Integrated Approach for Carbon Sequestration and Wastewater Treatment Using Algal–Bacterial Consortia: Opportunities and Challenges
Abstract
:1. Introduction
- (a)
- Reduced use of non-renewable energy sources including fossil-fuels.
- (b)
- Efficient use of low carbon renewable energy sources such as solar, wind, hydro, and nuclear, as well as carbon-neutral alternative energy sources such as biomass.
- (c)
- Adoption of post-treatment process such as carbon-capture and storage (CCS) technology.
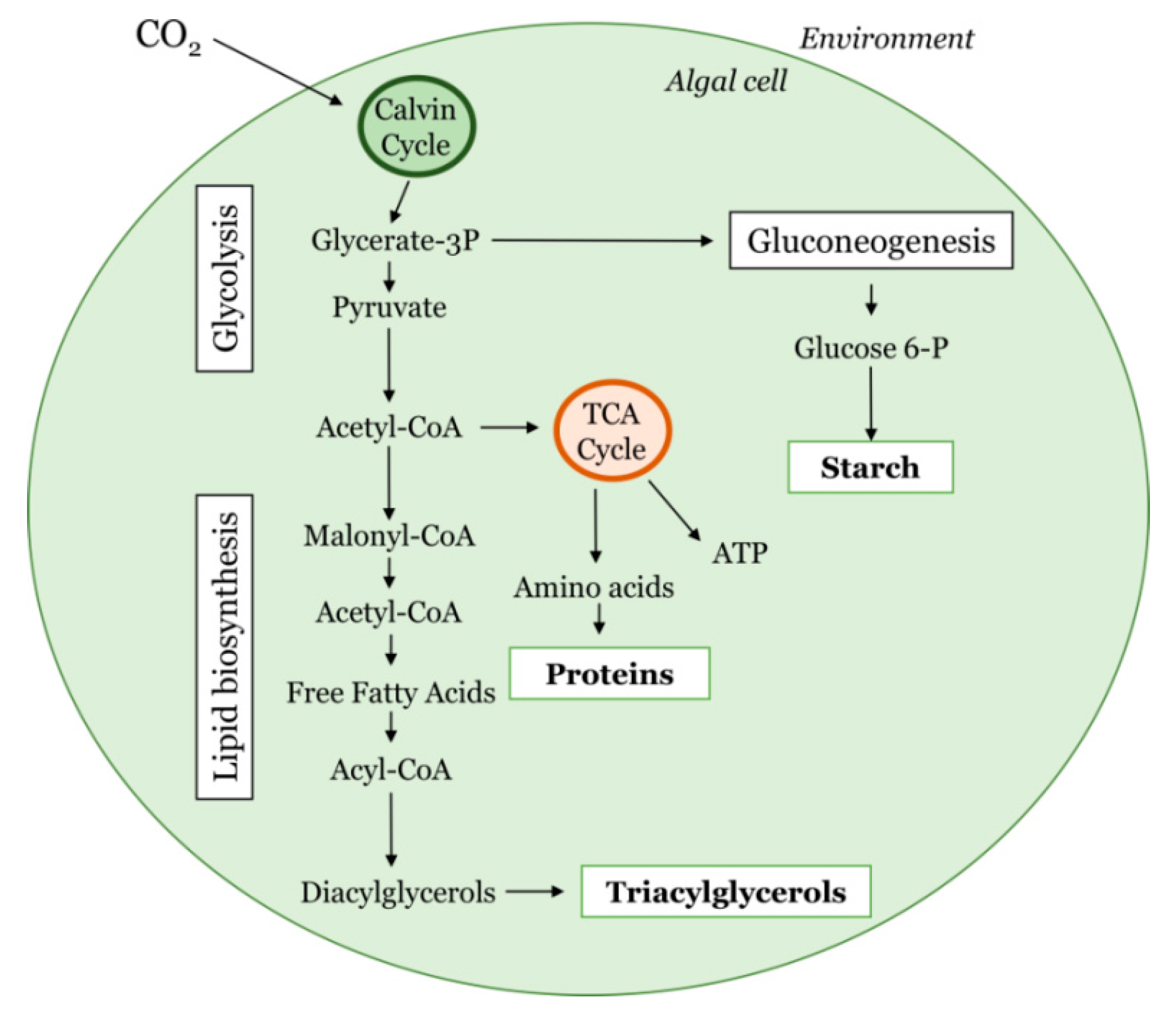
2. CO2 Capture and Fixation by Microalgae
3. Microalgae-Based Wastewater Treatment
4. Factors Influencing Algal Cultivation in Wastewater and CO2 Uptake
4.1. Algal Strains
4.2. Mode of Microalgal Growth
4.3. Nutrients
4.4. Temperature
4.5. pH
4.6. CO2 Concentration
4.7. Composition of Flue Gas
4.8. Light
5. Microalgae in Wastewater Treatment
Synergistic Effect of Algal–Bacterial Co-Cultivation in Wastewater
6. Algal Cultivation Systems and Possibilities
6.1. Open Pond Hybrid Design
6.2. Photobioreactor Design for CO2 Fixation
- Efficient collection of solar radiation due to narrow gauge of tubes or channels.
- High areal yields because of high culture densities.
- Very low contamination risk as they are efficient in maintaining sterility.
- High biomass yield ensured by proper nutrient mixing and better CO2 conversion.
- Often easy to operate with process monitoring and control systems.
6.3. Merits and Demerits
7. Challenges and Limitations
7.1. Algal–Bacterial Co-Cultivation
7.2. Efficient CO2 Sparging and Mixing Systems
7.3. Heavy Metals and Other Toxicants in the Effluents
7.4. Need for Efficient Algae Harvesting Techniques
7.5. Post-Harvest Preservation and Storage
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NOAA Report. Available online: https://www.giss.nasa.gov/research/news/20170118/ (accessed on 2 October 2020).
- Hossain, M.M.; de Lasa, H.I. Reactivity and stability of Co–Ni/Al2O3 oxygen carrier in a multicycle CLC. AIChE J. 2007, 53, 1817–1829. [Google Scholar] [CrossRef]
- Sayer, R. Microalgae: The potential for carbon capture. Bioscience 2010, 60, 722–727. [Google Scholar] [CrossRef]
- IEA. Global Energy & CO2 Status Report 2019; IEA: Paris, France, 2019; Available online: https://www.iea.org/reports/global-energy-co2-status-report-2019 (accessed on 5 November 2020).
- United Nations Climate Change Annual Report. United Nations Framework Convention on Climate Change (UNFCCC). 2019, p. 62. Available online: https://unfccc.int/sites/default/files/resource/unfccc_annual_report_2019.pdf (accessed on 20 November 2020).
- Wang, B.; Li, Y.; Wu, N.; Lan, C.Q. CO2 bio-mitigation using microalgae. Appl. Microbiol. Biotechnol. 2008, 79, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Khairul, S.N.K. Synthesis and Modification of Micro and Mesoporous Materials as CO2 Adsorbents. 2009. Available online: http://eprints.utm.my/id/eprint/9749/1/78207.pdf (accessed on 6 October 2019).
- IPCC. Climate change 2001: Impacts, Adaptation and Vulnerability, Contribution of Working Group II to the Third Assessment Report of the Intergovernmental Panel on Climate Change; McCarthy, J.J., Canziani, O.F., Leary, N.A., Dokken, D.J., White, K.S., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2001; p. 1032. [Google Scholar]
- Bhola, V.; Swalaha, F.; Ranjith Kumar, R.; Singh, M.; Bux, F. Overview of the potential of microalgae for CO2 sequestration. Intl. J. Environ. Sci. Technol. 2014, 11. [Google Scholar] [CrossRef] [Green Version]
- Sydney, E.B.; Sturm, W.S.; de Carvalho, J.C.; Thomaz-Soccol, V.; Larroche, C.; Pandey, A.; Soccol, C.R. Potential carbon dioxide fixation by industrially important microalgae. Bioresour. Technol. 2010, 101, 5892–5896. [Google Scholar] [CrossRef]
- Weerahandi, N.; Ngo, V.; Fagan, J.M. Alternative Fuels for Vehicle. An overview of various approaches for reducing greenhouse gas emissions and ending oil dependence. 2012; 1–13. [Google Scholar]
- Atomi, H. Microbial enzymes involved in carbon dioxide fixation. J. Biosci. Bioeng. 2002, 94, 497–505. [Google Scholar] [CrossRef]
- Parry, M.A.; Andralojc, P.J.; Mitchell, R.A.; Madgwick, P.J.; Keys, A.J. Manipulation of Rubisco: The amount, activity, function and regulation. J. Exp. Bot. 2003, 54, 1321–1333. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Show, P.L.; Chang, J.S.; Ling, T.C.; Juan, J.C. Biosequestration of atmospheric CO2 and flue gas containing CO2 by microalgae. Bioresour. Technol. 2015, 184, 190–201. [Google Scholar] [CrossRef]
- Miura, Y.; Yamada, W.; Hirata, K.; Miyamoto, K.; Kiyohara, M. Stimulation of hydrogen production in algal cells grown under high CO2 concentration and low temperature. Appl. Biochem. Biotechnol. 1993, 39/40, 753–761. [Google Scholar] [CrossRef]
- Hanagata, N.; Takeuchi, T.; Fukuju, Y.; Barnes, D.J.; Karube, I. Tolerance of microalgae to high CO2 and high temperature. Phytochem. 1992, 31, 3345–3348. [Google Scholar] [CrossRef]
- Fulke, A.B.; Chambhare, K.; Giripunje, M.D.; Sangolkar, L.; Krishnamurthi, K. Potential of wastewater grown algae for biodiesel production and CO2 sequestration. Afr. J. Biotechnol. 2013, 12, 2939–2948. [Google Scholar]
- Fulke, A.B.; Mudliar, S.N.; Yadav, R.; Shekh, A.; Srinivasan, N.; Ramanan, R.; Krishnamurthi, K.; Saravana Devi, S.; Chakrabarti, T. Bio-mitigation of CO2, calcite formation and simultaneous biodiesel precursors production using Chlorella sp. Bioresour. Technol. 2010, 101, 8473–8476. [Google Scholar] [CrossRef]
- Jin, H.F.; Lim, B.R.; Lee, K. Influence of nitrate feeding on carbon dioxide fixation by microalgae. J. Environ. Sci. Health A Tox. Hazard. Subs. Environ. Eng. 2006, 41, 2813–2824. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.J.; Oh, K.K.; Kim, Y.S. Optimization of the influential factors for the improvement of CO2 utilization efficiency and CO2 mass transfer rate. J. Indus. Engg. Chem. 2009, 15, 471–475. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, Y.; Xiong, K.; Zhang, Z.; Hao, X. Effect of cultivation mode on microalgal growth and CO2 fixation. Chem. Eng. Res. Design 2011, 89, 1758–1762. [Google Scholar] [CrossRef]
- de Morais, M.G.; Costa, J.A.V. Carbon-dioxide fixation by Chlorella kessleri, C. vulgaris, Scenedesmus obliquus and Spirulina sp. cultivated in flasks and vertical tubular photobioreactors. Biotechnol. Lett. 2007, 29, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.; Kao, C.; Shen, C.; Kuan, T.; Ong, S.; Lin, S. Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinous photobioreactor. Bioresour. Technol. 2008, 99, 3389–3396. [Google Scholar] [CrossRef]
- Tang, D.; Han, W.; Miao, X.; Zhong, J. CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour. Technol. 2011, 102, 3071–3076. [Google Scholar] [CrossRef]
- Yeh, K.L.; Chang, J.S. Nitrogen starvation strategies and photobioreactor design for enhancing lipid content and lipid production of a newly isolated microalga Chlorella vulgaris ESP-31: Implications for biofuels. Biotechnol. J. 2011, 6, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Kurano, N.; Ikemoto, H.; Miyashita, H.; Hasegawa, T.; Hata, H.; Miyachi, S. Fixation and utilization of carbon-dioxide by microalgal photosynthesis. Energy Convers. Manag. 1995, 36, 689–692. [Google Scholar] [CrossRef]
- Kodama, M.; Ikemoto, H.; Miyachi, S. A new species of highly CO2-tolreant fast-growing marine microalga suitable for high-density culture. J. Marine Biotechnol. 1993, 1, 21–25. [Google Scholar]
- Seckbach, J.; Gross, H.; Nathan, M.B. Growth and photosynthesis of Cyanidium caldarium cultured under pure CO2. Isr. J. Bot. 1971, 20, 84–90. [Google Scholar]
- Kishimoto, M.; Okakura, T.; Nagashima, H.; Minowa, T.; Yokoyama, S.Y. CO2 fixation and oil production using microalgae. J. Ferment. Bioeng. 1994, 78, 479–482. [Google Scholar] [CrossRef]
- Francisco, E.C.; Neves, D.B.; Jacob-Lopes, E.; Franco, T.T. Algae as feedstock for biofuel production: Carbon dioxide sequestration, lipid production and biofuel quality. J. Chem. Technol. Biotechnol. 2010, 85, 395–403. [Google Scholar] [CrossRef]
- Nakano, Y.; Miyatake, K.; Okuno, H.; Hamazaki, K.; Takenaka, S.; Honami, N.; Kiyota, M.; Aiga, I.; Kondo, J. Growth of photosynthetic algae Euglena in high CO2 conditions and its photosynthetic characteristics. Acta Hort. 1996, 440, 49–54. [Google Scholar] [CrossRef]
- Huntley, M.; Redalje, D. CO2 mitigation and renewable oil from photosynthetic microbes: A new appraisal. Mitig. Adapt. Strat. Glob. Chang. 2007, 12, 573–608. [Google Scholar] [CrossRef]
- Yoshihara, K.; Nagase, H.; Eguchi, K.; Hirata, K.; Miyamoto, K. Biological elimination of nitric oxide and carbon dioxide from flue gas by marine microalga NOA-113 cultivation in a long tubular photobioreactor. J. Ferment. Bioeng. 1996, 82, 351–354. [Google Scholar] [CrossRef]
- Ho, S.H.; Chen, C.Y.; Yeh, K.L.; Chen, W.M.; Lin, C.Y. Characterization of photosynthetic carbon-dioxide fixation ability of indigenous Scenedesmus obliquus isolates. Biochem. Eng. J. 2010, 53, 57–62. [Google Scholar] [CrossRef]
- Ho, S.H.; Li, P.J.; Liu, C.C.; Chang, J.S. Bioprocess development on microalgae-based CO2 fixation and bioethanol production using Scenedesmus obliquus CNW-N. Bioresour. Technol. 2013, 145, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Miyairi, S. CO2 assimilation in a thermophilic cyanobacterium. Energy Convers. Manag. 1995, 36, 763–766. [Google Scholar] [CrossRef]
- Sivakumar, M.; Ranjithkumar, R.; Shashirekha, V.; Seshadri, S. Influence of carbon-dioxide on the growth of Spirulina sp. (MCRC-A0003) isolated from Muttukadu backwaters, South India. World J. Microbiol. Biotechnol. 2014, 30, 2775–2781. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Yuan, X.; Sahu, A.K.; Erga, S.J.; Langenhove, V. A hollow fiber membrane photo-bioreactor for CO2 sequestration from combustion gas coupled with wastewater treatment: A process engineering approach. J. Chem. Technol. Biotechnol. 2010, 85, 387–394. [Google Scholar] [CrossRef]
- Matsumoto, H.; Shioji, N.; Hamasaki, A.; Ikuta, Y.; Fukuda, Y.; Sato, M.; Endo, N.; Tsukamoto, T. Carbon dioxide fixation by microalgae photosynthesis using actual flue gas discharged from a boiler. Appl. Biochem. Biotechnol. 1995, 51/52, 681–692. [Google Scholar] [CrossRef]
- Nagase, H.; Eguchi, K.; Yoshihara, K.; Hirata, K.; Miyamoto, K. Improvement of microalgal NOx removal in bubble column and airlift reactors. J. Ferment. Bioeng. 1998, 86, 421–423. [Google Scholar] [CrossRef]
- Ramanan, R.; Kannan, K.; Deshkar, A.; Yadav, R.; Chakrabarti, T. Enhanced algal CO2 sequestration through calcite deposition by Chlorella sp. and Spirulina platensis in a mini-raceway pond. Bioresour. Technol. 2010, 101, 2616–2622. [Google Scholar] [CrossRef]
- Ramirez-Perez, J.C.; Janes, H.W. Carbon-dioxide sequestration by Spirulina platensis in photo-bioreactors. Habitation 2009, 12, 65–77. [Google Scholar] [CrossRef]
- Jones, C.S.; Mayfield, S.P. Algae biofuels: Versatility for the future of bioenergy. Curr. Opin. Biotechnol. 2012, 23, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Ferro, L. Wastewater Treatment and Biomass Generation by Nordic Microalgae: Growth in Subarctic Climate and Microbial Interactions (PhD Dissertation, Umeå University). 2019. Available online: http://urn.kb.se/resolve?urn=urn:nbn:se:umu:diva-156470 (accessed on 27 July 2021).
- Larsson, M.; Lindblom, J. Algal flue gas sequestration and wastewater treatment: An industrial experiment. Master’s Thesis, KTH Industrial Engineering and Management Machine Design, Industrial Ecology, Royal Institute of Technology, Stockholm, Sweden, 2011; p. 75. [Google Scholar]
- Lizzul, A.M.; Hellier, P.; Purton, S.; Baganz, F.; Ladommatos, N.; Campos, L. Combined remediation and lipid production using Chlorella sorokiniana grown on wastewater and exhaust gases. Bioresour. Technol. 2014, 151, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Oswald, W.J.; Golueke, C. Biological transformation of solar energy. Adv. Appl. Microbiol. 1960, 2, 223–262. [Google Scholar] [PubMed]
- Tam, N.F.Y.; Wong, Y.S. Wastewater Treatment with Microorganisms; The Commercial Press (H.K.) Ltd.: Hong Kong, China, 1995. [Google Scholar]
- McGinn, P.J.; Dickinson, K.E.; Bhatti, S.; Frigon, J.C.; Guiot, S.R.; O’Leary, S.J.B. Integration of microalgae cultivation with industrial waste remediation for biofuel and bioenergy production: Opportunities and limitations. Photosynth Res. 2011, 109, 231–247. [Google Scholar] [CrossRef]
- Posadas, E.; Alcántara, C.; García-Encina, P.A.; Gouveia, L.; Guieysse, B.; Norvill, Z.; Acién, F.G.; Markou, G.; Congestri, R.; Koreiviene, J.; et al. Microalgae cultivation in wastewater. Chapter 3. In Microalgae-Based Biofuels and Bioproducts; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing Series in Energy; Woodhead Publishing: Chicago, IL, USA, 2017; ISBN 9780081010235. [Google Scholar] [CrossRef]
- Muñoz, R.; Guieysse, B. Algal–bacterial processes for the treatment of hazardous contaminants: A review. Water Res. 2006, 40, 2799–2815. [Google Scholar] [CrossRef]
- Khiewwijit, R.; Temmink, H.; Rijnaarts, H. Energy and nutrient recovery for municipal wastewater treatment: How to design a feasible plant layout? Environ. Model. Softw. 2015, 68, 156–165. [Google Scholar] [CrossRef]
- Gu, Y.; Li, Y.; Li, X.; Luo, P.; Wang, H.; Wang, X.; Wu, J.; Li, F. Energy self-sufficient wastewater treatment plants: Feasibilities and challenges. Energy Procedia. 2017, 105, 3741–3751. [Google Scholar] [CrossRef]
- Mallick, N. Biotechnological potential of immobilized algae for wastewater N, P and metal removal: A review. BioMetals 2002, 15, 377–390. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.J.; Chang, J.S. Integration of anaerobic digestion and microalgal cultivation for digestate bioremediation and biogas upgrading. Bioresour. Technol. 2019, 290, 121804. [Google Scholar] [CrossRef] [PubMed]
- Pittman, J.K.; Dean, A.P.; Osundeko, O. The potential of sustainable algal biofuel production using wastewater resources. Bioresour. Technol. 2011, 102, 17–25. [Google Scholar] [CrossRef]
- Ansari, A.A.; Khoja, A.H.; Nawar, A.; Qayyum, M.; Ali, E. Wastewater treatment by local microalgae strains for CO2 sequestration and biofuel production. Appl. Water Sci. 2017, 7, 4151–4158. [Google Scholar] [CrossRef] [Green Version]
- Koutra, E.; Grammatikopoulos, G.; Kornaros, M. Microalgal posttreatment of anaerobically digested agro-industrial wastes for nutrient removal and lipids production. Bioresour. Technol. 2017, 224, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, X.; Otero, A. Nutrient removal from the centrate of anaerobic digestion of high ammonium industrial wastewater by a semicontinuous culture of Arthrospira sp. and Nostoc sp. PCC 7413. J. Appl. Phycol. 2020. [Google Scholar] [CrossRef]
- Chuka-ogwude, D.; Ogbonna, J.; Borowitzka, M.A.; Moheimani, N.R. Screening, acclimation and ammonia tolerance of microalgae grown in food waste digestate. J. Appl. Phycol. 2020, 32, 3775–3785. [Google Scholar] [CrossRef]
- Krzemińska, I.; Oleszek, M.; Wiącek, D. Liquid anaerobic digestate as a source of nutrients for lipid and fatty acid accumulation by Auxenochlorella protothecoides. Molecules 2019, 24, 3582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kisielewska, M.; Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Quattrocelli, P.; Bordiean, A. Effects of liquid digestate treatment on sustainable microalgae biomass production. Bioenergy Res. 2021, 1–14. [Google Scholar] [CrossRef]
- Wang, B.; Lan, C.Q. Biomass production and nitrogen and phosphorus removal by the green alga Neochloris oleoabundans in simulated wastewater and secondary municipal wastewater effluent. Bioresour. Technol. 2011, 102, 5639–5644. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, C.; Acién, F.G.; Gómez, C.; Jiménez-Ruíz, N.; Riquelme, C.; Molina-Grima, E. Utilization of centrate for the production of the marine microalgae Nannochloropsis gaditana. Algal. Res. 2015, 9, 107–116. [Google Scholar] [CrossRef]
- Garbisu, C.; Hall, D.O.; Serra, J.L. Removal of phosphate from water by foam-immobilized Phormidium laminosum in batch and continuous-flow bioreactors. J. Chem. Technol. Biotechnol. 1993, 57, 181–189. [Google Scholar] [CrossRef]
- Shashirekha, V.; Sivakumar, M.; Seshadri, S. Effective C-N-P ratio for growth and nutrient removal efficiency of Scenedesmus obliquus in sugar mill effluent. Energy Ecol. Environ. 2016, 1, 283–295. [Google Scholar] [CrossRef]
- Shashirekha, V.; Sudhakar, M.P. Microalgae: Potential agents for CO2 mitigation and bioremediation of wastewaters. Chapter 8. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbes in Soil, Crop and Environmental Sustainability; Singh, J.S., Singh, D.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 129–148. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Barbosa, M.J. An outlook on microalgal biofuels. Science 2010, 329, 796–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudhakar, M.P.; Shashirekha, V. Algae as a sustainable and renewable bioresource for bio-fuel production. Chapter 6. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Biotechnology in Agro-Environmental Sustainability; Singh, J.S., Singh, D.P., Eds.; Elsevier: Amsterdam, The Netherland, 2019; pp. 77–84. ISBN 9780444641915. [Google Scholar] [CrossRef]
- Park, J.B.K.; Craggs, R.J.; Shilton, A.N. Wastewater treatment high rate algal ponds for biofuel production. Bioresour. Technol. 2011, 102, 34–42. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J.P. Impact of microalgae characteristics on their conversion to biofuel. Part II: Focus on biomethane production. Biofuel Bioprod. Bioref. 2012, 6, 205–218. [Google Scholar] [CrossRef]
- Wilkie, A.C.; Edmundson, S.J.; Duncan, J.G. Indigenous algae for local bioresource production: Phycoprospecting. Energy Sustain. Dev. 2011, 15, 365–371. [Google Scholar] [CrossRef]
- Eric, F.; Christophe, V.; Christophe, L.; Emilie, L.F.; Claire, C.; Bruno, M.; Jean-Philippe, S.; Bruno, S. Coupling algal biomass production and anaerobic digestion: Production assessment of some native temperate and tropical microalgae. Biomass Bioenergy 2014, 70, 564–569. [Google Scholar]
- Razzak, S.A.; Hossain, M.M.; Lucky, R.A.; Bassi, A.S.; de Lasa, H. Integrated CO2 capture, wastewater treatment and biofuel production by microalgae culturing—A review. Renew. Sustain. Energy Rev. 2013, 27, 622–653. [Google Scholar] [CrossRef]
- Cardozo, K.H.M.; Guaratini, T.; Barros, M.P.; Vanessa, R.F.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P.; et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2007, 146, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Yafei, S. Carbon dioxide bio-fixation and wastewater treatment via algae photochemical synthesis for biofuels production. RSC Adv. 2014, 92. [Google Scholar]
- Gupta, P.L.; Lee, S.M.; Choi, H.J. A mini review: Photobioreactors for large scale algal cultivation. World J. Microbiol. Biotechnol. 2015, 31, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Borowitzka, M.A. Limits to growth. In Wastewater Treatment with Algae; Wong, Y.S., Tam, N.F.Y., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 203–226. [Google Scholar]
- Saad, M.G.; Dosoky, N.S.; Zoromba, M.S.; Shafik, H.M. Algal Biofuels: Current Status and Key Challenges. Energies 2019, 12, 1920. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, A.; Chinnasamy, S.; Singh, M.; Das, K.C. Renewable biomass production by mixotrophic algae in the presence of various carbon sources and wastewaters. Appl. Energy 2011, 88, 3425–3431. [Google Scholar] [CrossRef]
- Márquez-Rocha, F.J. Reassessment of the bioenergetic yield of Arthrospira platensis using continuous culture. World J. Microbiol. Biotechnol. 1999, 15, 235–238. [Google Scholar] [CrossRef]
- Ashraf, A.; Ramamurthy, R.; Sayavedra, S.M.; Bhatt, P.; Gangola, S.; Noor, T.; Desmarais, M.; Rabbani, A.; Rene., E.R. Integrating photobioreactor with conventional activated sludge treatment for nitrogen removal from sidestream digestate: Current challenges and opportunities. J. Environ. Chem. Eng. 2021, 3, 106171. [Google Scholar] [CrossRef]
- Qin, L.; Wang, B.; Feng, P.; Cao, Y.; Wang, Z.; Zhu, S. Treatment and resource utilization of dairy liquid digestate by nitrification of biological aerated filter coupled with assimilation of Chlorella pyrenoidosa. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.E.; Jimenez, J.M.; Yousfi, E.L. Influence of phosphorus concentration and temperature on growth and phosphorus uptake by the microalga Scenedesmus obliquus. Bioresour. Technol. 1999, 67, 233–240. [Google Scholar] [CrossRef]
- Weissman, J.C.; Tillett, D.M. Design and Operation of an Outdoor Microalgae Test Facility: Large-Scale System Results. In Aquatic Species Project Report (FY 1989–90); NREL/TP-232-4174; Brown, L.M., Sprague, S., Eds.; National Renewable Energy Laboratory: Golden, CO, USA, 1992; pp. 32–56. [Google Scholar]
- Becker, E.W. Microalgae: Biotechnology & Microbiology; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Dalrymple, O.K.; Halfhide, T.; Udom, I.; Gilles, B.; Wolan, J.; Zhang, Q.; Ergas, S. Wastewater use in algae production for generation of renewable resources: A review and preliminary results. Aquat. Biosyst. 2013, 9, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Marin, A.; Mendoza-Espinosa, L.G.; Stephenson, T. Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour. Technol. 2010, 101, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, M.; Chinnasamy, S. Chlorella minutissima—A promising fuel alga for cultivation in municipal wastewaters. Appl. Biochem. Biotechnol. 2010, 161, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Anand, M.N. Microalgal bioremediation of nutrients in wastewater and carbon-dioxide in flue gas. Master’s Thesis, Environmental Engineering, Missouri University of Science and Technology, Rolla, MO, USA, 2010. [Google Scholar]
- Gao, F.; Li, C.; Yang, Z.H.; Zeng, G.M.; Feng, L.J.; Liu, J.Z.; Liu, M.; Cai, H.W. Continuous microalgae cultivation in aquaculture wastewater by a membrane photobioreactor for biomass production and nutrients removal. Ecol. Eng. 2016, 92, 55–61. [Google Scholar] [CrossRef]
- Khatoon, H.; Banerjee, S.; Syahiran, M.S.; Noordin, N.B.M.; Bolong, A.M.A.; Endut, A. Re-use of aquaculture wastewater in cultivating microalgae as live feed for aquaculture organisms. Desalin. Water Treat. 2016, 57, 29295–29302. [Google Scholar] [CrossRef]
- Hena, S.; Fatimah, S.; Tabassum, S. Cultivation of algae consortium in a dairy farm wastewater for biodiesel production. Water Resour. Ind. 2015, 10, 1–14. [Google Scholar]
- Kaushik, R.; Prasanna, R.; Joshi, H.C. Utilization of anaerobically digested distillery effluent for the production of Spirulina platensis (ARM 730). J. Sci. Ind. Res. 2006, 65, 521–525. [Google Scholar]
- Chi, Z.; Zheng, Y.; Jiang, A.; Chen, S. Lipid production and culturing oleaginous yeast and algae with food waste and municipal wastewater in an integrated process. Appl. Biochem. Biotechnol. 2011, 165, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Li, Y.; Min, M.; Hu, B.; Zhang, H.; Ma, X.; Li, L.; Cheng, Y.; Chen, P.; Ruan, R. Growing wastewater-borne microalga Auxenochlorella protothecoides UMN280 on concentrated municipal wastewater for simultaneous nutrient removal and energy feedstock production. Appl. Energy 2012, 98, 433–440. [Google Scholar] [CrossRef]
- Putri, E.V.; Din, M.F.M.; Ahmed, Z.; Jamaluddin, H.; Chelliapan, S. Investigation of Microalgae for High Lipid Content Using Palm Oil Mill Effluent (POME) as Carbon Source; IACSIT Press: Singapore, 2011; Volume 12, pp. 85–89. [Google Scholar]
- Hadiyanto, H.; Nur, M.M.A. Potential of palm oil mill effluent (POME) as medium growth of Chlorella sp. for bioenergy production. Intl. J. Environ. Bioenergy 2012, 3, 67–74. [Google Scholar]
- Saikia, M.K.; Kalita, S.; Sarma, G.C. An experimental investigation on growth stimulation (+) and inhibition (−) of algae (Oscillatoria chlorina and Scenedesmus quadricauda) treated with pulp and paper mill effluents. Int. J. Appl. Biol. Pharm. Technol. 2011, 2, 87–94. [Google Scholar]
- Usha, M.T.; Chandra, T.S.; Sarada, R.; Chauhan, V.S. Removal of nutrients and organic pollution load from pulp and paper mill effluent by microalgae in outdoor open pond. Bioresour. Technol. 2016, 214, 856–860. [Google Scholar] [CrossRef]
- Singh, M.; Reynolds, D.; Das, K.C. Microalgal system for treatment of effluent from poultry litter anaerobic digestion. Bioresour. Technol. 2011, 102, 10841–10848. [Google Scholar] [CrossRef]
- Toyub, M.A.; Miah, M.I.; Habib, M.A.B.; Rahman, M.M. Growth performance and nutritional value of Scenedesmus obliquus cultured in different concentrations of sweetmeat factory waste media. Bangladesh J. Anim. Sci. 2008, 37, 86–93. [Google Scholar] [CrossRef]
- Hu, B.; Min, M.; Zhou, W.; Du, Z.; Mohr, M.; Chen, P.; Zhu, J.; Cheng, Y.; Liu, Y.; Ruan, R. Enhanced mixotrophic growth of microalga Chlorella sp. on pretreated swine manure for simultaneous biofuel feedstock production and nutrient removal. Bioresour. Technol. Adv. Biol. Waste Treat. Bioconver. Technol. 2012, 126, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Michelon, W.; Da Silva, M.L.B.; Mezzari, M.P.; Pirolli, M.; Prandini, J.M.; Soares, H.M. Effects of nitrogen and phosphorus on biochemical composition of microalgae polyculture harvested from phycoremediation of piggery wastewater digestate. Appl. Biochem. Biotechnol. 2016, 178, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Hasan, R.; Zhang, B.; Wang, L.J.; Shahbazi, A. Bioremediation of swine wastewater and biofuel potential by using Chlorella vulgaris, Chlamydomonas reinhardtii and Chlamydomonas debaryana. J. Pet. Environ. Biotechnol. 2014, 5, 175. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.H.; Kang, Z.; Ramanan, R.; Choi, J.E.; Cho, D.H.; Oh, H.M.; Kim, H.S. Nutrient removal and biofuel production in high rate algal pond (HRAP) using real municipal wastewater. J. Microbiol. Biotechnol. 2014, 24, 1123–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abinandan, S.; Shashirekha, V.; Vedaraman, N.; Khambhaty, Y.; Seshadri, S.; Sridharan, M.R. Feasibility of microalgal cultivation in soak liquors from leather processing unit. Indian Hydrobiol. 2014, 16, 145–152. [Google Scholar]
- Pulgarin, A.; Kapeller, A.G.; Tarik, M.; Egloff, S.; Mariotto, M.; Ludwig, C.; Refardt, D. Cultivation of microalgae at high-density with pretreated liquid digestate as a nitrogen source: Fate of nitrogen and improvements on growth limitations. J. Clean. Prod. 2021, 324, 129238. [Google Scholar] [CrossRef]
- de Morais, M.G.; de Morais, E.G.; Duarte, J.H.; Deamici, K.M.; Mitchell, B.G.; Costa, J.A.V. Biological CO2 mitigation by microalgae: Technological trends, future prospects and challenges. World J. Microbiol. Biotechnol. 2019, 35, 78. [Google Scholar] [CrossRef]
- Satyanarayana, K.G.; Mariano, A.B.; Vargas, J.V.C. A review on microalgae, a versatile source for sustainable energy and materials. Int. J. Ener. Res. 2011, 35, 291–311. [Google Scholar] [CrossRef]
- Bauer, L.; Ranglová, K.; Masojídek, J.; Drosg, B.; Meixner, K. Digestate as sustainable nutrient source for microalgae—Challenges and Prospects. Appl. Sci. 2021, 11, 1056. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Harker, M.; Tsavalos, A.J.; Young, A.J. Use of response surface methodology to optimisecarotenogenesis in the microalga, Haematococcuspluvialis. J. Appl. Phycol. 1995, 7, 399–406. [Google Scholar] [CrossRef]
- Shi, T.; Francis, X.; Cunningham, F.X., Jr.; Youmans, M.; Grabowski, B.; Sun, Z.; Gantt, E. Cytochrome f loss in astaxanthin-accumulating cells of Haematococcus pluvialis (Chlorophyceae): Comparison of photosynthetic activity, photosynthetic enzymes and thylakoid membrane polypeptides in red and green cells. J. Phycol. 1995, 51, 897–905. [Google Scholar]
- Fan, L.; Vonshak, A.; Boussiba, S. Effect of temperature and irradiance on growth of Haematococcus pluvialis (Chlorophyceae). J. Phycol. 1994, 30, 829–833. [Google Scholar] [CrossRef]
- Torzillo, G.; Vonshak, A. Effect of light and temperature on the photosynthetic activity of the cyanobacterium Spirulina platensis. Biomass Bioenerg. 1994, 6, 399. [Google Scholar] [CrossRef]
- Kaplan, A.; Badger, M.R.; Berry, J.A. Photosynthesis and the intracellular inorganic carbon pool in the blue green alga Anabaena variabilis: Response to external CO2 concentration. Planta 1980, 149, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Rachlin, J.W.; Grosso, A. The effects of pH on the growth of Chlorella vulgaris and its interactions with Cadmium toxicity. Arch. Environ. Contam. Toxicol. 1991, 20, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Daliry, S.; Hallajisani, A.; Mohammadi, R.J.; Nouri, H.; Golzary, A. Investigation of optimal condition for Chlorella vulgaris microalgae growth. Glob. J. Environ. Sci. Manag. 2017, 3, 217–230. [Google Scholar]
- Raeesossadati, M.J.; Ahmadzadeh, H.; McHenry, M.P.; Moheimani, N.R. CO2 bioremediation by microalgae in photobioreactors: Impacts of biomass and CO2 concentrations, light, and temperature. Algal Res. 2014, 6, 78–85. [Google Scholar] [CrossRef]
- Patil, L.; Kaliwal, B. Effect of CO2 concentration on growth and biochemical composition of newly isolated indigenous microalga Scenedesmus bajacalifornicus BBKLP-07. Appl. Biochem. Biotechnol. 2017, 182, 335. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.D.; Lee, J.S.; Shin, C.S.; Park, S.C. Isolation of a new highly CO2 tolerant fresh water microalga Chlorella sp. KR-1. Korean J. Chem. Eng. 1998, 15, 449–450. [Google Scholar] [CrossRef]
- Benemann, J.R. CO2 mitigation with microalgae systems. Energy Convers. Manag. 1997, 38, S475–S479. [Google Scholar] [CrossRef]
- Douskova, I.; Doucha, J.; Livansky, K.; Machat, J.; Novak, P.; Umysova, D.; Zachleder, V.; Vitova, M. Simultaneous flue gas bioremediation and reduction of microalgal biomass production costs. Appl. Microbiol. Biotechnol. 2009, 82, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K.K. Photoprotection revisited: Genetic and molecular approaches. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 333–359. [Google Scholar] [CrossRef]
- Wollman, F.A. State transitions reveal the dynamics and flexibility of the photosynthetic apparatus. EMBO J. 2001, 20, 3623–3630. [Google Scholar] [CrossRef] [Green Version]
- Lemeille, S.; Rochaix, J.D. State transitions at the crossroad of thylakoid signalling pathways. Photosynth Res. 2010, 106, 33–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niyogi, K.K.; Truong, T.B. Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr. Opin. Plant Biol. 2013, 16, 307–314. [Google Scholar] [CrossRef]
- Richmond, A. Handbook of Microalgal Culture: Biotechnology and Applied Phycology, 1st ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Morales-Amaral, M.M.; Gómez-Serrano, C.; Acién, F.G.; Fernández-Sevilla, J.M.; Molina-Grima, E. Production of microalgae using centrate from anaerobic digestion as the nutrient source. Algal Res. 2015, 9, 297–305. [Google Scholar] [CrossRef]
- Navarro-López, E.; Ruíz-Nieto, A.; Ferreira, A.; Acién, F.G.; Gouveia, L. Biostimulant potential of Scenedesmus obliquus grown in brewery wastewater. Molecules 2020, 25, 664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranglová, K.; Lakatos, G.E.; Câmara Manoel, J.A.; Grivalský, T.; Suárez Estrella, F.; Acién Fernández, F.G.; Molnár, Z.; Ördög, V.; Masojídek, J. Growth, biostimulant and biopesticide activity of the MACC-1 Chlorella strain cultivated outdoors in inorganic medium and wastewater. Algal Res. 2020, 102136. [Google Scholar] [CrossRef]
- Gutzeit, G.; Lorch, D.; Weber, A. Bioflocculent algal–bacterial biomass improves low-cost wastewater treatment. Water Sci. Technol. 2005, 52, 9–18. [Google Scholar] [CrossRef]
- Subashchandrabose, S.R.; Ramakrishnan, B.; Megharaj, M. Consortia of cyanobacteria/microalgae and bacteria: Biotechnological potential. Biotechnol. Adv. 2011, 29, 896–907. [Google Scholar] [CrossRef]
- Aspatwar, A.; Haapanen, S.; Parkkila, S. An uptake on the metabolic roles of carbonic anhydrases in the model alga Chlamydomonas reindardtii. Metabolites 2018, 8, 22. [Google Scholar] [CrossRef] [Green Version]
- Hoang, C.V.; Chapman, K.D. Biochemical and molecular inhibition of plastidial carbonic anhydrase reduces the incorporation of acetate into lipids in cotton embryos and tobacco cell suspensions and leaves. Plant Physiol. 2002, 128, 1417–1427. [Google Scholar] [CrossRef] [Green Version]
- Hopkinson, B.M.; Meile, C.; Shen, C. Quantification of extracellular carbonic anhydrase activity in two marine diatoms and investigation of its role. Plant Physiol. 2013, 162, 1142–1152. [Google Scholar] [CrossRef] [Green Version]
- Moroney, J.V.; Ma, Y.; Frey, W.D.; Fusilier, K.A.; Pham, T.T.; Simms, T.A.; DiMario, R.J.; Yang, J.; Mukherjee, B. The carbonic anhydrase isoforms of Chlamydomonas reinhardtii: Intracellular location, expression, and physiological roles. Photosynth. Res. 2011, 109, 133–149. [Google Scholar] [CrossRef]
- Higgins, B.T.; Gennity, I.; Fitzgerald, P.S.; Ceballos, S.J.; Fiehn, O.; VanderGheynst, J.S. Algal–bacterial synergy in treatment of winery wastewater. NPJ Clean Water 2016, 1, 1–9. [Google Scholar] [CrossRef]
- Mouget, J.L.; Dakhama, A.; Lavoie, M.C.; la Noüe, J. Algal growth enhancement by bacteria: Is consumption of photosynthetic oxygen involved? FEMS Microbiol. Ecol. 1995, 18, 35–43. [Google Scholar] [CrossRef]
- Cho, D.H.; Ramanan, R.; Heo, J.; Kang, Z.; Kim, B.H.; Ahn, C.Y. Organic carbon, influent microbial diversity and temperature strongly influence algal diversity and biomass in raceway ponds treating raw municipal wastewater. Bioresour. Technol. 2015, 191, 481–487. [Google Scholar] [CrossRef]
- Sahoo, K.; Sahoo, R.K.; Gaur, M.; Subudhi, E. Algal-bacterial system: A novel low-cost biotechnological initiative in wastewater treatment. In The Role of Microalgae in Wastewater Treatment; Sukla, L.B., Subudhi, E., Pradhan, D., Eds.; Springer: Singapore, 2019; pp. 115–127. [Google Scholar]
- Ayre, J.M.; Mickan, B.S.; Jenkins, S.N.; Moheimani, N.R. Batch cultivation of microalgae in anaerobic digestate exhibits functional changes in bacterial communities impacting nitrogen removal and wastewater treatment. Algal Res. 2021, 57, 102338. [Google Scholar] [CrossRef]
- van der Ha, D.; Nachtergaele, L.; Kerckhof, F.M.; Rameiyanti, D.; Bossier, P.; Verstraete, W. Conversion of biogas to bioproducts by algae and methane oxidizing bacteria. Environ. Sci. Technol. 2012, 46, 13425–13431. [Google Scholar] [CrossRef]
- Avagyan, A. Microalgae production development global prospects and profitable technology wasterwater purification by the use microalgae. Water Wastewater Int. 2008, 24. [Google Scholar]
- Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Megharaj, M. Nutrient removal and biomass production: Advances in microalgal biotechnology for wastewater treatment. Critic. Rev. Biotechnol. 2018, 38, 1244–1260. [Google Scholar] [CrossRef] [PubMed]
- Subashchandrabose, S.R.; Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Mixotrophic cyanobacteria and microalgae as distinctive biological agents for organic pollutant degradation. Environ. Int. 2013, 51, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Pedroni, P.; Davison, J.; Beckert, H.; Bergman, P.; Benemann, J. A proposal to establish an international network on biofixation of CO2 and greenhouse gas abatement with microalgae. J. Ener. and Environ. Res. 2001, 1, 136–150. [Google Scholar]
- Seshadri, C.V.; Thomas, S. Mass culture of Spirulina using low-cost nutrients. Biotechnol Lett. 1979, 1, 278–291. [Google Scholar] [CrossRef]
- Jeeji Bai, N.; Beena, B.N.; Shashirekha, V. Spirulina (Arthrospira): Taxonomy, Biology and Applications; Bishen Singh Mahendra Pal Singh: Dehradun, India, 2018; p. 112. ISBN 978-81-211-0953-6. [Google Scholar]
- Tredici, M.R. Mass production of microalgae: Photobioreactors. In Handbook of Microalgal Culture Biotechnology and Appld. Phycol.; Richmond, A., Ed.; Wiley-Blackwell: Oxford, UK, 2004; pp. 273–280. [Google Scholar]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Sustainable biomass production from microalgae for food, feed and biofuels: An integrated approach. Biosci. Biotechnol. Res. Comm. 2016, 9, 729–736. [Google Scholar]
- Moroney, J.V.; Ynalvez, R.A. Proposed carbon-dioxide concentrating mechanism in Chlamydomonas reinhardtii. Eukaryotic Cell 2007, 6, 1251–1259. [Google Scholar]
- Cheng, J.; Yang, Z.; Huang, Y.; Huang, L.; Hu, L.; Xu, D.; Cen, K. Improving growth rate of microalgae in a 1191 m2 raceway pond to fix CO2 from flue gas in a coal-fired power plant. Bioresour. Technol. 2015, 190, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Workshop Summary Report. Algae Cultivation for Carbon Capture and Utilization. 2017. Available online: https://www.osti.gov/biblio/1413880 (accessed on 28 August 2021).
- Chai, X.; Zhao, X. Enhanced removal of carbon dioxide and alleviation of dissolved oxygen accumulation in photobioreactor with bubble tank. Biores. Technol. 2012, 116, 360–365. [Google Scholar] [CrossRef]
- Zheng, Q.; Xu, X.; Martin, G.J.O.; Kentish, S.E. Critical review of strategies for CO2 delivery to large-scale microalgae cultures. Chinese J. Chem. Eng. 2018, 26, 2219–2228, ISSN 1004-9541. [Google Scholar]
- Breida, M.; Younssi, S.A.; Ouammou, M.; Bouhria, M.; Hafsi, M. Pollution of water sources from agricultural and industrial effluents: Special attention to NO3–, Cr(VI) and Cu(II). In Water Chemistry; Eyvaz, M., Yüksel, E., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Butterfi, B.A.; Jones, J. Harvesting of algae grown in agricultural wastewaters. Trans. Geophys. Union. 1969, 50, 612. [Google Scholar]
- Grima, E.M.; Belarbi, E.H.; Fernández, F.A.; Medina, A.R.; Chisti, Y. Recovery of microalgal biomass and metabolites: Process options and economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]
- Weissman, J.C.; Goebel, R.P. Design and Analysis of Microalgal Open Pond Systems for the Purpose of Producing Fuels: A Subcontract Report, SERI/STR-231-2840, Solar Energy Research Institute: Golden, CO, United States. 1987. Available online: https://www.osti.gov/servlets/purl/6546458 (accessed on 28 August 2021).
- Chen, C.L.; Chang, J.S.; Lee, D.J. Dewatering and drying methods for microalgae. Dry. Technol. 2015, 33, 443–454. [Google Scholar] [CrossRef]
- Bosma, R.; van Spronsen, W.A.; Tramper, J.; Wijffels, R.H. Ultrasound, a new separation technique to harvest microalgae. J. Appl. Phycol. 2003, 15, 143–153. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- de-Bashan, L.E.; Bashan, Y. Immobilized microalgae for removing pollutants: Review of practical aspects. Bioresour. Technol. 2010, 101, 1611–1627. [Google Scholar] [CrossRef]
- Nelson, J.A. Postharvest Degradation of Microalgae: Effect of Temperature and Water Activity; Utah State University: Logan, UT, USA, 2015; p. 4458. [Google Scholar] [CrossRef]
- Hosseinizand, H.; Sokhansanj, S.; Lim, J. Studying the drying mechanism of microalgae Chlorella vulgaris and the optimum drying temperature to preserve quality characteristics. Drying Technol. 2017, 36, 1049–1060. [Google Scholar] [CrossRef]
| Microalgae Species | CO2 Concentration (%) | CO2 Fixation Rate (g L−1 day−1) | Reference |
|---|---|---|---|
| Chlamydomonas sp. | 15 | - | [15] |
| Chlorella sp. | 40 | - | [16] |
| 0.03 | 1.62 | [17] | |
| 15 | - | [18] | |
| 15 | 0.46 | [19] | |
| 5 | 0.7 | [20] | |
| - | 1.38 | [21] | |
| Chlorella kessleri | 18 | 0.16 | [22] |
| Chlorella (marine) | 2–15 | 2.14–4.69 | [23] |
| Chlorella pyrenoidosa SJTU-2 | 5–50 | 0.029–0.71 | [24] |
| Chlorella vulgaris | 10 | 0.25 | [10] |
| 2 | 0.43 | [25] | |
| 18 | - | [22] | |
| Chlorococcum littorale | 20 | 0.246 | [26] |
| 60 | - | [27] | |
| Chroococcus cohaerens | 0.03 | 0.78 | [17] |
| Cyanidium caldarium | 100 | - | [28] |
| Dunaliella sp. | 3 | 0.31 | [29] |
| Dunaliella tertiolecta | 10 | 0.27 | [10] |
| 15 | 5.82 | [30] | |
| Eudorina sp. | 20 | - | [16] |
| Euglena gracilis | 45 | - | [31] |
| Haematococcus pluvialis | 34 | 0.14 | [32] |
| Microcystis aeruginosa | 15 | 0.134 | [19] |
| Microcystis ichthyoblabe | 15 | 0.142 | [19] |
| Nannochloris sp. | 15 | - | [33] |
| Phaeodactylum tricornitum | 15 | 0.59 | [30] |
| Phormidium sp. | 15 | 7.39 | [30] |
| Scenedesmus sp. | 80 | - | [16] |
| 15 | 0.61 | [19] | |
| Scenedesmus dimorphus | 0.03 | 1.27 | [17] |
| Scenedesmus incrassatulus | 0.03 | 1.50 | [17] |
| Scenedesmus obliquus | 15 | 4.6 | [30] |
| 10 | 0.55 | [34] | |
| 10 | 0.29 | [24] | |
| 2.5 | 1.19 | [35] | |
| 18 | - | [22] | |
| Synechococcus elongatus | 60 | - | [36] |
| Spirulina sp. | 20 | 0.14 | [37] |
| Spirulina platensis | 15 | 0.92 | [38] |
| Tetraselmis sp. | 14 | - | [39] |
| Nature of Effluent | Microalga | References |
|---|---|---|
| Agricultural run-off | Chlorella vulgaris | [91] |
| Agro-industrial wastewater | [50] | |
| Aquaculture wastewater | Chlorella vulgaris, Scenedesmus obliquus | [92] |
| Aquaculture wastewater | Chaetoceros calcitrans, Nannochloris maculate, Tetraselmischuii | [93] |
| Dairy farm wastewater | Algae consortium—Chlorella saccharophila UTEX 2911, Chlamydomonas pseudococcum UTEX 214, Scenedesmus sp. UTEX 1185 | [94] |
| Digested distillery effluent | Spirulina platensis | [95] |
| Food waste and municipal wastewater | Chlorella sorokiniana | [96] |
| Municipal wastewater | Chlorella minutissima | [90] |
| Auxenochlorella protothecoides UMN280 | [97] | |
| Scenedesmus sp. AMDD | [49] | |
| Palm oil mill effluent | Chlorella sorokiniana | [98] |
| Chlorella sp. | [99] | |
| Paper and pulp mill effluent | Oscillatoria chlorina, Scenedesmus quadricauda | [100] |
| Scenedesmus sp. | [101] | |
| Poultry litter anaerobic digestion effluent | Chlorella minutissima, Chlorella sorokiniana, Scenedesmus bijuga | [102] |
| Sugar mill effluent | Scenedesmus obliquus | [66] |
| Sweetmeat factory waste media | Scenedesmus obliquus | [103] |
| Swine wastewater | Chlorella sp. | [104,105] |
| Chlorella vulgaris, Chlamydomonas reinhardtii, Chlamydomonas debaryana | [106] | |
| Scenedesmus sp. | [107] | |
| Tannery—soak liquor | Spirulina sp., Nannochloropsis sp. | [108] |
| Parameter | Relative Benefit | Remarks |
|---|---|---|
| Contamination risk | Raceway > PBR | Reduced or nil in PBR |
| Space requirement | Raceway ~ PBR | Depends on productivity |
| Water loss | Raceway > PBR | Depends on cooling system |
| CO2 loss | Raceway ~ PBR | Depends on pH, alkalinity of medium |
| O2 inhibition | Raceway < PBR | Often encountered in PBR |
| Process control | Raceway < PBR | Better in PBR |
| Biomass productivity | Raceway < PBR | 3–5 times more in PBR |
| Capex and Opex | Raceway << PBR | 3–10 times cheaper in Raceways |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viswanaathan, S.; Perumal, P.K.; Sundaram, S. Integrated Approach for Carbon Sequestration and Wastewater Treatment Using Algal–Bacterial Consortia: Opportunities and Challenges. Sustainability 2022, 14, 1075. https://doi.org/10.3390/su14031075
Viswanaathan S, Perumal PK, Sundaram S. Integrated Approach for Carbon Sequestration and Wastewater Treatment Using Algal–Bacterial Consortia: Opportunities and Challenges. Sustainability. 2022; 14(3):1075. https://doi.org/10.3390/su14031075
Chicago/Turabian StyleViswanaathan, Shashirekha, Pitchurajan Krishna Perumal, and Seshadri Sundaram. 2022. "Integrated Approach for Carbon Sequestration and Wastewater Treatment Using Algal–Bacterial Consortia: Opportunities and Challenges" Sustainability 14, no. 3: 1075. https://doi.org/10.3390/su14031075
APA StyleViswanaathan, S., Perumal, P. K., & Sundaram, S. (2022). Integrated Approach for Carbon Sequestration and Wastewater Treatment Using Algal–Bacterial Consortia: Opportunities and Challenges. Sustainability, 14(3), 1075. https://doi.org/10.3390/su14031075






