Eco-Friendly Biocontrol of Moniliasis in Ecuadorian Cocoa Using Biplot Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungi Strains
2.2. Culture Media
2.3. Mycelial Growth
2.4. Antagonistic Capacity
2.5. Experimental Test
2.6. Statistical Analysis
2.6.1. PCA Biplot
2.6.2. GGE Biplot
3. Results and Discussion
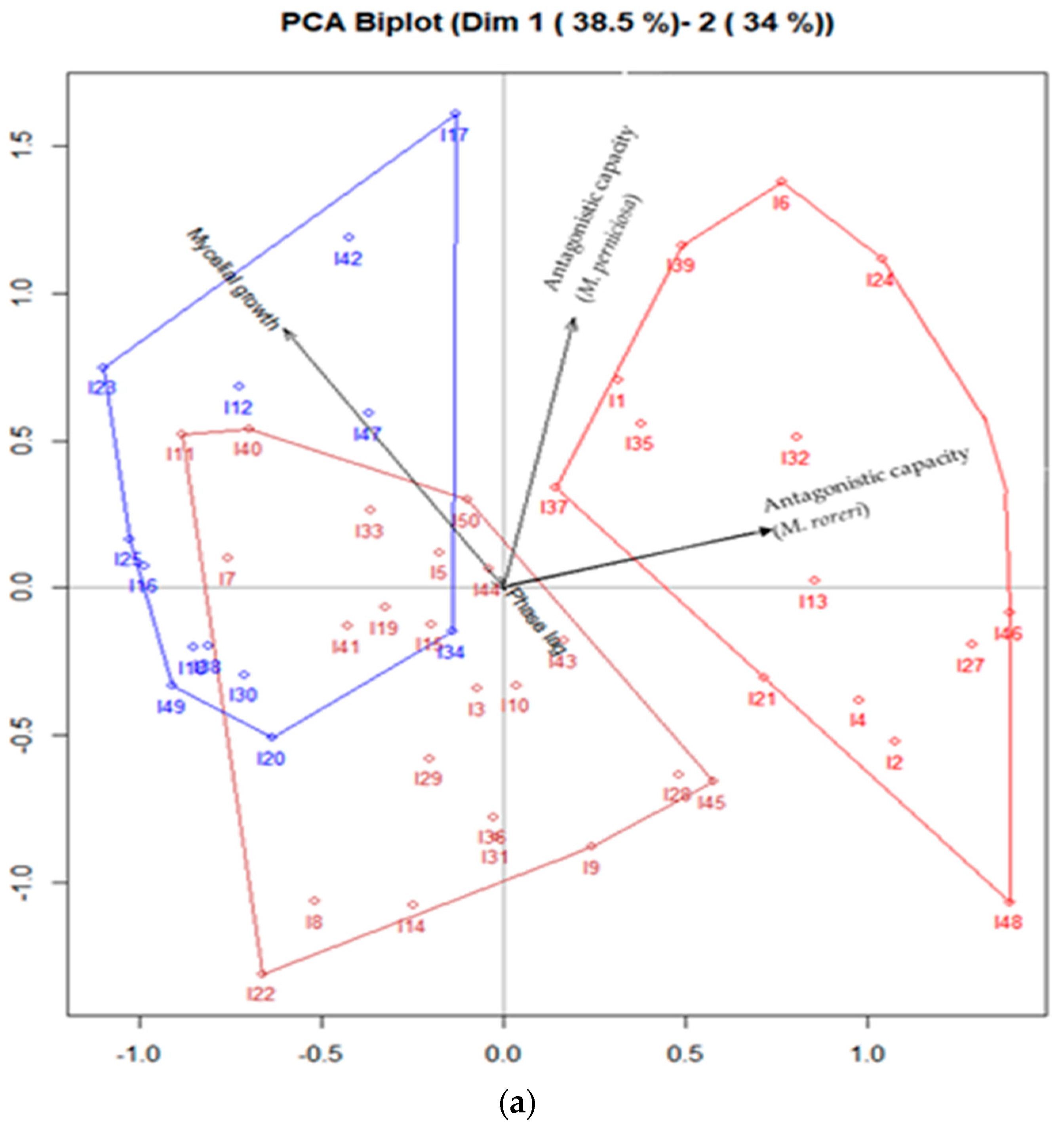
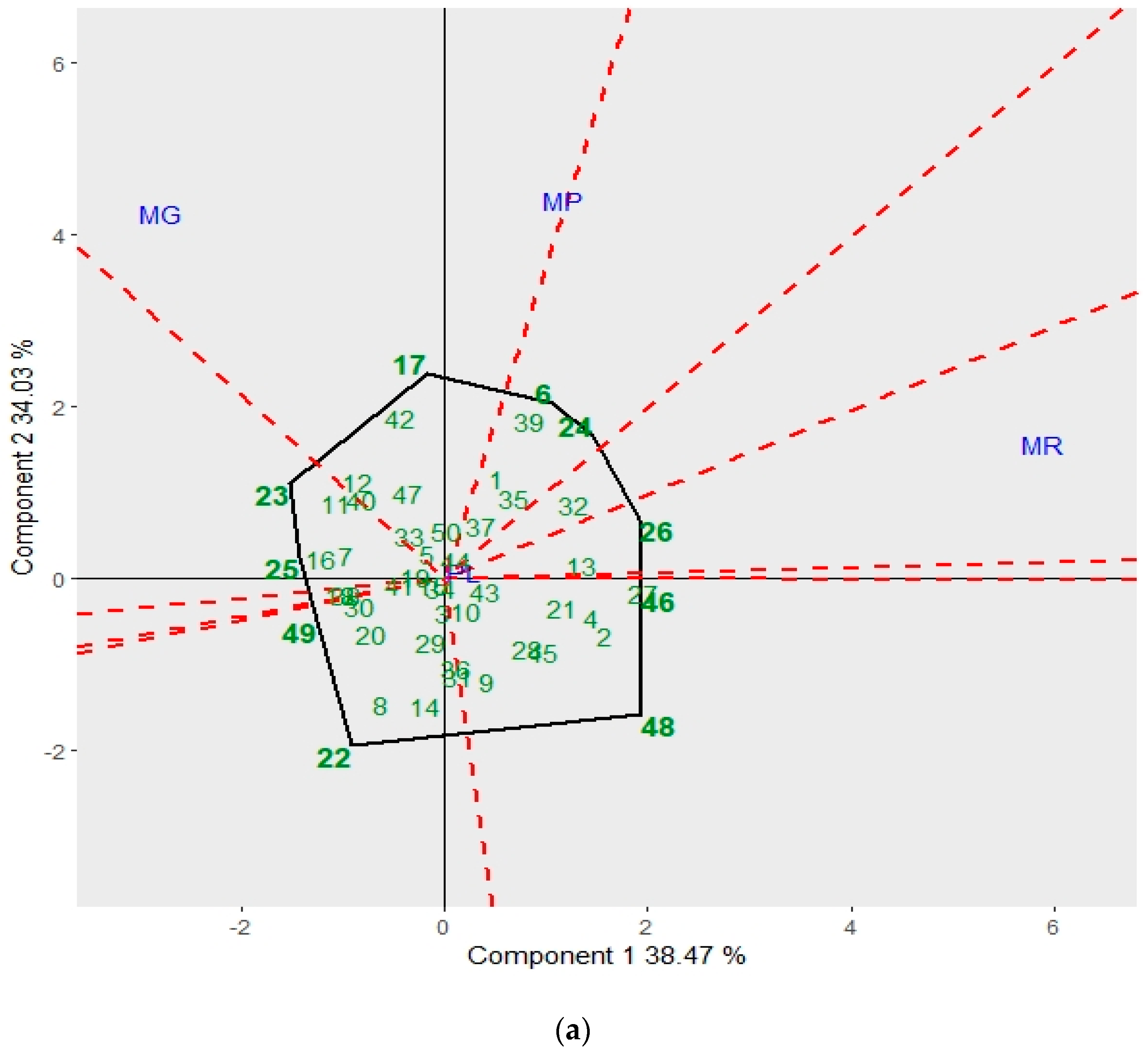
3.1. PCA Biplot Algorithm for Mycelial Characteristics and Antagonistic Capacity of Trichoderma spp.
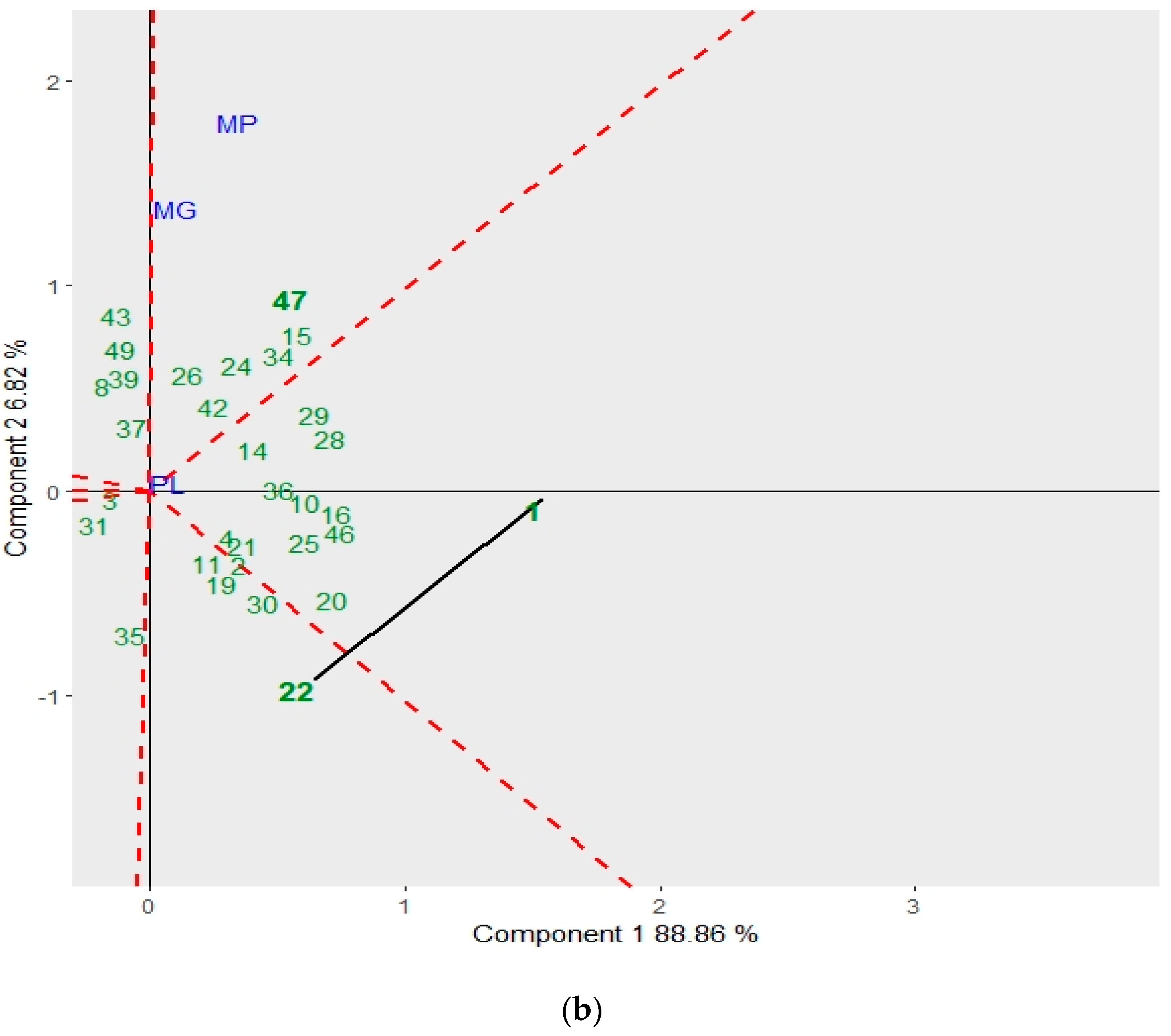
3.2. GGE Biplot for Mycelial Characteristics and the Antagonistic Capacity of Trichoderma spp.
3.3. Field Responses of Trichoderma spp. against Monialisis Caused by Moniliophthora perniciosa
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ceccarelli, V.; Lastra, S.; Loor Solorzano, R.G.; Chacón, W.W.; Nolasco, M.; Sotomayor Cantos, I.A.; Plaza Avellán, L.F.; López, D.A.; Fernández Anchundia, F.M.; Dessauw, D.; et al. Conservation and use of genetic resources of cacao (Theobroma cacao L.) by gene banks and nurseries in six Latin American countries. Genet. Resour. Crop Evol. 2022, 69, 1283–1302. [Google Scholar] [CrossRef]
- Ramírez, C.H.E.; Gaona, O.D.J.C.; Londoño, G.A.C.; Bustamante, E.G.M. YIield of cocoa under different agroforestry systems in a dry tropical forest in western Colombia. Bioagro 2022, 34, 39–50. [Google Scholar] [CrossRef]
- Wattnem, T.; Wiegel, J.; González, C.; Reyes, B. Who Defines Fine Chocolate? The Construction of Global Cocoa Quality Standards from Latin America. Int. J. Sociol. Agric. Food 2022, 28, 73–87. [Google Scholar]
- García, A.; Pico, B.; Jaimez, R. La cadena de producción del Cacao en Ecuador: Resiliencia en los diferentes actores de la producción. Novasinergia 2021, 4, 152–172. [Google Scholar]
- Villacis, A.H.; Alwang, J.R.; Barrera, V.; Dominguez, J. Prices, specialty varieties, and postharvest practices: Insights from cacao value chains in Ecuador. AgriBus 2022, 38, 426–458. [Google Scholar] [CrossRef]
- Jensch, C.; Schmidt, A.; Strube, J. Versatile Green Processing for Recovery of Phenolic Compounds from Natural Product Extracts towards Bioeconomy and Cascade Utilization for Waste Valorization on the Example of Cocoa Bean Shell (CBS). Sustainability 2022, 14, 3126. [Google Scholar] [CrossRef]
- Valenzuela-Cobos, J.D.; Guevara-Viejó, F.; Vicente-Galindo, P.; Galindo-Villardón, P. Food Sustainability Study in Ecuador: Using PCA Biplot and GGE Biplot. Sustainability 2022, 14, 13033. [Google Scholar] [CrossRef]
- Armengot, L.; Ferrari, L.; Milz, J.; Velásquez, F.; Hohmann, P.; Schneider, M. Cacao agroforestry systems do not increase pest and disease incidence compared with monocultures under good cultural management practices. Crop Prot. 2020, 130, 105047. [Google Scholar] [CrossRef]
- Anzules-Toala, V.; Pazmiño-Bonilla, E.; Alvarado-Huamán, L.; Borjas-Ventura, R.; Castro-Cepero, V.; Julca-Otiniano, A. Control of cacao (Theobroma cacao) diseases in Santo Domingo de los Tsachilas, Ecuador. Agron. Mesoam. 2022, 33, 45939. [Google Scholar] [CrossRef]
- Leiva, S.; Rubio, K.; Díaz-Valderrama, J.R.; Granda-Santos, M.; Mattos, L. Phylogenetic Affinity in the Potential Antagonism of Trichoderma spp. against Moniliophthora roreri. Agronomy 2022, 12, 2052. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Musumeci, M.A. Trichoderma as biological control agent: Scope and prospects to improve efficacy. World J. Microbiol. Biotechnol. 2021, 37, 90. [Google Scholar] [CrossRef]
- Verma, A.; Johri, B.N.; Prakash, A. Antagonistic Evaluation of Bioactive Metabolite from Endophytic Fungus, Aspergillus flavipes KF671231. J. Mycol. 2014, 2014, 371218. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.R.; Juthy, P.S.; Haque, M.M.; Rahman, M.M. Identification of plant growth promoting antagonistic bacteria against Xanthomonas oryzae pv. oryzae in Bangladesh. Fundam. Appl. Agric. 2019, 4, 1068–1080. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, A.; Sansinenea, E. The role of beneficial microorganisms in soil quality and plant health. Sustainability 2022, 14, 5358. [Google Scholar] [CrossRef]
- Rajani, P.; Rajasekaran, C.; Vasanthakumari, M.M.; Olsson, S.B.; Ravikanth, G.; Shaanker, R.U. Inhibition of plant pathogenic fungi by endophytic Trichoderma spp. through mycoparasitism and volatile organic compounds. Microbiol. Res. 2021, 242, 126595. [Google Scholar] [CrossRef]
- Sarker, I.H. Data science and analytics: An overview from data-driven smart computing, decision-making and applications perspective. SN Comput. Sci. 2021, 2, 377. [Google Scholar] [CrossRef]
- Niazian, M.; Niedbała, G. Machine learning for plant breeding and biotechnology. Agriculture 2020, 10, 436. [Google Scholar] [CrossRef]
- Valenzuela-Cobos, J.D.; Rodríguez-Grimón, R.O.; Zied, D.C.; Grijalva-Endara, A.; Garcés-Moncayo, M.F.; Garín-Aguilar, M.E.; Sánchez-Hernandez, A.; Valencia del Toro, G. Chemical composition and biological properties of Pleurotus spp. cultivated on different concentrations of peat moss and wheat straw. Emir. J. Food Agric. 2019, 31, 830–836. [Google Scholar] [CrossRef]
- Hernández, A.A.S.; Cobos, J.D.V.; Martínez, J.H.; Arce, R.V.; Gomez, Y.d.G.y.; Segura, P.B.Z.; Aguilar, M.E.G.; Lara, H.L.; del Toro, G.V. Characterization of Pleurotus djamor neohaplonts recovered by production of protoplasts and chemical dedikaryotization. 3 Biotech 2019, 9, 24. [Google Scholar] [CrossRef]
- Wawrzyniak, J. Model of Fungal Development in Stored Barley Ecosystems as a Prognostic Auxiliary Tool for Postharvest Preservation Systems. Food Bioprocess Technol. 2021, 14, 298–309. [Google Scholar] [CrossRef]
- Liu, H.; Chen, N.; Feng, C.; Tong, S.; Li, R. Impact of electro-stimulation on denitrifying bacterial growth and analysis of bacterial growth kinetics using a modified Gompertz model in a bio-electrochemical denitrification reactor. Bioresour. Technol. 2017, 232, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Cobos, J.D.V.; Páramo, D.; Aguilar, M.E.G.; Hernández, A.S.; Lara, H.L.; del Toro, G.V. Production of hybrid strains among Pleutorus and Lentinula and evaluation of their mycelial growth kinetics on malt extract agar and wheat grain using the Gompertz and Hill models. Emir. J. Food Agric. 2017, 29, 927–935. [Google Scholar]
- Urbez-Torres, J.R.; Tomaselli, E.; Pollard-Flamand, J.; Boule, J.; Gerin, D.; Pollastro, S. Characterization of Trichoderma isolates from southern Italy, and their potential biocontrol activity against grapevine trunk disease fungi. Phytopathol. Mediterr. 2020, 59, 425–439. [Google Scholar]
- Leiva, S.; Oliva, M.; Hernández, E.; Chuquibala, B.; Rubio, K.; García, F.; Torres de la Cruz, M. Assessment of the Potential of Trichoderma spp. Strains Native to Bagua (Amazonas, Peru) in the Biocontrol of Frosty Pod Rot (Moniliophthora roreri). Agronomy 2020, 10, 1376. [Google Scholar] [CrossRef]
- Greenacre, M.; Groenen, P.J.; Hastie, T.; d’Enza, A.I.; Markos, A.; Tuzhilina, E. Principal component analysis. Nat. Rev. Methods Primers. 2022, 2, 100. [Google Scholar] [CrossRef]
- Gabriel, K.R. The biplot-graphical display of matrices with applications to principal component analysis. Biometrica 1971, 58, 453–467. [Google Scholar] [CrossRef]
- Galindo, M.P. Una alternativa de representación simultánea: HJ-Biplot. Qüestiió 1986, 10, 13–23. [Google Scholar]
- Khan, M.M.H.; Rafii, M.Y.; Ramlee, S.I.; Jusoh, M.; Al Mamun, M. AMMI and GGE biplot analysis for yield performance and stability assessment of selected Bambara groundnut (Vigna subterranea L. Verdc.) genotypes under the multi-environmental trials (METs). Sci. Rep. 2021, 11, 22791. [Google Scholar] [CrossRef]
- Burgueño, J.; Crossa, J.; Vargas, M. SAS Programs for Graphing GE and GGE Biplot. The AMMI Analysis and Graphing the Biplot; Biometrics and Statistics Unit CIMMYT: Mexico City, Mexico, 2001; pp. 29–36. [Google Scholar]
- Arzate-Vega, J.; Santos-Eméstica, O.A.; Michel-Aceves, A.C.; Domínguez-Márquez, V.M. Antagonismo de Trichoderma spp. sobre Mycosphaerella fijiensis Morelet, agente causal de la Sigatoka Negra del plátano (Musa sp.) in vitro e invernadero. Rev. Mex. Fitopatol. 2006, 24, 98–104. [Google Scholar]
- Mejía, L.C.; Rojas, E.I.; Maynard, Z.; Van Bael, S.; Arnold, A.E.; Hebbar, P.; Samuels, G.J.; Robbins, N.; Herre, E.A. Endophytic fungi as biocontrol agents of Theobroma cacao pathogens. Biol. Control 2008, 46, 4–14. [Google Scholar] [CrossRef]
- Krauss, U.; Hidalgo, E.; Bateman, R.; Adonijah, V.; Arroyo, C.; García, J.; Crozier, J.; Brown, N.; Ten Hoopen, G.M.; Holmes, K.A. Improving the formulation and timing of application of endophytic biocontrol and chemical agents against frosty pod rot (Moniliophthora roreri) in cocoa (Theobroma cacao). Biol. Control 2010, 54, 230–240. [Google Scholar] [CrossRef]
- Villamil Carvajal, J.E.; Blanco Valbuena, J.O.; Viteri Rosero, S.E. Evaluación in vitro de Microorganismos Nativos por su Antagonismo contra Moniliophthora roreri Cif & Par en Cacao (Theobroma cacao L.). Rev. Fac. Nac. Agron. Medellín 2012, 65, 6305–6315. [Google Scholar]
- Ketta, H.A.; Hewedy, O.A.E.R. Biological control of Phaseolus vulgaris and Pisum sativum root rot disease using Trichoderma species. Egypt. J. Biol. Pest Control 2021, 31, 96. [Google Scholar] [CrossRef]
- Nayak, P.; Solanki, H. Pesticides and Indian agriculture—A review. Int. J. Res. Granthaalayah 2021, 9, 250–263. [Google Scholar] [CrossRef]
- Sousa Filho, H.R.; de Jesus, R.M.; Bezerra, M.A.; Santana, G.M.; de Santana, R.O. History, dissemination, and field control strategies of cocoa witches’ broom. Plant Pathol. 2021, 70, 1971–1978. [Google Scholar] [CrossRef]
- Grey, A.B.J.; Cadelis, M.M.; Diao, Y.; Park, D.; Lumley, T.; Weir, B.S.; Copp, B.R.; Wiles, S. Screening of Fungi for Antimycobacterial Activity Using a Medium-Throughput Bioluminescence-Based Assay. Front. Microbiol. 2021, 12, 739995. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Navarro-Cruz, A.R.; Vera-López, O.; Palou, E.; Avila-Sosa, R. Growth modeling to control (in vitro) Fusarium verticillioides and Rhizopus stolonifer with thymol and carvacrol. Rev. Argent. Microbiol. 2018, 50, 70–74. [Google Scholar] [CrossRef]
- Illescas, M.; Pedrero-Méndez, A.; Pitorini-Bovolini, M.; Hermosa, R.; Monte, E. Phytohormone production profiles in Trichoderma species and their relationship to wheat plant responses to water stress. Pathogens 2021, 10, 991. [Google Scholar] [CrossRef]
- Valenzuela Cobos, J.; Ramírez Grimón, R.; Vargas Farías, C.; Grijalva-Endara, A.; Mercader-Camejo, O. Biodegradation of plantain rachis using phytopathogenic fungi for composting. Rev. Mex. Ing. Quim. 2019, 19, 533–541. [Google Scholar]
- Mulatu, A.; Alemu, T.; Megersa, N.; Vetukuri, R.R. Optimization of Culture Conditions and Production of Bio-Fungicides from Trichoderma Species under Solid-State Fermentation Using Mathematical Modeling. Microorganisms 2021, 9, 1675. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, R.S.; Lima, H.L.; Pinto, G.A.; Gava, C.A.; Rodrigues, S. Effect of moisture on Trichoderma conidia production on corn and wheat bran by solid state fermentation. Food Bioprocess Technol. 2008, 1, 100–104. [Google Scholar] [CrossRef]
- Hamrouni, R.; Molinet, J.; Miché, L.; Carboué, Q.; Dupuy, N.; Masmoudi, A.; Roussos, S. Production of coconut aroma in solid-state cultivation: Screening and identification of Trichoderma strains for 6-pentyl-alpha-pyrone and conidia production. J. Chem. 2019, 2019, 8562384. [Google Scholar] [CrossRef] [Green Version]
- Serna-Díaz, M.G.; Mercado-Flores, Y.; Jiménez-González, A.; Anducho-Reyes, M.A.; Medina-Marín, J.; Tuoh-Mora, J.S.; Téllez-Jurado, A. Use of barley straw as a support for the production of conidiospores of Trichoderma harzianum. Biotechnol. Rep. 2020, 26, e00445. [Google Scholar] [CrossRef]
- Hashem, M.; Mostafa, Y.S.; Alamri, S.; Abbas, A.M.; Eid, E.M. Exploitation of Agro-Industrial Residues for the Formulation of a New Active and Cost Effective Biofungicide to Control the Root Rot of Vegetable Crops. Sustainability 2021, 13, 9254. [Google Scholar] [CrossRef]
- Loguercio, L.L.; de Carvalho, A.C.; Niella, G.R.; De Souza, J.T.; Pomella, A.W.V. Selection of Trichoderma stromaticum isolates for efficient biological control of witches’ broom disease in cacao. Biol. Control 2009, 51, 130–139. [Google Scholar] [CrossRef]
- Krauss, U.; Soberanis, W. Effect of fertilization and biocontrol application frequency on cocoa pod diseases. Biol. Control 2002, 24, 82–89. [Google Scholar] [CrossRef]
- Lyubenova, A.; Rusanova, M.; Nikolova, M.; Slavov, S.B. Plant extracts and Trichoderma spp.: Possibilities for implementation in agriculture as biopesticides. Biotechnol. Biotechnol. Equip. 2023, 37, 159–166. [Google Scholar] [CrossRef]
- Grzegorczyk, M.; Kancelista, A.; Łaba, W.; Piegza, M.; Witkowska, D. The effect of lyophilization and storage time on the survival rate and hydrolytic activity of Trichoderma strains. Folia Microbiol. 2018, 63, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Pettitt, T.R.; Buenfeld, N.; Smith, S.R. A critical review of the physiological, ecological, physical and chemical factors influencing the microbial degradation of concrete by fungi. Build. Environ. 2022, 214, 108925. [Google Scholar] [CrossRef]
- Ruangwong, O.-U.; Pornsuriya, C.; Pitija, K.; Sunpapao, A. Biocontrol Mechanisms of Trichoderma koningiopsis PSU3-2 against Postharvest Anthracnose of Chili Pepper. J. Fungi. 2021, 7, 276. [Google Scholar] [CrossRef]
- Gunjal, A.B. Trichoderma: An Eco-Friendly Biopesticide for Sustainable Agriculture. In Organic Farming for Sustainable Development; Apple Academic Press: Palm Bay, FL, USA, 2023; pp. 3–21. [Google Scholar]
- Schweiger, R.; Padilla-Arizmendi, F.; Nogueira-López, G.; Rostás, M.; Lawry, R.; Brown, C.; Hampton, J.; Steyaert, J.M.; Müller, C.; Mendoza-Mendoza, A. Insights into metabolic changes caused by the Trichoderma virens-maize root interaction. Mol. Plant Microbe Interact. 2021, 34, 524–537. [Google Scholar] [CrossRef]

| Treatment | Incidence (%) | Efficiency (%) | Yield (kg/ha) |
|---|---|---|---|
| Control | 18.2 a | - | 533.50 c |
| T1 | 5.8 c | 70.4 b | 721.80 a |
| T2 | 10.1 b | 54.3 c | 615.20 b |
| T3 | 3.1 d | 75.1 a | 753.10 a |
| T4 | 9.7 b | 60.8 c | 630.50 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzuela-Cobos, J.D.; Guevara-Viejó, F.; Vicente-Galindo, P.; Galindo-Villardón, P. Eco-Friendly Biocontrol of Moniliasis in Ecuadorian Cocoa Using Biplot Techniques. Sustainability 2023, 15, 4223. https://doi.org/10.3390/su15054223
Valenzuela-Cobos JD, Guevara-Viejó F, Vicente-Galindo P, Galindo-Villardón P. Eco-Friendly Biocontrol of Moniliasis in Ecuadorian Cocoa Using Biplot Techniques. Sustainability. 2023; 15(5):4223. https://doi.org/10.3390/su15054223
Chicago/Turabian StyleValenzuela-Cobos, Juan Diego, Fabricio Guevara-Viejó, Purificación Vicente-Galindo, and Purificación Galindo-Villardón. 2023. "Eco-Friendly Biocontrol of Moniliasis in Ecuadorian Cocoa Using Biplot Techniques" Sustainability 15, no. 5: 4223. https://doi.org/10.3390/su15054223







