The Pandemic Puzzle—Reviewing the Existing Pieces, Searching for the Missing Ones
Abstract
:1. Introduction
1.1. Theoretical Background—Mirroring the Present and the Future in the Context of (Re)emerging Infectious Diseases
1.2. Aim of the Research
2. Materials and Methods
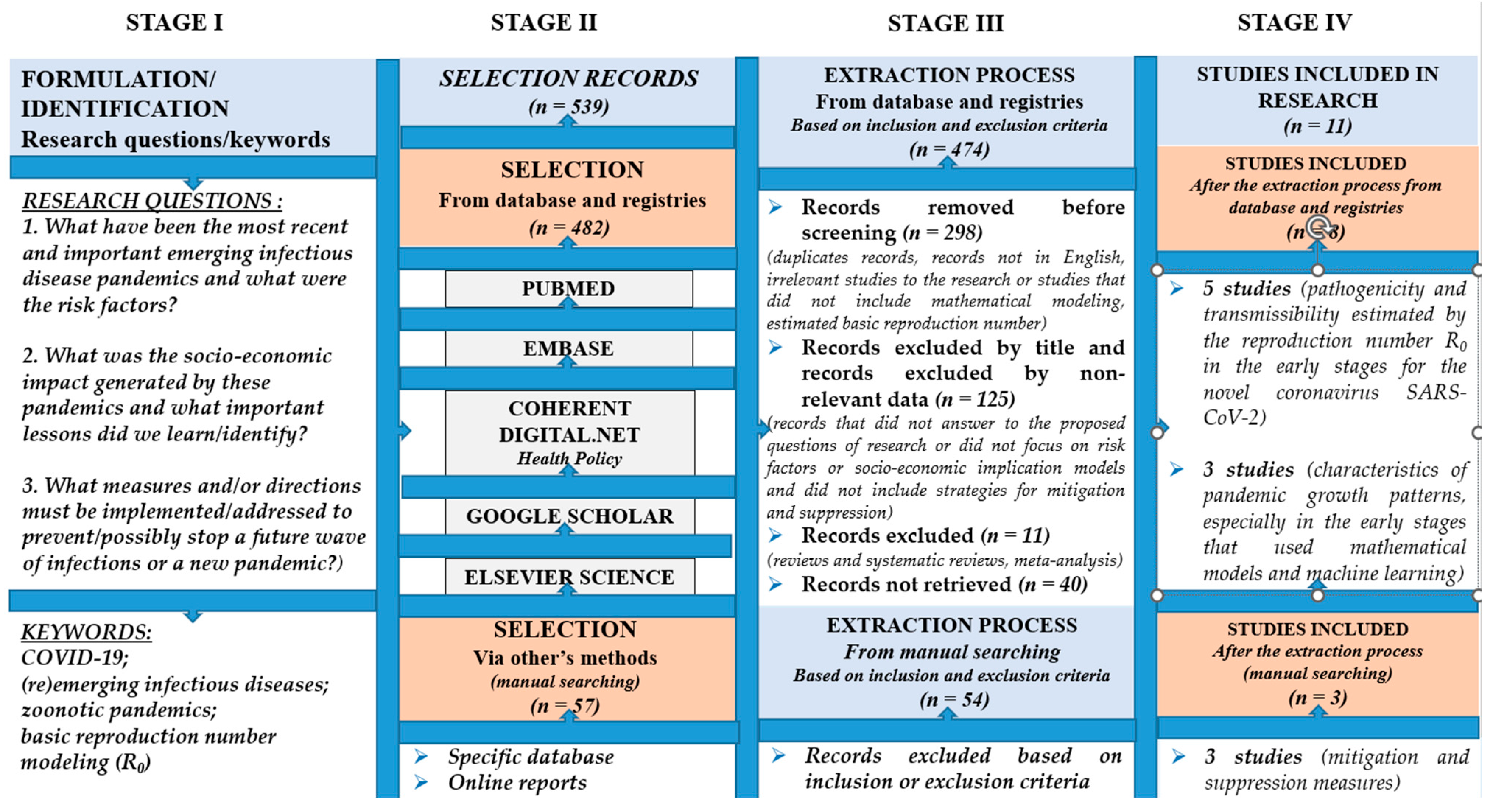
2.1. Search Method
2.2. Selection Criteria
- Studies published between January 2017 and December 2022.
- Relevant articles according to the year of publication, the name of the researcher, the category of the research, the title of the source and the list of journals.
- Studies published in English.
- Studies based on mathematical modeling to track the parameters of transmissibility (estimated by R0) and severity (health indicators) of (re)emerging infectious diseases in early phases of the outbreaks.
- Studies based on the implications and prevention plans applied to prevent/combat zoonotic pandemics (avian, porcine, those due to the Coronavirus family as well as some emerging viruses considered possible future threats (Ebola, Nipah and Hantavirus)).
- Studies focused on risk factors that can trigger pandemics.
- Studies regarding the evolution of (re)emerging infectious diseases and the socioeconomic implication models.
- Articles that were irrelevant to the research (symposia, books, workshops or discussion papers).
- Duplicates and articles that were not in English.
- Studies that did not include mathematical modeling or estimated of the basic reproduction number (R0) of (re)emerging infectious diseases in early phases of the outbreaks.
- Studies that did not answer the proposed research questions.
- Studies that did not focus on risk factors that can trigger pandemics or to evolution and socioeconomic implication models.
- Studies that did not include implications and prevention plans applied to prevent/combat zoonotic pandemics (avian, porcine, those due to the Coronavirus family as well as some emerging viruses considered possible future threats (Ebola, Nipah and Hantavirus)).
- Systematic reviews and meta-analysis.
3. Results
3.1. Brief History of Pathogens That Have Influenced Medical Research
3.2. Risk Factors Involved in the Development of Emerging Infectious Diseases
| Risk Factors | Variables |
|---|---|
| Virus [19] |
|
| Human host [19] |
|
| External factors (medium) [20] |
|
3.3. The Characteristics of Pandemics and the Socioeconomic Implications Generated by (Re)emerging Infectious Disease
3.3.1. Characteristics
| Years | Virus/ Continent/ Epicentre | Items of Interest | Severity Criteria/ Risk of Spread (R0) | Estimated Costs |
|---|---|---|---|---|
| 2019 | Coronavirus SARS-CoV-2/Asia (China) | Member of the Coronaviridae Family. Origin: bats. Transmission: respiratory droplets and aerosol. Vulnerable groups: elderly | R0: 3.3–5.5 [9] R0: 3.6–4.0 [10] R0: 4.71(2019) and 2.08 (2020) [11] CFR: 3%; R0: 1.4–5.5 [12] 6,679,319 deaths worldwide [30] | €40.5 billion [18] |
| 2012–2015 | Coronavirus MERS-Co-V/Asia (Saudi Arabia) | Origin: bats. Coronaviridae family. Transmission: respiratory droplets. Vulnerable groups: elderly | CFR: 40%; 2502 cases and R0 < 1 [12] 2193 cases of infections, 858 deaths alone in Saudi Arabia and 2600 of cases with 935 deaths in 27 countries, CFR 34.4% [31] | $US 2.6 billion in lost tourism revenue [26] |
| 2003 | Coronavirus SARS-CoV-1/Asia (China) | Spread in 29 countries on 5 continents. The main factor that led to the spread of the virus was the high mobility of the population and the lack of measures to isolate the outbreak. Transmission: respiratory droplets. Vulnerable groups: elderly | R0: 2–5 and CFR 10% [12] 800 deaths worldwide and CFR 9–10% [32,33] | $US 6 billion [26] close to $40 billion [33] |
| 2001–2008 | Nipah virus/Asia (Malaysia and Singapore) | Paramyxoviridae family related to Hendra virus. Origin: bats. Transmission: bodily fluids, contaminate food. People can also develop asymptomatic infection. Symptoms include moderate to severe respiratory infection, and inflammation of the brain. | CFR: 40–70% and 700 cases from 1999–2018 WHO Southeast Asia [34] | |
| 1999 | Nipah virus/Asia (Bangladesh and India) | |||
| 2014-2016 | Ebola virus/ Africa (Sierra Leone, Guinea, Congo) | Member of the Filoviridae family Origin: Fruits bats, porcupines, and non-human primates. Transmission: bodily fluids. Vulnerable groups: health workers, women, and young children | R0: 1.5–2.5; CFR 70% [12] R0: 1.2–2.5 [35] CFR: 50%; Over 30,000 cases and 2100 deaths [36] | $US 4.3 billion to fight outbreaks. [18,36] |
| 1993 | Hantavirus/ US | Exposure to certain types of infected rodents can lead to infectious diseases. Pulmonary syndrome—detected in United States. Transmission: respiratory droplets and bodily fluids | 27 cases; R0 = 1.2 (0.8–1.6) [37] | |
| 2009 | Influenza virus—H1N1/ latin america (Mexico) | Origin: pig; Risk factors for the emergence of influenza viruses in humans are urban agglomerations (like South China Southeast Asia), where people live near animals. Transmission: Respiratory droplets. Vulnerable groups: teenagers, young adults. | CFR 0.03%; R0 = 1.2–1.6 [12] More than 150,000 people died into the first year and between 0.001–0.007% of the world’s population died due to respiratory problems in the first year the virus circulated [38] | $US 2.8 billion [26] |
| 1968-1969 | Influenza virus—H3N2 Hong Kong/Asia | Origin: porcin; Hong Kong Influenza A H2N2; Transmission: respiratory droplets. Vulnerable groups: elderly. | 1.1 million deaths 0.03 CFR [38] | |
| 1957-1958 | Influenza Influenza virus—H2N2 Asia (Southern China) | Origin: avian; bird flu; Influenza A H3N2. Transmission: respiratory droplets. Vulnerable groups: children and elderly | 1 million deaths 0.03 CFR [38,39] | |
| 1918–1919 | Influenza virus—H1N1 US | Origin: avian; bird flu. Influenza A H1N1. Transmission: respiratory droplets. Vulnerable groups: adults and pregnant women | CFR: 3%; R0: 1.4–3.8 [12] Over 50 million deaths and 3%CFR [38,40] |
3.3.2. The Measures Implemented and the Socioeconomic Implications
4. Discussion
5. Future Suggestions and Lessons for the Future Pandemics
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Prioritizing Diseases for Research and Development in Emergency Contexts. 2018. Available online: https://www.who.int/activities/prioritizing-diseases-for-research-and-development-in-emergency-contexts (accessed on 21 April 2020).
- Burkle, F.M., Jr. Globalization and Disasters: Issues of Public Health, State Capacity and Political Action. J. Int. Aff. 2006, 59, 241–265. [Google Scholar]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Akin, L.; Gözel, M.G. Understanding dynamics of pandemics. Turk. J. Med. Sci. 2020, 50, 515–519. Available online: http://journals.tubitak.gov.tr/medical/ (accessed on 8 April 2020). [CrossRef] [PubMed]
- Schar, D.L.; Yamey, G.M.; Machalaba, C.C.; Karesh, W. A framework for stimulating economic investments to prevent emerging diseases. Bull. World Health Organ. 2017, 96, 138–140. [Google Scholar] [CrossRef]
- Heesterbeek, H.; Anderson, R.M.; Andreasen, V.; Bansal, S.; De Angelis, D.; Dye, C.; Eames, K.T.D.; Edmunds, W.J.; Frost, S.D.W.; Funk, S.; et al. Threats. Science 2015, 347, aaa4339. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Bi, Q.; Wu, Y.; Mei, S.; Ye, C.; Zou, X.; Zhang, Z.; Liu, X.; Wei, L.; Truelove, S.A.; Zhang, T.; et al. Epidemiology and Transmission of COVID-19 in Shenzhen China: Analysis of 391 Cases and 1286 of Their Close Contacts. 2020. Available online: https://www.medrxiv.org/content/10.1101/2020.03.03.20028423v3 (accessed on 8 April 2020).
- Zhao, S.; Lin, Q.; Ran, J.; Musa, S.S.; Yang, G.; Wang, W.; Lou, Y.; Gao, D.; Yang, L.; He, D.; et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: A data-driven analysis in the early phase of the outbreak. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Read, J.M.; Bridgen, J.R.; Cummings, D.A.; Ho, A.; Jewell, C.P. Novel coronavirus 2019-nCoV: Early estimation of epidemiological parameters and epidemic predictions. medRixiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.; Peng, Z.; Xiao, Y. Modelling the epidemic trend of the 2019 novel coronavirus outbreak in China. bioRxiv 2020, in press. [Google Scholar] [CrossRef]
- Chen, J. Pathogenicity and transmissibility of 2019-nCoV-A quick overview and comparison with other emerging viruses. Microbes Infect. 2020, 22, 69–71. [Google Scholar] [CrossRef]
- Wu, J.T.; Leung, K.; Bushman, M.; Kishore, N.; Niehus, R.; de Salazar, P.M.; Cowling, B.J.; Lipsitch, M.; Leung, G.M. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat. Med. 2020, 26, 506–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, S.; Feng, Z.; Ling, C.; Chang, C.; Feng, Z. Prediction of the COVID-19 epidemic trends based on SEIR and AI models. PLoS ONE 2021, 16, e0245101. [Google Scholar] [CrossRef] [PubMed]
- Winskill, P.; Whittaker, C.; Walker, P.; Watson, O.; Laydon, D.; Imai, N.; Cuomo-Dannenburg, G.; Ainslie, K.; Baguelin, M.; Bhatt, S. Equity in Response to the COVID-19 Pandemic: An Assessment of the Direct and Indirect Impacts on Disadvantaged and Vulnerable Populations in Low- and Lower Middle-Income Countries. Imperial College London (12-05-2020). Available online: https://spiral.imperial.ac.uk/handle/10044/1/78965 (accessed on 18 December 2022).
- Walker, P.; Whittaker, C.; Watson, O.; Baguelin, M.; Ainslie, K.; Bhatia, S.; Bhatt, S.; Boonyasiri, A.; Boyd, O.; Cattarino, L.; et al. The Global Impact of COVID-19 and Strategies for Mitigation and Suppression. Imperial College London (26-03-2020). Available online: https://spiral.imperial.ac.uk/handle/10044/1/77735 (accessed on 18 December 2022).
- Ferguson, N.; Laydon, D.; Nedjati Gilani, G.; Imai, N.; Ainslie, K.; Baguelin, M.; Bhatia, S.; Boonyasiri, A.; Cucunuba Perez, Z.; Cuomo-Dannenburg, G.; et al. Impact of Non-Pharmaceutical Interventions (NPIs) to Reduce COVID-19 Mortality and Healthcare Demand. Imperial College London (16-03-2020). Available online: https://spiral.imperial.ac.uk/handle/10044/1/77482 (accessed on 18 December 2022).
- European Council. Coiuncil of the European Union. Global Solidarity during the COVID-19 Pandemic. Available online: https://www.consilium.europa.eu/en/policies/coronavirus/global-solidarity/ (accessed on 3 August 2022).
- Van Damme, W.; Dahake, R.; Delamou, A.; Ingelbeen, B.; Wouters, E.; Vanham, G.; Van De Pas, R.; Dossou, J.-P.; Ir, P.; Abimbola, S.; et al. The COVID-19 pandemic diverse contexts; different epidemics-how and why? BMJ Glob. Health 2020, 5, e003098. [Google Scholar] [CrossRef] [PubMed]
- Findlater, A.; Bogoch, I.I. Human Mobility and the Global Spread of Infectious Diseases: A Focus on Air Travel. Trends Parasitol. 2018, 34, 772–783. [Google Scholar] [CrossRef]
- Heffernan, J.M.; Smith, R.J.; Wahl, L.M. Perspectives on the basic reproductive ratio. J. R. Soc. Interface R. Soc. 2005, 2, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Barril, C.; Calsina, A.; Ripoll, J. A practical approach to R0 in continuous-time ecological models. Math. Meth. Appl. Sci. 2017, 41, 8432–8445. [Google Scholar] [CrossRef]
- Breda, D.; Florian, F.; Ripoll, J.; Vermiglio, R. Efficient numerical computation of the basic reproduction number for structured populations. J. Comput. Appl. Math. 2021, 384, 113165. [Google Scholar] [CrossRef]
- Last, J.M. (Ed.) . A Dictionary of Epidemiology, 4th ed.; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Mercer, G.; Glass, K.; Beckers, N. Effective reproduction numbers are commonly overestimated early in a disease outbreak. Stat. Med. 2011, 30, 984–994. [Google Scholar] [CrossRef]
- European Parliamentary Research Service. Economic Impact of Epidemics and Pandemics. Available online: https://www.europarl.europa.eu/thinktank/en/document/EPRS_BRI(2020)646195 (accessed on 3 August 2022).
- Imperial College COVID-19 Response Team. Available online: http://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/ (accessed on 18 December 2022).
- Lessler, J.; Azman, A.S.; Grabowski, M.K.; Salje, H.; Rodriguez-Barraquer, I. Trends in the mechanistic and dynamic modeling of infectious diseases. Curr. Epidemiol. Rep. 2016, 3, 212–222. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Prioritizing COVID-19 Contact Tracing Mathematical Methods and Findings. Available online: www.cdc.gov/coronavirus/2019-ncov/php/contact-tracing/contact-tracingresources.htm (accessed on 8 August 2022).
- Worldmater. COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/coronavirus/ (accessed on 16 December 2022).
- World Health Organization. Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON422 (accessed on 16 December 2022).
- Saywell, T.; Fowler, G.A.; Crispin, S.W.; Borsuk, R.; Cohen, M. The cost of SARS: $11 billion and rising. Dow Jones Far East. Econ. Rev. 2003, 166, 12–17. [Google Scholar]
- Flu Outbreaks Reminder of Pandemic Threat; The World Bank: Washington, DC, USA, 2013; Available online: https://www.worldbank.org/en/news/feature/2013/03/05/flu-outbreaks-reminder-of-pandemic-threat (accessed on 18 April 2020).
- World Health Organization. Health Topics. Nipah Virus Infection, Overview. Symptoms. Treatment. Available online: https://www.who.int/health-topics/nipah-virus-infection#tab=tab_1 (accessed on 3 August 2022).
- Van Kerkhove, M.D.; Bento, A.I.; Mills, H.L.; Ferguson, N.M.; Donnelly, C.A. A review of epidemiological parameters from Ebola outbreaks to inform early public health decision-making. Sci. Data 2015, 2, 150019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Bank. Pandemics. Brief. Ebola outbreak in Democratic Republic of Congo. Available online: https://www.worldbank.org/en/topic/pandemics/brief/fact-sheet-world-bank-support-to-10th-ebola-outbreak-in-democratic-republic-of-congo (accessed on 18 April 2021).
- Centers for Disease Control and Prevention. Hantavirus. Reportet Cases of Hantavirius. Available online: https://www.cdc.gov/hantavirus/index.html (accessed on 20 December 2022).
- Centers for Disease Control and Prevention. 2009 H1N1 Pandemic. (H1N1 pdm09 Virus). Available online: https://www.cdc.gov/flu/pandemic-resources/2009-h1n1-pandemic.html (accessed on 20 December 2022).
- Centers for Disease Control and Prevention 1957–1958 Pandemic (H2N2 Virus). Available online: https://www.cdc.gov/flu/pandemic-resources/1957-1958-pandemic.html (accessed on 20 December 2022).
- Centers for Disease Control and Prevention. History of 1918 Flu Pandemics. Available online: https://www.cdc.gov/flu/pandemic-resources/1918-commemoration/1918-pandemic-history.htm (accessed on 20 December 2022).
- European Council. Council of the European Union. EU’s Response to COVID-19. An International Treaty on Pandemic, Prevention, and Preparedness. Available online: https://www.consilium.europa.eu/en/policies/coronavirus/pandemic-treaty/ (accessed on 3 August 2022).
- WHO Director-General’s Opening Remarks at the Global Preparedness Monitoring Board (GPMB) Board Meeting Hosted/Organised by: GPMB Secretariat. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-global-preparedness-monitoring-board-(gpmb)-board-meetinghosted-organised-by--gpmb-secretariat (accessed on 30 September 2022).
- Remarks of General Secretary of ONU, Summit 26 March 2020. Available online: www.un.org/sg/en/content/sg/statement/2020-03-26/secretary-generals-remarks-g-20-virtual-summit-the-covid-19-pandemic (accessed on 18 April 2021).
- Carrol, D.; Watson, B.; Togami, E.; Daszak, P.; Mczet, J.A.; Chrisman, C.J.; Rubin, E.M.; Wolfe, N.; Morel, C.M.; Gao, G.F.; et al. Building a global atlas of zoonotic viruses. Bull. World Health Organ. 2018, 96, 292–294. [Google Scholar] [CrossRef] [PubMed]

| Authors/References | Virus/Year | Type of Study/Contribution/Results |
|---|---|---|
| Li Q, Guan X et al., 2020 [7] | SARS-CoV-2 (2019) | 425 cases with confirmed pneumonia, with average age of 59 years from which 56% were men; 55% of cases with onset before 1 January 2020, associated with epicenter Wuhan Seafood Market, Hubei, China R0 = 2.2 (95% CI 1.4–3.9), mean 7.5 days (95% CI, 5.3–19). Prevention and transmission reduction measures should be implemented in the population at risk |
| Bi Qifang, Yong Sheng Wu et al., 2020 [8] | SARS-CoV2 (2019) | Data collected from Shenzhen Center for Disease Control and Prevention, Hubei, China from 14 January to 12 February 2020. From 391 confirmed cases, 204 were women and 187 men with average age of 45 years; 91% of cases with moderate clinical signs; cases were isolated on average 4.6 days after the development of symptoms. R0 = 0.4 |
| Zaho S et al., 2020 [9] | SARS-CoV-2 (2019) | Modeling the epidemic curve, China, 10–24 January 2020; have demonstrated the high potential to generate outbreaks of infection. Mean R0 ranged from 2.24 (95% CI 1.96–2.55) to 3.58 (95% CI 2.89–4.39) in the early phases, values significantly greater than 1. |
| Read J et al., 2020 [10] | SARS-CoV-2 (2019) | Early estimate of reproductive rate and case fatality rate (CFR%) for cases in Wuhan, China R0 = 3.1 (95%CI; 2.39–4.13) 58–76% transmission should be prevented to decrease risk of infection; the R0 estimates were comparable to SARS and MERS in the early periods. |
| Shen et al., 2020 [11] | SARS-CoV-2 (2019) | Diagnosis and the establishment of non-pharmacological interventions are essential in controlling the epidemic curve. R0 = 4.71 (4.50–4.92) at the beginning of the pandemic then decreased to 2.08 (1.99–2.18) on 22 January 2020 Comparing SARS and MERS had similar values for SARS (4.91) in Beijing, China (2003) and 3.5–6.7 for MERS in Saudi Arabia (2014). |
| Chen et al., 2020 [12] | SARS-CoV-2 (2019) | SEIR model, Pathogenicity and transmissibility of 2019-nCoV- compared to other emerging viruses. CFRSARS-CoV-2 = 3, R0 = 1.4–5.5 |
| SARS-CoV (2003) | CFR 10%; R0 = 2–5. | |
| MERS-CoV (2009) | CFR 40%; R0 < 1. | |
| H5N1/H1N1 (2009) | CFR 0.03%; R0 = 1.2–1.6. | |
| H1N1 (1918) | CFR 3%; R0 = 1.4–3.8. | |
| Ebola virus (2014) | CFR 70%; R0 = 1.5–2.5 | |
| Wu JT et al., 2020 [13] | SARS-CoV-2 (2019) | SEIR model, sensitivity analysis to understand infectious spread trend for China. R0 = 2.7, 95% CI, for Wuhan, China. CFR 1.4% (95% CI 0.9%–2.1%); the risk of symptomatic infections increases with age |
| S. Feng, Z. Feng [14] | SARS-CoV-2 (2019) | Prediction trends epidemics of COVID-19 on the basis SEIR and AI models Estimate transmission rates and death rates |
| Peter Winskill, Charlie Whittaker, Patrick Walker et al. Imperial College London (12 May 2020) [15] | SARS-CoV-2 (2019) | Report 22: Equity as a response the direct and indirect impact due to evolution the COVID-19 pandemic, on populations vulnerable in the countries with low and moderate incomes. The growth probability of death through COVID-19 by 32% in the poor population, with 12.4% for the populations that had limited access to medical services, with 9.2% for those with limitation to running water and by 6% due to their job—unable to social distancing. |
| Patrick GT Walker, Charles Whittaker, Oliver Watson et al. Imperial College London (26 March 2020) [16] | SARS-CoV-2 (2019) | Report 12: The Global Impact of COVID-19 and Strategies for Mitigation and Suppression The research combine data on age-specific contact patterns and COVID-19 severity to project the health impact in 202 countries (in the countries with low-income the risk profile for COVID-19 could be different from that observed in China, Europe, and North America). It was estimated that without interventions there would be 7.0 billion infections and 40 million deaths worldwide in one year and with interventions based on decreasing transmission such as a 40% decrease in social contacts, 20 million lives could be saved and through the early introduction of isolation, quarantine, testing and vaccination measures, 30.7 million lives could be saved. |
| Neil M Ferguson, Daniel Laydon, Gemma Nedjati-Gilani et al. Imperial College London (16 March 2020) [17] | SARS-CoV-2 (2019) | Report 9: microsimulation model performed in UK (Great Britain) and US: Impact of Non-Pharmaceutical Interventions (NPIs) on Reducing COVID-19 Mortality and Healthcare Demand. Two fundamental strategies were debated: -mitigation using NPIs (isolation, quarantine, social distancing), strategy adopted by some US cities in 1918, and by the world in 1957, 1968 and 2009 influenza pandemic, which, according to estimates, would lead to a 50% decrease in requests for medical assistance and -suppression (decrease R0 to 1 or below 1, as for SARS or Ebola) to reduce cases and implicitly eliminate human-to-human transmission, which should be maintained until immunity is obtained or until the virus circulates. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ene, G.-V.B.; Mănuc, D.; Bordianu, A.; Todea, D.A. The Pandemic Puzzle—Reviewing the Existing Pieces, Searching for the Missing Ones. Sustainability 2023, 15, 5214. https://doi.org/10.3390/su15065214
Ene G-VB, Mănuc D, Bordianu A, Todea DA. The Pandemic Puzzle—Reviewing the Existing Pieces, Searching for the Missing Ones. Sustainability. 2023; 15(6):5214. https://doi.org/10.3390/su15065214
Chicago/Turabian StyleEne, Gianina-Valentina Băcescu, Daniela Mănuc, Anca Bordianu, and Doina Adina Todea. 2023. "The Pandemic Puzzle—Reviewing the Existing Pieces, Searching for the Missing Ones" Sustainability 15, no. 6: 5214. https://doi.org/10.3390/su15065214
APA StyleEne, G.-V. B., Mănuc, D., Bordianu, A., & Todea, D. A. (2023). The Pandemic Puzzle—Reviewing the Existing Pieces, Searching for the Missing Ones. Sustainability, 15(6), 5214. https://doi.org/10.3390/su15065214






