The Optimization of Operational Variables of Electrochemical Water Disinfection Using Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methodology
2.1. Sample Collection and Experimental Setup
2.2. Response Surface Methodology
2.3. Electrochemical Disinfection
2.4. Microbial Analysis
3. Results and Discussion
3.1. Pretreatment Experiment
3.2. Optimization of Designed Parameters through RSM
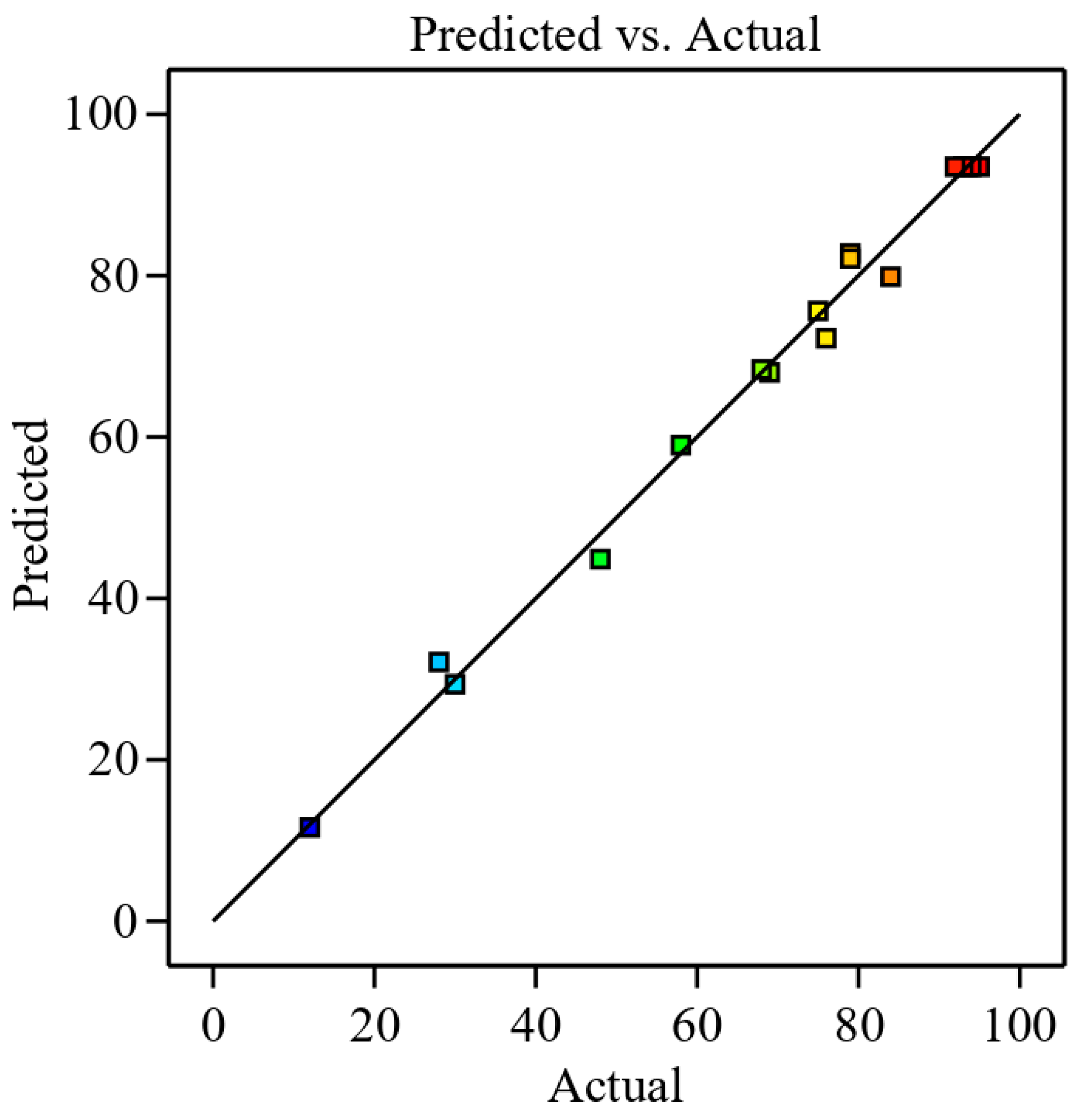
3.2.1. Adequacy of Mathematical Model
3.2.2. Effect of Process Variables on the Disinfection Efficiency
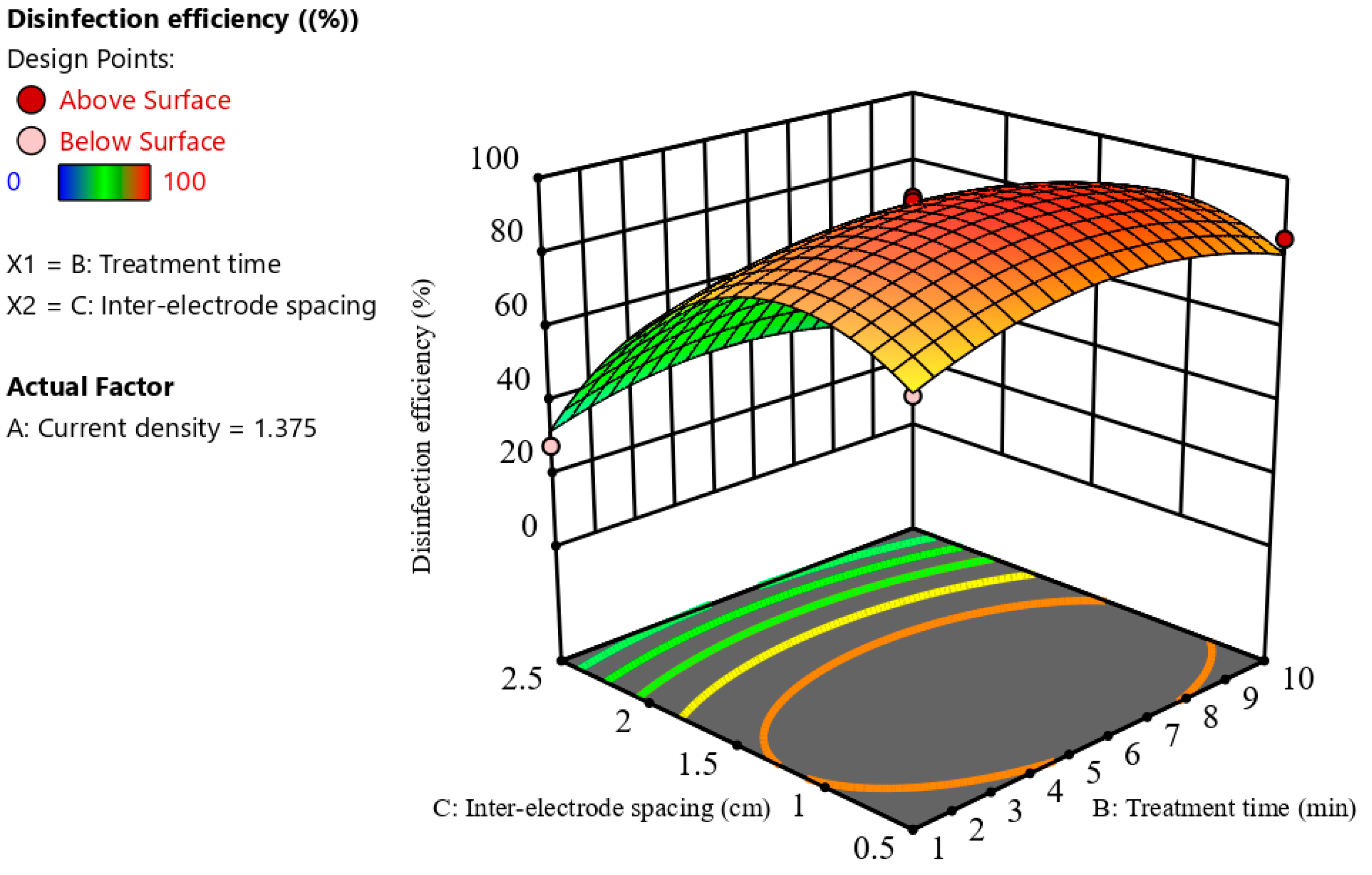
3.2.3. Combined Effect of Process Variables on Disinfection Efficiency
3.2.4. Optimization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plappally, A.K.; Lienhard, J.H.V. Energy requirements for water production, treatment, end use, reclamation, and disposal. Renew. Sustain. Energy Rev. 2012, 16, 4818–4848. [Google Scholar] [CrossRef]
- Hoekstra, A.Y.; Chapagain, A.K. The water footprints of Morocco and the Netherlands: Global water use as a result of domestic consumption of agricultural commodities. Ecol. Econ. 2007, 64, 143–151. [Google Scholar] [CrossRef]
- Czarny, J.; Präbst, A.; Spinnler, M.; Biek, K.; Sattelmayer, T. Development and simulation of decentralised water and energy supply concepts—Case study of rainwater harvesting at the angkor centre for conservation of biodiversity in cambodia. J. Sustain. Dev. Energy Water Environ. Syst. 2017, 5, 626–644. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, B.; Wei, W.; Shao, L.; Li, Z.; Jiang, W.; Chen, G. Global water use associated with energy supply, demand and international trade of China. Appl. Energy 2020, 257, 113992. [Google Scholar] [CrossRef]
- Abu Hasan, H.; Muhammad, M.H.; Ismail, N.I. A review of biological drinking water treatment technologies for contaminants removal from polluted water resources. J. Water Process Eng. 2020, 33, 101035. [Google Scholar] [CrossRef]
- Sonone, S.S.; Jadhav, S.V.; Sankhla, M.S.; Kumar, R. Water Contamination by Heavy Metals and their Toxic Effect on Aquaculture and Human Health through Food Chain. Lett. Appl. NanoBioSci. 2020, 10, 2148–2166. [Google Scholar] [CrossRef]
- Saleh, I.A.; Zouari, N.; Al-Ghouti, M.A. Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Environ. Technol. Innov. 2020, 19, 101026. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Nienhauser, A.B.; Fajardo, A.S.; Bansal, R.; Conrad, C.L.; Fortner, J.D.; Marcos-Hernández, M.; Rogers, T.; Villagran, D.; Wong, M.S.; et al. Disparities between experimental and environmental conditions: Research steps toward making electrochemical water treatment a reality. Curr. Opin. Electrochem. 2020, 22, 9–16. [Google Scholar] [CrossRef]
- Bharadwaj, K.K. Water Pollution: A Threatening Alarm to The Mankind. Innov. Res. Thoughts 2018, 4, 204–209. [Google Scholar]
- Ogbo, F.A.; Agho, K.; Ogeleka, P.; Woolfenden, S.; Page, A.; Eastwood, J.; Homaira, N.; Burrett, S.; Zwi, K.; Schaefer, M.; et al. Infant feeding practices and diarrhoea in sub-Saharan African countries with high diarrhoea mortality. PLoS ONE 2017, 12, e0171792. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Brillas, E. Electrochemical alternatives for drinking water disinfection. Angew. Chem. Int. Ed. 2008, 47, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Guerra, O.J.; Reklaitis, G.V. Advances and challenges in water management within energy systems. Renew. Sustain. Energy Rev. 2018, 82, 4009–4019. [Google Scholar] [CrossRef]
- Kraft, A. Electrochemical water disinfection: A short review. Platin. Met. Rev. 2008, 52, 177–185. [Google Scholar] [CrossRef]
- Chakrabarti, M.H.; Saleem, M.; Irfan, M.F.; Raza, S.; Hasan, D.B.; Daud, W.M.A.W. Application of waste derived activated carbon felt electrodes in minimizing NaCl use for electrochemical disinfection of water. Int. J. Electrochem. Sci. 2011, 6, 4470–4480. [Google Scholar]
- Cossali, G.; Routledge, E.J.; Ratcliffe, M.S.; Blakes, H.; Fielder, J.E.; Karayiannis, T.G. Inactivation of E. coli, Legionella, and Pseudomonas in Tap Water Using Electrochemical Disinfection. J. Environ. Eng. 2016, 142, 04016063. [Google Scholar] [CrossRef]
- Chaplin, B.P. Advantages, Disadvantages, and Future Challenges of the Use of Electrochemical Technologies for Water and Wastewater Treatment; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128131602. [Google Scholar]
- Kerwick, M.I.; Reddy, S.M.; Chamberlain, A.H.L.; Holt, D.M. Electrochemical disinfection, an environmentally acceptable method of drinking water disinfection? Electrochim. Acta 2005, 50, 5270–5277. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, C.; Yoon, J. The effect of electrode material on the generation of oxidants and microbial inactivation in the electrochemical disinfection processes. Water Res. 2009, 43, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.T.; Makwana, A.R.; Ahammed, M.M. The use of response surface methodology for modelling and analysis of water and wastewater treatment processes: A review. Water Sci. Technol. 2014, 69, 464–478. [Google Scholar] [CrossRef]
- Darvishmotevalli, M.; Zarei, A.; Moradnia, M.; Noorisepehr, M.; Mohammadi, H. Optimization of saline wastewater treatment using electrochemical oxidation process: Prediction by RSM method. MethodsX 2019, 6, 1101–1113. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.Y.; Cho, M.; Choi, W.; Yoon, J. Inactivation of Escherichia coli in the electrochemical disinfection process using a Pt anode. Chemosphere 2007, 67, 652–659. [Google Scholar] [CrossRef]
- Ndjomgoue-Yossa, A.C.; Nanseu-Njiki, C.P.; Kengne, I.M.; Ngameni, E. Effect of electrode material and supporting electrolyte on the treatment of water containing Escherichia coli by electrocoagulation. Int. J. Environ. Sci. Technol. 2015, 12, 2103–2110. [Google Scholar] [CrossRef]
- Riyanto; Agustiningsih, W.A. Electrochemical disinfection of coliform and Escherichia coli for drinking water treatment by electrolysis method using carbon as an electrode. IOP Conf. Ser. Mater. Sci. Eng. 2018, 349, 012053. [Google Scholar] [CrossRef]
- Samarghandi, M.R.; Nemattollahi, D.; Asgari, G.; Shokoohi, R.; Ansari, A.; Dargahi, A. Electrochemical process for 2,4-D herbicide removal from aqueous solutions using stainless steel 316 and graphite Anodes: Optimization using response surface methodology. Sep. Sci. Technol. 2019, 54, 478–493. [Google Scholar] [CrossRef]
- Casqueira, R.G.; Torem, M.L.; Kohler, H.M. The removal of zinc from liquid streams by electroflotation. Miner. Eng. 2006, 19, 1388–1392. [Google Scholar] [CrossRef]
- Delaedt, Y.; Daneels, A.; Declerck, P.; Behets, J.; Ryckeboer, J.; Peters, E.; Ollevier, F. The impact of electrochemical disinfection on Escherichia coli and Legionella pneumophila in tap water. Microbiol. Res. 2008, 163, 192–199. [Google Scholar] [CrossRef]
- Hyde, K. Turbidity measurement: Its application for water resource recycling in buildings. Process Saf. Environ. Prot. 2021, 146, 629–638. [Google Scholar] [CrossRef]
- Malik, Q.H. Performance of alum and assorted coagulants in turbidity removal of muddy water. Appl. Water Sci. 2018, 8, 40. [Google Scholar] [CrossRef]
- Agbovi, H.K.; Wilson, L.D. Optimisation of orthophosphate and turbidity removal using an amphoteric chitosan-based flocculant-ferric chloride coagulant system. Environ. Chem. 2019, 16, 599–612. [Google Scholar] [CrossRef]
- Lee, H.J.; Halali, M.A.; Baker, T.; Sarathy, S.; de Lannoy, C.F. A comparative study of RO membrane scale inhibitors in wastewater reclamation: Antiscalants versus pH adjustment. Sep. Purif. Technol. 2020, 240, 116549. [Google Scholar] [CrossRef]
- Baptisttella, A.M.S.; Araújo, A.A.D.; Barreto, M.C.; Madeira, V.S.; da Motta Sobrinho, M.A. The use of metal hydroxide sludge (in natura and calcined) for the adsorption of brilliant blue dye in aqueous solution. Environ. Technol. 2019, 40, 3072–3085. [Google Scholar] [CrossRef]
- Daryabeigi, A.; Baghvand, A.; Zand, A.D.; Mehrdadi, N.; Karbassi, A. Optimizing Coagulation Process for Low to High Turbidity Waters Using Aluminum and Iron Salts. Am. J. Environ. Sci. 2010, 6, 442–448. [Google Scholar]
- Ditta, A.; Ullah, K.S.; Farhat, I.; Zehra, U.; Ayoub, H.; Ahtisham, M.; Imtiaz, H.; Tabish, A.N. The Effect of Operational Variables on the Performance of Electrochemical Water Treatment for Drinking Purposes. In Proceedings of the 2021 4th International Conference on Energy Conservation and Efficiency (ICECE), Lahore, Pakistan, 16–17 March 2021. [Google Scholar] [CrossRef]
- Ghasemian, S.; Asadishad, B.; Omanovic, S.; Tufenkji, N. Electrochemical disinfection of bacteria-laden water using antimony-doped tin-tungsten-oxide electrodes. Water Res. 2017, 126, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.J.K.; Aziz, H.A.; Yusoff, M.S.; Adlan, M.N. Application of response surface methodology (RSM) for optimization of ammoniacal nitrogen removal from semi-aerobic landfill leachate using ion exchange resin. Desalination 2010, 254, 154–161. [Google Scholar] [CrossRef]
- GilPavas, E.; Arbeláez, P.; Medina, J.D.; Dobrosz-Gómez, I.; Gómez-García, M.Á. The electrochemical elimination of coliforms from water using BBD/Ti or graphite anodes: A comparative study. Water Sci. Technol. Water Supply 2018, 18, 408–417. [Google Scholar] [CrossRef]
- Gómez-López, V.M.; Gobet, J.; Selma, M.V.; Gil, M.I.; Allende, A. Operating conditions for the electrolytic disinfection of process wash water from the fresh-cut industry contaminated with E. coli o157:H7. Food Control 2013, 29, 42–48. [Google Scholar] [CrossRef]
- Qi, X.; Wang, T.; Long, Y.; Ni, J. Synergetic antibacterial activity of reduced graphene oxide and boron doped diamond anode in three dimensional electrochemical oxidation system. Sci. Rep. 2015, 5, 10388. [Google Scholar] [CrossRef]
- Flox, C.; Cabot, P.L.; Centellas, F.; Garrido, J.A.; Rodríguez, R.M.; Arias, C.; Brillas, E. Electrochemical combustion of herbicide mecoprop in aqueous medium using a flow reactor with a boron-doped diamond anode. Chemosphere 2006, 64, 892–902. [Google Scholar] [CrossRef]
- Thirugnanasambandham, K.; Sivakumar, V.; Maran, J.P. Response surface modelling and optimization of treatment of meat industry wastewater using electrochemical treatment method. J. Taiwan Inst. Chem. Eng. 2015, 46, 160–167. [Google Scholar] [CrossRef]
- WHO. A Global Overview of National Regulations and Standards for Drinking-Water Quality. 2018. Available online: http://apps.who.int/bookorders (accessed on 15 July 2021).






| Coded Factors, x | |||||
|---|---|---|---|---|---|
| −1 | 0 | 1 | |||
| Variables | Units | Minimum | Mean | Maximum | Std. Dev. |
| Current density (i) | mA/cm2 | 0.25 | 1.38 | 2.50 | 0.77 |
| Treatment time (t) | min | 1.00 | 5.50 | 10.0 | 3.09 |
| Interelectrode spacing (d) | cm | 0.50 | 1.50 | 2.50 | 0.69 |
| Run | Independent Variables | Response Disinfection Efficiency (%) | ||||
|---|---|---|---|---|---|---|
| i (mA/cm2) | t (min) | d (cm) | Experimental | Predicted | Residual | |
| 1 | 1.375 | 10 | 0.5 | 84.0 | 79.88 | 4.13 |
| 2 | 1.375 | 1 | 0.5 | 75.0 | 75.63 | −0.63 |
| 3 | 2.5 | 5.5 | 0.5 | 68.0 | 68.38 | −0.38 |
| 4 | 0.25 | 5.5 | 0.5 | 79.0 | 82.13 | −3.13 |
| 5 | 1.375 | 5.5 | 1.5 | 93.0 | 93.50 | −0.5 |
| 6 | 1.375 | 5.5 | 1.5 | 92.0 | 93.50 | −1.50 |
| 7 | 1.375 | 5.5 | 1.5 | 95.0 | 93.50 | 1.5 |
| 8 | 1.375 | 5.5 | 1.5 | 93.5 | 93.50 | 0.00 |
| 9 | 1.375 | 5.5 | 1.5 | 94.0 | 93.50 | 0.50 |
| 10 | 2.5 | 10 | 1.5 | 79.0 | 82.75 | −3.75 |
| 11 | 0.25 | 1 | 1.5 | 76.0 | 72.25 | 3.75 |
| 12 | 0.25 | 10 | 1.5 | 58.0 | 59.00 | −1.0 |
| 13 | 2.5 | 1 | 1.5 | 69.0 | 68.00 | 1 |
| 14 | 1.375 | 1 | 2.5 | 28.0 | 32.13 | −4.13 |
| 15 | 0.25 | 5.5 | 2.5 | 12.0 | 11.63 | 0.38 |
| 16 | 2.5 | 5.5 | 2.5 | 48.0 | 44.88 | 3.13 |
| 17 | 1.375 | 10 | 2.5 | 30.0 | 29.38 | 0.63 |
| Factor | Coefficient Estimate | Standard Error | F-Ratio | p-Value |
|---|---|---|---|---|
| Intercept (β0) | 93.5 | 1.60 | 90.48 | <0.0001 |
| A: Current density (β1) | 4.87 | 1.27 | 14.83 | 0.0063 |
| B: Treatment time (β3) | 0.38 | 1.27 | 0.088 | 0.7757 |
| C: Interelectrode spacing (β2) | −23.5 | 1.27 | 344.58 | <0.0001 |
| AB (β12) | 7 | 1.79 | 15.29 | 0.0058 |
| AC (β13) | 11.75 | 1.79 | 43.07 | 0.0003 |
| BC (β23) | −1.75 | 1.79 | 0.96 | 0.3609 |
| A2 (β11) | −12.75 | 1.75 | 53.39 | 0.0002 |
| B2 (β22) | −10.25 | 1.75 | 34.50 | 0.0006 |
| C2 (β33) | −29 | 1.75 | 276.18 | <0.0001 |
| R2 | 0.991 | -- | -- | -- |
| Adjusted R2 | 0.981 | -- | -- | -- |
| Current Density (mA/cm2) | Interelectrode Spacing (cm) | Treatment Time (min) | Disinfection Efficiency (%) | Desirability |
|---|---|---|---|---|
| 1.52 | 1.13 | 6.35 | 98.08 | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ditta, A.; Tabish, A.N.; Farhat, I.; Razzaq, L.; Fouad, Y.; Miran, S.; Mujtaba, M.A.; Kalam, M.A. The Optimization of Operational Variables of Electrochemical Water Disinfection Using Response Surface Methodology. Sustainability 2023, 15, 4390. https://doi.org/10.3390/su15054390
Ditta A, Tabish AN, Farhat I, Razzaq L, Fouad Y, Miran S, Mujtaba MA, Kalam MA. The Optimization of Operational Variables of Electrochemical Water Disinfection Using Response Surface Methodology. Sustainability. 2023; 15(5):4390. https://doi.org/10.3390/su15054390
Chicago/Turabian StyleDitta, Allah, Asif Nadeem Tabish, Iqra Farhat, Luqman Razzaq, Yasser Fouad, Sajjad Miran, Muhammad Abbas Mujtaba, and Muhammad Abul Kalam. 2023. "The Optimization of Operational Variables of Electrochemical Water Disinfection Using Response Surface Methodology" Sustainability 15, no. 5: 4390. https://doi.org/10.3390/su15054390
APA StyleDitta, A., Tabish, A. N., Farhat, I., Razzaq, L., Fouad, Y., Miran, S., Mujtaba, M. A., & Kalam, M. A. (2023). The Optimization of Operational Variables of Electrochemical Water Disinfection Using Response Surface Methodology. Sustainability, 15(5), 4390. https://doi.org/10.3390/su15054390










