1. Introduction
Land use and/or the modification of the natural landscape by humans has resulted in a significant transformation of the terrain. These changes can contribute to disrupting the balance of ecosystems including the soil environment, leading to its acidification and nutrient depletion or leaching, i.e., loss of soil organic matter (SOM) [
1].
Mining is an economic sector that exerts particularly high pressure on the environment. Negative externalities caused by the mining industry include the removal of native vegetation, loss of biodiversity, changes in water relations, soil and water pollution, generating large amounts of waste, and occupation and degradation of large areas of soil [
2]. Of particular note is the devastation of soils, which are the supporting matrix of terrestrial ecosystems [
3].
Today, growing concerns about the environmental impact of mining highlight the importance of reclamation in the study of mined soils [
4]. Furthermore, land reclamation strategy is a key aspect of degraded area management [
5]. The economic use of degraded areas requires the restoration of healthy soils on them [
6,
7,
8]. The main objective of rehabilitating post-mining landscapes is to reconstruct the soil, increase the soil nutrient and organic matter content, and restore the biological activity of the topsoil, which is necessary for the development of soil-forming processes [
9].
A common environmental practice for the remediation of degraded soils is the use of organic waste [
10,
11,
12] and fertilizers produced from these wastes [
13,
14,
15,
16,
17].
A form of waste that can be used for this purpose is municipal sewage sludge, the use of which for agricultural, non-agricultural, and soil remediation purposes is contingent on the identification of its physical, chemical, and microbiological properties [
18,
19].
Sewage sludge contains nutrients and trace elements necessary for plant growth [
20] and organic matter that can act as a soil conditioner [
21].
However, sewage sludge cannot be applied in a way that is harmful to the soil and, consequently, to the environment [
22,
23]. In its raw state, sewage sludge contains high concentrations of readily decomposable organic matter, heavy metals [
24], pharmaceuticals [
25], microplastic contamination [
26] and pathogens, therefore, their direct disposal or introduction into the soil can cause serious risks such as groundwater and soil contamination, as well as odors [
27].
In order to reduce the negative impact of sewage sludge on the environment, stabilization is carried out by methane fermentation [
28] and adding calcium carbonate or waste with high calcium content, such as fly ash from power plants [
29,
30]. One factor limiting the use of sewage sludge for fertilization and soil remediation is the potential threat from pathogenic microorganisms, which is why sewage sludge must be subjected to hygienizing processes. One effective way to hygienize sewage sludge is to compost it [
31,
32]. Composting leads to a safe and stable bioproduct that can be used as an organic fertilizer and the high temperatures achieved during this process eliminate pathogens [
33].
The quality of sludge compost depends on the properties of the materials used and the conditions under which the composting process takes place. Due to the high moisture content, sludge for composting should be mixed with dry materials. The materials used as fillers (BA) during the composting of sewage treatment sludge include organic fractions of municipal solid waste [
34,
35], sawdust, wood chips, and other agricultural bio-waste and mineral materials such as zeolites [
36], bentonite [
37], pumice [
38], and fly ash [
39].
Fly ash resulting from the combustion of coal in thermal power plants is considered a problematic solid waste [
40] needing disposal. Numerous studies indicate the wide potential of fly ash in enhancing soil productivity and remediation of degraded land [
41,
42,
43,
44,
45], and the fertilizer potential of fly ash has been reported in the literature on acid mine spoil reclamation [
46,
47]. Fly ash is a source of almost all macro- and micro-nutrients [
48] and its addition increases the content of essential plant nutrients in the soil [
49].
Studies also indicate that fly ash can be used to optimize the composting process of organic wastes [
50,
51], including sewage sludge, and improve the fertilizing properties of the resulting composts [
52,
53,
54,
55]. The addition of fly ash reduces the content of soluble P in compost, which reduces the risk of eutrophication after the application of SS in the soil [
53]. Moreover, FA can increase the nutrient content of nutrient-poor composts.
In many countries, legal regulations impose an obligation to recultivate post-mining areas [
56]. However, there are no regulations regarding the assessment of the effectiveness of the performed reclamation procedures.
Indicators based on the physical, chemical, and biological properties of soils are used to assess the effects of fertilization and reclamation. Although soil biological properties are considered more difficult to measure, predict, and quantify, they are potentially more sensitive to changes in the soil environment [
57,
58].
Soil enzymes are natural mediators and act as catalysts in many important soil processes such as the decomposition of organic matter released into the soil during plant vegetation, the reactions of soil humus formation and decomposition, the release and availability of mineral substances to plants, the fixation of molecular nitrogen, and the movement of carbon, nitrogen, and other basic elements of the biochemical cycle [
59]. Soil enzymes respond to changes in soil management more rapidly than other variables and can, therefore, be useful as early indicators of biological change [
60].
Based on enzyme activity, the biochemical potential of the soil can be estimated, and thus the capacity of the soil to carry out a range of processes important for ecosystem functioning and resilience can be measured [
61]. Therefore, enzyme activity tests have been proposed as an integrated measure of soil quality [
62,
63]. Numerous authors have indicated that enzyme activity reflects soil productivity and is considered a sensitive indicator of soil fertility and the quality of reclaimed land [
59,
64,
65]. The usefulness of enzymatic activity for assessing the remediation effects of degraded soils was confirmed by Araujo et al. [
66], Zhang et al. [
67], Kabiri et al. [
68], and Russel et al. [
69].
Studies on the effect of sewage sludge compost on the properties of arable and reclaimed soils are found in the literature, but there are few studies on sludge-ash composts. In addition, the studies most often cover a period of 2–3 years. This study evaluated the impact of two composts (from 100% sewage sludge and 70% sewage sludge + 30% fly ash), 12, 13, and 14 years after their introduction to degraded post-mining soil, on the effectiveness of reclamation.
The aim of this study was to evaluate the subsequent effects of sewage sludge composts and sewage sludge with fly ash composts on the enzymatic activity of reclaimed post-mining soil. Differences in dehydrogenase, phosphatase, and urease activities were assessed 12, 13, and 14 years after reclamation.
4. Discussion
The research was carried out on soils degraded by borehole sulphur mining at the Jeziórko Mine. As a result of the mining activities, these soils were strongly acidified [
89]. They are also characterized by low organic matter and nutrient content, and are often devoid of plant cover, resulting in a reduced or total lack of biological activity [
16].
The reclamation of soils devastated by mining activities aims to restore biologically active soil capable of performing ecosystem services. As indicated by studies presented by Baran et al. [
90] and Joniec et al. [
15,
16], wastes such as sewage sludge, tailings lime, and rockwool have a positive effect on a number of properties of soils degraded by the sulphur mining industry. The significant impact of sewage sludge on biological life in the soil environment is related to the strong positive effects of this type of waste on organic matter, nutrient content, soil porosity, bulk density, aggregate structure, and water-holding capacity [
21]. Soil quality parameters indicate that the application of sewage sludge results in significant improvements in organic matter and nutrient content of modified technosols, as reported in various works [
47,
91,
92].
Another waste with significant fertilizer potential is fly ash. Options for the use of coal fly ash include the reclamation of wasteland to increase nutrient content and the revitalization of degraded land [
93].
Studies indicate that ash and municipal sewage sludge, due to their high nutrient content, can be used jointly as fertilizers in biomass production or the biological restoration of degraded land [
45,
94,
95].
The introduction of sewage sludge or ash into the soil carries a risk of environmental pollution [
27,
96]. As studies show, this risk can be reduced by composting the waste [
36]. Samars et al. [
97], based on physicochemical parameters, indicated that the addition of FA to SS could be considered as an alternative sludge-stabilizing agent; hence, in the present study, compost from sewage sludge and sewage sludge with ash was used to rehabilitate a devastated post-mining soil.
The effect of land reclamation in mining areas can be assessed by open (vegetation, biodiversity) and invisible (soil properties, microorganisms) methods [
98]. In our study, the evaluation was based on a determination of the activity of selected enzymes (Adh, Apf, and AU) 12, 13, and 14 years after the reclamation process.
Enzyme activity reflects the advancement of soil-forming processes [
64], illustrates the trends of pedogenic processes in post-mining soils, and enables the assessment of the effectiveness of reclamation treatments being applied [
62]. The literature shows that enzymatic activity has been repeatedly used to study the state of soil environments affected by different degrees of anthropopression [
16,
99,
100,
101,
102].
Enzyme activity is correlated with soil organic matter content, as the latter plays a key role as a precursor for enzyme synthesis (increases soil microbial biomass, which is the source of enzymes) and in the physical stabilization of enzymes [
103].
The results showed that the composts applied to fertilize the reclaimed soil significantly increased the TOC content, compared to that in the CS. On the first study date, SSC-fertilized soil had a significantly higher TOC content than SSFAC. At the end of the study, the TOC content was significantly higher in soil treated with SSFAC. The results obtained confirm the thesis known from the literature that, in soil reclamation, the introduction of external organic matter and its quality are important for organic matter accumulation processes [
104]. With a dose of 180 Mg·ha
−1 SSC, more TOC was introduced into the soil than with SSFAC. The higher TOC content found 14 years after reclamation in the S + SSFAC variant indicates that the organic matter of this compost was more stable, which is confirmed in studies by other authors, indicating the stabilizing effect of fly ash on the organic matter of sewage sludge and other organic wastes [
97].
In the soil fertilized with the composts, the content of TN was significantly higher than in CS on all study dates. The observed changes in the TN content under the influence of the applied composts are consistent with the research results [
105].
The results showed significant differences in dehydrogenase, phosphatase, and urease activities between the evaluated variants as well as the test dates.
Dehydrogenases are considered to be indicators of total microbial activity in the soil, as they are found exclusively in living cells where they catalyse oxidoreductive processes [
106]. Adh indicates the presence of physiologically active microorganisms [
107]. Adh is strongly associated with carbon cycles and soil organic matter (SOM) [
107], and is also associated with the activity of other soil enzymes, e.g., catalase and β-glucosidase, and the presence of nitrogen. Adh plays an important role in the biological oxidation processes in soil [
93]. Soil dehydrogenases have been widely studied and, in relation to other soil parameters, have been found to be a reliable, sensitive, and useful indicator of changes in soil quality [
108].
The results showed that on the first test date, Adh in CS-, SSC-, and SSFAC- amended soil dremained similar and was approximately 0.25 cm
3 H
2∙kg
−1∙d
−1. On subsequent test dates, Adh significantly increased in compost-amended soil, with a significantly higher Adh in soil treated with SSC, compared to the soil of the S + SSFAC object. Adh is strongly associated with carbon cycling and soil organic matter (SOM) [
94], which, with respect to the results obtained in our study, was confirmed by PCA analysis.
The observed changes in Adh under the influence of applied composts are confirmed by studies evaluating Adh under the influence of sewage sludge composts applied to arable soils [
109,
110].
Phosphatases are active soil enzymes that are sensitive to changes in the soil environment and, as indicated by Krämer et al. [
111], phosphatase activity can be a good indicator of organic phosphorus mineralization potential and soil biological activity. The results showed that fertilization of the post-mining soil with composts (SSC and SSFAC) increased the phosphatase activity. The Aph of the soil of the control object took the lowest values in each term and ranged from 19.34 to 25.37 mmol PNP·kg
−1·h
−1 in the first and last terms of the study, respectively. Compared to the CS, fertilization of the reclaimed soil with composts (S + SSC and S + SSFAC) significantly increased Aph. The soil of the S + SSFAC variant was characterized by a significantly higher Aph within the soil fertilized with the composts.
Phosphatases play an important role in the conversion of organic P into inorganic forms that are available to plants [
112]. Global studies have shown that soil TN content is a good predictor of phosphatase activity. A high TN content supports high phosphatase activity, as phosphatase synthesis requires a high amount of N [
113,
114,
115]. The results obtained are consistent with this view. The PCA analysis showed a significant positive correlation between Aph and TN content.
The direction of changes in Aph on successive dates was influenced by the type of compost used. The soil of the S + SSC variant showed a significant decrease in Aph in terms II and III and an increase in Term IV. The soil of the S + SSFAC variant showed a significant increase in Aph in Term II, a decrease in the next term, and then an increase in Term IV. Similar sweeps of Aph were recorded by Kaur et al. [
110] when the soil was incubated with the addition of sewage sludge and compost. The increase in Aph in the soil evaluated with the composts in Term I was probably partly due to the direct contribution of phosphatase activity from the composts and the stimulation of phosphatase production by the microflora [
116]. The observed reduction in Aph may have been due to the fact that quantities of C and N were introduced into the soil along with the composts, which increased microbial activity and thus P demand and phosphatase release. With time, the soil C content decreases, resulting in lower microbial activity, a lower P demand, and thus lower phosphatase activity [
117].
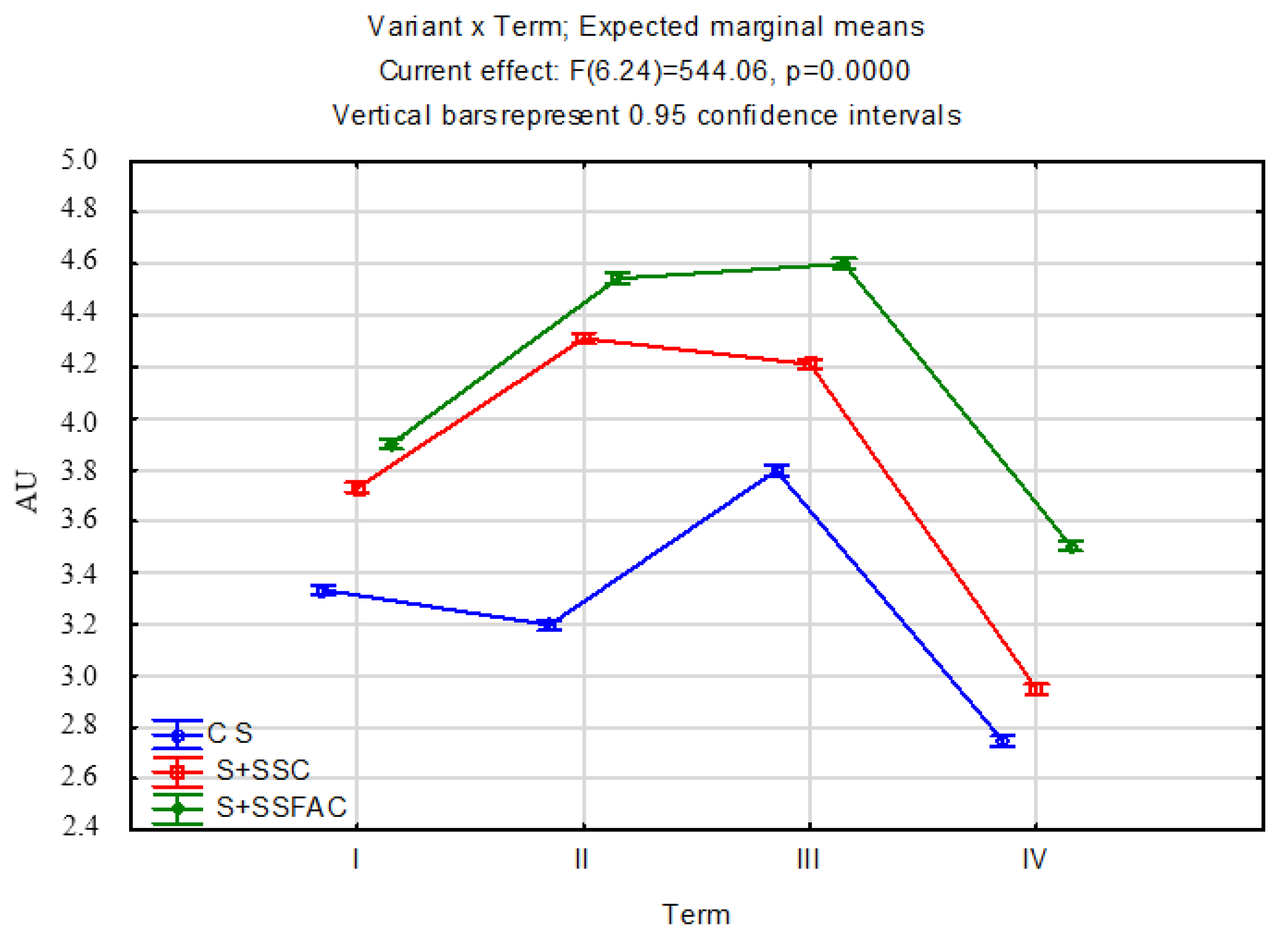
Of the many soil enzymes, urease (urea amidohydrolase, EC 3.5.1.5), closely related to metabolism, biological cycling, and the bioavailability of nitrogen [
87,
118], is a key enzyme. The results showed that the composts had a significant effect on increasing AU in the remediated soil. Within the composts evaluated, the soil of the S + SSFAC site had higher AU than the soil of the S + SSC variant. The PCA analysis showed that AU was significantly positively correlated with TN and TOC content, which is consistent with the results of Vaheda et al. [
119]. Urease is an extracellular enzyme that is made stable by forming complexes with organic and mineral colloids [
120,
121]. High soil UA is believed to be a direct indicator of improved soil fertility, helping to increase nitrogen uptake by plants [
87,
122].
5. Conclusions
The obtained results showed that sewage sludge composts and sewage sludge with the addition of fly ash have a beneficial subsequent effect on the enzymatic activity of the reclaimed post-mining soil.
Compared to the control soil, fertilization with compost significantly increased the activity of the assessed enzymes. The extent of increasing enzymatic activity in soils formed on reclaimed land was significantly dependent on the composition of the applied compost. Within the assessed composts, significantly higher dehydrogenase activity was found in the soil to which compost made from sewage sludge was applied, whereas the soil on which sewage sludge and fly ash compost was applied was characterized by higher phosphatase and urease activity.
All the analysed enzymes, i.e., dehydrogenases, phosphatase, and urease, were sensitive indicators of long-term changes caused by the application of the composts to degraded post-mining soil; therefore, the activity of these enzymes can be used in monitoring and assessing the effects of reclamation of degraded soils.
Enzymatic activity, its changes in the analysed period of research, and the content of total organic carbon and total nitrogen indicate that the soil fertilized with sewage sludge and ash compost was characterized by significantly better properties. Our research proves that a one-time application of composts made of sewage sludge and sludge with the addition of ash, as well as the introduction of plant cover—a mixture of grasses, which allows for a lasting reclamation effect. A single application of a high dose (180 Mg·ha−1) of composts, especially with the addition of sewage sludge and fly ash, can be recommended as an effective technology for the reclamation of degraded areas. An additional ecological advantage of this reclamation model is waste management (SS and FA), which is part of the circular economy strategy.