Rapid Screening for Mycosporine-like Amino Acids (MAAs) of Irish Marine Cyanobacteria and Their Antioxidant Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Cyanobacteria Strains and Cultivation Conditions
2.2. Biodiscovery Screening of Cyanobacteria for MAAs Content
2.3. Optimisation of MAAs Extraction
2.4. Large-Scale Extraction of MAAs from Cyanobacteria Candidates
2.5. Quantification of Specific MAAs Content
2.6. LC-MS Identification of Specific MAAs
2.7. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
2.8. Ferric Reducing Antioxidant Power (FRAP) Assay
2.9. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.10. Statistical Analysis
3. Results
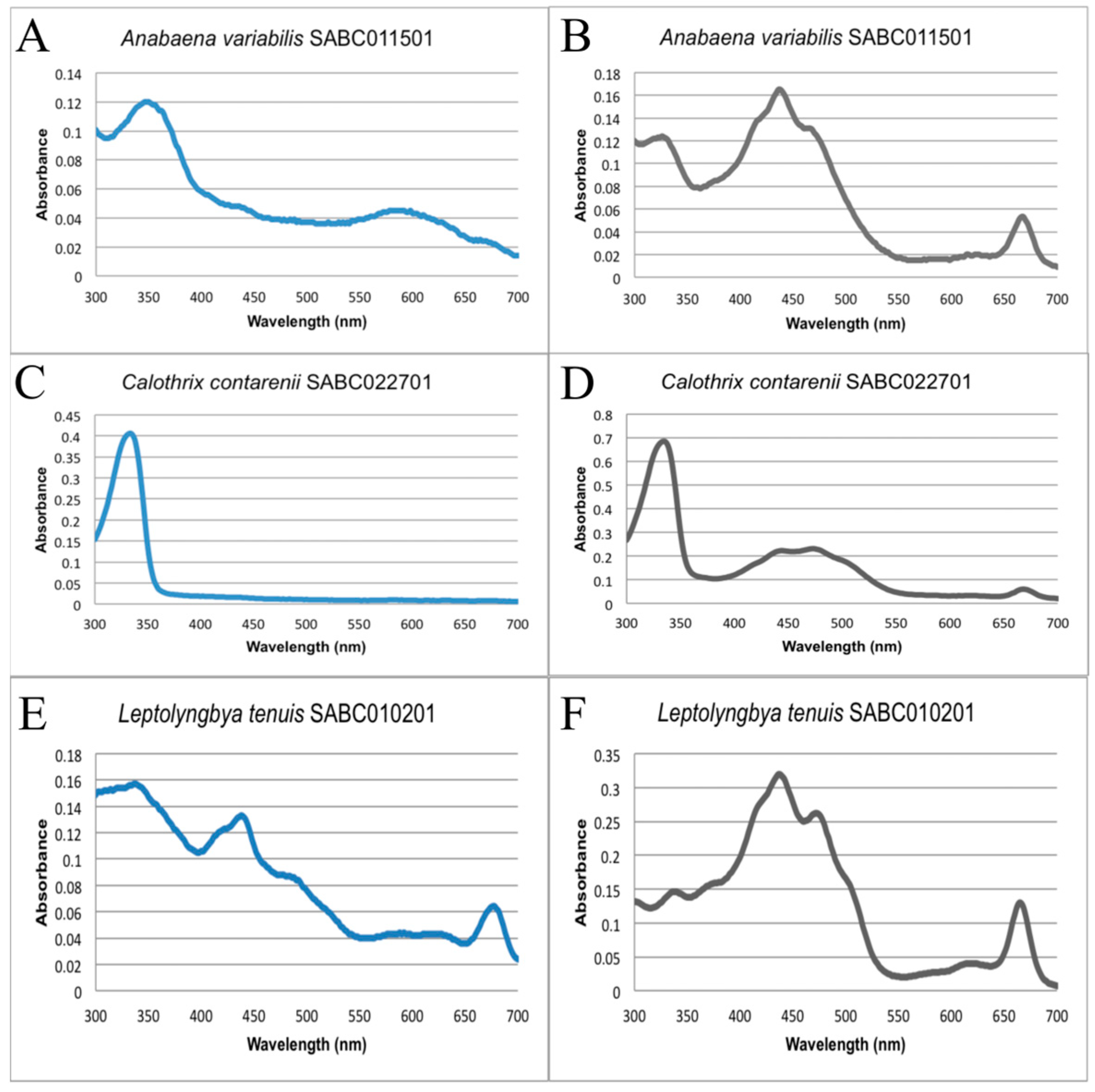
3.1. Biodiscovery Screen and Cellular Morphology
3.2. Determination of Specific MAAs Content
3.3. Identification of Specific MAAs by LC-MS
3.4. Antioxidant Capacity of Total MAAs Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amadu, A.A.; deGraft-Johnson, K.A.A.; Ameka, G.K. Industrial Applications of Cyanobacteria. In Cyanobacteria—Recent Advances in Taxonomy and Applications; Hozzein, W.N., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Rasmussen, B.; Fletcher, I.R.; Brocks, J.J.; Kilburn, M.R. Reassessing the first appearance of eukaryotes and cyanobacteria. Nature 2008, 455, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Deng, S.; Li, C.; Liu, Y.; Chen, L.; Hu, C. Damage to DNA caused by UV-B radiation in the desert cyanobacterium Scytonema javanicum and the effects of exogenous chemicals on the process. Chemosphere 2012, 88, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Richa; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic. Acids. 2010, 2010, 592980. [Google Scholar] [CrossRef] [PubMed]
- Dubey, G.; Prasad, S.M. Differential display of antioxidants in mitigating adverse effects of UV-B radiation in Nostoc muscorum and Phormidium foveolarum photoacclimated to different irradiances. Appl. Biochem. Biotechnol. 2015, 175, 2703–2728. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Singh, S.P.; Häder, D.P.; Sinha, R.P. Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2′,7′-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem. Bioph. Res. Commun. 2010, 397, 603–607. [Google Scholar] [CrossRef]
- Li, P.; Liu, W.; Gao, K. Effects of temperature, pH, and UV radiation on alkaline phosphatase activity in the terrestrial cyanobacterium Nostoc flagelliforme. J. Appl. Phycol. 2013, 25, 1031–1038. [Google Scholar] [CrossRef]
- Shrivastava, A.K.; Chatterjee, A.; Yadav, S.; Singh, P.K.; Singh, S.; Rai, L.C. UV-B stress induced metabolic rearrangements explored with comparative proteomics in three Anabaena species. J. Proteomics. 2015, 127, 122–133. [Google Scholar] [CrossRef]
- Kottuparambil, S.; Shin, W.; Brown, M.T.; Han, T. UV-B affects photosynthesis, ROS production and motility of the freshwater flagellate, Euglena agilis Carter. Aquat. Toxicol. 2012, 122, 206–213. [Google Scholar] [CrossRef]
- Barbato, R.; Frizzo, A.; Friso, G.; Rigoni, F.; Giacometti, G.M. Degradation of the D1 protein of photosystem-II reaction centre by ultraviolet-B radiation requires the presence of functional manganese on the donor side. Eur. J. Biochem. 1995, 227, 723–729. [Google Scholar] [CrossRef]
- Newton, J.W.; Tyler, D.D.; Slodki, M.E. Effect of ultraviolet-B (280 to 320 nm) radiation on blue-green algae (cyanobacteria), possible biological indicators of stratospheric ozone depletion. Appl. Environ. Microbiol. 1979, 37, 1137–1141. [Google Scholar] [CrossRef]
- Bhatia, S.; Garg, A.; Sharma, K.; Kumar, S.; Sharma, A.; Purohit, A.P. Mycosporine and mycosporine-like amino acids: A paramount tool against ultra violet irradiation. Pharmacogn. Rev. 2011, 5, 138–146. [Google Scholar] [CrossRef]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. The deactivation pathways of the excited-states of the mycosporine-like amino acids shinorine and porphyra-334 in aqueous solution. Photoch. Photobiol. Sci. 2004, 3, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Kilsch, M.; Sinha, R.P.; Richter, P.R.; Häder, D.P. Mycosporine-like amino acids (MAAs) protect against UV-B-induced damage in Gyrodinium dorsum Kofoid. Plant. Physiol. 2001, 158, 1449–1454. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Incharoensakdi, A. UV radiation-induced biosynthesis, stability and antioxidant activity of mycosporine-like amino acids (MAAs) in a unicellular cyanobacterium Gloeocapsa sp. CU2556. J. Photochem. Photobiol. B. 2014, 130, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Babele, P.K.; Sinha, R.P.; Tyagi, M.B.; Kumar, A. Enzymatic and non-enzymatic defense mechanisms against ultraviolet-B radiation in two Anabaena species. Process. Biochem. 2013, 48, 796–802. [Google Scholar] [CrossRef]
- Matsui, K.; Nazifi, E.; Kunita, S.; Wada, N.; Matsugo, S.; Sakamoto, T. Novel glycosylated mycosporine-like amino acids with radical scavenging activity from the cyanobacterium Nostoc commune. J. Photochem. Photobiol. B. 2011, 105, 81–89. [Google Scholar] [CrossRef]
- Nazifi, E.; Wada, N.; Yamaba, M.; Asano, T.; Nishiuchi, T.; Matsugo, S.; Sakamoto, T. Glycosylated porphyra-334 and palythine-threonine from the terrestrial cyanobacterium Nostoc commune. Mar. Drugs 2013, 11, 3124–3154. [Google Scholar] [CrossRef]
- Sinha, R.P.; Singh, S.P.; Häder, D.P. Database on mycosporines and mycosporine-like amino acids (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. J. Photochem. Photobiol. B. 2007, 89, 29–35. [Google Scholar] [CrossRef]
- Athukorala, Y.; Trang, S.; Kwok, C.; Yuan, Y.V. Antiproliferative and antioxidant activities and mycosporine-like amino acid profiles of wild-harvested and cultivated edible Canadian marine red macroalgae. Molecules 2016, 21, 119. [Google Scholar] [CrossRef]
- de la Coba, F.; Aguilera, J.; Figueroa, F.L.; De Gálvez, M.V.; Herrera, E. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J. Appl. Phycol. 2008, 21, 161–169. [Google Scholar] [CrossRef]
- Suh, S.S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.S.; Lee, J.H.; Moh, S.H.; Lee, T.K. Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef]
- Balskus, E.P.; Walsh, C.T. The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 2010, 329, 1653–1656. [Google Scholar] [CrossRef]
- Browne, N.; Donovan, F.; Murray, P.; Saha, S.K. Cyanobacteria as bio-factories for production of UV-screening compounds. OA Biotechnol. 2014, 12, 6. [Google Scholar]
- Garcia-Pichel, F.; Castenholz, R.W. Occurrence of UV-absorbing, mycosporine-like compounds among cyanobacterial isolates and an estimate of their screening capacity. Appl. Environ. Microbiol. 1993, 59, 163–169. [Google Scholar] [CrossRef]
- Al-Utaibi, A.A.; Niaz, G.R.; Al-Lihaibi, S.S. Mycosporine-like amino acids in six scleractinian coral species. Oceanologia 2009, 51, 93–104. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Wingard, C.E.; Castenholz, R.W. Evidence regarding the UV sunscreen role of a mycosporine-like compound in the cyanobacterium Gloeocapsa sp. Appl. Environ. Microbiol. 1993, 59, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Roullier, C.; Chollet-Krugler, M.; Pferschy-Wenzig, E.M.; Maillard, A.; Rechberger, G.N.; Legouin-Gargadennec, B.; Bauer, R.; Boustie, J. Characterization and identification of mycosporines-like compounds in cyanolichens. Isolation of mycosporine hydroxyglutamicol from Nephroma laevigatum Ach. Phytochemistry 2011, 72, 1348–1357. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending applicability of the Oxygen Radical Absorbance Capacity (ORAC-Fluorescein) assay. J. Agric. Food. Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Shiels, K.; Browne, N.; Donovan, F.; Murray, P.; Saha, S.K. Molecular Characterization of Twenty-Five Marine Cyanobacteria Isolated from Coastal Regions of Ireland. Biology 2019, 8, 59. [Google Scholar] [CrossRef]
- Keshari, N.; Das, S.K.; Adhikary, S.P. Identification of cyanobacterial species with overlapping morphological features by 16S rRNA gene sequencing. Eur. J. Phycol. 2015, 50, 395–399. [Google Scholar] [CrossRef]
- Svenning, M.M.; Eriksson, T.; Rasmussen, U. Phylogeny of symbiotic cyanobacteria within the genus Nostoc based on 16S rRNA sequence analyses. Arch. Microbiol. 2005, 183, 19–26. [Google Scholar] [CrossRef]
- Řeháková, K.; Johansen, J.R.; Casamatta, D.A.; Xuesong, L.; Vincent, J. Morphological and molecular characterization of selected desert soil cyanobacteria: Three species new to science including Mojavia pulchra gen. et sp. Nov. Phycologia 2007, 46, 481–502. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Wu, Q. Protective effects of mycosporine-like amino acids of Synechocystis sp. PCC 6803 and their partial characterization. J. Photochem. Photobiol. B 2007, 86, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Pathak, J.; Richa, R.; Sonker, A.S.; Kannaujiya, V.K.; Sinha, R.P. Isolation and partial purification of scytonemin and mycosporine-like amino acids from biological crusts. J. Chem. Pharm. Res. 2015, 7, 362–371. [Google Scholar]
- Palinska, K.A.; Deventer, B.; Hariri, K.; Lotocka, M. A taxonomic study on Phormidium-group (cyanobacteria) based on morphology, pigments, RAPD molecular markers and RFLP analysis of the 16S rRNA gene fragment. Fottea 2011, 11, 41–55. [Google Scholar] [CrossRef]
- D’Agostino, P.M.; Javalkote, V.S.; Mazmouz, R.; Pickford, R.; Puranik, P.R.; Neilan, B.A. Comparative profiling and discovery of novel glycosylated mycosporine-like amino acids in two strains of the cyanobacterium Scytonema cf. crispum. Appl. Environ. Microbiol. 2016, 82, 5951–5959. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.P.; Ambasht, N.K.; Sinha, J.P.; Klisch, M.; Häder, D.P. UV-B-induced synthesis of mycosporine-like amino acids in three strains of Nodularia (cyanobacteria). J. Photochem. Photobiol. B. 2003, 71, 51–58. [Google Scholar] [CrossRef]
- Liu, Z.; Häder, D.P.; Sommaruga, R. Occurrence of mycosporine-like amino acids (MAAs) in the bloom-forming cyanobacterium Microcystis aeruginosa. J. Plankton. Res. 2004, 26, 963–966. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumari, S.; Rastogi, R.P.; Singh, K.L.; Sinha, R.P. Mycosporine-like amino acids (MAAs): Chemical structure, biosynthesis and significance as UV-absorbing/screening compounds. Indian. J. Exp. Biol. 2008, 46, 7–17. [Google Scholar]
- Hartmann, A.; Becker, K.; Karsten, U.; Remias, D.; Ganzera, M. Analysis of mycosporine-like amino acids in selected algae and cyanobacteria by hydrophilic interaction liquid chromatography and a novel MAA from the red alga Catenella repens. Mar. Drugs 2015, 13, 6291–6305. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D.; Incharoensakdi, A. Characterization and antioxidant functions of mycosporine-like amino acids in the cyanobacterium Nostoc sp. R76DM. Algal Res. 2016, 16, 110–118. [Google Scholar] [CrossRef]
- Hossain, M.F.; Ratnayake, R.R.; Meerajini, K.; Wasantha Kumara, K.L. Antioxidant properties in some selected cyanobacteria isolated from fresh water bodies of Sri Lanka. Food. Sci. Nutr. 2016, 4, 753–758. [Google Scholar] [CrossRef]
- Price, J.A.; Sanny, C.G.; Shevlin, D. Application of manual assessment of oxygen radical absorbent capacity (ORAC) for use in high throughput assay of ‘‘total’’ antioxidant activity of drugs and natural products. J. Pharmacol. Toxicol. Methods 2006, 54, 56–61. [Google Scholar] [CrossRef] [PubMed]




| Cyanobacterial Isolates | MAAs Content (A* mg DW−1 ± SD) | GenBank Accession Number |
|---|---|---|
| Anabaena variabilis SABC011501 | 0.155 ± 0.009 | KX765290 |
| Calothrix contarenii SABC022701 | 0.445 ± 0.007 | KT740998 |
| Leptolyngbya africana SABC021601 | 0.412 ± 0.006 | KT740999 |
| Leptolyngbya tenuis SABC010201 | 0.511 ± 0.014 | KX765288 |
| Phormidium angustissimum SABC020801 | 0.114 ± 0.024 | KT740997 |
| Phormidium angustissimum SABC022612 | 0.156 ± 0.011 | KX765287 |
| Phormidium sp. SABC022903 | 0.123 ± 0.025 | KT741000 |
| Schizothrix sp. SABC022401 | 0.199 ± 0.004 | KX765289 |
| Phormidium angustissimum SABC020801 | Phormidium angustissimum SABC022612 | Leptolyngbya africana SABC021601 | Leptolyngba tenuis SABC010201 | Phormidium sp. SABC022903 | Schizothrix sp. SABC022401 | Anabaena variabilis SABC011501 | Calothrix contarenii SABC022701 | |
|---|---|---|---|---|---|---|---|---|
| Mycosporine-serinol | 1.198 260.9086 310 nm | - | - | - | - | - | - | - |
| Mycosporine-taurine | 1.186 317.0687 309 nm | 1.188 317.0521 309 nm | 1.174 317.0504 309 nm | 1.170 317.0615 309 nm | 1.214 317.0611 309 nm | 1.194 317.0607 309 nm | - | 1.196 317.0624 309 nm |
| Usujirene | 24.239 283.2665 357 nm | 24.229 283.2656 357 nm | 24.144 283.2660 357 nm | 24.175 283.2675 357 nm | 24.172 283.2655 357 nm | 24.459 283.2652 357 nm | - | - |
| Mycosporine-glutamicol | - | - | 2.744 302.0658 310 nm | 2.645 302.0706 310 nm | - | - | - | - |
| Mycosporine-glutaminol-glucoside | - | 3.159 463.2186 310 nm | - | 3.094 463.2215 310 nm | - | - | - | - |
| Palythine | 3.311 243.1741 320 nm | - | - | - | - | - | - | - |
| Palythine-threonine-sulphate | - | 27.18 367.3812 322 nm | 27.13 367.3663 322 nm | - | - | - | - | - |
| Porphya-334 | - | - | - | - | - | - | 1.301 345.1368 334 nm | 1.196 345.9219 334 nm |
| UC (M-314) | 2.862 219.0557 314 nm C6H12N4O3S | 2.764 219.0531 314 nm C9H8N4O3 | ||||||
| UC (M-326) | 1.347 289.0730 326 nm C9H15ClN6O | 1.43 289.0557 326 nm C6H15ClN4O7 | ||||||
| UC (M-330) | 19.705 309.2061 330 nm C11H30N6O2S | |||||||
| UC (M-346) | 15.489 321.0005 346 nm C13H10N2O4S |
| Cyanobacterial Isolates | DPPH (μM TE g DW−1) | FRAP (μM TE g DW−1) | ORAC (μM TE g DW−1) |
|---|---|---|---|
| Anabaena variabilis SABC011501 | 343.1579 ± 0.0269 | 325.8333 ± 0.0098 | 180,405.99 ± 12,615.73 |
| Calothrix contarenii SABC022701 | 504.5614 ± 0.0190 | 510.5556 ± 0.0172 | 111,593.23 ± 381.742 |
| Leptolyngbya africana SABC021601 | 641.4035 ± 0.0038 | 603.6111 ± 0.0065 | 216,537.81 ± 8019.166 |
| Leptolyngbya tenuis SABC010201 | 388.7719 ± 0.0045 | 323.0556 ± 0.0136 | 230,336.89 ± 8985.885 |
| Phormidium angustissimum SABC020801 | 351.9298 ± 0.0108 | 410.5556 ± 0.0127 | 233,694.25 ± 5125.243 |
| Phormidium angustissimum SABC022612 | 469.4737 ± 0.0026 | 445.2778 ± 0.0050 | 107,705.11 ± 554.054 |
| Phormidium sp. SABC022903 | 764.2105 ± 0.0075 | 727.2222 ± 0.0015 | 246,140.97 ± 9370.107 |
| Schizothrix sp. SABC022401 | 167.7193 ± 0.0030 | 223.05556 ± 0.0084 | 241,224.56 ± 8580.119 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Browne, N.; Otero, P.; Murray, P.; Saha, S.K. Rapid Screening for Mycosporine-like Amino Acids (MAAs) of Irish Marine Cyanobacteria and Their Antioxidant Potential. Sustainability 2023, 15, 3792. https://doi.org/10.3390/su15043792
Browne N, Otero P, Murray P, Saha SK. Rapid Screening for Mycosporine-like Amino Acids (MAAs) of Irish Marine Cyanobacteria and Their Antioxidant Potential. Sustainability. 2023; 15(4):3792. https://doi.org/10.3390/su15043792
Chicago/Turabian StyleBrowne, Norma, Paz Otero, Patrick Murray, and Sushanta Kumar Saha. 2023. "Rapid Screening for Mycosporine-like Amino Acids (MAAs) of Irish Marine Cyanobacteria and Their Antioxidant Potential" Sustainability 15, no. 4: 3792. https://doi.org/10.3390/su15043792
APA StyleBrowne, N., Otero, P., Murray, P., & Saha, S. K. (2023). Rapid Screening for Mycosporine-like Amino Acids (MAAs) of Irish Marine Cyanobacteria and Their Antioxidant Potential. Sustainability, 15(4), 3792. https://doi.org/10.3390/su15043792








