Preparation and Characterization of Geopolymers Based on Metakaolin with the Addition of Organic Phase PVA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods and Characterization
2.2.1. X-ray Fluorescence Analysis (XRF)
2.2.2. X-ray Diffraction (XRD)
2.2.3. Fourier-Transform Infrared Spectroscopy (FTIR)
2.2.4. Ultraviolet-Visible Spectroscopy (UV/VIS) Analysis
2.2.5. Scanning Electron Microscope with Energy Dispersive Spectroscopy (SEM/EDS)
3. Results and Discussion
3.1. Influence of Temperature on Thermodynamic Parameters of Alkaline Activator
3.2. Surface Groups and Chemical Composition of Geopolymer and Hybrid Materials
3.3. Characterization of Geopolymer and Hybrid Materials
3.3.1. XRD Analysis of Hybrid Geopolymers
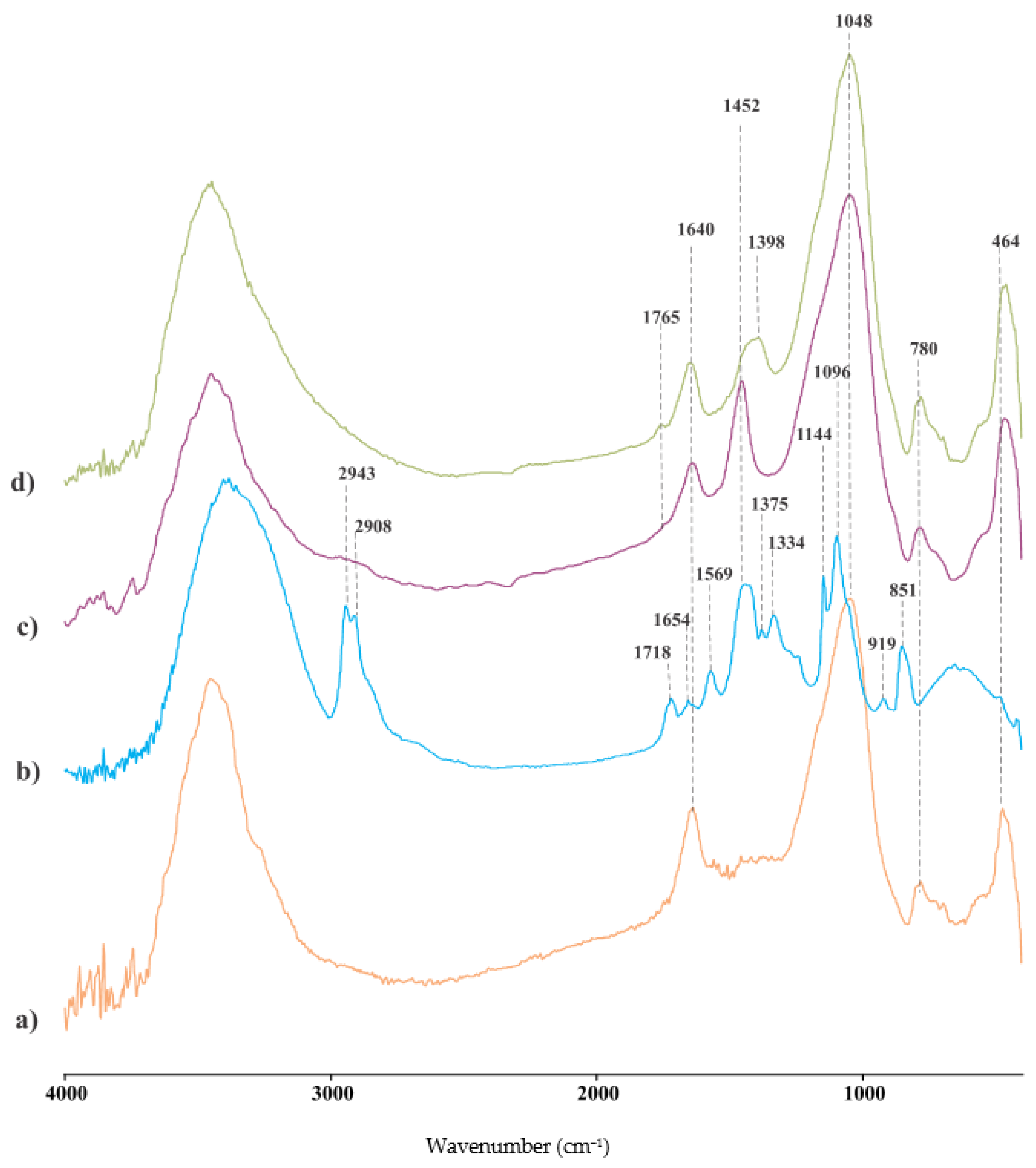
3.3.2. FTIR Analysis of Hybrid Geopolymers
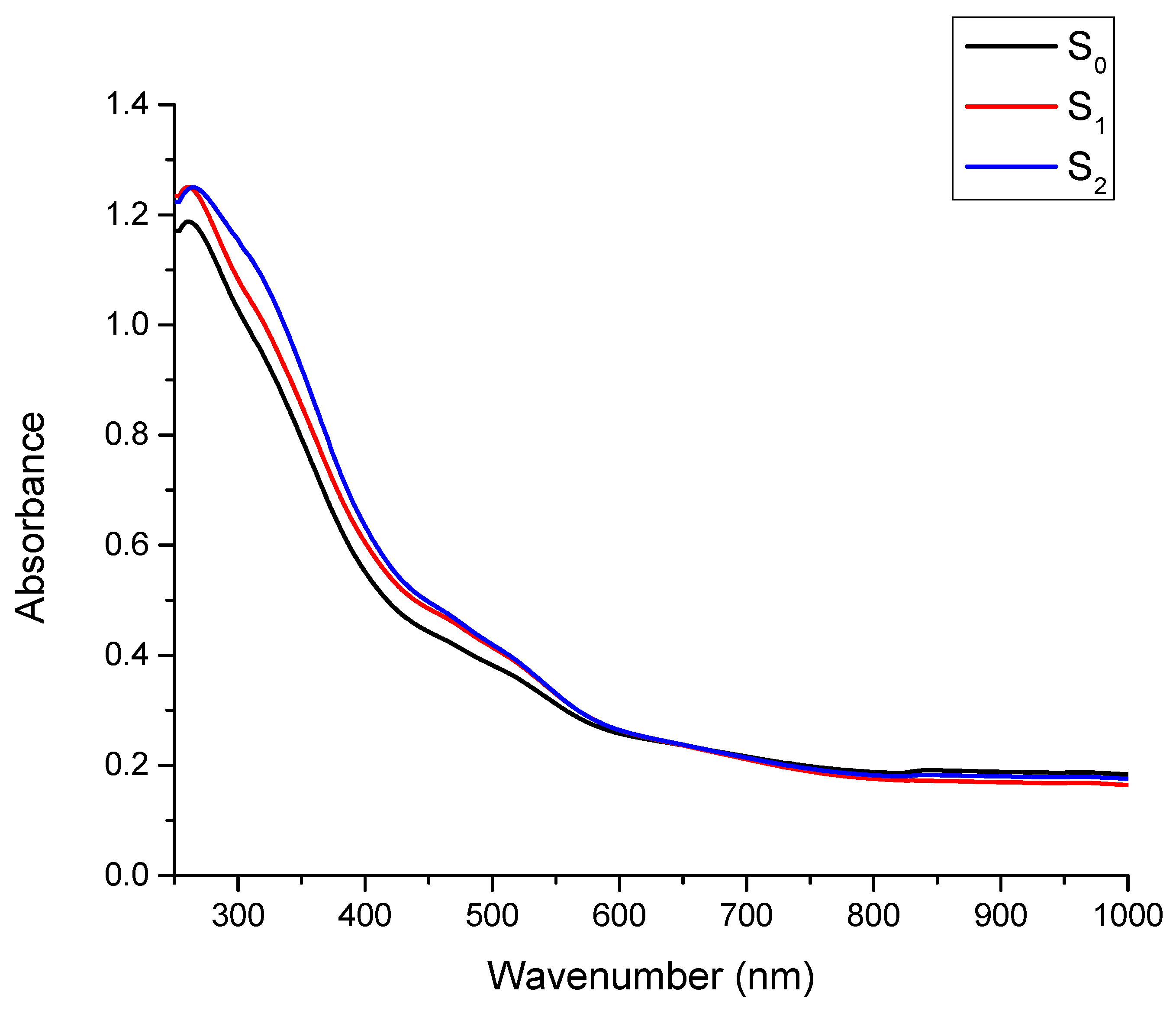
3.3.3. UV/VIS Spectroscopy Analysis
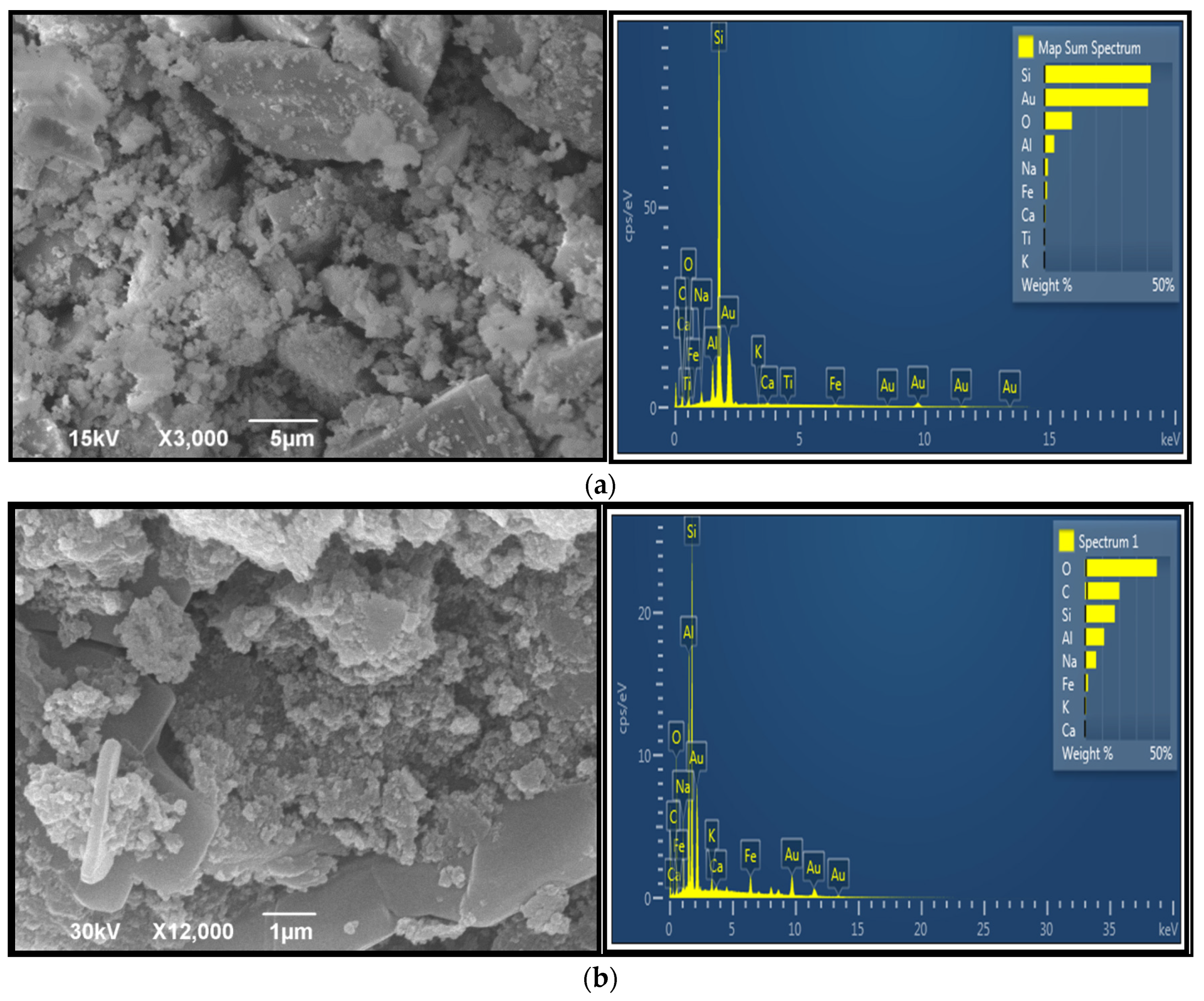
3.3.4. SEM/EDS Analysis of Organic–Inorganic Hybrid Geopolymers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, C.; Fernández Jiménez, A.; Palomo, A. New cements for the 21st century: Thepursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Van Deventer, J.S.J.; Provis, J.L.; Duxson, P. Technical and commercial progress in the adoption of geopolymer cement. Min. Eng. 2012, 29, 89–104. [Google Scholar] [CrossRef]
- Hussain, M.; Varley, R.J.; Cheng, Y.B.; Mathys, Z.; Simon, G.P. Synthesis and thermal behavior of inorganic–organic hybrid geopolymer composites. J. Appl. Polym. Sci. 2005, 96, 112–121. [Google Scholar] [CrossRef]
- Sugama, T.; Brothers, L.E.; Van de Putte, T.R. Acidresistant cements for geothermal wells: Sodium silicate activated slag/fly ash blends. Adv. Cem. Res. 2005, 17, 65–75. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. Compendium of chemical terminology. In The Gold Book, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar]
- De Santis, R.; Catauro, M.; Di Lucy, S.; Manto, L.; Raucci, M.G.; Ambrosio, L.; Nicolais, L. Effects of polymer amount and processing conditions on the in vitro behaviour of hybrid titanium dioxide/polycaprolactone composites. Biomaterials 2007, 28, 2801–2809. [Google Scholar] [CrossRef] [PubMed]
- Kickelbick, G. Introduction to Hybrid Materials. In Hybrid Materials: Synthesis, Characterization, and Applications; Kickelbick, G., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar] [CrossRef] [Green Version]
- Zahid, M.; Shafiq, N.; Nooriza, S.; Razakc, A.; Tufail, R.F. Investigating the effects of NaOH molarity and the geometry of PVA fibers on the post-cracking and the fracture behavior of engineered geopolymer composite. Constr. Build. Mater. 2020, 265, 120295. [Google Scholar] [CrossRef]
- Mohamed, I.M.A.; Yasin, A.S.; Barakat, N.A.M.; Song, S.A.; Lee, H.E.; Kim, S.S. Electrocatalytic behavior of a nanocomposite of Ni/Pd supported by carbonized PVA nanofibers towards formic acid, ethanol and urea oxidation: A physicochemical and electro-analysis study. Appl. Surf. Sci. 2018, 435, 122–129. [Google Scholar] [CrossRef]
- Finch, C.A. Polyvinyl Alcohol—Developments; John Wiley & Sons Ltd.: London, UK, 1992. [Google Scholar]
- Markert, B.; Friese, K. Trace Elements-Their Distribution and Effects in the Environment; Elsevier Science: Amsterdam, The Netherlands, 2000; Volume 4, pp. 187–213. [Google Scholar]
- Maingi, F.M.; Mbuvi, H.M.; Ng’ang’a, M.M.; Mwangi, H. Adsorption of Cadmium Ions on Geopolymers Derived from Ordinary Clay and Rice Husk Ash. Int. J. Mater. Chem. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Onutai, S.; Kobayashi, T.; Thavorniti, P.; Jiemsirilers, S. The dsorption of Cadmium Ions on Fly Ash Based Geopolymer Particles. Key Eng. Mater. 2018, 766, 65–70. [Google Scholar] [CrossRef]
- Mladenović, N.; Kljajević, L.; Nenadović, S.; Ivanović, M.; Čalija, B.; Gulicovski, J.; Trivunac, K. The Applications of New Inorganic Polymer for Adsorption Cadmium from Waste Water. J. Inorg. Organomet. Polym. Mater. 2020, 30, 554–563. [Google Scholar] [CrossRef]
- Marouane, E.A.; Saliha, A.; Mohammed, E.A.; M’hamed, T. Preparation, Characterization, and Application of Metakaolin-Based Geopolymer for Removal of MethyleneBlue from Aqueous Solution. J. Chem. 2019, 2019, 4212901. [Google Scholar] [CrossRef]
- Ivanović, M.; Nenadović, S.; Pavlović, V.P.; Radović, I.; Kijevčanin, M.; Pavlović, V.B.; Kljajević, L. The influence of thermodynamic parameters on alkaline activators of geopolymers and the structure of geopolymers. Maced. J. Chem. Chem. Eng. 2021, 40, 107–117. [Google Scholar] [CrossRef]
- Boehm, H.P. Chemical identification of surface groups. Adv. Catal. 1966, 16, 179–274. [Google Scholar]
- PDXL, version 2.8.3.0, Integrated X-ray Powder Diffraction Software. Rigaku Corporation: Tokyo, Japan, 2011.
- Powder Diffraction File P-D, Announcement of New Data-Base Release 2012; International Centrefor Diffraction Data (ICDD): Newtown Square, PA, USA, 2012.
- Cong, P.; Cheng, Y. Advances in geopolymer materials: A comprehensive review. J. Traffic Transp. Eng. Engl. Ed. 2021, 8, 283–314. [Google Scholar] [CrossRef]
- Ivanović, M.; Kljajević, L.; Gulicovski, J.; Petkovic, M.; Jankovic-Castvan, I.; Bučevac, D.; Nenadović, S. The effect of the concentration of alkaline activator and aging time on the structure of metakaolin based geopolymer. Sci. Sinter. 2020, 52, 219–229. [Google Scholar] [CrossRef]
- Guo, L.; Wu, Y.; Xu, F.; Song, X.; Ye, J.; Duan, P.; Zhang, Z. Sulfate resistance of hybrid fiber reinforced metakaolin geopolymer composites. Compos. Part B Eng. 2020, 183, 107689. [Google Scholar] [CrossRef]
- Badogiannis, E.; Tsivilis, S.; Papadakis, V.; Chaniotakis, E. The effect of metakaolin on concrete properties. In Proceedings of the International Congress: Challenges of Concrete Construction, Scotland, UK, 9–11 September 2002; Dhir, R.K., Hewlett, P.C., Cetenyi, L.J., Eds.; Thomas Telford: Telford, UK, 2002; pp. 81–89. [Google Scholar]
- Ilić, B.U. ticaj termički i mehanohemijski aktivirane kaolinske gline na mehanička svojstva i strukturu cementnih kompozita. Ph.D. Thesis, Fakultet Tehničkih Nauka, Univerzitet u Novom Sadu, Novi Sad, Serbia, 2016. [Google Scholar]
- He, J.; Zhang, J.; Yu, Y.; Zhang, G. The strength and microstructure of two geopolymers derived from metakaolin and red mud-fly ash admixture: A comparative study. Constr. Build. Mater. 2012, 30, 80–91. [Google Scholar] [CrossRef]
- Zhang, P.; Han, X.; Hu, S.; Wang, J.; Wang, T. High-temperature behavior of polyvinyl alcohol fiber-reinforced metakaolin/fly ash-based geopolymer mortar. Compos. Part B Eng. 2022, 244, 110171. [Google Scholar] [CrossRef]
- Kljajević, L.; Nenadović, M.; Ivanović, M.; Bučevac, D.; Mirković, M.; Nikolić, N.M.; Nenadović, S. Heat Treatment of Geopolymer Samples Obtained by Varying Concentration of Sodium Hydroxide as Constituent of Alkali Activator. Gels 2022, 8, 333. [Google Scholar] [CrossRef]
- Lee, S.V.; Halim, N.A.; Arof, A.K.; Abidin, Z.H.Z. Characterisation of poly(vinyl alcohol) coating mixed with anthocyanin dye extracted from roselle flower with different nitrate salt. Pigment Resin Techn. 2013, 42, 146–151. [Google Scholar] [CrossRef]
- Zhang, S.; Gong, K.; Lu, J. Novel modification method for inorganic geopolymer by using water soluble organic. Mater. Lett. 2004, 58, 1292–1296. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogen with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2020, 28, 539–548. [Google Scholar] [CrossRef]
- Delanu, I.M.; Stoica, A.; Stroescu, M.; Dobre, L.M.; Dobre, T.; Jinga, S.; Tardei, C. Potassium sorbate release from poly(vinyl alcohol)–bacterial cellulose films. Chem. Pap. 2012, 66, 138–143. [Google Scholar] [CrossRef]
- Karlsson, C.; Zanghellini, E.; Swenson, J.; Roling, B.; Bowron, D.T.; Börjesson, L. Structure of mixed alkali/alkaline-earth silicate glasses from neutron diffraction and vibrational spectroscopy. Phys. Rev. B 2005, 72, 064206. [Google Scholar] [CrossRef]
- Electronic Supplementary Material (ESI) for RSC Advances. Available online: http://www.rsc.org/suppdata/c5/ra/c5ra25983e/c5ra25983e1.pdf (accessed on 15 August 2022).
- Asran, A.S.; Henning, S.; Michler, G.H. Poly vinylalcohol–collagen–hydroxyapatite biocompositenano fibrous scaffold: Mimicking the key features of natural bone at the nanoscale level. Polymer 2010, 51, 868–876. [Google Scholar] [CrossRef]
- Fan, J.; Li, G.; Deng, S.; Wang, Z. Mechanical Properties and Microstructure of Polyvinyl Alcohol (PVA) Modified Cement Mortar. Appl. Sci. 2019, 9, 2178. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, S.; Guo, Y.; Xu, D.; Gong, Y.; Tang, X. Supercritical water oxidation of polyvinyl alcohol and desizing wastewater: Influence of NaOH on the organic decomposition. J. Environ. Sci. 2013, 25, 1583–1591. [Google Scholar] [CrossRef]
- Xiao, R.; Ma, Y.; Jiang, X.; Zhang, M.; Zhang, Y.; Wang, Y.; Huang, B.; He, Q. Strength, microstructure, efflorescence behavior and environmental impacts of wasteglass geopolymers cured at ambient temperature. J. Clean. Prod. 2020, 252, 119610. [Google Scholar] [CrossRef]
- Hebeish, A.; Abdel-Gawad, I.I.; Basily, I.K.; El-Bazza, S. Degradation of poly(vinyl alcohol) in strongly alkaline solutions of hydrogen peroxide. J. Appl. Polym. Sci. 1995, 30, 2321–2327. [Google Scholar] [CrossRef]
- Kim, J.H.; Robertson, R.E. Effects of Polyvinyl Alcohol on Aggregate-Paste Bond Strength and the Interfacial Transition Zone. Adv. Cem. Based Mater. 1998, 8, 66–76. [Google Scholar] [CrossRef]



| Geopolymer Material | Acidic Groups (mmol g−1) | Basic Groups (mmol g−1) |
|---|---|---|
| S0 | 4.50 | 6.83 |
| S1 | 5.68 | 6.62 |
| S2 | 4.42 | 6.43 |
| Oxides (mas %) | SiO2 | Al2O3 | Fe2O3 | Na2O | TiO2 | CaO | K2O | SO3 | L.O.I. * |
|---|---|---|---|---|---|---|---|---|---|
| MK | 55.03 | 35.44 | 3.89 | 0.89 | 0.62 | 1.38 | 2.07 | 0.68 | 0.44 |
| S0 | 62.23 | 23.83 | 3.15 | 5.90 | 0.90 | 0.91 | 1.35 | 0.80 | 0.93 |
| S1 | 64.23 | 19.39 | 3.05 | 5.69 | 0.99 | 0.94 | 3.01 | 0.94 | 1.76 |
| S2 | 65.03 | 18.26 | 3.09 | 5.75 | 1.01 | 0.93 | 2.96 | 0.96 | 2.01 |
| S0 | S1 | S2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Element | Wt% | Standard Label | Element | Wt% | Standard Label | Element | Wt% | Standard Label |
| C | / | C | 20.12 | C Vit | C | 20.57 | C Vit | |
| O | 10.78 | SiO2 | O | 42.09 | SiO2 | O | 45.39 | SiO2 |
| Na | 1.43 | Albite | Na | 6.53 | Albite | Na | 9.73 | Albite |
| Al | 3.88 | Al2O3 | Al | 11.23 | Al2O3 | Al | 5.83 | Al2O3 |
| Si | 41.50 | SiO2 | Si | 17.42 | SiO2 | Si | 17.00 | SiO2 |
| K | 0.17 | KBr | K | 0.65 | KBr | K | 0.53 | KBr |
| Ca | 0.44 | Wollastonite | Ca | 0.21 | Wollastonite | Ca | / | |
| Ti | 0.23 | Ti | Ti | / | Ti | / | ||
| Fe | 1.11 | Fe | Fe | 1.74 | Fe | Fe | 0.95 | Fe |
| Au | 40.46 | Au | Au | / | Au | / | ||
| Total: | 100.00 | Total: | Total: | 100.00 | Total: | 100.00 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanović, M.; Knežević, S.; Radović, I.; Kljajević, L.; Mirković, M.; Nenadović, M.; Nenadović, S. Preparation and Characterization of Geopolymers Based on Metakaolin with the Addition of Organic Phase PVA. Sustainability 2023, 15, 4441. https://doi.org/10.3390/su15054441
Ivanović M, Knežević S, Radović I, Kljajević L, Mirković M, Nenadović M, Nenadović S. Preparation and Characterization of Geopolymers Based on Metakaolin with the Addition of Organic Phase PVA. Sustainability. 2023; 15(5):4441. https://doi.org/10.3390/su15054441
Chicago/Turabian StyleIvanović, Marija, Sanja Knežević, Ivona Radović, Ljiljana Kljajević, Miljana Mirković, Miloš Nenadović, and Snežana Nenadović. 2023. "Preparation and Characterization of Geopolymers Based on Metakaolin with the Addition of Organic Phase PVA" Sustainability 15, no. 5: 4441. https://doi.org/10.3390/su15054441










