1. Introduction
Arsenic is a metalloid listed in group VA of the periodic chart. It exists in nature in the oxidation states +V (arsenate), +III (arsenite), 0 (arsenic), and -III (arsine) [
1].
Soils contain both organic and inorganic arsenic species. Inorganic As species include arsenite and arsenate, which are the most abundant forms found in the environment. The majority of As in aerated soils exist as H₂AsO₄
− (acid soils) or HAsO₄
2− (neutral species and basic). However, HAs₃O₃ is predominant in anaerobic soils [
2].
The oxidation state and its mobility are fundamentally controlled by redox conditions (redox potential, Eh) and pH [
3]. Arsenic migration is greatly limited due to the strong sorption by clays, hydroxides, and organic matter [
4,
5,
6].
Arsenic is a well-known toxic and carcinogenic metalloid that occurs naturally in soil, aquifer, and sediments [
7]. Elevated concentrations of arsenic have primarily resulted from natural sources, such as erosion and leaching from geological formations or anthropogenic sources. In addition, use of As for industrial purposes, and pesticides and fertilizers for mining and metal processing activities, are other major sources of contamination [
8].
The distribution of arsenic is strongly related to areas of active plate tectonics, magmatism and associated hydrothermal activity, and high rates of erosion. Sources of arsenic contamination are mainly hydrothermal water, sulfide and arsenide minerals, volcanic ash, and iron oxyhydroxide/oxide as weathering products [
9].
Arsenic has a great affinity to form or occur in many minerals, and of over 200 As-containing minerals, approximately 60% are arsenates, although As minerals and compounds are readily soluble [
10].
Surface sediments and soils in various parts of the world contain higher and more variable concentrations, ranging from 2 to 20 mg kg
−1 on average, than the average crustal concentration [
11]. Typically, the concentrations of As in non-contaminated soils vary from 0.1 to 10 mg kg
−1 [
12]. Natural and anthropogenic sources contribute to the levels of arsenic found in soil and sediments. Average concentrations in soil are often around 5 mg kg
−1 but can range from 1 mg kg
−1 to up to 40 mg kg
−1. This variation in arsenic levels that occurs naturally in soils is associated with the presence of geological formations. Soils contaminated with arsenic from anthropogenic sources can have arsenic concentrations ranging from 5 to 3000 mg kg
−1 [
13].
Arsenic exposure has likely been a longstanding problem in Latin America. Though this widespread contamination from both natural and anthropological sources has long been a threat to human health in Latin America, relatively little is known about occurrence, distribution, and exposed populations in countries other than Argentina, Brazil, Chile, and Mexico [
14].
Arsenic-contaminated areas in Latin America (14 countries in Central and South America) are concentrated in the circum-Pacific region, where they are associated with the “Ring of Fire”. The arsenic sources in this area are related to magmatic activity (including volcanic, hot springs, fumaroles, and geothermal well, volcanic ash, and sulfide deposits [
15].
Data for geothermal systems in Mexico, Guatemala, Honduras, El Salvador, Nicaragua, Costa Rica, Ecuador, Bolivia, and Chile are presented. Two sources of As can be recognized in the investigated sites: Arsenic partitioned into volcanic gases and emitted in plumes and fumaroles, and arsenic in the rocks of volcanic edifices that are leached by groundwaters enriched in volcanic gases [
16].
In the Andean range, Chile, Bolivia, Peru, and Ecuador, As is predominantly released by weathering of volcanic rocks and sulfide ore deposits, and leaching of their weathering products [
17]. Dissolution of volcanic rocks of the Andes contributes additional As to the overland flow and infiltrating water and transports it to the rivers and springs, respectively [
18].
Ecuador has many volcanic centers and geothermal systems. Geothermal waters with high As concentrations are located in the northcentral Andean region of Ecuador and the basin of Papallacta Lake in Quijos County [
16].
The arsenic content of geothermal hot springs and their sediments in the north-central Andean region of Ecuador has been investigated. The area of study includes five provinces: Carchi, Imbabura, Pichincha, Cotopaxi, and Tungurahua. The results indicate that the total arsenic in geothermal waters in this region has a range of 2–969 µg As L
−1, and sediments contain arsenic ranging from 1.6 to 717.6 mg kg
−1 [
19].
In the central part of the Andean region, natural As is present in Papallacta Lake. Sediment samples taken on the eastern and southeastern of lake shores obtain relatively high arsenic (540 and 613 mg kg
−1) in contrast to the lower sediment As levels on the northwestern and southwestern margins (60 and 72 mg kg
−1) [
16].
In addition to drinking water, food is another source of As in Latin American countries. A few studies from As endemic areas in Latin America have indicated that food contributes up to 50% of total As intake [
20,
21]. High amounts of As have been detected in fish, cow milk, grains, and vegetables including potato, onion, beet, pumpkin, radish, cabbage, and beans in Bolivia, Brazil, Chile, Ecuador, El Salvador, Honduras, Mexico, Nicaragua, and Peru. Cow milk from Argentina and Mexico are shown to have As [
22].
Due to lack of agreement on the definition of soil quality, there is currently no general consensus regarding the soils that should be considered of maximum quality. The different approaches to the latter point can be summarized as two options. The first considers that a maximum quality soil is the soil in equilibrium with all the components of the environment, i.e.
, a climax soil developed under climax vegetation. The second option considers that the maximum quality reference soils are soils capable of maintaining high productivity and of causing minimum environmental distortion [
23].
The population of Papallacta is considered exclusively rural, with a high dependence on food and water from local sources. In the study area, agricultural soils can also be contaminated with arsenic through the use of contaminated water for crop irrigation purposes, creating a potential risk for human health [
24].
Food security attainment has been one of the most important sustainable development goals in most developing nations. Agricultural soil serves as a storage reservoir for water and nutrients needed for plants’ growth. Therefore, heavy metals contamination in agricultural soils can be harmful to normal plants’ growth [
25].
The main objectives of this study are to assess the degree of As contamination in the agricultural soils of the Papallacta parish, environmental quality of soils, and ecological risk associated with the obtained levels of As using the enrichment factor (EF), contamination factor (CF), and geo-accumulation index (Igeo).
Ecological risk indices such as EF, CF, and Igeo have been conveniently employed as diagnostic tools by a number of researchers [
26,
27,
28,
29,
30] for assessing the pollution status of arsenic and metals Pb, Cd, Ni, Fe, Cu, Mn, Co, and Zn contaminated agricultural soils and sediments.
2. Materials and Methods
2.1. Study Area
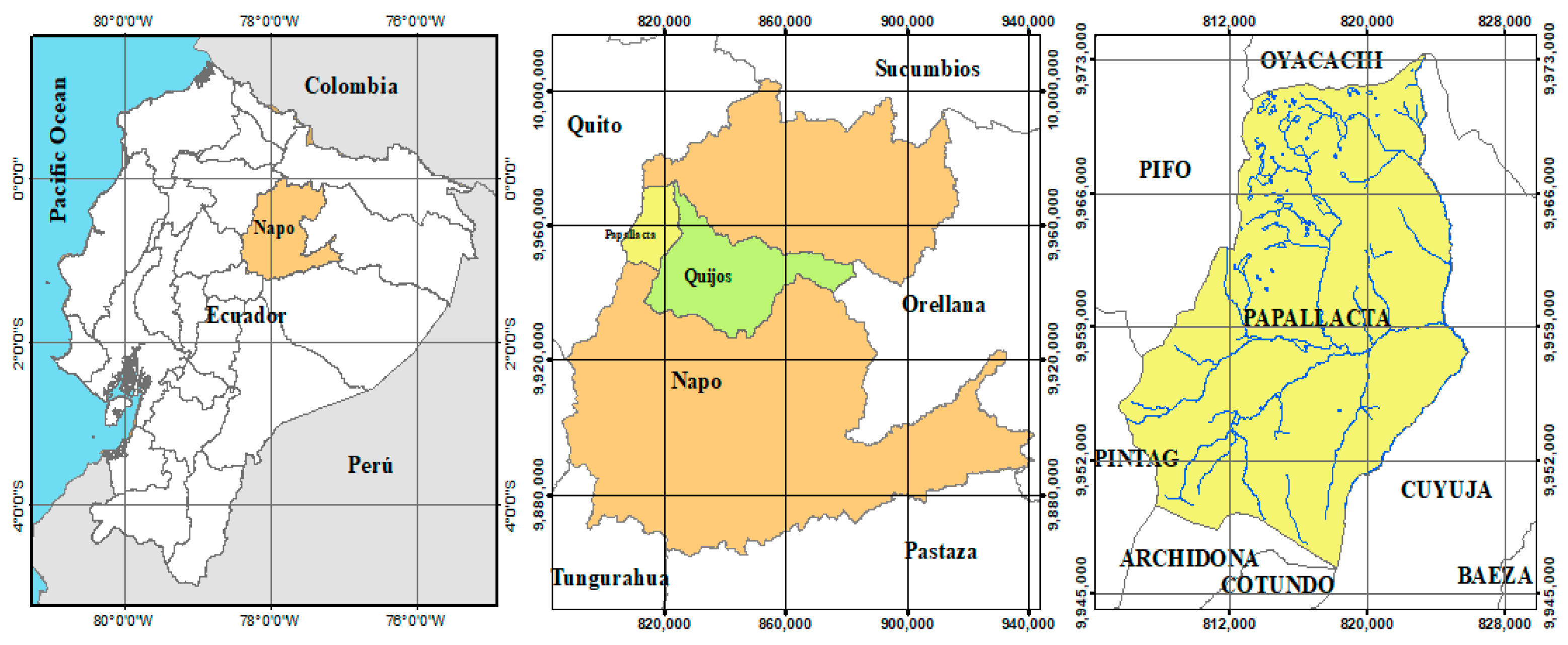
Papallacta is a rural parish located in Quijos County, Napo province, in northeastern Ecuador, 67 km east of Quito city (
Figure 1). Altitudinally, it is located in the eastern range of the Andes Mountain at 3156 m above sea level; the parish has an area of 319.6 km
2 [
31].
The parish and its human settlements are located at altitudes that range from 2800 to 4500 m above sea level. The annual average precipitation is from 1000 to 1500 mm and the annual average temperature is 7–15 °C.
According to the Ecuador National Institute of Census Statistics [
32], in the 2010 population census, the parish of Papallacta has a population of 920 inhabitants.
2.2. Geography, Geological and Soil Setting of Study Area
Papallacta in Ecuador is divided into five morphotectonic regions [
33]: (1) the coastal lowlands, which is covered by Paleogene to Neogene forearc deposits, (2) the Western Cordillera, which is composed of mafic and intermediate volcanic and intrusive rocks, (3) the Interandean Depression, which lies east of the Western Cordillera and hosts thick Pliocene to Pleistocene volcanic deposits [
34], (4) the Eastern Cordillera (Cordillera Real in Ecuador) is composed of Paleozoic metamorphic rocks and Mesozoic granitoids [
35], and (5) the Oriente Basin, including the Subandean zone, is a Late Cretaceous–Holocene foreland basin that developed on the South American plate in response to growth of the Eastern Cordillera [
36].
Papallacta parish is located on the crest of the Eastern Cordillera, in the central part of the Chacana Caldera, between the Antisana and Cayambe volcanoes.
The Chacana Caldera comprises the largest rhyolitic volcanic complex in the quaternary of the Northern Andes. This favors the presence of highly differentiated magmas emplaced at shallow crustal levels, which induce strong and consistent thermal anomalies in order to feed geothermal systems [
37].
The Chacana Caldera is underlain by a complex metamorphic basement and both geologic regimes are affected by ongoing tectonic activity that includes NE-trending transpressive faulting as well as eastward-trending thrust faulting. The caldera’s dimensions are large: the N-S diameter across the entire structure measures 50 km and the E-W width is truncated but measures at least 30 km. Outward-sloping packages of ignimbrites, lava flows, and tuffs comprise the north, west, and southwest outer flanks [
38].
The central depression is filled everywhere, first by ignimbrites, tuffs, and breccias of siliceous nature associated with the volcanism of the outer flanks, and later by post-collapse volcanism consisting of lava emissions of intermediate composition, and subsequently, by detrital sediments [
38].
About 20 intracaldera volcanic vents of rock composition varying from acid andesites to dacites and to rhyolites are scattered in an area of 30 km (NS) and 7 km (EW) and have assumed ages of less than 450,000 years; the last eruption occurred only 240 years ago [
37].
The Chacana Caldera has emitted two lava flows in the last 300 years. One of them is the Pinantura Flow, which is located on the outer flank of the caldera, and the other is the Papallacta Flow, which is located inside the caldera and forms the Papallacta Lagoon [
39].
Furthermore, Palaeozoic and Precambrian gneisses and amphibolites occur in the Cordillera Real at Papallacta [
40].
Andisols make up 94.93% of the parish area, while the rest of the area is represented by Histosols [
31,
41]. The main attributes of Andisols generally include a low degree of crystallinity minerals, high specific surface area, low bulk density values, high water-holding and infiltration capacity, and high contents of soil organic matter [
42,
43].
2.3. Sampling Site Description
The agricultural soil sampling was performed in four different districts (Cabecera Parroquial (S1), El Tambo (S2), Baños (S3), and (S4) Chalpi) of Papallacta parish (
Figure 2), where agriculture (potatoes, beans, corn, vegetables) and grazing activities are the primary occupation of the area and farmers in the village use the arsenic contaminated water for various purposes, especially for irrigation.
Sediments sampling was carried out at Papallacta Lake (S5); discharges of geothermal waters are the main natural sources of arsenic in the lake.
Approximately 0.5 kg composite soil samples were collected at a depth of about 10–35 cm from the arable surface.
Table 1 details a summary of the characteristics of agricultural soils and sediments samples taken of the five sampling areas of Papallacta parish, referring to the type of soil and land use.
MAG-SIGTIERRAS, the Ecuadorian entity in charge of soil mapping for the entire continental territory, adopts the US Soil Taxonomy System (Soil Survey Staff, 2014), which has reached the level of suborders [
44].
Most of the soil samples in the studied area correspond to the Andisol and Histosol order; according to the U.S. Soil Taxonomy System, Andisols soils derived from volcanic ejecta, especially volcanic ash, are characterized by high concentrations of OM [
45].
The main soil’s parent material is represented by andesitic to rhyolitic volcanic products and ash deposits (sulfur, iron, aluminum). The Histosols connotate soils dominated by organic materials at different stages of decomposition. They tend to be poorly drained, have low bulk density values, and high-water retention capacity [
42]. They occur only in S4 site (
Table 1).
The topography of the Papallacta parish area is very rugged, with dominant slopes ranging from undulating to mountainous (4–64)% [
31].
2.4. Sample Collection, Preparation, and Analysis
Agricultural soils (n = 30) and sediments samples from Papallacta lake (n = 6) were taken in the Papallacta parish area. The aim was to ensure that the composite sample better represented the selected field soil; collected samples were transported to the lab in sterile sealed polythene zipped bags for further processing and analysis.
The samples were kept in the shade for air drying for two to three weeks at room temperature. Air-dried soils were disaggregated, and any visible remains of organisms and debris were removed. The dried samples were then crushed in a mortar and passed through a 150 μm sieve to normalize particle sizes.
The total concentration of arsenic was analyzed by inductively coupled plasma-mass spectrometry (ICP-MS); the following operational phase was carried out.
In order to investigate the total concentration of As, each sample of homogenized soil (0.25 g) was digested in an aqua regia (4 mL) consisting of HNO3 and HCl (1 mL:3 mL) in digestion tubes and was heated at 70 °C on a shaker (brand, model) for 1 h.
After the digests cooled, 1 mL of the solution was extracted and deionized water (9 mL) was then added to the solution. An Agilent 7700 (Agilent Technologies, Tokyo, Japan) inductively coupled plasma mass spectrometer (ICP-MS) was used to determine the amount of As in digested sediment samples. The detection limit of As in solution matrix of the ICP-MS was 0.1 μg L−1.
For quality control, the precision and accuracy of the analytical methods were analyzed using a blank digest and standard reference materials (trace element, SRM 1643e) to verify the results of As in sediment. This Standard Reference Material (SRM) is primarily intended for use in evaluating methods used in the determination of trace elements. The SRM 1643e consists of approximately 250 mL of acidified water in a polyethylene bottle, which was sealed in an aluminized plastic bag to maintain stability. The SRM 1643e simulates the elemental composition of fresh water. Nitric acid is present at a concentration of approximately 0.8 mol/L to stabilize the trace elements and is used to verify the results of As in sediments. The certified values for arsenic in SRM 1643e are reported both as mass fractions (58.98 ± 0.70 µg/kg) and as mass concentrations (60.45 ± 0.72 µg/L). A certified value has the highest confidence in its accuracy, so all known or suspected sources of bias have been investigated and considered. The certified values are the average of the gravimetrically prepared values and a value determined by either inductively coupled plasma mass spectrometry (ICP-MS) or inductively coupled plasma optical emission spectrometry (ICP-OES) [
46].
Standard reference material was analyzed every 20 samples during analysis to check ICP-MS accuracy. The SRM sample was digested after utilizing the same procedure as sediments and the results gave good recovery of up to 96%.
2.5. Data Analyses
The degree of contamination and enrichment of As in the agricultural soils of Papallacta and in the surface sediments of Papallacta Lake were evaluated using three ecological risk indices (EF, CF, and Igeo).
The data obtained for As in sediments and agricultural soils were compared with the sediment quality guidelines of the United States Environmental Protection Agency (USEPA) and with the permissible limits of As (12 mg kg
−1) for agricultural soils proposed by the Ministry of Environment of Ecuador [
47,
48].
2.5.1. Enrichment Factor
Enrichment factor (EF) of arsenic in agricultural soils can distinguish metal pollution caused by indigenous sources of metal, anthropogenic activities, and combination of both [
49]. The metals EF is a useful indicator reflecting the status and degree of environmental contamination [
50].
The EF calculations compare each value with a given background level, either from the local site, using older deposits formed under similar conditions but without anthropogenic impact, or from a regional or global average composition [
26,
51].
The enrichment factor for each metal (arsenic) is calculated by dividing its ratio to the normalizing element (
Fe) by the same ratio found in the chosen baseline Equation (1) [
49]. Thus, EF is computed using the relationship below:
where (
Me/
Fe) sample is the ratio of As to Fe in the sample and (
Me/
Fe) background is the ratio of As to Fe concentrations in the background. In the study, the background concentration was taken from the control sites.
Iron was chosen as the reference element to normalize sediment As concentrations and to alleviate the variations produced by heterogeneous sediments. This is because Fe is the most abundant naturally occurring and highly refractory metal and the levels of Fe are not generally influenced by anthropogenic sources [
26,
52].
According to the value obtained in the relationship, the level of enrichment is characterized in seven proposed categories as shown in
Table 2 [
53].
The origin of the metal (arsenic) can be determined by means of the enrichment factor;
Table 3 presents the characteristics of each interval of the EF [
54].
2.5.2. Contamination Factor
Contamination factor (CF) is considered to be an effective parameter which monitors metal pollution over a period of time. The CF is the ratio obtained by dividing the concentration of each metal (arsenic) in the soil by the baseline or background value (concentration in unpolluted soil) [
55].
The level of contamination of sediments by As expressed in terms of CF was calculated as Equation (2):
where,
Cm sample is the concentration of As in sediments and
Cm background is the background value of As.
Values of this index are divided into four categories according to
Table 4 [
56,
57].
2.5.3. The Geo-Accumulation Index
The index of geo-accumulation (Igeo), originally introduced by Müller (1969), can be used to assess the environmental contamination status or current levels of metal concentrations compared with geochemical background concentrations or original pre-industrial concentrations in the soils [
29,
58].
The Igeo values were calculated for As using the Equation (3) of Muller (1969) [
26,
59,
60]:
In the above equation,
Cm sample is the concentration of arsenic in the present soil sample and
Cm background is the background value of the same metal. The factor 1.5 has been used with reference to possible variations in the background value. For the classification of various degrees of contamination level, a total of seven classes were reported in the geo-accumulation index,
Table 5 [
7,
61].
4. Conclusions
The results of the present study show an analysis of geogenic arsenic concentrations in the agricultural soils around the Papallacta parish in Ecuador.
Analysis of soil samples and sediments from five sampling point sites of the Papallacta parish show enrichment of arsenic. The mean concentrations of arsenic in soil were higher than the safe limit set (12 mg kg−1) for agricultural purposes by the Ministry of Environment of Ecuador.
The sediments of Papallacta Lake have higher concentrations of arsenic than the agricultural soils, for which it is concluded that this enrichment is due to an additional source to the parent rock, coming from the interaction of the sediments with the high concentrations of arsenic found in the geothermal discharges of the main tributaries of the lake.
Soil pollution in the present study was assessed using enrichment factor, contamination factor, and geo-accumulation index geochemical approaches. The EF and CF of the arsenic show that the soils in the study area are moderately to considerably polluted.
The Igeo results illustrate that soils and sediments in the study area are uncontaminated to moderately contaminated with respect to arsenic; the identified values are probably a result of natural enrichment and are not influenced by anthropogenic activities that may contribute to arsenic enrichment in the Papallacta parish.
The study reveals that the elevated concentrations of As in soils were mostly encountered in areas that are influenced by geothermal discharges and are in a coordination environment with Fe oxides.
The sampling area in this study is primarily agricultural land, therefore, regular monitoring of water, which is a main source of irrigation, should also be assessed for As content. Arsenic toxicity in agricultural soils is a global menace that needs urgent intervention.
As the relief in most conditions of the studied sites is very rugged, although the Andisols have very high aggregates stability and very high infiltration capacity, and consequently low potential for runoff generation, erosion should be considered a potential risk for As redeposition and contamination in lower areas of the landscape, and preventive conservationist planning should receive attention and monitoring.
These results illustrate the importance of more research regarding mobility, distribution speciation, and bioaccessibility of As to create treatment/management strategies for As-containing soils to enable flexible land use in the future.