; Bajón-Fernández, Y.; Koch, K. Effects of CO2 Enrichment on the Anaerobic Digestion of Sewage Sludge in Continuously Operated Fermenters. Bioresour. Technol. 2021, 332, 125147. [Google Scholar] [CrossRef] [PubMed]
- Alimahmoodi, M.; Mulligan, C.N. Anaerobic Bioconversion of Carbon Dioxide to Biogas in an Up-flow Anaerobic Sludge Blanket Reactor. J. Air Waste Manag. Assoc. 2008, 58, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Salomoni, C.; Caputo, A.; Bonoli, M.; Francioso, O.; Rodriguez-Estrada, M.T.; Palenzona, D. Enhanced Methane Production in a Two-Phase Anaerobic Digestion Plant, after CO2 Capture and Addition to Organic Wastes. Bioresour. Technol. 2011, 102, 6443–6448. [Google Scholar] [CrossRef]
- Bajón Fernández, Y.; Soares, A.; Koch, K.; Vale, P.; Cartmell, E. Bioconversion of Carbon Dioxide in Anaerobic Digesters for On-Site Carbon Capture and Biogas Enhancement–A Review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1555–1580. [Google Scholar] [CrossRef]
- Hepburn, A.J.; Daugulis, A.J. The Use of CO2 for Reversible pH Shifting, and the Removal of Succinic Acid in a Polymer-Based Two-Phase Partitioning Bioreactor. J. Chem. Technol. Biotechnol. 2012, 87, 42–50. [Google Scholar] [CrossRef]
- Kim, K.H.; Hong, J. Supercritical CO2 Pretreatment of Lignocellulose Enhances Enzymatic Cellulose Hydrolysis. Bioresour. Technol. 2001, 77, 139–144. [Google Scholar] [CrossRef]
- Zhuang, Q.; Clements, B.; Li, Y. International Journal of Greenhouse Gas Control From Ammo-nium Bicarbonate Fertilizer Production Process to Power Plant CO2 Capture. Int. J. Greenh. Gas Control 2012, 10, 56–63. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A. Utilisation of CO2 as a Chemical Feedstock: Opportunities and Challenges. J. Chem. Society. Dalton Trans. 2007, 28, 2975–2992. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Modestra, J.A.; Amulya, K.; Butti, S.K.; Velvizhi, G. A Circular Bioeconomy with Biobased Products from CO2 Sequestration. Trends Biotechnol. 2016, 34, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, J.K.; Valgepea, K.; de Souza Pinto Lemgruber, R.; Casini, I.; Plan, M.; Tappel, R.; Simp-son, S.D.; Köpke, M.; Nielsen, L.K.; Marcellin, E. Enhancing CO2-Valorization Using Clostridium Autoethanogenum for Sustainable Fuel and Chemicals Production. Front. Bioeng. Biotechnol. 2020, 8, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vongvichiankul, C.; Deebao, J.; Khongnakorn, W. Relationship between PH, Oxidation Reduction Potential (ORP) and Biogas Production in Mesophilic Screw Anaerobic Digester. Energy Procedia 2017, 138, 877–882. [Google Scholar] [CrossRef]
- Weißbach, M.; Drewes, J.E.; Koch, K. Application of the Oxidation Reduction Potential (ORP) for Process Control and Monitoring Nitrite in a Coupled Aerobic-Anoxic Nitrous Decomposition Operation (CANDO). Chem. Eng. J. 2018, 343, 484–491. [Google Scholar] [CrossRef]
- Su, H.; Tan, F.; Xu, Y. Enhancement of Biogas and Methanization of Citrus Waste via Biodegradation Pretreatment and Subsequent Optimized Fermentation. Fuel 2016, 181, 843–851. [Google Scholar] [CrossRef]
- ISO 10694 (1995); Soil Quality—Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis). Beuth: Berlin, Germany, 1995.
- EN 13654-1 (2001); Soil Improvers and Growing Media—Determination of Nitrogen—Part 1—Modified Kjeldahl Method, Nitrogen. European Committee for Standardization: Brussels, Belgium, 2001.
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the Anaerobic Digestion of Agricultural Resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Kobayashi, T.; Li, Y.Y.; Xu, K.; Zhao, Y. Mesophilic Anaerobic Co-Digestion of Waste Activated Sludge and Egeria Densa: Performance Assessment and Kinetic Analysis. Appl. Energy 2015, 148, 78–86. [Google Scholar] [CrossRef]
- Chakraborty, D.; Karthikeyan, O.P.; Selvam, A.; Palani, S.G.; Ghangrekar, M.M.; Wong, J.W.C. Two-Phase Anaerobic Digestion of Food Waste: Effect of Semi-Continuous Feeding on Acidogenesis and Methane Production. Bioresour. Technol. 2022, 346, 126396. [Google Scholar] [CrossRef]
- Rao, M.S.; Singh, S.P. Bioenergy Conversion Studies of Organic Fraction of MSW: Kinetic Studies and Gas Yield-Organic Loading Relationships for Process Optimisation. Bioresour. Technol. 2004, 95, 173–185. [Google Scholar] [CrossRef]
- Möller, K. Effects of Anaerobic Digestion on Soil Carbon and Nitrogen Turnover, N Emissions, and Soil Biological Activity. A Review. Agron. Sustain. Dev. 2015, 35, 1021–1041. [Google Scholar] [CrossRef]
- Jiang, Y.; McAdam, E.; Zhang, Y.; Heaven, S.; Banks, C.; Longhurst, P. Ammonia Inhibition and Toxicity in Anaerobic Digestion: A Critical Review. J. Water Process Eng. 2019, 32, 100899. [Google Scholar] [CrossRef]
- Provolo, G.; Perazzolo, F.; Mattachini, G.; Finzi, A.; Naldi, E.; Riva, E. Nitrogen Removal from Digested Slurries Using a Simplified Ammonia Stripping Technique. Waste Manag. 2017, 69, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Rafique, R.; Poulsen, T.G.; Nizami, A.S.; Asam, Z.u.Z.; Murphy, J.D.; Kiely, G. Effect of Thermal, Chemical and Thermo-Chemical Pre-Treatments to Enhance Methane Production. Energy 2010, 35, 4556–4561. [Google Scholar] [CrossRef]
- Samani, S.; Abdoli, M.A.; Karbassi, A. Role of Ph, Oxidation Reduction Potential and Temperature Change in Biogas Yield during Anaerobic Digestion of Cattle Manure. Int. J. Recent Sci. Res. 2017, 8, 16137–16140. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Wei, Y.; Leng, X. Improving Biogas Production Using Additives in Anaerobic Digestion: A Review. J. Clean. Prod. 2021, 297, 126666. [Google Scholar] [CrossRef]
- Qiao, W.; Yan, X.; Ye, J.; Sun, Y.; Wang, W.; Zhang, Z. Evaluation of Biogas Production from Different Biomass Wastes with/without Hydrothermal Pretreatment. Renew Energy 2011, 36, 3313–3318. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ahring, B.K. Methods for Increasing the Biogas Potential from the Recalcitrant Organic Matter Contained in Manure. Water Sci. Technol. 2000, 41, 189–194. [Google Scholar] [CrossRef]
- Ramos-Suárez, J.L.; Gómez, D.; Regueiro, L.; Baeza, A.; Hansen, F. Alkaline and Oxidative Pre-treatments for the Anaerobic Digestion of Cow Manure and Maize Straw: Factors Influencing the Process and Preliminary Economic Viability of an Industrial Application. Bioresour. Technol. 2017, 241, 10–20. [Google Scholar] [CrossRef]
- Zahan, Z.; Othman, M.Z. Effect of Pre-Treatment on Sequential Anaerobic Co-Digestion of Chicken Litter with Agricultural and Food Wastes under Semi-Solid Conditions and Comparison with Wet Anaerobic Digestion. Bioresour. Technol. 2019, 281, 286–295. [Google Scholar] [CrossRef]
- Park, H.S.; Jung, Y.M.; You, J.K.; Hong, W.H.; Kim, J.N. Analysis of the CO2 and NH3 Reaction in an Aqueous Solution by 2D IR COS: Formation of Bicarbonate and Carbamate. J. Phys. Chem. A 2008, 112, 6558–6562. [Google Scholar] [CrossRef]
- Costa, J.C.; Barbosa, S.G.; Alves, M.M.; Sousa, D.Z. Thermochemical Pre- and Biological Co-Treatments to Improve Hydrolysis and Methane Production from Poultry Litter. Bioresour. Technol. 2012, 111, 141–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
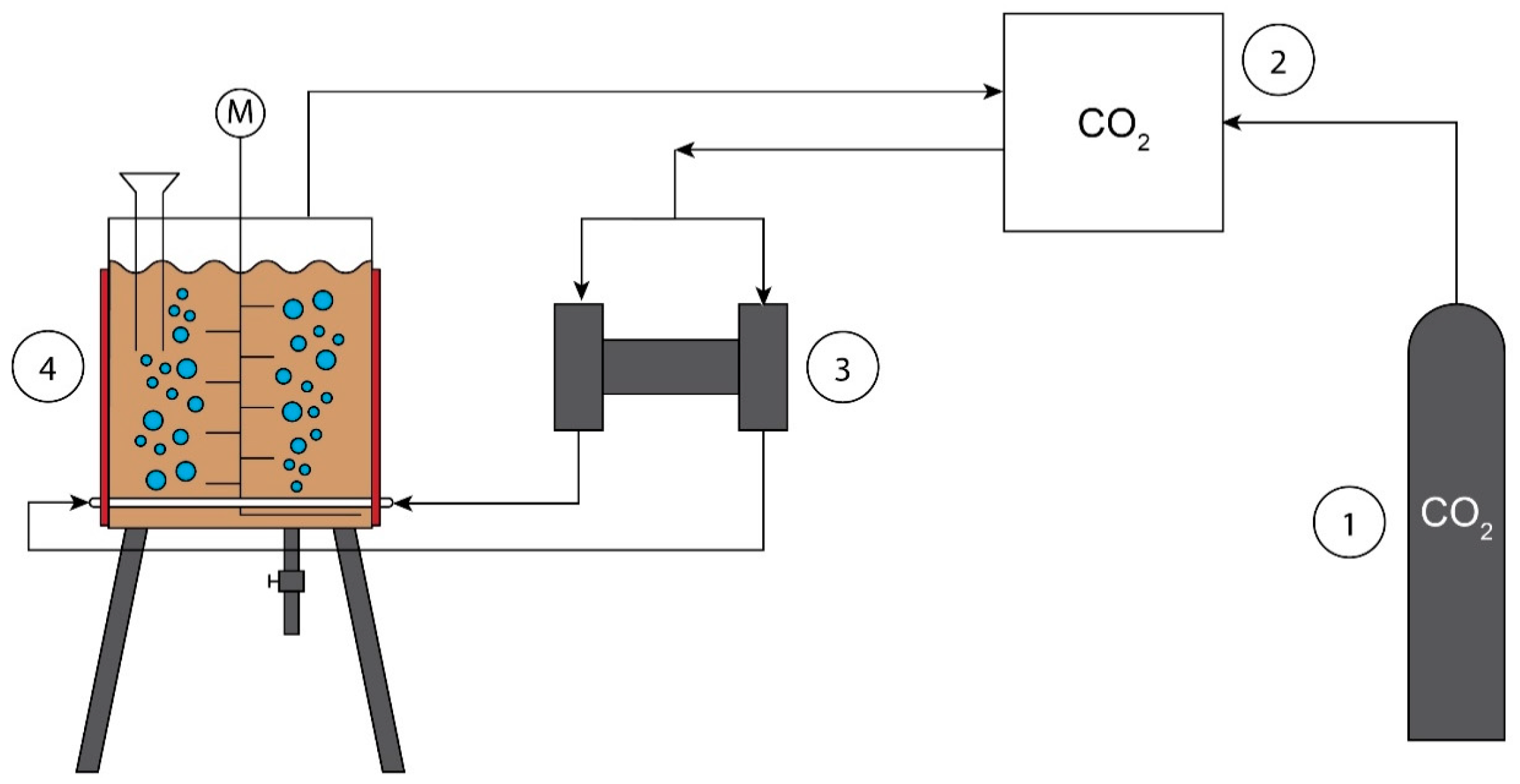
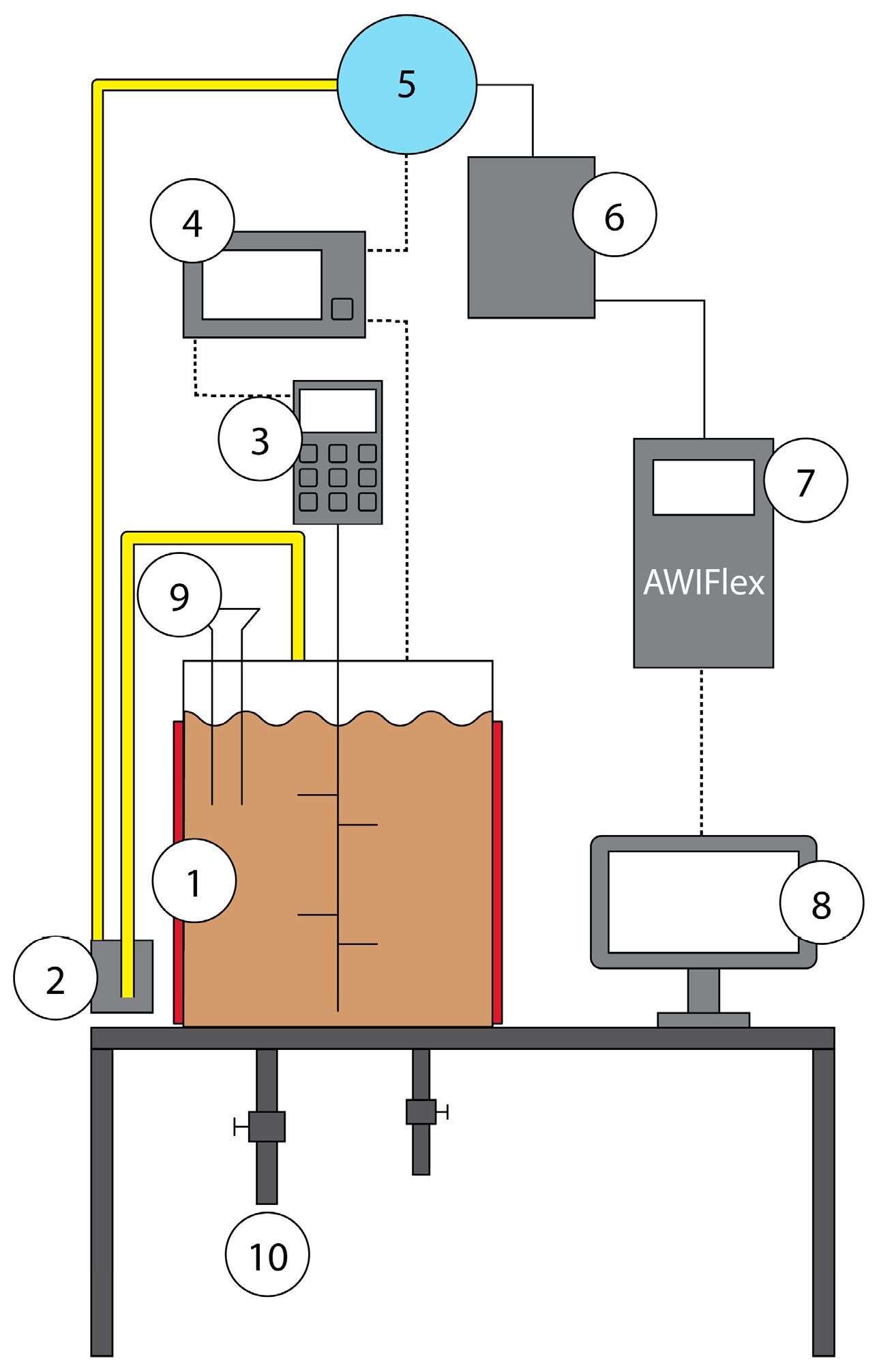
| Feedstock | CoM | ChM | PM |
|---|---|---|---|
| Total solid, % | 7.42 | 37.75 | 7.5 |
| Volatile solid, % | 5.83 | 26.46 | 5.33 |
| Total carbon, % in TS | 34.3 | 26.8 | 28.9 |
| Total nitrogen, % in TS | 0.39 | 2.36 | 0.691 |
| Carbon/nitrogen ratio (C/N) | 87.9 | 11.4 | 41.8 |
| Characteristic | CoM | ChM | PM |
|---|---|---|---|
| Daily mass of the feedstock loaded in the digester, g | 457 | 74.2 | 500 |
| Water amount used for dilution, g | 0 | 240.7 | 0 |
| Stable biogas yield and composition research duration with raw feedstock, days | 3 | 6 | 6 |
| Stable biogas yield and composition research duration with CO2-pretreated feedstock, days | 7 | 11 | 9 |
| Total biogas yield and composition research duration, days | 49 | 80 | 56 |
| Pretreatment reactor organic load rate, kg VS/(m3·d) | 8.86 | 14 | 8.88 |
| Pretreatment reactor hydraulic load rate, kg/m3 | 152 | 166 | 166 |
| Digester organic load rate, kg VS/(m3·d) | 1.4 | 1.4 | 1.4 |
| Digester hydraulic load rate, kg/m3 | 24.1 | 28.5 | 26.3 |
| Indicator | PM | CoM | ChM |
|---|---|---|---|
| pH of raw feedstock | 8.24 ± 0.14 | 7.91 ± 0.1 | 6.33 ± 0.04 |
| pH of pretreated feedstock | 7.3 ± 0.04 | 7.25 ± 0.05 | 5.72 ± 0.09 |
| Influence of pretreatment on feedstock pH, % | −11.4 | −8.4 | −9.6 |
| pH of the digestate using raw feedstock | 8.03 ± 0.02 | 7.79 ± 0.03 | 7.95 ± 0.05 |
| pH of the digestate using pretreated feedstock | 8.07 ± 0.02 | 7.77 ± 0.02 | 8.09 ± 0.02 |
| Influence of pretreatment on substrate pH, % | 0.42% | −0.26% | 1.7% |
| ORP of raw feedstock, mV | −413.4 ± 12.8 | −227 ± 25 | 54.2 ± 67.6 |
| ORP of pretreated feedstock, mV | −391 ± 16.8 | −313 ± 20 | −225 ± 39 |
| Indicator | Raw PM | Pretreated PM | Raw CoM | Pretreated CoM | Raw ChM | Pretreated ChM |
|---|---|---|---|---|---|---|
| Bioreactor organic load kg VS/(m3·d) | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 |
| Biogas yield from feedstock, L/kg | 21.66 ± 1.06 | 26.52 ± 1.22 | 8.32 ± 0.20 | 10.34 ± 0.23 | 123.09 ± 2.35 | 137.56 ± 2.94 |
| Biogas yield feedstock total solids, L/kg | 288.76 ± 14.34 | 353.60 ± 16.30 | 112.06 ± 3.05 | 139.34 ± 3.05 | 531.52 ± 10.15 | 593.99 ± 12.68 |
| Biogas yield from feedstock volatile solids, L/kg | 406.33 ± 20.17 | 497.56 ± 22.94 | 142.87 ± 3.36 | 177.65 ± 3.89 | 758.31 ± 14.48 | 847.44 ± 18.08 |
| Methane concentration in biogas, % | 66.69 ± 0.66 | 66.35 ± 0.34 | 54.70 ± 0.06 | 58.40 ± 1.51 | 54.73 ± 0.70 | 63.05 ± 0.45 |
| Energetic value of biogas, MJ/m3 | 23.54 ± 0.23 | 23.42 ± 0.12 | 19.31 ± 0.02 | 20.77 ± 0.45 | 19.32 ± 0.25 | 22.26 ± 0.16 |
| Biomethane yield from feedstock volatile solids, L/kgVS | 192.64 ± 5.71 | 234.62 ± 10.93 | 61.3 ± 1.5 | 82.01 ± 3.19 | 290.88 ± 5.74 | 374.53 ± 9.27 |
| Energy obtained from feedstock, MJ/kg | 0.51 ± 0.03 | 0.62 ± 0.03 | 0.16 ± 0.01 | 0.21 ± 0.01 | 2.38 ± 0.05 | 3.06 ± 0.08 |
| Influence of the pretreatment on biomethane yield, % | +21.78 | +33.78 | +28.76 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žalys, B.; Venslauskas, K.; Navickas, K.; Buivydas, E.; Rubežius, M. The Influence of CO2 Injection into Manure as a Pretreatment Method for Increased Biogas Production. Sustainability 2023, 15, 3670. https://doi.org/10.3390/su15043670
Žalys B, Venslauskas K, Navickas K, Buivydas E, Rubežius M. The Influence of CO2 Injection into Manure as a Pretreatment Method for Increased Biogas Production. Sustainability. 2023; 15(4):3670. https://doi.org/10.3390/su15043670
Chicago/Turabian StyleŽalys, Bronius, Kęstutis Venslauskas, Kęstutis Navickas, Egidijus Buivydas, and Mantas Rubežius. 2023. "The Influence of CO2 Injection into Manure as a Pretreatment Method for Increased Biogas Production" Sustainability 15, no. 4: 3670. https://doi.org/10.3390/su15043670
APA StyleŽalys, B., Venslauskas, K., Navickas, K., Buivydas, E., & Rubežius, M. (2023). The Influence of CO2 Injection into Manure as a Pretreatment Method for Increased Biogas Production. Sustainability, 15(4), 3670. https://doi.org/10.3390/su15043670







