Constructed Technosols as a Soil Rebuilding Technique to Reclaim Abandoned Limestone Quarries in the Mediterranean Region: A Field Study
Abstract
1. Introduction
2. Materials and Methods
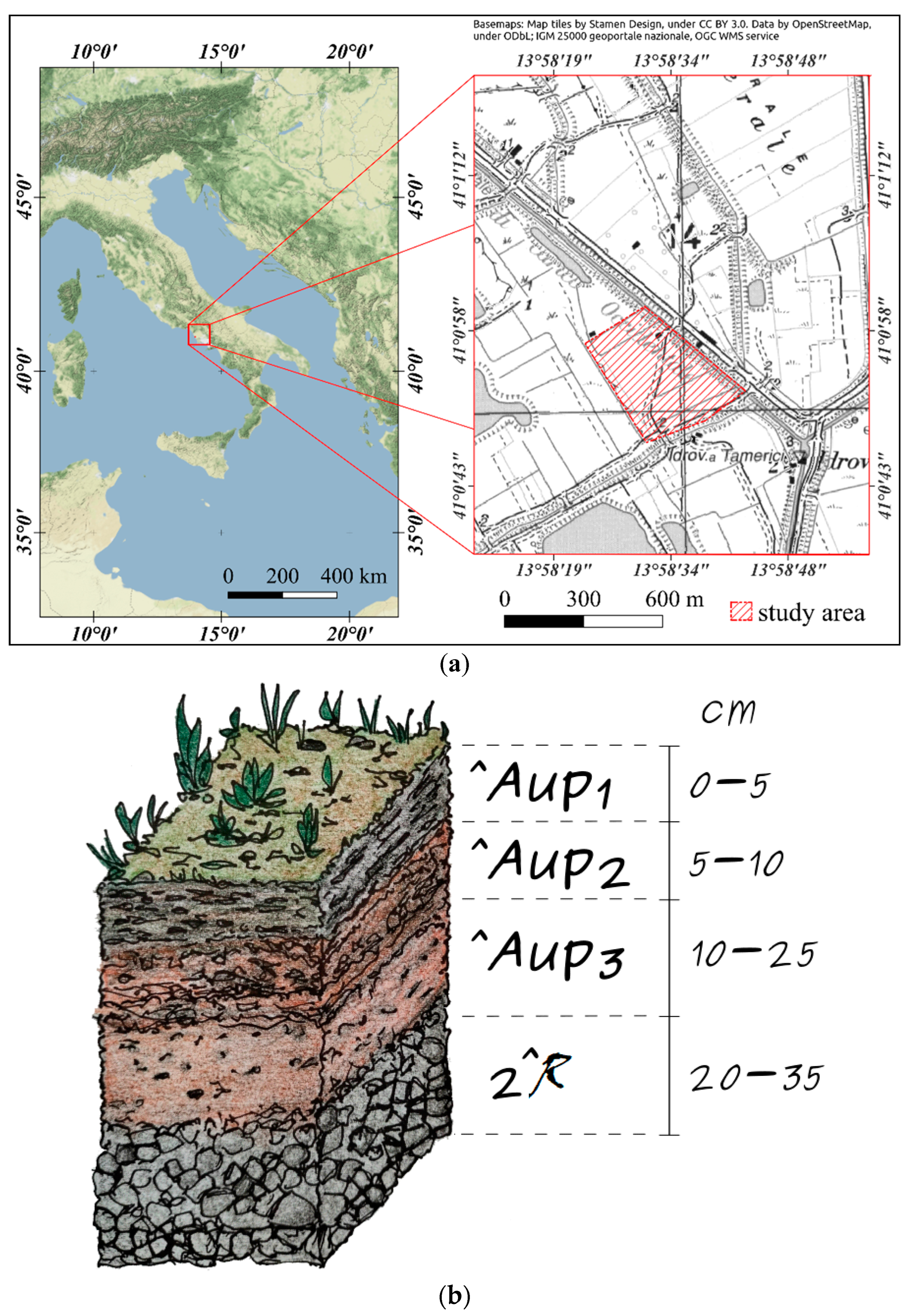
2.1. Study Area
2.2. Experimental Design
- A (re)constructed Technosol (CT) mixed with a common commercial organic amendment (OA) at a ratio of 60:40 w/w: CTOA. It was mainly derived from urban organic wastes, agrozootechnical activities, composted sewage sludge, and “green wastes” such as pruning and cutting;
- A (re)constructed Technosol (CT) treated with conventional mineral fertilization (CF) according to both plant-specific requirements and manufacturer’s recommendations (vide infra): CTCF. It mainly consisted of NPK-based fertilizers. In particular, N was added as NO3−N, NH4+−N (as ammonium nitrate), and CH4N2O, P as P2O5 (triple superphosphate), and K as K2O (potassium oxide). Fertilizer was broadcast added at the beginning of the experiment at the recommended dose of 200 kg ha−1 [1]. After one month, a second CF dose was added at a rate of 300 kg ha−1 for rosemary and 600 kg ha−1 for olive and grape. At the time of vegetative wakening (spring), a third dose was added as 500 kg ha−1 for rosemary + pasture and 800 kg ha−1 for olive or grape + pasture.
2.3. Pedotechnosystems Preparation
2.4. Plant Characterization
Metabolomics
2.5. Physical-Chemical Characterization of Pedotechnosystems
2.6. Statistical Analyses
3. Results and Discussion
3.1. Pasture Yield and Nutrient Concentrations
3.2. Pedotechnosystem Behaviour and Development
3.3. Crop Characterization and Metabolic Profile
3.4. Multivariate Statistic
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buondonno, A.; Capra, G.F.; Di Palma, D.; Grilli, E.; Vigliotti, R.C. Pedotechnologies for the environmental reclamation of limestone quarries. A protocol proposal. Land Use Policy 2018, 71, 230–244. [Google Scholar] [CrossRef]
- Grilli, E.; Vigliotti, R.C.; Rossetti, L.; Scognamiglio, M.; Fiumano, V.; Fiorentino, A.; Leone, N.; Nogueira, T.A.R.; Abreu-Junior, C.H.; Jani, A.R.; et al. Restoration of quarry areas in Mediterranean regions through a low-cost soil rebuilding technique for profitable pedotechnosystems development. Soil Tillage Res. 2021, 209, 104936. [Google Scholar] [CrossRef]
- Zhendi, H.; Peijun, W.; Jing, L. Ecological Restoration of Abandoned Mine Land in China. J. Resour. Ecol. 2012, 3, 289–296. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, L. The current policy and problems about land reclamation of Chinese mainland. J. Am. Soc. Min. Reclam. 2015, 4, 117–136. [Google Scholar] [CrossRef]
- Heneghan, L.; Miller, S.P.; Baer, S.; Callaham, M.A., Jr.; Montgomery, J.; Pavao-Zuckerman, M.; Rhoades, C.C.; Richardson, S. Integrating soil ecological knowledge into restoration management. Rest. Ecol. 2008, 16, 608–617. [Google Scholar] [CrossRef]
- Chenot, J.; Jaunatre, R.; Buisson, E.; Bureau, F.; Dutoit, T. Impact of quarry exploitation and disuse on pedogenesis. Catena 2018, 160, 354–365. [Google Scholar] [CrossRef]
- Ruiz, F.; Cherubin, M.R.; Ferreira, T.O. Soil quality assessment of constructed Technosols: Towards the validation of a promising strategy for land reclamation, waste management and the recovery of soil functions. J. Environ. Manage. 2020, 276, 111344. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, F.; Resmini, L.; Valdomiro, S.; de Souza, S.; Cheyson, J.J.; dos Santos, B.; Ferreira, T.O. Fast pedogenesis of tropical Technosols developed from dolomitic limestone mine spoils (SE-Brazil). Geoderma 2020, 374, 114439. [Google Scholar] [CrossRef]
- Jordán, M.M.; Garcıá-Sánchez, E.; Almendro-Candel, M.B.; Navarro-Pedrenõ, J.; Gómez- Lucas, I.; Melendez, I. Geological and environmental implications in the reclamation of limestone quarries in Sierra de Callosa (Alicante, Spain). Environ. Earth Sci. 2009, 59, 687–694. [Google Scholar] [CrossRef]
- Domene, X.; Mattana, S.; Ramírez, W.; Colón, J.; Jiménez, P.; Balanyà, T.; Alcañiz, J.M.; Bonmatí, M. Bioassays prove the suitability of mining debris mixed with sewage sludge for land reclamation purposes. J. Soils Sediments 2010, 10, 30–44. [Google Scholar] [CrossRef]
- Cohen-Fernández, A.C.; Naeth, M.A.; Wilkinson, S.R. Anthroposol development from limestone quarry substrates. Can. J. Soil Sci. 2013, 93, 555–566. [Google Scholar] [CrossRef]
- Buondonno, A.; Grilli, E.; Capra, G.F.; Glorioso, C.; Langella, A.; Leone, A.P.; Leone, N.; Odierna, P.; Vacca, S.; Vigliotti, R.C. Zeolitized tuffs in Pedotechnique for the reclamation of abandoned quarries. A case study in the Campania region (Italy). J. Environ. Manage. 2013, 122, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Josa, R.; Jorba, M.; Vallejo, V.R. Opencast mine restoration in a Mediterranean semi-arid environment: Failure of some common practices. Ecol. Eng. 2012, 42, 183–191. [Google Scholar] [CrossRef]
- Ferreira, C.S.S.; Seifollahi-Aghmiuni, S.; Destouni, G.; Ghajarnia, N.; Kalantari, Z. Soil degradation in the European Mediterranean region: Processes, status and consequences. Sci. Total Environ. 2022, 805, 150106. [Google Scholar] [CrossRef]
- Fanning, D.S.; Fanning, M.C.B. Soil: Morphology, Genesis, and Classification; John Wiley & Sons: New York, NY, USA, 1989. [Google Scholar]
- Capra, G.F.; Duras, M.G.; Vacca, S.; Grilli, E.; Buondonno, A. Issues concerning soils treated with wastewater: Pedotechnical management with zeolitized tuffs as an option for turning N and P pollutants into potential fertilizers. Microporous Mesoporous Mater. 2013, 167, 22–29. [Google Scholar] [CrossRef]
- Dazzi, C.; Lo Papa, G. Taxonomic and environmental implication of pedotechnique in large scale farming. Int. Soil Water Conserv. Res. 2016, 4, 137–141. [Google Scholar] [CrossRef]
- Clemente, A.S.; Werner, C.; Máguas, C.; Cabral, M.S.; Martins-Loução, M.A.; Correia, O. Restoration of a limestone quarry: Effect of soil amendments on the establishment of native Mediterranean sclerophyllous shrubs. Restor. Ecol. 2014, 12, 20–28. [Google Scholar] [CrossRef]
- Pitz, C.; Mahy, G.; Harzé, M.; Uyttenbroeck, R.; Monty, A. Comparison of mining spoils to determine the best substrate for rehabilitating limestone quarries by favoring native grassland species over invasive plants. Ecol. Eng. 2019, 127, 510–518. [Google Scholar] [CrossRef]
- Soria, R.; Ortega, R.; Bastida, F.; Miralles, I. Role of organic amendment application on soil quality, functionality and greenhouse emission in a limestone quarry from semiarid ecosystems. Appl. Soil. Ecol. 2021, 164, 103925. [Google Scholar] [CrossRef]
- Luna, L.; Miralles, I.; Lázaro, R.; Contreras, S.; Solé-Benet, A. Effect of soil properties and hydrologic characteristics on plants in a restored calcareous quarry under a transitional arid to semiarid climate. Ecohydrology 2018, 11, e1896. [Google Scholar] [CrossRef]
- Capra, G.F.; Tidu, S.; Lovreglio, R.; Certini, G.; Salis, M.; Bacciu, V.; Ganga, A.; Filzmoser, P. The impact of wildland fires on calcareous Mediterranean pedosystems (Sardinia Italy)—An integrated multiple approach. Sci. Total Environ. 2018, 624, 1152–1162. [Google Scholar] [CrossRef]
- Mkhonza, N.P.; Buthelezi-Dube, N.N.; Muchaonyerwa, P. Effects of lime application on nitrogen and phosphorus availability in humic soils. Sci. Rep. 2020, 10, 8634. [Google Scholar] [CrossRef]
- Pastor, J.L.; Tomás, R.; Cano, M.; Riquelme, A.; Gutiérrez, E. Evaluation of the improvement effect of limestone powder waste in the stabilization of swelling clayey soil. Sustainability 2019, 11, 679. [Google Scholar] [CrossRef]
- Capra, G.F.; Coppola, E.; Odierna, P.; Grilli, E.; Vacca, S.; Buondonno, A. Occurrence and distribution of key potentially toxic elements (PTEs) in agricultural soils: A paradigmatic case study in an area affected by illegal landfills. J. Geochem. Explor. 2014, 145, 169–180. [Google Scholar] [CrossRef]
- Kumar, R.; Bohra, A.; Pandey, A.K.; Pandey, M.K.; Kumar, A. Metabolomics for plant improvement: Status and prospects. Front. Plant Sci. 2017, 8, 1302. [Google Scholar] [CrossRef] [PubMed]
- Lankadurai, B.P.; Nagato, E.G.; Simpson, M.J. Environmental metabolomics: An emerging approach to study organism responses to environmental stressors. Environ. Rev. 2013, 21, 180–205. [Google Scholar] [CrossRef]
- Costantini, E.A.C.; Urbano, F.; L’Abate, G. Soil Regions of Italy. Available online: www.soilmaps.it (accessed on 28 October 2022).
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2014. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-NRCS. US Gov. Print. Office: Washington, DC, USA, 2014.
- Ministero per le Politiche Agricole e Forestali. Metodi di Analisi Chimica del Suolo; Collana di Metodi Analitici per l’Agricoltura; Angeli, F., Ed.; Ministero per le Politiche Agricole e Forestali: Milano, Italy, 2000. [Google Scholar]
- Campbell, C.R.; Plank, C.O. Preparation of plant tissue for laboratory analysis. In Handbook of Methods for Plant Analysis; Kalra, Y.P., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Oxford, UK, 1998; pp. 37–49. [Google Scholar]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based metabolomic analysis of plants. Nat. Protoc. 2010, 5, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, M.; Fiumano, V.; D′Abrosca, B.; Esposito, A.; Choi, Y.H.; Verpoorte, R.; Fiorentino, A. Chemical interactions between plants in Mediterranean vegetation: The influence of selected plant extracts on Aegilops geniculata metabolome. Phytochemistry 2014, 106, 69–85. [Google Scholar] [CrossRef]
- Scognamiglio, M.; D’Abrosca, B.; Fiumano, V.; Golino, M.; Esposito, A.; Fiorentino, A. Seasonal phytochemical changes in Phillyrea angustifolia L.: Metabolomic analysis and phytotoxicity assessment. Phytochem. Lett. 2014, 8, 163–170. [Google Scholar] [CrossRef]
- Dell’Abate, M.T.; Benedetti, A.; Trinchera, A.; Dazzi, C. Humic substances along the profile of two Typic Haploxerert. Geoderma 2002, 107, 281–296. [Google Scholar] [CrossRef]
- Rubino, M.; Benedetti, A.; Coppola, E.; Dell’Abate, M.T.; Buondonno, A. A new oxidation method for determination of soil extracted an humic substances. In The Soils of Tomorrow: Soils Changing in a Changing World; Advances in Geoecology; Dazzi, C., Costantini, E., Eds.; Catena Verlag: Reiskirchen, German, 2008; Volume 39, pp. 719–728. [Google Scholar]
- Gardner, W.H. Water content. In Methods of Soil Analysis, Part 1. Physical and Mineralogical Methods—Agronomy Monograph No 9, 2nd ed.; Klute, A., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1998; pp. 493–544. [Google Scholar]
- Rossel, R.A.V.; Minasny, B.; Roudier, P.; McBratney, A.B. Colour space models for soil science. Geoderma 2006, 133, 320–337. [Google Scholar] [CrossRef]
- RStudio. Integrated Development for R. RStudio; RStudio Team. Inc.: Boston, MA, USA, 2021; Available online: http://www.rstudio.com/ (accessed on 28 October 2022).
- Larney, F.J.; Angers, D.A. The role of organic amendments in soil reclamation: A review. Can. J. Soil Sci. 2012, 92, 19–38. [Google Scholar] [CrossRef]
- Jones, J.B., Jr.; Wolf, B.; Mills, H.A. Plant Analysis Handbook: A Practical Sampling, Preparation, Analysis, and Interpretation Guide; Micro-Macro Publishing: Athens, Greece, 1991. [Google Scholar]
- Van Alfen, N.K. Encyclopedia of Agriculture and Food Systems; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Luna, L.; Pastorelli, R.; Bastida, F.; Hernández, T.; García, C.; Mirallesd, I.; Solé-Benet, A. The combination of quarry restoration strategies in semiarid climate induces different responses in biochemical and microbiological soil properties. Appl. Soil Ecol. 2016, 107, 33–47. [Google Scholar] [CrossRef]
- Huang, M.; Zhu, Y.; Li, Z.; Huang, B.; Luo, N.; Liu, C.; Zeng, G. Compost as a soil amendment to remediate heavy metal-contaminated agricultural soil: Mechanisms, efficacy, problems, and strategies. Water Air Soil Pollut. 2016, 227, 359. [Google Scholar] [CrossRef]
- Norris, E.N.; Congreves, K.A. Alternative management practices improve soil health indices in intensive vegetable cropping systems: A review. Front. Environ. Sci. 2018, 6, 50. [Google Scholar] [CrossRef]
- Ampong, K.; Thilakaranthna, M.S.; Gorim, L.Y. Understanding the Role of Humic Acids on Crop Performance and Soil Health. Front. Agron. 2022, 4, 848621. [Google Scholar] [CrossRef]
- Aitkenhead, M.J.; Coull, M.; Towers, W.; Hudson, G.; Black, H.I.J. Prediction of soil characteristics and colour using data from the National Soils Inventory of Scotland. Geoderma 2019, 200–201, 99–107. [Google Scholar] [CrossRef]
- Uchida, R. Essential nutrients for plant growth. In Plant Nutrient Management in Hawaii’s Soils, Approaches for Tropical and Subtropical Agriculture College of Tropical Agriculture and Human Resources; Silva, J.A., Uchida, R., Eds.; University of Hawaii at Manoa: Manoa, HI, USA, 2014; pp. 31–55. [Google Scholar]
- Umar, K.; Ayub, M.A.; Ur Rehman, M.Z.; Ahmad, A.R.; Farooqi, Z.R.; Shahzad, A.; Rehman, U.; Mustafa, A.; Nadeem, M. Nitrogen and phosphorus use efficiency in agroecosystems. In Resources Use Efficiency in Agriculture; Kumar, S., Meena, R.S., Jhariya, M.K., Eds.; Springer: Singapore, 2020; pp. 213–257. [Google Scholar]
- Volpin, F.; Chekli, L.; Phuntsho, P.; Cho, J.; Ghaffour, N.; Vrouwenvelder, J.S.; Shon, H.K. Simultaneous phosphorous and nitrogen recovery from source-separated urine: A novel application for fertiliser drawn forward osmosis. Chemosphere 2018, 203, 482–489. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Flavonoids and Phenolic Acids from Oregano: Occurrence, Biological Activity and Health Benefits. Plants (Basel) 2018, 7, 2. [Google Scholar] [CrossRef]
- Lattanzio, V.; Cardinali, A.; Ruta, C.; Fortunato, I.M.; Lattanzio, V.M.T.; Linsalata, V.; Cicco, N. Relationship of secondary metabolism to growth in oregano (Origanum vulgare L.) shoot cultures under nutritional stress. Environ. Exp. Bot. 2009, 65, 54–62. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E.A. Principles of Plant Nutrition, 4th ed.; International Potash Institute: Bern, Switzerland, 1987. [Google Scholar]
- Petridis, A.; Therios, I.; Samouris, G.; Tananaki, C. Salinity-induced changes in phenolic compounds in leaves and roots of four olive cultivars (Olea europaea L.) and their relationship to antioxidant activity. Environ. Exp. Bot. 2012, 79, 37–43. [Google Scholar] [CrossRef]
- Martinelli, F.; Remorini, D.; Saia, S.; Massai, R.; Tonutti, P. Metabolic profiling of ripe olive fruit in response to moderate water stress. Sci. Hortic. 2013, 159, 52–58. [Google Scholar] [CrossRef]
- Pais, I.; Jones, J.B., Jr. The Handbook of Trace Elements; St. Lucie Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Muller, B.; Pantin, F.; Génard, M.; Turc, O.; Freixes, S.; Piques, M.; Gibon, Y. Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. J. Exp. Bot. 2011, 62, 1715–1729. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Trovato, M.; Mattioli, R.; Costantino, P. Multiple roles of proline in plant stress tolerance and development. Rend. Lincei 2008, 19, 325–346. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, D.; Korpelainen, H.; Li, C. Metabolic and physiological analyses reveal that Populus cathayana males adopt an energy-saving strategy to cope with phosphorus deficiency. Tree Physiol. 2019, 39, 1630–1645. [Google Scholar] [CrossRef]
- del Baño, M.J.; Lorente, J.; Castillo, J.; Benavente-García, O.; del Río, J.A.; Ortuño, A.; Quirin, K.-W.; Gerard, D. Phenolic Diterpenes, Flavones, and Rosmarinic Acid Distribution during the Development of Leaves, Flowers, Stems, and Roots of Rosmarinus officinalis. Antioxidant Activity. J. Agric. Food Chem. 2003, 51, 4247–4253. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: London, UK, 2002. [Google Scholar]

| CT | pH-H2O | EC | OC | HA + FA-C | HUM-C | NHC | N | C/N | HI | DH | HR | HU | PM3 | KM3 | WHC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dS m−1 | g kg−1 | % | mg kg−1 | g kg−1 | % | ||||||||||
| Control | 9.5 ± 0.1 | 0.22 ± 0.01 | 0.6 ± 0.0 | 0.05 ± 0.00 | 0.42 ± 0.02 | 0.05 ± 0.01 | 0.01 ± 0.00 | 91 ± 1 | 1.0 ± 0.0 | 80 ± 3 | 10 ± 0 | 90 ± 3 | 1.05 ± 0.05 | 0.03 ± 0.00 | 42 ± 1 |
| CTOA | 7.9 ± 0.1 | 2.76 ± 0.05 | 129.8 ± 1.2 | 28.52 ± 1.15 | 85.89 ± 0.18 | 15.40 ± 0.45 | 6.84 ± 0.25 | 19 ± 0 | 0.5 ± 0.0 | 65 ± 1 | 22 ± 1 | 88 ± 2 | 152.47 ± 2.55 | 0.38 ± 0.05 | 130 ± 4 |
| CTCF | 9.5 ± 0.2 | 0.22 ± 0.01 | 0.6 ± 0.0 | 0.05 ± 0.00 | 0.42 ± 0.03 | 0.05 ± 0.01 | 0.03 ± 0.01 | 48 ± 1 | 1.0 ± 0.0 | 80 ± 2 | 10 ± 0 | 90 ± 3 | 3.62 ± 0.65 | 0.04 ± 0.00 | 42 ± 1 |
| Constructed Technosol (CT) | Pedotechnosystems (PTS) | |
|---|---|---|
| Acronym | Meaning | |
| Control: CT without additional treatments | CTr | Pasture species + Rosemary on CT |
| CTo | Pasture species + Olive on CT | |
| CTsg | Pasture species + Sangiovese on CT | |
| CTtb | Pasture species + Trebbiano on CT | |
| CTOA: CT + organic amendment | CTOAr | Pasture species + Rosemary on CTOA |
| CTOAo | Pasture species + Olive on CTOA | |
| CTOAsg | Pasture species + Sangiovese on CTOA | |
| CTOAtb | Pasture species + Trebbiano on CTOA | |
| CTCF: CT + conventional fertilization | CTCFr | Pasture species + Rosemary on CTCF |
| CTCFo | Pasture species + Olive on CTCF | |
| CTCFsg | Pasture species + Sangiovese on CTCF | |
| CTCFtb | Pasture species + Trebbiano on CTCF | |
| PTS | TDM | N | P | K |
|---|---|---|---|---|
| g kg−1 | ||||
| Rosemary | ||||
| CTr | 0.03 b [0.01] | 25.67 a [1.58] | 0.71 b [0.18] | 9.67 a [0.74] |
| CTOAr | 0.16 a [0.02] | 25.83 a [1.82] | 1.79 a [0.03] | 9.23 a [2.56] |
| CTCFr | 0.05 a [0.02] | 22.70 a [0.07] | 1.11 b [0.06] | 15.04 a [1.08] |
| Olive, cv. Frantoio | ||||
| CTo | 0.08 b [0.00] | 26.82 a [2.38] | 2.29 a [0.83] | 10.10 a [1.54] |
| CTOAo | 0.56 a [0.21] | 27.24 a [1.34] | 1.69 a [0.06] | 13.71 a [1.45] |
| CTCFo | 0.05 b [0.01] | 26.09 a [0.40] | 1.44 a [0.03] | 10.76 a [0.50] |
| Grape, cv. Trebbiano | ||||
| CTtb | 0.02 b [0.00] | 29.53 a [0.75] | 2.27 b [0.08] | 12.42 b [0.18] |
| CTOAtb | 0.54 a [0.05] | 32.20 a [1.40] | 3.21 a [0.03] | 27.87 a [3.32] |
| CTCFtb | 0.05b a [0.00] | 28.16 a [4.29] | 2.99 ac [0.20] | 21.68 ab [2.75] |
| Grape, cv. Sangiovese | ||||
| CTsg | 0.01 b [0.00] | 12.52 a [1.28] | 2.04 b [0.02] | 11.02 b [0.24] |
| CTOAsg | 0.28 a [0.04] | 30.47 a [6.24] | 3.13 a [0.05] | 29.38 a [1.74] |
| CTCFsg | 0.11 b [0.05] | 26.05 a [6.30] | 2.54 ab [0.23] | 19.28 ab [3.78] |
| PTS | pH H2O | EC | OC | HA + FA-C | HUM-C | NHC | N | C/N | HI | DH | HR | HU | PM3 | KM3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dS m−1 | g kg−1 | % | mg kg−1 | g kg−1 | ||||||||||
| ___________________________________________________________________________ Rosemary ___________________________________________________________________________ | ||||||||||||||
| CTr | 9.4 a * [0.02] | 0.30 b * [0.01] | 0.75 b * [0.04] | 0.54 b * [0.02] | 0.07 b * [0.01] | 0.14 b * [0.02] | 0.05b * [0.00] | 15.9 a * [0.7] | 0.3 b * [0.1] | 79.2 a * [1.5] | 72.1 a * [1.9] | 81.1 a * [1.2] | 3.02 b [0.63] | 0.07 a * [0.004] |
| CTOAr | 8.1 b [0.04] | 0.82 a * [0.03] | 95.95 a * [ 0.94] | 25.54 a [3.74] | 47.14 a * [1.24] | 23.27 a [4.88] | 8.79 a * [0.26] | 10.9 b * [0.4] | 1.1 a [0.3] | 52.9 b [8.9] | 26.7 c [4.0] | 75.8 a [5.0] | 160.17 a [8.90] | 0.08 a * [0.005] |
| CTCFr | 9.3 a * [0.02] | 0.36 b * [0.02] | 2.44 b * [0.06] | 1.41 b * [0.07] | 0.30 b [0.08] | 0.73 b * [0.11] | 0.14 b * [0.01] | 17.1 a [1.2] | 0.5 b * [0.1] | 66.2 ab [4.5] | 57.5 b * [2.8] | 70.0 a * [4.6] | 1.31 b * [0.11] | 0.09 a * [0.005] |
| Olive, cv. Frantoio | ||||||||||||||
| Cto | 9.4 a [0.02] | 0.29 b [0.02] | 0.73 b [0.06] | 0.09 b [0.02] | 0.35 b [0.03] | 0.28 b * [0.02] | 0.05 b * [0.00] | 13.7 a * [0.6] | 3.4 a [0.7] | 24.6 c * [3.8] | 12.8 b [1.7] | 60.4 a * [2.9] | 1.33 b * [0.02] | 0.08 a * [0.01] |
| CTOAo | 8.0 b [0.04] | 0.68 a * [0.06] | 126.06 a [9.20] | 34.09 a [3.26] | 79.85 a [5.50] | 12.12 a [0.54] | 8.40 a [0.88] | 15.2 a * [0.5] | 0.4 b * [0.0] | 73.5 a * [1.2] | 26.9 a * [0.7] | 90.3 b [0.4] | 147.24 a [9.97] | 0.05 a * [0.01] |
| CTCFo | 9.3 a [0.05] | 0.31 b [0.03] | 1.37 b * [0.08] | 0.25 b [0.06] | 0.75 b [0.19] | 0.37 b * [0.06] | 0.18 b * [0.01] | 7.5 b * [0.3] | 1.6 b [0.2] | 38.4 b * [2.3] | 18.7 b [5.4] | 71.9 a [5.9] | 1.85 b * [0.19] | 0.06 a [0.01] |
| Grape, cv. Trebbiano | ||||||||||||||
| CTtb | 9.9 a * [0.02] | 0.40 b * [0.01] | 1.52 b * [0.07] | 0.35 b * [0.05] | 0.34 b [0.04] | 0.84 b * [0.03] | 0.18 b * [0.01] | 8.4 b * [0.3] | 2.6 a [0.4] | 29.2 b * [3.5] | 22.8 a * [2.7] | 44.7 b * [3.3] | 1.71 b * [0.06] | 0.06 b * [0.001] |
| CTOAtb | 8.3 b [0.11] | 1.14 a * [0.01] | 103.40 a * [3.76] | 29.01 a [1.29] | 55.16 a * [1.29] | 19.23 a [1.87] | 7.34 a [0.65] | 14.3 a * [0.8] | 0.7 b [0.0] | 60.4 a [1.3] | 28.0 a * [0.5] | 81.5 a * [1.2] | 193.34 a [14.77] | 0.62 a * [0.01] |
| CTCFtb | 9.6 a * [0.01] | 0.37 b * [0.03] | 1.42 b * [0.08] | 0.50 b * [0.05] | 0.39 b [0.07] | 0.53 b [0.17] | 0.14 b * [0.01] | 10.5 b [0.5] | 1.2 ab [0.5] | 52.2 ab [10.6] | 36.6 a * [5.5] | 63.9 ab [10.0] | 2.18 b * [0.09] | 0.08 b * [0.001] |
| Grape, cv. Sangiovese | ||||||||||||||
| CTsg | 9.6 a [0.05] | 0.54 b * [0.01] | 1.28 b * [0.03] | 0.32 b [0.10] | 0.16 b * [0.04] | 0.81 b * [0.15] | 0.08 b * [0.01] | 16.3 a * [1.3] | 4.2 a [1.8] | 29.6 a [10.1] | 25.4a [8.3] | 37.8 b * [10.3] | 2.83 b * [0.20] | 0.09 b [0.02] |
| CTOAsg | 8.1 b [0.06] | 1.14 a * [0.08] | 106.57 a * [1.72] | 31.26 a [1.68] | 51.15 a * [3.52] | 24.15 a * [0.60] | 9.58 a * [0.63] | 11.2 a * [0.6] | 0.8 a * [0.0] | 56.3 a * [0.9] | 29.4 a [1.9] | 77.3 a * [0.9] | 194.07 a [10.25] | 0.64 a * [0.03] |
| CTCFsg | 9.8 a * [0.02] | 0.34 c * [0.01] | 1.27 b * [0.05] | 0.40 b * [0.07] | 0.40 b * [0.07] | 0.81 b * [0.08] | 0.09 b [0.01] | 14.1 a [1.9] | 2.4 a [0.6] | 32.9 a [6.0] | 31.0 a [5.3] | 36.1 b * [6.3] | 3.27 b * [0.11] | 0.07 b * [0.01] |
| PTS | HueYR std | Value | Chroma | Munsell Soil Color Description |
|---|---|---|---|---|
| Starting PTS | ||||
| CTr; CTo; CTtb; CTsg; CTCFr; CTCFo; CTCFtb; CTCFsg | 10.00 | 7.90 | 1.79 | White |
| CTOAr; CTOAo; CTOAtb; CTOAsg | 10.84 | 4.02 | 1.23 | Dark gray |
| Final PTS | ||||
| Rosemary | ||||
| CTr | 10.03 | 6.81 | 2.24 | Light gray |
| CTOAr | 10.73 | 4.75 | 1.54 | Grayish brown |
| CTCFr | 10.54 | 6.33 | 2.07 | Light brownish gray |
| Olive, cv. Frantoio | ||||
| CTo | 10.03 | 7.42 | 2.17 | Light gray |
| CTOAo | 10.77 | 4.70 | 1.53 | Grayish brown |
| CTCFo | 11.60 | 7.37 | 3.97 | Very pale brown |
| Grape, cv. Trebbiano | ||||
| CTtb | 10.33 | 8.07 | 1.66 | White |
| CTOAtb | 10.26 | 4.49 | 1.21 | Gray |
| CTCFtb | 10.20 | 7.31 | 1.96 | Light gray |
| Grape, cv. Sangiovese | ||||
| CTsg | 10.29 | 7.59 | 2.02 | White |
| CTOAsg | 9.54 | 4.31 | 1.12 | Dark gray |
| CTCFsg | 10.12 | 7.48 | 2.04 | White |
| PTS | H | W | N | P | K | Rosmarinic Acid | Rosmanol |
|---|---|---|---|---|---|---|---|
| cm | g kg−1 | g kg−1 | |||||
| CTr | 45 a [2] | 39 a [1] | 11.96 a [2.21] | 0.87 a [0.09] | 3.77 a [1.61] | 49.04 a [4.09] | 29.22 a [9.20] |
| CTOAr | 54 a [1] | 48 b [2] | 12.88 ab [1.02] | 1.00 a [0.03] | 5.51 a [1.41] | 51.47 a [6.51] | 35.76 a [7.31] |
| CTCFr | 43 a [1] | 32 c [1] | 17.76 b [0.70] | 0.90 a [0.05] | 0.91 a [0.07] | Nd | 23.75 a [1.06] |
| PTSs | H | W | N | P | K | Glucose | Sucrose | Glc/Suc | Phenols |
|---|---|---|---|---|---|---|---|---|---|
| cm | g kg−1 | g kg−1 | % | ||||||
| CTo | 117 a [11] | 93 a [8] | 9.38 a [0.54] | 0.58 a [0.04] | 6.03 a [0.46] | 105.12 a [28.24] | 4.76 a [0.93] | 26.5 a [12.3] | 4.55 a [0.72] |
| CTOAo | 127 a [7] | 98 a [8] | 13.45 b [0.67] | 0.69 a [0.21] | 6.76 a [0.27] | 74.72 a [2.73] | 24.14 b [0.04] | 3.1 a [0.1] | 1.85 b [0.22] |
| CTCFo | 121 a [3] | 74 a [5] | 11.84 a [0.59] | 0.63 a [0.02] | 5.59 a [0.24] | 88.30 a [17.61] | 9.82 c [0.76] | 8.90 a [1.11] | 4.93 a [0.60] |
| PTS | H | W | N | P | K | T | A | C | Gm | P | Gl | Su | Ka | Qg | Q |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cm | g kg−1 | g kg−1 | |||||||||||||
| CTtb | 51 a [6] | 20 a [3] | 14.95 a [0.58] | 1.29 a [0.06] | 13.25 a [0.41] | 27.18 a [7.45] | 61.75 a [10.73] | 27.22 a [1.43] | nd | nd | 30.12 a [5.82] | 97.86 a [23.17] | nd | 34.71 a [1.28] | 28.15 a [2.61] |
| CTOAtb | 88 b [6] | 30 b [4] | 32.29 b [1.06] | 1.93 b [0.07] | 14.63 a [1.91] | 13.13 a [4.02] | 49.00 a [7.65] | 15.93 a [3.66] | nd | nd | 26.74 a [4.69] | 65.07 a [3.21] | nd | 4.64 b [3.35] | 7.69 a [5.56] |
| CTCFtb | 66 a [3] | 25 a [1] | 14.06 a [0.26] | 1.08 a [0.03] | 14.11 a [0.13] | 37.74 a [9.48] | 50.35 a [8.90] | 24.03 a [4.86] | nd | nd | 38.79 a [11.66] | 115.68 a [16.60] | nd | 18.09 b [1.89] | 19.27 a [2.01] |
| CTsg | 109 a [8] | 30a [2] | 16.40 a [1.06] | 1.05 a [0.05] | 20.44 a [0.37] | 27.75 a [5.67] | 46.58 a [0.12] | 22.43 a [0.00] | 8.51 a [0.39] | nd | 24.12 a [2.24] | 102.92 a [1.33] | 6.33 [0.95] | 35.80 a [6.74] | 32.13 a [3.95] |
| CTOAsg | 128 b [4] | 28 a [3] | 31.07 b [0.73] | 1.84 b [0.12] | 22.50 b [0.59] | 24.26 a [0.91] | 87.11 a [8.67] | 15.85 a [2.84] | 15.57 b [0.55] | 18.63 [0.66] | 32.72 a [4.29] | 50.69 a [17.24] | nd | nd | 3.10a [1.90] |
| CTCFsg | 107 a [8] | 35 a [4] | 14.70 a [0.37] | 1.31 a [0.03] | 23.45 b [0.90] | 32.32 a [1.37] | 48.70 a [17.64] | 29.27 a [5.82] | nd | nd | 34.36 a [7.74] | 133.79 a [32.87] | nd | 35.09 a [15.43] | 24.19 a [10.63] |
| Parameters | Factors | ||
|---|---|---|---|
| F1 | F2 | F3 | |
| WHC | 0.901 | 0.356 | 0.228 |
| pH | 0.900 | 0.379 | 0.197 |
| EC | 0.896 | 0.397 | 0.178 |
| OC | 0.901 | 0.342 | 0.244 |
| N | 0.995 | 0.071 | 0.021 |
| C/N | −0.632 | 0.548 | 0.445 |
| KM3 | 0.983 | 0.159 | 0.062 |
| PM3 | −0.990 | 0.019 | 0.070 |
| H | 0.618 | 0.780 | 0.029 |
| W | −0.563 | −0.608 | −0.439 |
| N | −0.013 | 0.953 | −0.109 |
| P | −0.445 | 0.313 | −0.792 |
| K | 0.326 | 0.930 | −0.073 |
| Rosmarinic acid | −0.010 | −0.052 | −0.974 |
| Rosmanol | 0.340 | 0.749 | 0.083 |
| TDM | 0.937 | 0.233 | −0.101 |
| N TDM | 0.051 | 0.132 | 0.173 |
| P TDM | −0.951 | 0.159 | −0.211 |
| K TDM | 0.071 | 0.733 | −0.040 |
| Variance (%) | 57 | 21 | 13 |
| Cumulative variance (%) | 57 | 78 | 91 |
| Eigenvalues | 10.855 | 4.018 | 2.453 |
| Parameters | Factors | ||
|---|---|---|---|
| F1 | F2 | F3 | |
| WHC | 0.965 | 0.014 | 0.259 |
| pH | 0.959 | −0.032 | 0.270 |
| EC | 0.950 | −0.077 | 0.280 |
| TOC | 0.967 | 0.043 | 0.252 |
| N | 0.722 | −0.008 | 0.678 |
| C/N | 0.115 | 0.052 | −0.942 |
| KM3 | 0.766 | −0.057 | 0.628 |
| PM3 | −0.611 | 0.035 | −0.769 |
| H | 0.267 | −0.833 | 0.388 |
| W | 0.363 | 0.079 | −0.237 |
| N leaf | −0.225 | −0.333 | −0.907 |
| P leaf | 0.940 | 0.327 | 0.094 |
| K leaf | −0.465 | −0.760 | 0.050 |
| Glu | −0.247 | 0.332 | −0.117 |
| Suc | 0.595 | −0.302 | 0.738 |
| Phenols | 0.815 | −0.501 | 0.092 |
| TDM | 0.963 | 0.007 | −0.119 |
| N TDM | −0.146 | −0.969 | −0.170 |
| P TDM | 0.098 | −0.990 | −0.097 |
| K TDM | −0.746 | 0.610 | −0.229 |
| Variance (%) | 56 | 24 | 15 |
| Cumulative variance (%) | 56 | 80 | 95 |
| Eigenvalues | 11.728 | 5.068 | 3.079 |
| Parameters | Factors (Trebbiano Grape) | Factors (Sangiovese Grape) | ||||
|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F1 | F2 | F3 | |
| WHC | 0.974 | 0.086 | 0.146 | 0.815 | 0.507 | 0.265 |
| pH | 0.964 | 0.082 | 0.182 | 0.827 | 0.502 | 0.233 |
| EC | 0.953 | 0.083 | 0.222 | 0.836 | 0.495 | 0.202 |
| TOC | 0.979 | 0.086 | 0.121 | 0.807 | 0.510 | 0.284 |
| N | 0.861 | 0.432 | 0.023 | 0.609 | 0.784 | 0.052 |
| C/N | −0.057 | −0.883 | 0.216 | 0.203 | −0.845 | 0.459 |
| KM3 | 0.886 | 0.387 | 0.061 | 0.634 | 0.759 | 0.083 |
| PM3 | −0.785 | −0.530 | 0.020 | −0.511 | −0.853 | 0.027 |
| H | 0.268 | 0.333 | 0.125 | 0.210 | 0.196 | 0.949 |
| W | −0.712 | −0.595 | −0.305 | 0.917 | −0.247 | 0.142 |
| N leaf | 0.976 | −0.044 | 0.200 | 0.830 | 0.283 | 0.421 |
| P leaf | 0.948 | −0.192 | 0.090 | 0.479 | 0.856 | 0.065 |
| K leaf | −0.127 | 0.033 | 0.339 | −0.118 | −0.572 | 0.808 |
| Tartaric acid | −0.019 | −0.962 | −0.273 | 0.751 | −0.180 | 0.253 |
| Aspartic acid | −0.104 | −0.246 | −0.370 | 0.914 | 0.183 | −0.263 |
| Caffeic acid | 0.615 | 0.212 | 0.342 | 0.544 | 0.219 | 0.765 |
| Glucose | −0.308 | 0.073 | −0.898 | −0.174 | 0.897 | 0.198 |
| Sucrose | 0.701 | 0.025 | 0.296 | 0.967 | 0.092 | −0.006 |
| Kaempferol | Nd | Nd | Nd | 0.109 | 0.947 | −0.288 |
| Quercetin 3 glucoside | 0.818 | 0.518 | 0.012 | 0.891 | 0.394 | −0.094 |
| Quercetin | 0.769 | 0.177 | −0.163 | 0.823 | 0.519 | −0.198 |
| Glutamine | Nd | Nd | Nd | −0.712 | 0.402 | −0.546 |
| Proline | Nd | Nd | Nd | −0.814 | −0.507 | −0.265 |
| TDM | −0.940 | −0.183 | −0.039 | −0.279 | −0.958 | −0.036 |
| N TDM | −0.166 | −0.191 | −0.951 | −0.509 | −0.451 | 0.567 |
| P TDM | −0.631 | −0.503 | 0.415 | −0.721 | −0.589 | 0.363 |
| K TDM | 0.510 | 0.571 | 0.123 | 0.681 | 0.612 | −0.399 |
| Variance (%) | 62 | 15 | 10 | 62 | 22 | 10 |
| Cumulative variance (%) | 62 | 77 | 87 | 62 | 85 | 95 |
| Eigenvalues | 14.904 | 3.594 | 2.429 | 16.843 | 6.045 | 2.830 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grilli, E.; Vigliotti, R.C.; Fiorentino, A.; Scognamiglio, M.; Rossetti, L.; Nogueira, T.A.R.; Jani, A.D.; Abreu-Junior, C.H.; Ribeiro Roder, L.; Ganga, A.; et al. Constructed Technosols as a Soil Rebuilding Technique to Reclaim Abandoned Limestone Quarries in the Mediterranean Region: A Field Study. Sustainability 2023, 15, 5036. https://doi.org/10.3390/su15065036
Grilli E, Vigliotti RC, Fiorentino A, Scognamiglio M, Rossetti L, Nogueira TAR, Jani AD, Abreu-Junior CH, Ribeiro Roder L, Ganga A, et al. Constructed Technosols as a Soil Rebuilding Technique to Reclaim Abandoned Limestone Quarries in the Mediterranean Region: A Field Study. Sustainability. 2023; 15(6):5036. https://doi.org/10.3390/su15065036
Chicago/Turabian StyleGrilli, Eleonora, Renata Concetta Vigliotti, Antonio Fiorentino, Monica Scognamiglio, Luigi Rossetti, Thiago Assis Rodrigues Nogueira, Arun Dilipkumar Jani, Cassio Hamilton Abreu-Junior, Ludmila Ribeiro Roder, Antonio Ganga, and et al. 2023. "Constructed Technosols as a Soil Rebuilding Technique to Reclaim Abandoned Limestone Quarries in the Mediterranean Region: A Field Study" Sustainability 15, no. 6: 5036. https://doi.org/10.3390/su15065036
APA StyleGrilli, E., Vigliotti, R. C., Fiorentino, A., Scognamiglio, M., Rossetti, L., Nogueira, T. A. R., Jani, A. D., Abreu-Junior, C. H., Ribeiro Roder, L., Ganga, A., & Capra, G. F. (2023). Constructed Technosols as a Soil Rebuilding Technique to Reclaim Abandoned Limestone Quarries in the Mediterranean Region: A Field Study. Sustainability, 15(6), 5036. https://doi.org/10.3390/su15065036











