Evolution of Hydrogeochemistry in the Haolebaojinao Watershed of the Ordos Basin, China
Abstract
:1. Introduction
2. Overview of the Study Area
3. Methods
3.1. Water Sample Collection
3.2. Analysis and Testing
4. Results
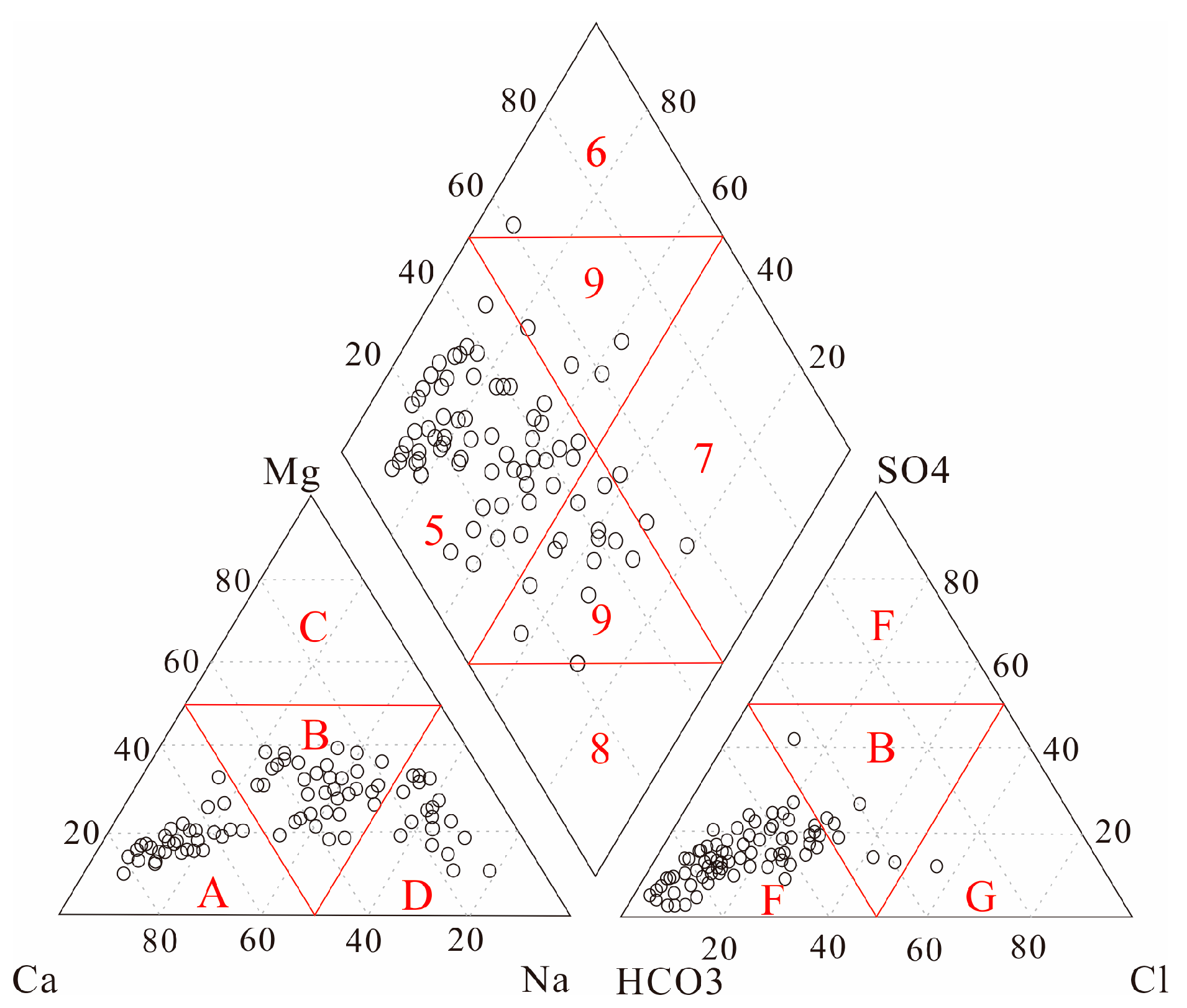
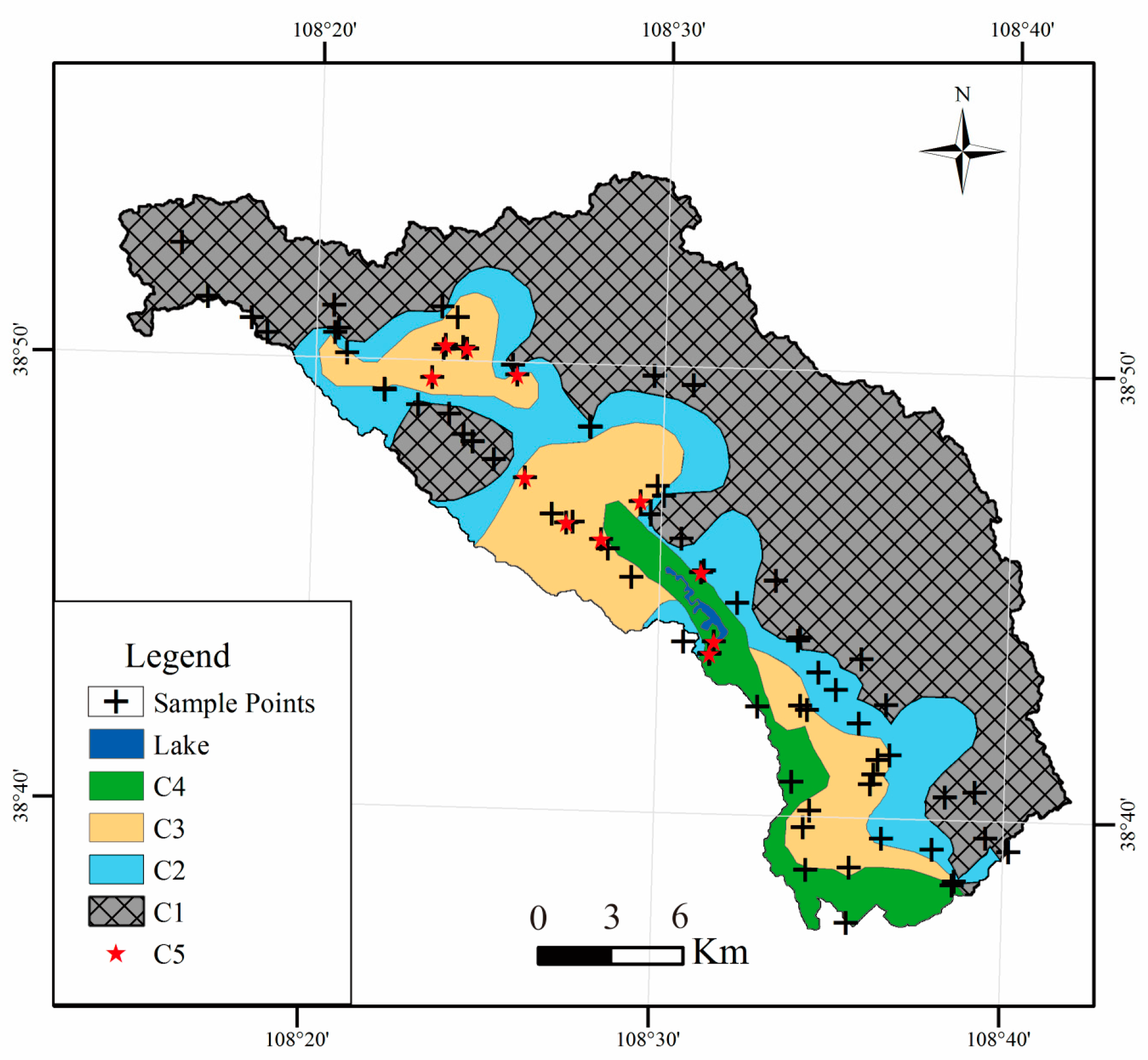
4.1. Water Chemistry
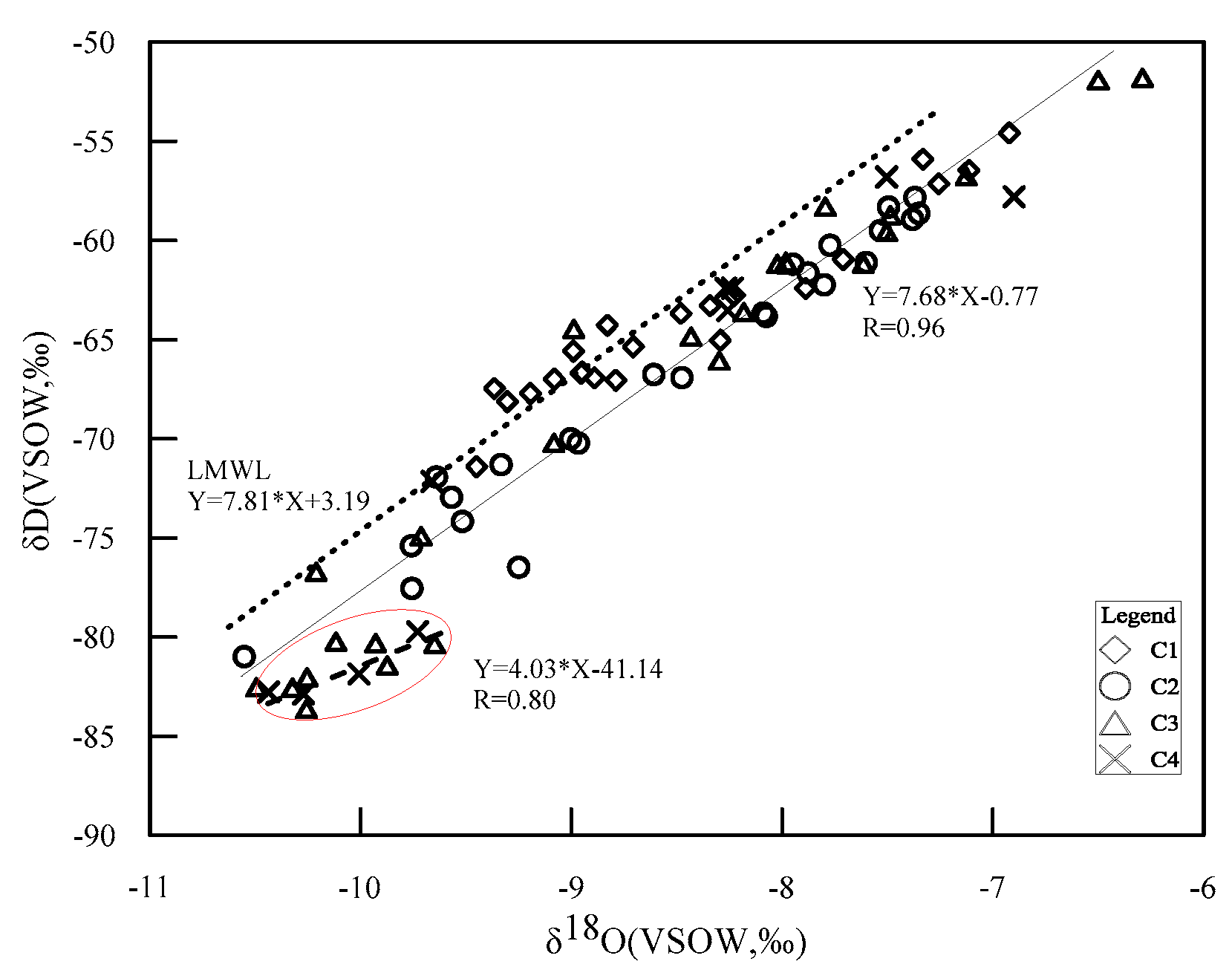
4.2. Isotopes
5. Discussion
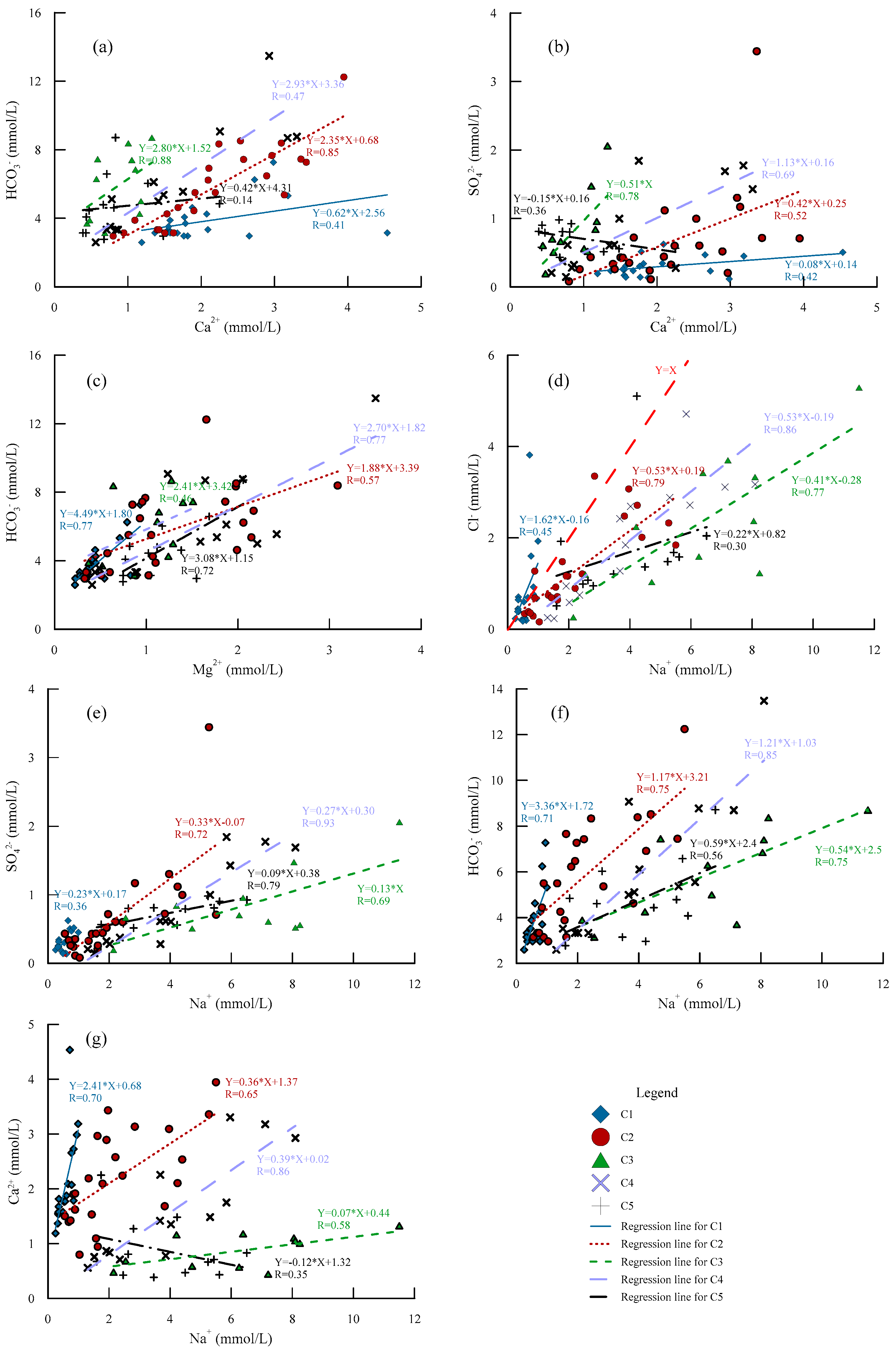
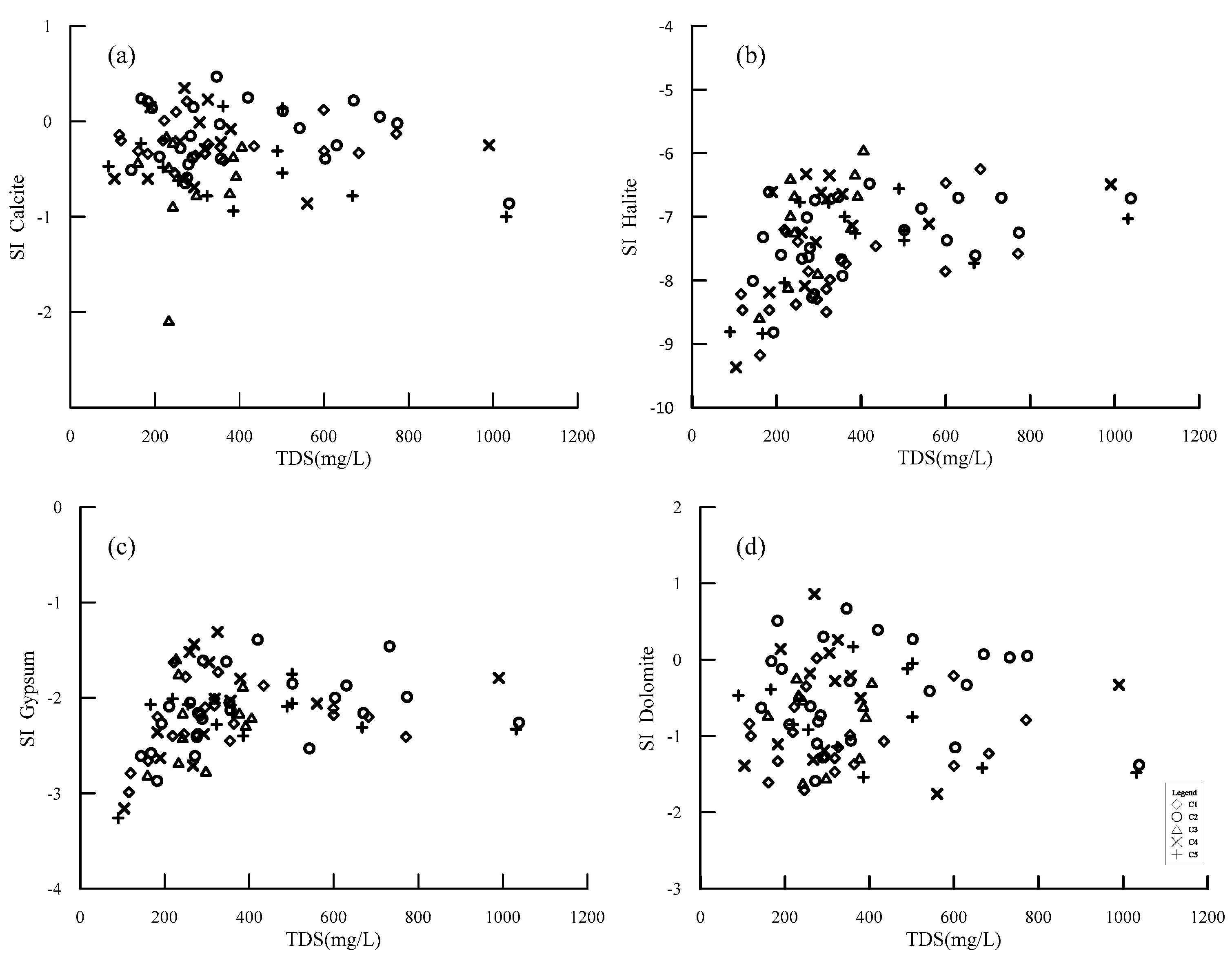
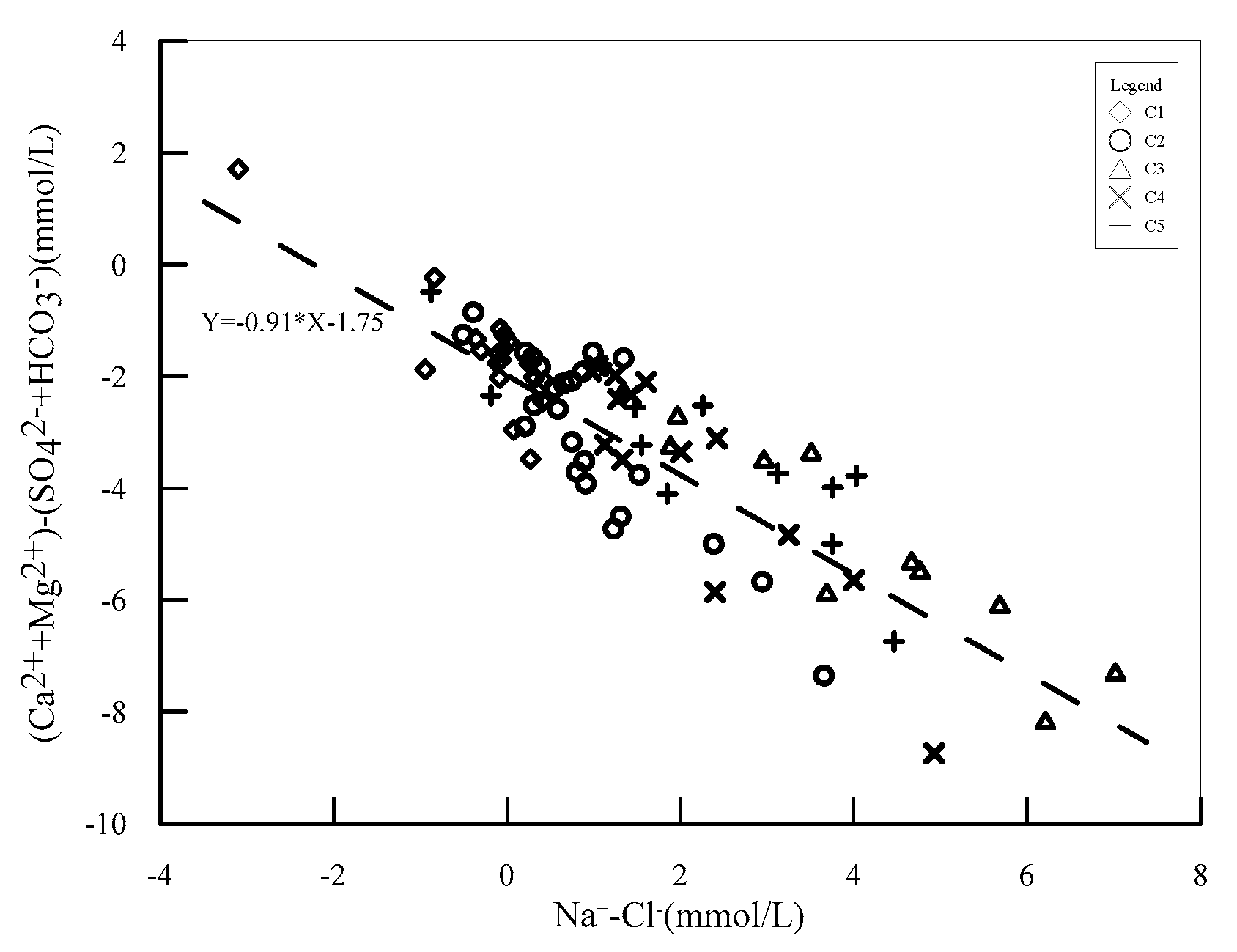
5.1. Hydrogeochemical Processes
5.2. Formation Mechanisms of the Water Chemistry
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, S.; Li, P.; Su, F.; Wang, D.; Ren, X. Identification and apportionment of shallow groundwater nitrate pollution in Weining Plain, northwest China, using hydrochemical indices, nitrate stable isotopes, and the new Bayesian stable isotope mixing model (MixSIAR). Environ. Pollut. 2022, 298, 118852. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, P.; Chen, W.; He, S.; Li, F.; Mu, D.; Elumalai, V. Impacts of land use/land cover patterns on groundwater quality in the Guanzhong Basin of northwest China. Geocarto. Int. 2022, 37, 1–17. [Google Scholar] [CrossRef]
- Liu, F.; Song, X.; Yang, L.; Han, D.; Zhang, Y.; Ma, Y.; Bu, H. The role of anthropogenic and natural factors in shaping the geochemical evolution of groundwater in the Subei Lake basin, Ordos energy base, Northwestern China. Sci. Total Environ. 2015, 538, 327–340. [Google Scholar] [CrossRef]
- Nakamura, T.; Nishida, K.; Kazama, F. Influence of a dual monsoon system and two sources of groundwater recharge on Kofu basin alluvial fans, Japan. Hydrol. Res. 2017, 48, 1071–1087. [Google Scholar] [CrossRef]
- Chen, J.; Qian, H.; Gao, Y.Y.; Wang, H.; Zhang, M. Insights into hydrological and hydrochemical processes in response to water replenishment for lakes in arid regions. J. Hydrol. 2020, 581, 124386. [Google Scholar] [CrossRef]
- Kamal, S.; Sefiani, S.; Laftouhi, N.; El Mandour, A.; Moustadraf, J.; Elgettafi, M.; Himi, M.; Casas, A. Hydrochemical and isotopic assessment for characterizing groundwater quality and recharge processes under a semi arid area: Case of the Haouz plain aquifer (Central Morocco). J. Afr. Earth Sci. 2021, 174, 104077. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, J.; Wang, P.; Wang, T.; Li, Y. Groundwater-fed oasis in arid Northwest China: Insights into hydrological and hydrochemical processes. J. Hydrol. 2021, 597, 123154. [Google Scholar] [CrossRef]
- Wang, D.; Li, P.; He, X.; He, S. Exploring the response of shallow groundwater to precipitation in the northern piedmont of the Qinling Mountains, China. Urban Clim. 2023, 47, 101379. [Google Scholar] [CrossRef]
- Wang, H.B.; Li, X.; Xiao, J.F.; Ma, M.; Tan, J.; Wang, X.; Geng, L. Carbon fluxes across alpine, oasis, and desert ecosystems in northwestern China: The importance of water availability. Sci. Total Environ. 2019, 697, 133978. [Google Scholar] [CrossRef]
- Yang, G.; Tian, L.; Li, X.; He, X.; Gao, Y.; Li, F.; Xue, L.; Li, P. Numerical assessment of the effect of water-saving irrigation on the water cycle at the Manas River Basin oasis, China. Sci. Total Environ. 2019, 691, 506–515. [Google Scholar] [CrossRef]
- Bruce, B.Y.; Sandow, M.Y.; Emnmnuel, N. Hydrocliemical analysis of groundwater using multivariate statistical methods-the Volta region. Can. J. Civ. Eng. 2010, 13, 55–63. [Google Scholar]
- Liu, Y.; Yamanaka, T. Tracing groundwater recharge sources in a mountain–plain transitional area using stable isotopes and hydrochemistry. J. Hydrol. 2012, 464, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Abel, O.T.; Moshood, N.T. Hydrochemical and stable isotopic characterization ofshallow groundwater system in the crystalline basement terrain of Ekitiarea, southwestern Nigeria. Appl. Water Sci. 2014, 3, 229–245. [Google Scholar]
- Reza, D.; Navid, S.H.; Jafar, S.S.; Taghipour, H. Integrated assessment of spatial and temporal variations of groundwater quality in the eastern area of Urmia Salt Lakefiasin using multivariate statistical anaIysis. Water Resour. Manag. 2015, 29, 1351–1364. [Google Scholar]
- Ayla, B. Assessment of the hydrogeochemical characteristics of groundwater in two aquifer systems in Cumra Plain, Central Anatolia. Environ. Earth Sci. 2016, 75, 674. [Google Scholar]
- Argamasilla, M.; Barbera, J.A.; Andreo, B. Factors controlling groundwater salinization and hydrogeochemical processes in coastal aquifers from southern Spain. Sci. Total Environ. 2018, 580, 50–68. [Google Scholar] [CrossRef]
- Bouteraa, O.; Mebarki, A.; Bouaicha, F.; Nouaceur, Z.; Laignel, B. Groundwater quality assessment using multivariate analysis, geostatistical modeling, and water quality index (WQI): A case of study in the Boumerzoug-El Khroub valley of Northeast Algeria. Acta Geochim. 2021, 38, 796–814. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Ma, H.; Wang, L.; Martín, J.D. Identification of the hydrogeochemical processes and assessment of groundwater quality using classic integrated geochemical methods in the Southeastern part of Ordos basin, China. Environ. Pollut. 2016, 218, 879–888. [Google Scholar] [CrossRef]
- Wu, C.; Su, X.; Guo, J.; Dong, W. Multivariate statistical analysis of hydrogeochemical evolution of groundwater in Cretaceous aquifer Ordos desert plateau. Glob. Geol. 2011, 30, 244–253. (In Chinese) [Google Scholar]
- Zhang, H.; Jiang, X.; Wan, L.; Ke, S.; Liu, S.; Han, G.; Guo, H.; Dong, A. Fractionation of Mg isotopes by clay formation and calcite precipitation in groundwater with long residence times in a sandstone aquifer, Ordos Basin, China. Geochim. Cosmochim. Acta 2018, 237, 261–274. [Google Scholar] [CrossRef]
- Zhang, R. Hydrochemical characteristics and influencing factors of Cretaceous groundwater in the northern Ordos Basin. Coal Chem. Ind. 2019, 42, 57–64. (In Chinese) [Google Scholar]
- Wang, X.R.; Yin, L.; Zhang, J.; Wang, X. Simulation and assessment on allowable groundwater exploitation in the Haolebaoji well field Based on ecological constraint. Geotech. Investig. Surv. 2021, 3, 36–42. (In Chinese) [Google Scholar]
- Wang, X.; Yin, L.; Fang, K.; Zhang, J.; Wang, X. Inspection and assessment of the environmental impacts of groundwater exploitation at the Haolebaoji wellfield in Inner Mongolia. Hydrogeol. Eng. Geol. 2019, 46, 5–12. (In Chinese) [Google Scholar]
- Greenpeace. Coals devouring water-A survey on the environmental impacts of Shenhua coal conversion to oil project in Ordos. Green Leaf 2013, 8, 88–92. (In Chinese) [Google Scholar]
- Jiang, X.W.; Wan, L.; Wang, J.Z.; Yin, B.; Fu, W.; Lin, C. Field identification of groundwater flow systems and hydraulic traps in drainage basins using a geophysical method. Geophys. Res. Lett. 2014, 41, 2812–2819. [Google Scholar] [CrossRef]
- Sun, F. Research on Groundwater Circulation and Environment Effect of Dusitu River in Ordos Basin; Chang’an University: Xi’an, China, 2010; (In Chinese with English Abstract). [Google Scholar]
- Piper, A.M. A Graphic Procedure in the Geochemical Interpretation of Water Analysis. In Environmental Isotopes in Hydrogeology; Fritz, I., Ed.; United States Department of the Interior, Geological Survey, Water Resources Division, Ground Water Branch: Washington, DC, USA; CRC Press: Boca Raton, FL, USA, 1953; p. 63. [Google Scholar]
- Zhang, X.; Guo, Q.; Liu, M.; Luo, J.; Yin, Z.; Zhang, C.; Zhu, M.; Guo, W.; Li, J.; Zhou, C. Hydrogeochemical processes occurring in the hydrothermal systems of the Gonghe–Guide Basin, northwestern China: Critical insights from a principal components analysis (PCA). Environ. Earth Sci. 2016, 75, 1–17. [Google Scholar] [CrossRef]
- Kang, X.; Niu, Y.; Yu, H.; Gou, P.; Hou, Q.; Lu, X.; Wu, Y. Effect of rainfall-runoff process on sources and transformations of nitrate using a combined approach of dual isotopes, hydrochemical and Bayesian model in the Dagang River basin. Sci. Total Environ. 2022, 837, 155674. [Google Scholar] [CrossRef]
- Kong, X.; Wang, S.; Liu, B.; Sun, H.; Sheng, Z. Impact of water transfer on interaction between surface water and groundwater in the lowland area of North China Plain. Hydrol. Process. 2018, 32, 2044–2057. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, Q.; Qian, H.; Li, M.; Hou, K. Characterization of geothermal water in the piedmont region of Qinling Mountains and Lantian-Bahe Group in Guanzhong Basin, China. Environ. Earth Sci. 2019, 78, 442. [Google Scholar] [CrossRef]
- Yin, Z.; Luo, Q.; Wu, J.; Xu, S.; Wu, J. Identification of the long-term variations of groundwater and their governing factors based on hydrochemical and isotopic data in a river basin. J. Hydrol. 2021, 592, 125604. [Google Scholar] [CrossRef]
- Shamsi, A.; Karami, G.H.; Hunkeler, D.; Taheri, A. Isotopic and hydrogeochemical evaluation of springs discharging from high-elevation karst aquifers in Lar National Park, northern Iran. Hydrogeol. J. 2019, 27, 655–667. [Google Scholar] [CrossRef]
- Lihe, Y.; Guangcai, H.; Zhengping, T.; Ying, L. Origin and recharge estimates of groundwater in the ordos plateau, People’s Republic of China. Environ. Earth Sci. 2010, 60, 1731–1738. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, X.; Wan, L.; Han, G.; Guo, H. Hydrogeochemical characterization of groundwater flow systems in the discharge area of a river basin. J. Hydrol. 2015, 527, 433–441. [Google Scholar] [CrossRef]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Appelo, C.A.J.; Willemsen, A. Geochemical calculations and observations on salt water intrusions, I. A combined geochemical/minxing cell model. J. Hydrol. 1987, 94, 313–330. [Google Scholar] [CrossRef]
- Duan, R.; Li, P.; Wang, L.; He, X.; Zhang, L. Hydrochemical characteristics, hydrochemical processes and recharge sources of the geothermal systems in Lanzhou City, northwestern China. Urban Clim. 2022, 43, 1–15. [Google Scholar] [CrossRef]
- Su, Y.H.; Zhu, G.F.; Feng, Q.; Li, Z.Z.; Zhang, F.P. Environmental isotopic and hydrochemical study of groundwater in the Ejina Basin, northwest China. Environ. Geol. 2009, 58, 601–614. [Google Scholar] [CrossRef]
- Bekele, E.; Zhang, Y.; Donn, M.; McFarlane, D. Inferring groundwater dynamics in a coastal aquifer near wastewater infiltration ponds and shallow wetlands (Kwinana, Western Australia) using combined hydrochemical, isotopic and statistical approaches. J. Hydrol. 2019, 568, 1055–1070. [Google Scholar] [CrossRef]
- Noble, J.; Ansari, M.A. Isotope hydrology and geophysical techniques for reviving a part of the drought prone areas of Vidarbha, Maharashtra, India. J. Hydrol. 2019, 570, 495–507. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, J.J.; Pu, J.; Wang, P.; Liang, X.; Yang, P.; He, Q.; Gou, P.; Yuan, D. Integrated understanding of the Critical Zone processes in a subtropical karst watershed (Qingmuguan, Southwestern China): Hydrochemical and isotopic constraints. Sci. Total Environ. 2020, 749, 141257. [Google Scholar] [CrossRef]
- Dugga, P.; Pervez, S.; Tripathi, M.; Siddiqui, M.N. Spatiotemporal variability and source apportionment of the ionic components of groundwater of a mineral-rich tribal belt in Bastar, India. Groundw. Sustain. Dev. 2020, 10, 100356. [Google Scholar] [CrossRef]
- Hou, G.; Zhao, M.; Wang, Y. Groundwater Investigation in the Ordos Basin; China Geological Survey: Beijing, China, 2006. (In Chinese)
- Yin, L.; Hou, G.; Dou, Y.; Tao, Z.; Li, Y. Hydrogeochemical and isotopic study of groundwater in the Habor Lake Basin of the Ordos Plateau, NW China. Environ. Earth 2009, 64, 1575–1584. [Google Scholar] [CrossRef]
- Magaritz, M.; Nadler, A.; Koyumdjisky, H.; Dan, J. The use of Na/Cl ratios to trace solute sources in a semiarid zone. Water Resour. Res. 1981, 17, 602–608. [Google Scholar] [CrossRef]
- Dixon, W.; Chiswell, B. The use of hydrochemical sections to identify recharge areas and saline intrusions in alluvial aquifers, southeast Queensland, Australia. J. Hydrol. 1992, 135, 259–274. [Google Scholar] [CrossRef]
- Belkhiri, L.; Boudoukha, A.; Mouni, L.; Baouz, T. Statistical categorization geochemical modeling of groundwater in Ain Azel plain (Algeria). J. Afr. Earth Sci. 2011, 59, 140–148. [Google Scholar] [CrossRef]
- Schoeller, H. Qualitative evaluation of groundwater resources. In Methods and Techniques of Groundwater Investigations and Development; UNESCO: Paris, France, 1956; pp. 54–83. [Google Scholar]
- Li, P.; Qian, H.; Wu, J.; Zhang, Y.; Zhang, H. Major ion chemistry of shallow groundwater in the Dongsheng Coalfield, Ordos Basin, China. Mine Water Environ. 2013, 32, 195–206. [Google Scholar] [CrossRef]
- Kumar, S.; Venkatesh, A.S.; Singh, R.; Udayabhanu, G.; Saha, D. Geochemical signatures and isotopic systematics constraining dynamics of fluoride contamination in groundwater across Jamui district, Indo-Gangetic alluvial plains, India. Chemosphere 2018, 205, 493–505. [Google Scholar] [CrossRef]
- Jampani, M.; Liedl, R.; Hülsmann, S.; Sonkamble, S.; Amerasinghe, P. Hydrogeochemical and mixing processes controlling groundwater chemistry in a wastewater irrigated agricultural system of India. Chemosphere 2020, 239, 124741. [Google Scholar] [CrossRef]
- Drever, J.I. The Geochemistry of Natural Waters: Surface and Groundwater Environments; Prentice Hall: Hoboken, NJ, USA, 1997. [Google Scholar]
- Kim, K.H.; Yun, S.T.; Yu, S.; Choi, B.; Kim, M.; Lee, K. Geochemical pattern recognitions of deep thermal groundwater in South Korea using self-organizing map: Identified pathways of geochemical reaction and mixing. J. Hydrol. 2020, 589, 125202. [Google Scholar] [CrossRef]
- Ghobadi, M.H.; Dehban Avan Stakhri, M.; Mirarabi, A. Investigating the hydrogeological properties of springs in a karstic aquifer in Dorfak region (Guilan Province, Iran). Environ. Earth Sci. 2018, 77, 96. [Google Scholar] [CrossRef]
- Manoj, S.; Thirumurugan, M.; Elango, L. Hydrogeochemical modelling to understand the surface water-groundwater interaction around a proposed uranium mining site. J. Earth Syst. Sci. 2019, 128, 49. [Google Scholar] [CrossRef] [Green Version]
- Yang, N.; Wang, G.C.; Shi, Z.M.; Zhao, D.; Jiang, W.; Guo, L.; Liao, F.; Zhou, P. Application of multiple approaches to investigate the hydrochemistry evolution of groundwater in an Arid Region: Nomhon, Northwestern China. Water 2018, 10, 1667. [Google Scholar] [CrossRef] [Green Version]
- Ma, R.; Shi, J.S.; Liu, J.C.; Gui, C. Combined use of multivariate statistical analysis and hydrochemical analysis for groundwater quality evolution: A case study in north chain plain. J. Earth Sci. 2014, 25, 587–597. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Marghade, D.; Malpe, D.B.; Zade, A.B. Major ion chemistry of shallow groundwater of a fast growing city of Central India. Environ. Monit. Assess. 2012, 184, 2405–2418. [Google Scholar] [CrossRef]
- Xing, L.; Guo, H.; Zhan, Y. Groundwater hydrochemical characteristics and processes along flow paths in the North China Plain. J. Asian Earth Sci. 2013, 70–71, 250–264. [Google Scholar] [CrossRef]
- Pant, R.R.; Zhang, F.; Rehman, F.U.; Wang, G.; Ye, M.; Zeng, C.; Tang, H. Spatiotemporal variations of hydrogeochemistry and its controlling factors in the Gandaki River Basin, Central Himalaya Nepal. Sci. Total Environ. 2018, 622–623, 770–782. [Google Scholar] [CrossRef]
- Winnick, M.J.; Maher, K. Relationships between CO2, thermodynamic limits on silicate weathering, and the strength of the silicate weathering feedback. Earth Planet. Sci. Lett. 2018, 485, 111–120. [Google Scholar] [CrossRef]




| Project | Minimum | Maximum | Median | Average Value |
|---|---|---|---|---|
| pH | 7.49 | 9.53 | 7.94 | 7.99 |
| TDS (mg/L) | 90 | 1038 | 305.4 | 366.15 |
| K+ (mg/L) | 0.41 | 19.61 | 2.46 | 2.88 |
| Na+ (mg/L) | 5.68 | 264.57 | 46.39 | 67.37 |
| Ca2+ (mg/L) | 15.445 | 181.45 | 61.4 | 67.08 |
| Mg2+ (mg/L) | 5.34 | 84.08 | 22.34 | 25.76 |
| Cl− (mg/L) | 5.71 | 187.79 | 41.25 | 52.37 |
| SO42− (mg/L) | 7.90 | 330.41 | 49.65 | 60.18 |
| HCO3− (mg/L) | 158.05 | 822.66 | 281.43 | 316.91 |
| CO32− (mg/L) | 0 | 33.31 | 0 | 0.71 |
| NO3− (mg/L) | 0 | 95.81 | 4.66 | 11.38 |
| δD (‰) | −83.61 | −51.79 | −65.19 | −67.04 |
| δ18O (‰) | −10.55 | −6.28 | −8.48 | −8.62 |
| Grouping | Group C1 Groundwater | Group C2 Groundwater | Group C3 Groundwater | Group C4 Groundwater | Group C5 Groundwater | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| project | F1 | F2 | F3 | F1 | F2 | F3 | F1 | F2 | F3 | F1 | F2 | F3 | F1 | F2 | F3 |
| Na+ | 0.67 | 0.57 | 0.07 | 0.86 | −0.15 | 0.11 | 0.72 | 0.41 | −0.49 | 0.33 | 0.89 | 0.11 | 0.92 | 0.00 | 0.28 |
| Ca2+ | 0.91 | 0.27 | 0.02 | 0.72 | 0.48 | 0.28 | 0.18 | 0.41 | 0.65 | 0.85 | 0.25 | 0.18 | 0.01 | −0.20 | 0.94 |
| Mg2+ | 0.83 | 0.48 | 0.16 | 0.86 | 0.16 | −0.25 | 0.77 | 0.22 | 0.51 | 0.94 | 0.11 | 0.14 | 0.73 | 0.24 | 0.58 |
| Cl− | 0.88 | −0.04 | −0.04 | 0.91 | −0.10 | −0.13 | 0.93 | 0.04 | 0.29 | 0.17 | 0.91 | 0.12 | 0.06 | −0.16 | 0.91 |
| SO42− | 0.86 | −0.01 | 0.01 | 0.77 | −0.05 | −0.08 | 0.97 | 0.00 | 0.10 | 0.52 | 0.72 | 0.23 | 0.86 | 0.01 | −0.30 |
| HCO3− | 0.37 | 0.80 | 0.07 | 0.72 | 0.37 | 0.38 | 0.30 | 0.83 | −0.03 | 0.88 | 0.42 | 0.11 | 0.89 | 0.27 | 0.11 |
| pH | −0.32 | −0.05 | −0.78 | −0.08 | −0.74 | −0.04 | −0.17 | 0.06 | −0.88 | −0.16 | 0.06 | −0.83 | 0.23 | 0.61 | −0.21 |
| δ18O | 0.04 | 0.96 | 0.07 | −0.04 | 0.94 | 0.06 | 0.12 | 0.88 | 0.26 | −0.07 | 0.45 | 0.82 | 0.07 | 0.96 | 0.04 |
| δD | 0.02 | 0.93 | 0.10 | −0.02 | 0.96 | 0.01 | 0.17 | 0.90 | 0.23 | 0.10 | 0.49 | 0.79 | −0.01 | 0.94 | −0.28 |
| Eigenvalues | 3.75 | 3.08 | 1.34 | 3.95 | 2.78 | 1.22 | 3.13 | 2.88 | 1.94 | 2.87 | 2.83 | 2.50 | 3.10 | 2.53 | 2.45 |
| Contribution rate/% | 37.52 | 30.75 | 13.42 | 39.48 | 27.77 | 12.23 | 31.32 | 28.84 | 19.39 | 28.73 | 28.25 | 25.02 | 31.04 | 25.30 | 24.52 |
| Cumulative contribution rate/% | 37.52 | 68.27 | 81.68 | 39.48 | 67.24 | 79.47 | 31.32 | 60.16 | 79.54 | 28.73 | 56.99 | 82.00 | 31.04 | 56.34 | 80.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Zhang, R.; Han, P.-F.; Wang, J.; Wan, L. Evolution of Hydrogeochemistry in the Haolebaojinao Watershed of the Ordos Basin, China. Sustainability 2023, 15, 5091. https://doi.org/10.3390/su15065091
Zhang B, Zhang R, Han P-F, Wang J, Wan L. Evolution of Hydrogeochemistry in the Haolebaojinao Watershed of the Ordos Basin, China. Sustainability. 2023; 15(6):5091. https://doi.org/10.3390/su15065091
Chicago/Turabian StyleZhang, Baoyun, Ruolin Zhang, Peng-Fei Han, Junzhi Wang, and Li Wan. 2023. "Evolution of Hydrogeochemistry in the Haolebaojinao Watershed of the Ordos Basin, China" Sustainability 15, no. 6: 5091. https://doi.org/10.3390/su15065091







