Influence of Peat Soil Environment on Mechanical Properties of Cement-Soil and Its Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- The soil used in the experiment is cohesive soil from the north slope of Chenggong dormitory at the Kunming University of Technology. The soil sample has a low organic matter content and has little impact on the test results. The fundamental physical indicators are shown in Table 1. The test soil is naturally air-dried, ground, passed through a 2.00 mm geotechnical sieve, and boxed for later use. The chemical composition of the test soil and the mass fraction of each composition are tested using the X-ray fluorescence (XRF) test. As shown in Table 2, the main chemical components of the test soil are SiO2, Fe2O3, and Al2O3. The X-ray diffraction (XRD) test is carried out on the soil for testing to determine its phase composition. As shown in Figure 2, the main phases of the soil sample are quartz, kaolinite, mica, goethite, and anatase. The results of the soil composition analysis show that the soil conforms to the general characteristics of cohesive soil, and the organic matter content is low. When studying the influence of peat soil environment on the mechanical properties of cement-soil, using peat soil can minimize the influence of raw materials on the test results.
- 2.
- The cement used is Shilin brand 42.5# ordinary Portland cement produced by Yunnan Huaxin Cement Co., Ltd. The cement company is located in Kunming, China.
- 3.
- The humic acid produced by Tianjin Guangfu Chemical Reagent Factory is selected, which is sealed and stored through a 2.00 mm geotechnical sieve. The chemical reagent plant is located in Tianjin, China.
- 4.
- Fulvic acid (FA) is selected from the biochemical FA reagent produced by Pingxiang Red Land Humic Group Co., Ltd. The humic group company is located in Pingxiang City, China.
- 5.
- The test water is deionized water produced by Hugke Water treatment Equipment (Xi’ bei) Co., Ltd. The deionized water production company is located in Shenzhen, China.
2.2. Experimental Design and Sample Preparation
2.3. Experimental Procedure
- UCS test
- 2.
- Acid consumption and ion leaching test
- 3.
- SEM and XRD
3. Results and Analysis
3.1. Influence of Peat Soil Environment on Cement-Soil Strength
3.2. Test Results and Analysis of Ion Leaching and Acid Consumption
3.3. SEM and XRD Results and Analysis
3.3.1. SEM Results and Analysis
3.3.2. SEM Results and Analysis
4. Conclusions
- HA can significantly reduce the UCS of cement-soil. FA reduced the UCS of cement-soil containing less than 18% HA. However, when the content of HA is more than 18%, FA can weaken the negative influence of HA on the UCS of cement-soil, and make the UCS slightly increase. Under FA and HA, cement-soil samples’ deformation and failure type gradually changed from brittle shear failure to plastic shear failure.
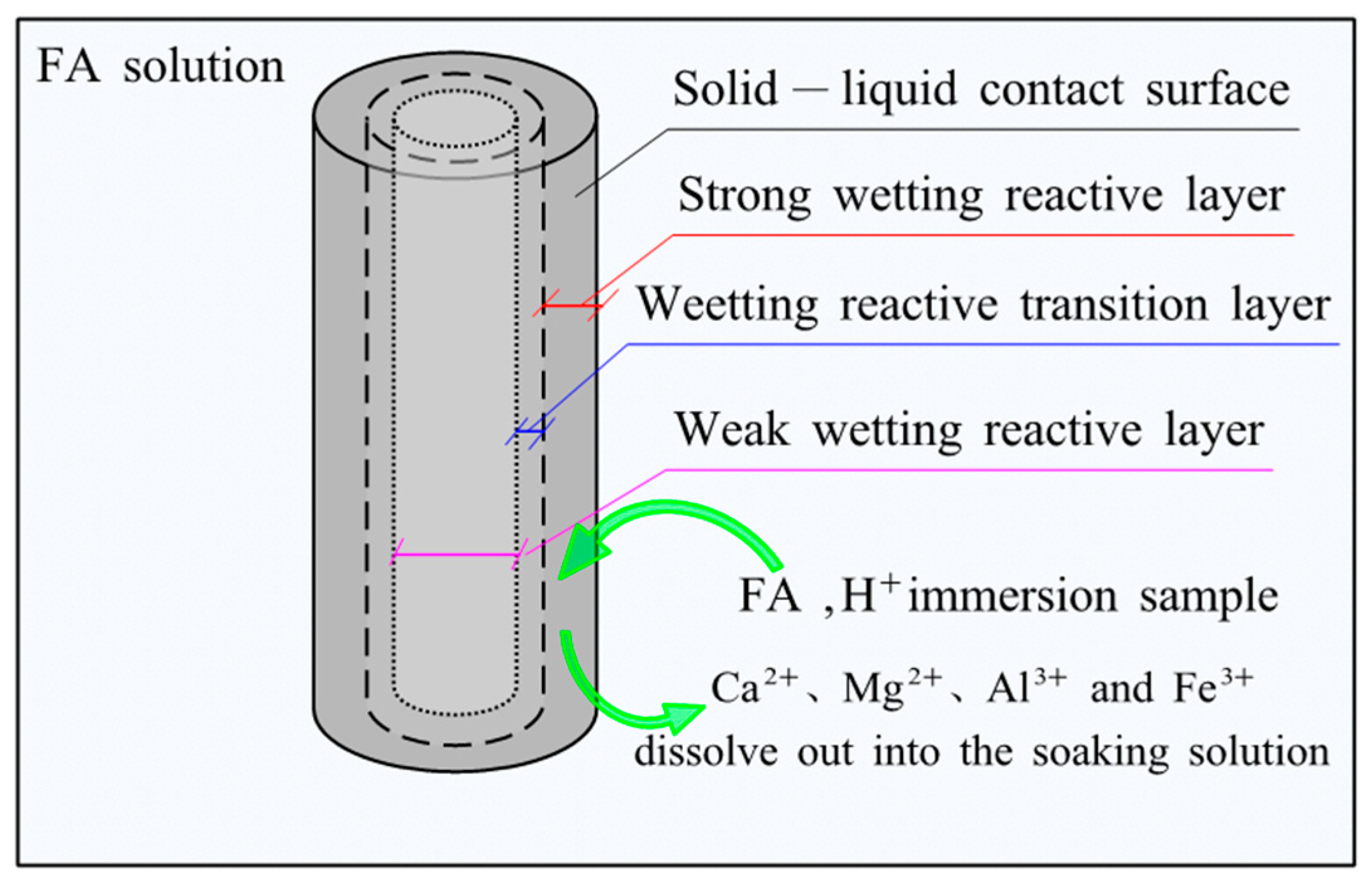
- FA reacted with cement hydration products, which made Ca2+, Mg2+, Al3+, and Fe3+ in the cement-soil gradually dissolve into the soaking solution, increasing the content of four ions. The accumulative acid consumption of cement-soil increased continuously, but the increasing rate gradually decreased with the soaking time, indicating that the infiltration rate of FA in cement-soil slowed down. In practical engineering, designers should take certain anti-corrosion measures. For example, the use of admixtures to cement-soil becomes dense. In addition, an anticorrosive coating can be applied to the outer surface to weaken the infiltration effect of FA on cement-soil.
- The SEM images indicated that HA increased the macropores of cement-soil and enhanced the connectivity. There were no prominent fibrous and flocculent hydration products in the SWRL of cement-soil, but massive amorphous hydration products can be observed, and hexagonal massive hydration products can be observed in the WWRL. Part of the pores of the two wetting reaction layers is filled with FA with colloidal properties. XRD showed the existence of CSH and Ca(OH)2 hydration products in cement-soil. However, FA and HA reacted with hydration products, decreasing diffraction peak strength.
- The PSE has the twofold effect of infiltration enhancement and infiltration weakening on the UCS of cement-soil simultaneously. Fibrous and flocculent hydration products cannot be observed in cement-soil treated with FA and HA, but massive hydration products can still be observed. The structure of HA particles has a particularity and is easily strengthened by FA. In addition, FA has a filling and cementing effect on the interlayer of cohesive particles. The structure of cement-soil with HA content greater than 18% is enhanced, and its UCS is relatively improved. When HA content is less than 18%, there are more small pores in the cement-soil. The interaction between FA and HA is weak. Therefore, the effect of FA on the UCS of cement-soil is mainly a weakening effect, which leads to a relative decrease in its UCS.
- The increase in cement mixing ratio can weaken the influence of PSE on the UCS of cement-soil, and the effect is very significant at 20%. Fortunately, the cement mixing ratio in this study is not high for the peat soil foundation. Therefore, the pH value and HA content of peat soil can be measured in engineering practice. According to the test results of this paper, following the concept of economic saving and environmental friendliness, other curing agents are reasonably selected to be used in combination with cement, and the amount of cement is appropriately reduced. These measures can better realize the development and sustainable development of the peat soil foundation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gui, Y.; Fu, J.; Wu, C.K.; Cao, J.; Gao, Y.F. Hydraulic conductivity of lacustrine peaty soil in plateau areas and its mechanism analysis. Rock Soil Mech. 2016, 37, 3197–3207. [Google Scholar]
- Wang, D.W.; Wang, Q.; Chen, J.P. Research on the distribution regularity and the engineering geological property of peat soil in Dianchi basin. In Proceedings of the The 2nd National Geotechnical and Engineering Academic Conference; Science Press: Beijing, China, 2006; pp. 831–835. [Google Scholar]
- Ye, C. Experimental Study on Permeability Characteristics of Peat Soil in Yunnan Province. Master’s Thesis, Beijing Jiaotong University, Beijing, China, 2020. [Google Scholar]
- Gong, X.N. Ground Treatment Handbook, 3rd ed.; China Architecture & Building Press: Beijing, China, 2008. [Google Scholar]
- Huang, X.; Zhou, G.J. Hardening mechanism of cement-stabilized soil. Chin. J. Geotech. Eng. 1994, 16, 62–68. [Google Scholar]
- Sprengel, C. About plant humus, humic acid and humic acid salts. Arch. Fur Die Gesammte Nat. 1826, 8, 145–220. [Google Scholar]
- Schmeide, K.; Sachs, S.; Bubner, M.; Reich, T.; Heise, K.H.; Bernhard, G. Interaction of uranium(VI) with various modified and unmodified natural and synthetic humic substances studied by EXAFS and FTIR spectroscopy. Inorg. Chim. Acta 2003, 351, 133–140. [Google Scholar] [CrossRef]
- Xiongtian, G.Y. Soil Organic Matter Chemistry; Science Press: Beijing, China, 1984. [Google Scholar]
- Gong, G.Q.; Xu, L.W.; Zhang, Y.J.; Liu, W.X.; Wang, M.; Zhao, Y.F.; Yuan, X.; Li, Y.J. Extraction of fulvic acid from lignite and characterization of its functional groups. ACS Omega 2020, 5, 27953–27961. [Google Scholar] [CrossRef]
- Valls, S.; Vazquez, E. Stabilisation and solidification of sewage sludges with portland cement. Cem. Concr. Res. 2000, 30, 1671–1678. [Google Scholar] [CrossRef]
- Kamon, M.; Tomohisa, S.; Sawa, K. On stabilization of hedoro by using cement group hardening materials. J. Soc. Mater. Sci. 1989, 38, 1092–1097. [Google Scholar] [CrossRef]
- Xun, Y. The anti-influences and influences of containing organic matter on cement soil strength. Sichuan Build. Sci. 2000, 26, 58–60. [Google Scholar]
- Tremblay, H.; Duchesne, J.; Locat, J.; Leroueil, S. Influence of the nature of organic compounds on fine soil stabilization with cement. Can. Geotech. 2002, 39, 535–546. [Google Scholar] [CrossRef]
- Zeng, W.D.; Tang, X.Y.; He, M.Z. Application of cement deep-mixing method in treatment of peat soil. J. Geol. Hazards Environ. Preserv. 2002, 13, 67–69,79. [Google Scholar]
- Zhu, W.; Chiu, C.F.; Zhang, C.L.; Zeng, K.L. Effect of humic acid on the behaviour of solidified dredged material. Can. Geotech. J. 2009, 46, 1093–1099. [Google Scholar] [CrossRef]
- Zhang, S.B.; Wang, Q.; Chen, J.P.; Fan, J.H. Tests for effect of soil humid acid components on strengte of cemented soft soils. J. Eng. Geol. 2009, 17, 842–846. [Google Scholar]
- Kang, G.O.; Tsuchida, T.; Kim, Y.S.; Beak, W. Influence of humic acid on the strength behavior of cement-treated clay during various curing stages. J. Mater. Civil. Eng. 2017, 29, 04017057. [Google Scholar] [CrossRef]
- Qi, T.; Li, S.J.; Zhang, J.R.; Feng, D.L. study on strength characteristics of cement-soil containing humus. Guangzhou Archit. 2019, 47, 21–24. [Google Scholar]
- Cao, J.; Hu, Y.C.; Liu, H.M. Experimental Study on Corrosivity of CFG Pile Material in Fulvic Acid. Bull. Chin. Silic. Soc. 2019, 38, 3990–3998. [Google Scholar]
- Cao, J.; Li, S.P.; Liu, H.M.; Kong, C. On the strength of cement soil affected and effected by the humic acid and its mechanism. J. Saf. Environ. 2021, 21, 2571–2576. [Google Scholar]
- Cao, J.; Huang, S.Y.; Liu, W.L.; Gao, Y.; Song, Y.F.; Li, S.P. Study on Strength Development and Microstructure of Cement-Solidified Peat Soil Containing Humic Acid of Dianchi Lake. Adv. Mater. Sci. Eng. 2022, 2022, 8136852. [Google Scholar] [CrossRef]
- Wang, X.; Cui, J.; Wu, Y.; Zhu, C.Q.; Wang, X.Z. Mechanical properties of calcareous silts in a hydraulic fill island-reef. Mar. Georesour. Geotechnol. 2021, 39, 1–14. [Google Scholar] [CrossRef]
- Shen, J.H.; Hu, M.J.; Wang, X.; Zhang, C.Y.; Xu, D.S. SWCC of calcareous silty sand under different fines contents and dry densities. Front. Environ. Sci. 2021, 9, 682907. [Google Scholar] [CrossRef]
- Cao, J.; Huang, S.Y.; Liu, W.L.; Kong, C.; Gao, Y.; Liu, F.Y. Study on Simulation Test of Peat Soil Environment in Dianchi Lake. Adv. Civ. Eng. 2022, 2022, 1437733. [Google Scholar] [CrossRef]
- Shao, Y.F. Study on Humus-Containing Soil Stabilization. Ph.D. Thesis, Zhejiang University, Zhejiang, China, 2006. [Google Scholar]
- GB/T 50123-2019; Standard for Geotechnical Testing Method. Ministry of Water Resources of the People’s Republic of China. China Planning Press: Beijing, China, 2019.
- Huang, J.W.; Li, Z. X-ray Diffraction of Polycrystalline Materials: Experimental Principles, Methods and Applications; Metallurgical Industry Press: Beijing, China, 2012. [Google Scholar]
- Dudas, M.J.; Pawluk, S. Naturally occurring organo-clay complexes of orthic black chernozems. Geoderma 1969, 3, 5–17. [Google Scholar] [CrossRef]
- Liao, P.F.; Wu, P.X.; Wu, W.M.; Chen, Q.Q.; Xu, Y.F. Study of the sorption of humic acids on the clays. Bull. Mineral. Petrol. Geochem. 2009, 28, 272. [Google Scholar]
- Lea, F.M. Chemistry of Cement and Concrete; Chemical publisher: New York, NY, USA, 1971; pp. 177–249. [Google Scholar]
- Mounanga, P.; Khelidj, A.; Loukili, A.; Baroghel-Bouny, V. Predicting Ca(OH)₂ content and chemical shrinkage of hydrating cement pastes using analytical approach. Cem. Concr. Res. 2004, 34, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.L.; Kuo, L.J.; Wang, H.L.; Hsieh, P.C. Effects of ionic strength on the binding of phenanthrene and pyrene to humic substances: Three-stage variation model. Water Res. 2003, 37, 4250–4258. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Feng, M.M.; Mao, X.B.; Xu, J.M.; Zhang, W.L.; Ni, X.Y.; Han, G.S. Particle size distribution of aggregate effects on mechanical and structural properties of cemented rockfill: Experiments and modeling. Constr. Build. Mater. 2018, 193, 295–311. [Google Scholar] [CrossRef]
- Wu, J.Y.; Jing, H.W.; Gao, Y.; Meng, Q.B.; Yin, Q.; Du, Y. Effects of carbon nanotube dosage and aggregate size distribution on mechanical property and microstructure of cemented rockfill. Cement. Concrete. Comp. 2022, 127, 104408. [Google Scholar] [CrossRef]
- Tombácz, E. Colloidal properties of humic acids and spontaneous changes of their colloidal state under variable solution conditions. Soil Sci. 1999, 164, 814–824. [Google Scholar] [CrossRef]
- Jones, M.N.; Bryan, N.D. Colloidal properties of humic substances. Adv. Colloid Interface 1998, 78, 1–48. [Google Scholar] [CrossRef]
- Xu, D.P.; Wu, Y.; Miao, D.; Li, X.B.; Li, H.L. Migration characteristics of fulvic acid and its influence on heavy metal migration in porous media. Appl. Chem. Ind. 2017, 46, 1700–1704. [Google Scholar]
- Gong, X.N. Soil Mechanics; China Architecture & Building Press: Beijing, China, 2002. [Google Scholar]










| Test Soil | Natural Water Content (%) | Liquid Limit wL (%) | Plastic Limit wP (%) | Natural Density (g·cm−3) | Grain Specific Gravity Gs |
|---|---|---|---|---|---|
| Cohesive soil | 18.60 | 39.20 | 23.00 | 1.96 | 2.73 |
| Test Soil | The Chemical Composition and Its Mass Fraction (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Fe2O3 | Al2O3 | TiO2 | K2O | MgO | CaO | Na2O | MnO | P2O5 | LOI | |
| Cohesive soil | 46.57 | 21.22 | 20.80 | 8.90 | 0.48 | 0.48 | 0.16 | 0.04 | 0.14 | 0.57 | 0.64 |
| No. | Test Items | Cement Mixing Ratio (β)/% | Soaking Solution | HA Content (λ)/% | Soaking Time/d |
|---|---|---|---|---|---|
| 1 | UCS test | 15, 0, 25 | FA solution (pH = 6), Deionized water | 0, 15, 20, 25, 30 | 90 |
| 2 | Acid consumption test | 20 | FA solution (pH = 6) | 0, 15, 20, 25, 30 | |
| 3 | Ion leaching test | 20 | FA solution (pH = 6), Deionized water | 20 | |
| 4 | SEM, XRD | 20 | FA solution (pH = 6), Deionized water | 20 | |
| FA solution (pH = 6) | 0, 20, 30 |
| Type of Soaking Solution | Mass of Each Material Before Soaking/(g) | Total Dry Matter Mass of the Sample Before Soaking/(g) | Quality of Water Required for Complete Curing of Cement/(g) | The Total Mass of the Drying Sample After Soaking/(g) | Increase in Sample Dry Substances/(g) | ||
|---|---|---|---|---|---|---|---|
| Cohesive Soil | HA | Cement | |||||
| Deionized water | 83.07 | 20.82 | 20.82 | 124.71 | 5.00 | 125.03 | −4.68 |
| FA solution (pH = 6) | 145.14 | 15.43 | |||||
| Type of Soaking Solution | Ion Content/(mg·L−1) | |||
|---|---|---|---|---|
| Ca2+ | Mg2+ | Al3+ | Fe3+ | |
| FA solution (pH = 6) | 232.00 | 75.30 | 296.00 | 20.20 |
| Deionized water | 41.30 | 1.30 | 3.36 | 0.035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Cao, J.; Ding, W.; Song, Z.; Liu, H.; Huang, S.; Zhu, W. Influence of Peat Soil Environment on Mechanical Properties of Cement-Soil and Its Mechanism. Sustainability 2023, 15, 4580. https://doi.org/10.3390/su15054580
Song Y, Cao J, Ding W, Song Z, Liu H, Huang S, Zhu W. Influence of Peat Soil Environment on Mechanical Properties of Cement-Soil and Its Mechanism. Sustainability. 2023; 15(5):4580. https://doi.org/10.3390/su15054580
Chicago/Turabian StyleSong, Yunfei, Jing Cao, Wenyun Ding, Zhigang Song, Hong Liu, Siyang Huang, and Weiming Zhu. 2023. "Influence of Peat Soil Environment on Mechanical Properties of Cement-Soil and Its Mechanism" Sustainability 15, no. 5: 4580. https://doi.org/10.3390/su15054580






