Experimental Study on the Effective Production of Biocement for Soil Solidification and Wind Erosion Control
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain
2.2. Biomass and Activity Determinations
2.3. Sample Preparation and Microbial Treatment
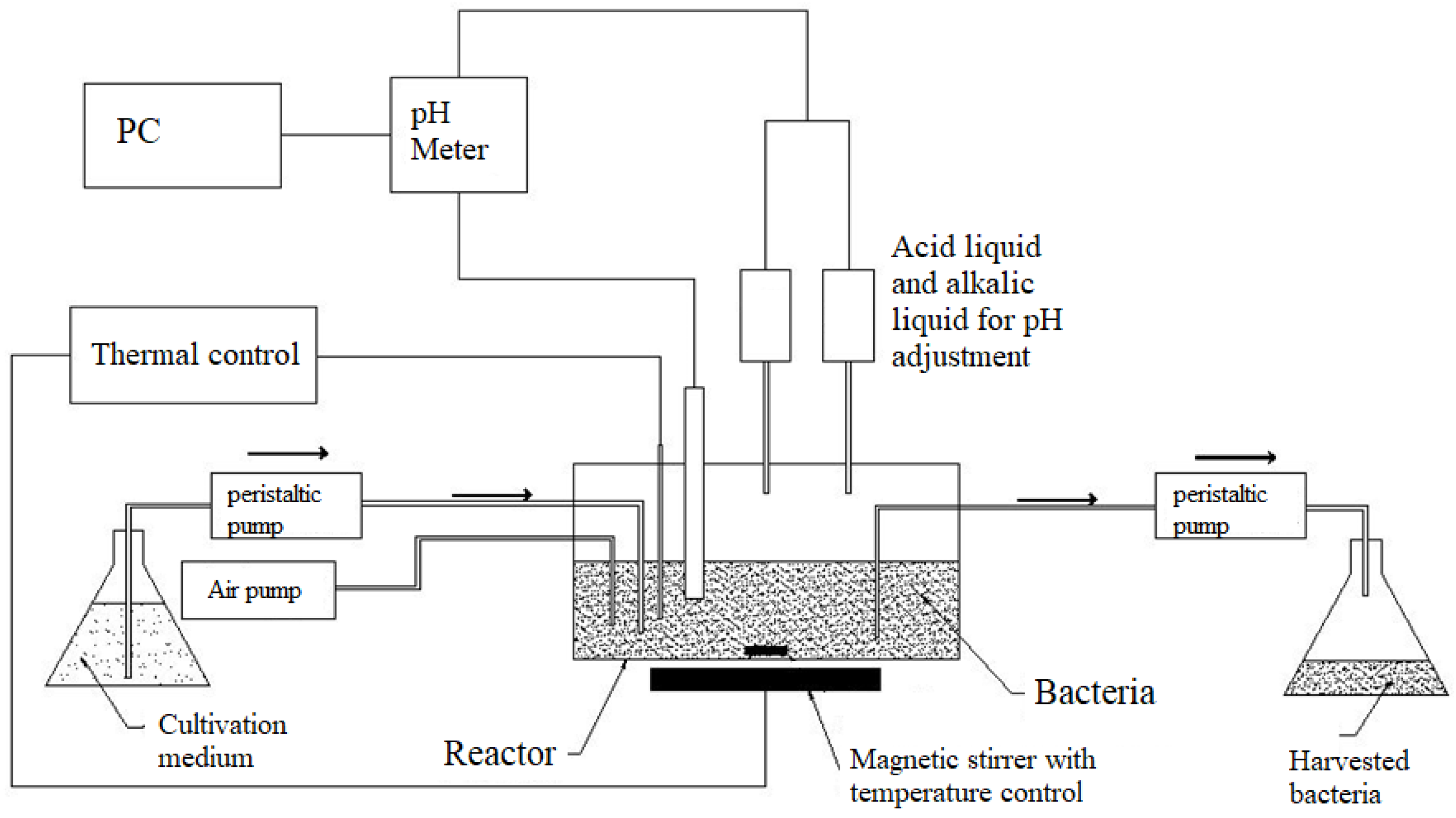
2.4. Chemostat Cultivation
2.5. Alternative Carbon Sources
2.6. Soil Stabilization Test
3. Results and Discussion
3.1. Effect of Nitrogen Sources
3.2. Effects of the Initial pH Value
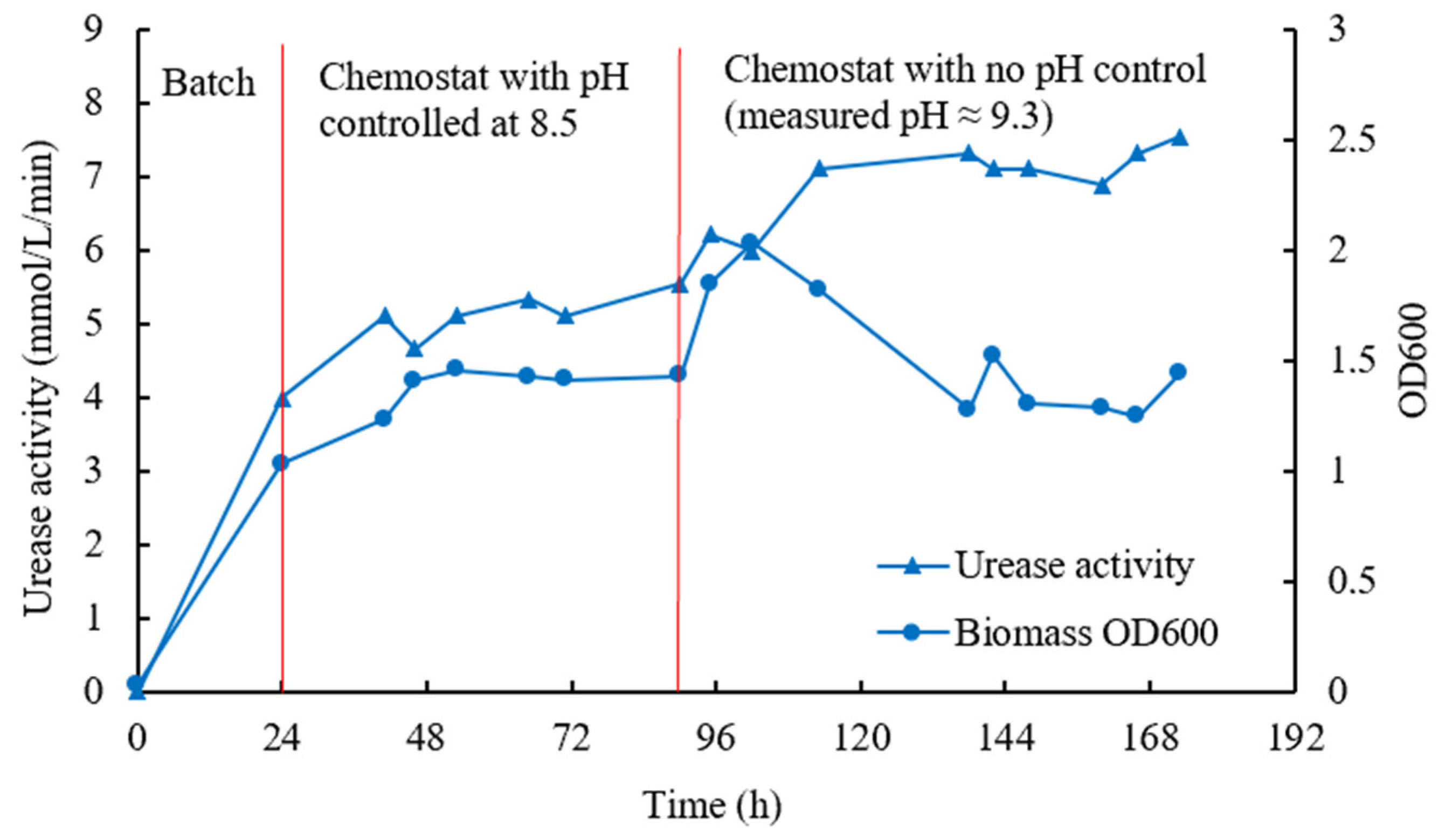
3.3. Chemostat Cultivation
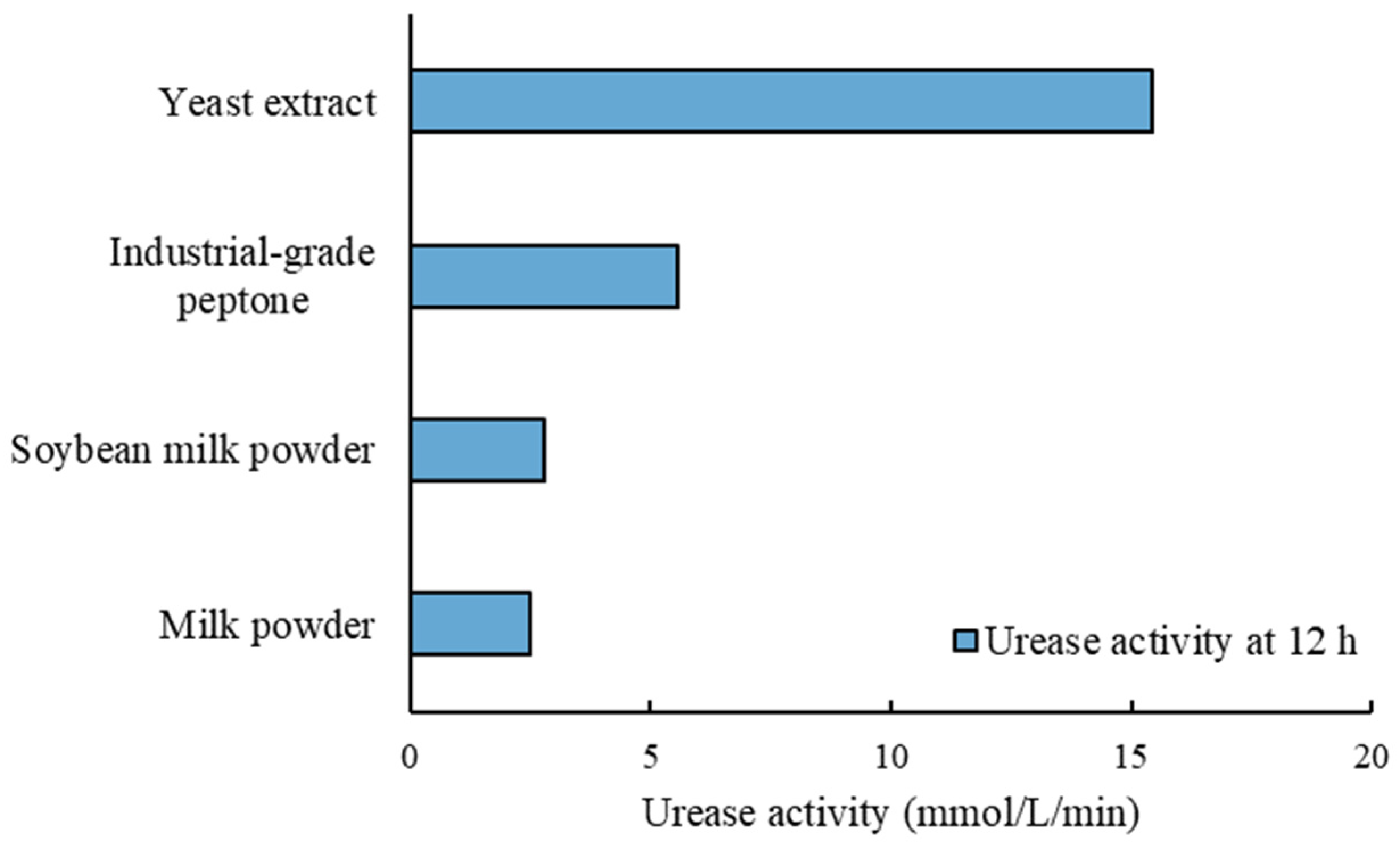
3.4. Alternative Carbon Sources
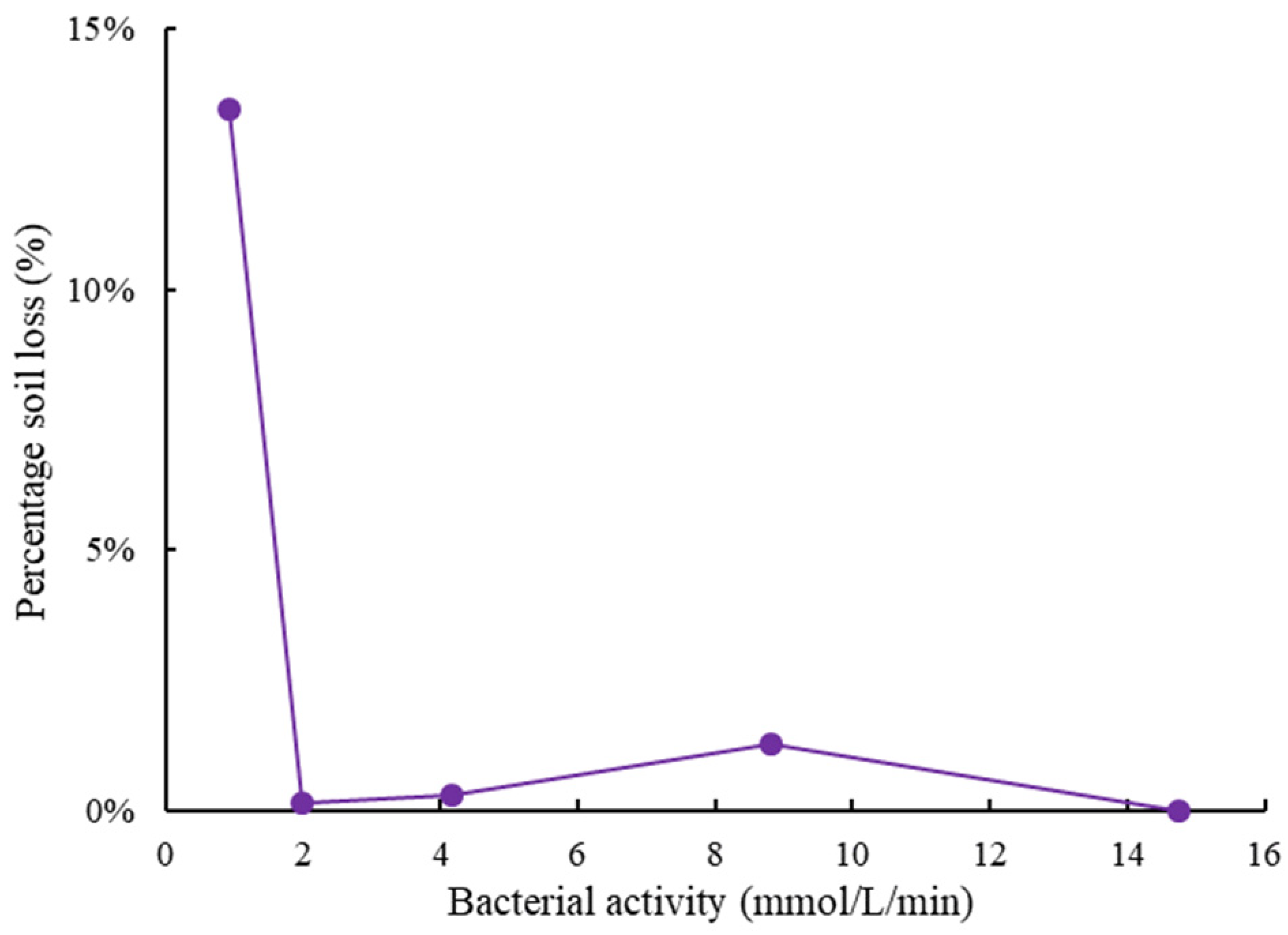
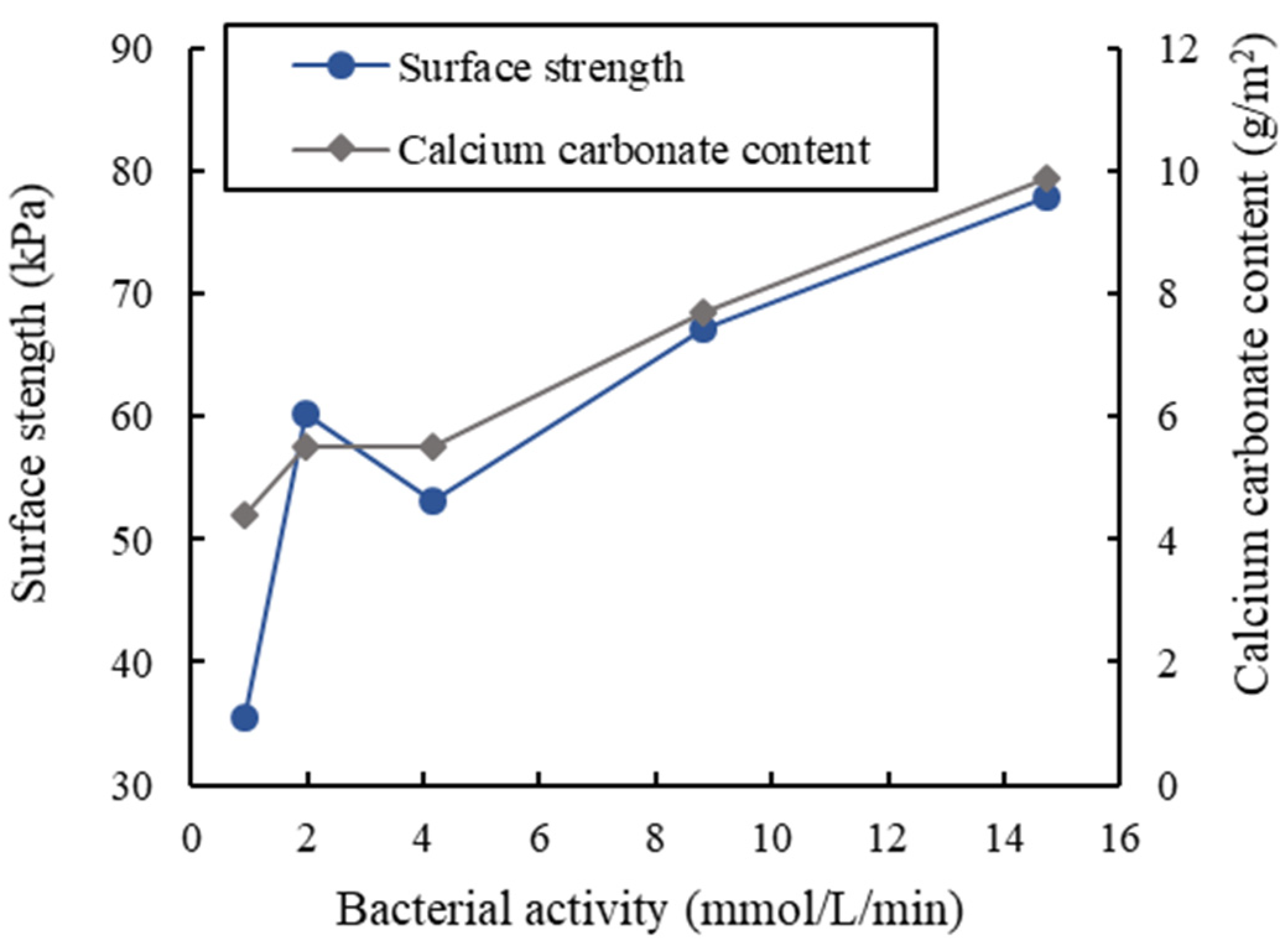
3.5. Soil Stabilization Tests
4. Discussions
4.1. Urea or Ammonium Salt as Nitrogen Source
4.2. Effect of pH
4.3. Chemostat Cultivation
4.4. Large-Scale Cultivation Methods
5. Conclusions
- In the tests with different nitrogen source types, the cultivation medium with pure urea had the highest biomass yield, urease activity, and specific urease activity compared with the other tests with pure NH4Cl or both NH4Cl and urea. The use of urea as the nitrogen source in the media also led to an increase in pH, which was not found in the test with pure NH4Cl;
- In the tests with different urea concentrations, the tests with a higher urea concentration had a higher biomass yield, urease activity, and pH value;
- In the tests with different pH values. the test with 8.5 initial pH value had a higher biomass yield, urease activity, and specific urease activity than the tests with 7.5 and 9.5 initial pH values;
- In the chemostat condition with continuous feeding into and harvesting from the bioreactor, the ureolytic bacteria could be steadily produced with OD600 of around 1.3 and urease activity of around 7 mmol/L/min;
- When using soybean milk powder or milk powder as the carbon sources, the urease activity was around 2.5 mmol/L/min. Although it was lower than that using the laboratory-grade or industrial-grade carbon sources, this level of urease activity was high enough to be used for MICP soil cementation, as demonstrated by the soil stabilization test.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ivanov, V.; Chu, J. Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev. Environ. Sci. Biotechnol. 2008, 7, 139–153. [Google Scholar] [CrossRef]
- Gao, Y.F.; Hang, L.; He, J.; Chu, J. Mechanical behaviour of biocemented sands at various treatment levels and relative densities. Acta Geotech. 2019, 14, 697–707. [Google Scholar] [CrossRef]
- Gao, Y.F.; He, J.; Tang, X.Y.; Chu, J. Calcium carbonate precipitation catalyzed by soybean urease as an improvement method for fine-grained soil. Soils Found. 2019, 59, 1631–1637. [Google Scholar] [CrossRef]
- Blauw, M.; Lambert, J.W.M.; Latil, M.N. Biosealing: A method for in situ sealing of leakages. In Proceedings of the International Symposium on Ground Improvement Technologies and Case Histories, Singapore, 9–12 December 2009; pp. 125–130. [Google Scholar]
- Chu, J.; Ivanov, V.; Stabnikov, V.; Li, B. Microbial method for construction of aquaculture pond in sand. Géotechnique 2013, 63, 871–875. [Google Scholar] [CrossRef]
- Hamdan, N.; Kavazanjian, E.J. Enzyme-induced carbonate mineral precipitation for fugitive dust control. Géotechnique 2016, 66, 546–555. [Google Scholar] [CrossRef]
- Jiang, N.J.; Soga, K.; Kuo, M. Microbially induced carbonate precipitation for seepage-induced internal erosion control in sand–clay mixtures. J. Geotech. Geoenviron. Eng. 2017, 143, 04016100. [Google Scholar] [CrossRef]
- Fattahi, S.M.; Soroush, A.; Huang, N. Biocementation control of sand against wind erosion. J. Geotech. Geoenviron. Eng. 2020, 146, 04020045. [Google Scholar] [CrossRef]
- Hang, L.; Yang, E.J.; Zhou, Y.D.; Song, W.Z.; He, J. Microbially induced calcite precipitation (MICP) for stabilization of desert sand against the wind-induced erosion: A parametric study. Sustainability 2022, 14, 11409. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Hua, C.; Ke, T. Field Test on Soybean-Urease Induced Calcite Precipitation (SICP) for Desert Sand Stabilization against the Wind-Induced Erosion. Sustainability 2022, 14, 15474. [Google Scholar] [CrossRef]
- DeJong, J.T.; Soga, K.; Kavazanjian, E.; Burns, S.; Van Paassen, L.A. Biogeochemical processes and geotechnical applications: Progress, opportunities and challenges. Géotechnique 2013, 63, 287–301. [Google Scholar] [CrossRef]
- Whiffin, V.S. Microbial CaCO3 precipitation for the production of biocement. Ph.D. Thesis, Murdoch University, Perth, Australia, 2004. [Google Scholar]
- Achal, V.; Mukherjee, A.; Reddy, M.S. Biocalcification by Sporosarcina pasteurii using corn steep liquor as nutrient source. Ind. Biotechnol. 2010, 6, 170–174. [Google Scholar] [CrossRef]
- Cheng, L.; Cord-Ruwisch, R. Selective enrichment and production of highly urease active bacteria by non-sterile (open) chemostat culture. J. Ind. Microbiol. Biotechnol. 2013, 40, 1095–1104. [Google Scholar] [CrossRef]
- Cuzman, O.A.; Richter, K.; Wittig, L.; Tiano, P. Alternative nutrient sources for biotechnological use of Sporosarcina pasteurii. World J. Microbiol. Biotechnol. 2015, 31, 897–906. [Google Scholar] [CrossRef]
- Williams, S.L.; Kirisits, M.J.; Ferron, R.D. Optimization of growth medium for Sporosarcina pasteurii in bio-based cement pastes to mitigate delay in hydration kinetics. J. Ind. Microbiol. Biotechnol. 2016, 43, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Yoosathaporn, S.; Tiangburanatham, P.; Bovonsombut, S.; Chaipanich, A.; Pathom-aree, W. A cost-effective cultivation medium for biocalcification of Bacillus pasteurii KCTC 3558 and its effect on cement cubes properties. Microbiol. Res. 2016, 186, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, I.O.; Lock, H.N.; Dominic, E.L.O.; Peter, M.N. Low-cost cultivation of Sporosarcina pasteurii strain in food-grade yeast extract medium for microbially induced carbonate precipitation (MICP) application. Biocatal. Agric. Biotechnol. 2019, 17, 247–255. [Google Scholar]
- Chu, J.; Stabnikov, V.; Ivanov, V. Microbially induced calcium carbonate precipitation on surface or in the bulk of soil. Geomicrobiol. J. 2012, 29, 544–549. [Google Scholar] [CrossRef]
- Al Qabany, A.; Soga, K. Effect of chemical treatment used in MICP on engineering properties of cemented soils. Geotechnique 2013, 63, 331–339. [Google Scholar] [CrossRef]
- He, J.; Gao, Y.F.; Gu, Z.X.; Chu, J.; Wang, L.Y. Characterization of Crude Bacterial Urease for CaCO3 Precipitation and Cementation of Silty Sand. J. Mater. Civ. Eng. 2020, 32, 04020071. [Google Scholar] [CrossRef]
- Whiffin, V.S.; Van Paassen, L.V.; Harkes, M.P. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 2007, 24, 411–423. [Google Scholar] [CrossRef]
- Stabnikov, V.; Chu, J.; Ivanov, V.; Li, V. Halotolerant, alkaliphilic urease-producing bacteria from different climate zones and their application for biocementation of sand. World J. Microbiol. Biotechnol. 2013, 29, 1453–1460. [Google Scholar] [CrossRef]
- Li, B. Geotechnical properties of biocement treated sand and clay. Ph.D. Thesis, Nan Yang Technological University, Perth, Australia, 2015. [Google Scholar]
- Mobley, H.; Hausinger, R.; Mobley, H.; Hausinger, R. Microbial ureases: Significance, regulation, and molecular characterization. Microbiol. Rev. 1989, 53, 85–108. [Google Scholar] [CrossRef]
- Masover, G.K.; Razin, S.; Hayflick, L. Localization of enzymes in Ureaplasma urealyticum (T-strain mycoplasma). J. Bacteriol. 1977, 130, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Romano, N.; Tolone, G.; Ajello, F.; Licata, R.L. Adenosine 5′-triphosphate synthesis induced by urea hydrolysis in Ureaplasma urealyticum. J. Bacteriol. 1980, 144, 830–832. [Google Scholar] [CrossRef]
- Slonczewski, J.L.; Fujisawa, M.; Dopson, M.; Krulwich, T.A. Cytoplasmic pH Measurement and Homeostasis in Bacteria and Archaea. Adv. Microb. Physiol. 2019, 55, 1–79. [Google Scholar]
- Jahns, T. Ammonium/urea-dependent generation of a proton electrochemical potential and synthesis of ATP in Bacillus pasteurii. J. Bacteriol. 1996, 178, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Wiley, W.R.; Stokes, J.L. Effect of pH and ammonium ions on the permeability of Bacillus pasteurii. J. Bacteriol. 1963, 86, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.; Jahns, T. Regulation of leucine transport by intracellular pH in Bacillus pasteurii. Arch. Microbiol. 1996, 165, 265–271. [Google Scholar] [CrossRef]
- Yang, F. Study on the optimization of biological soil cementation. Master’s Thesis, Hohai University, Nanjing, China.

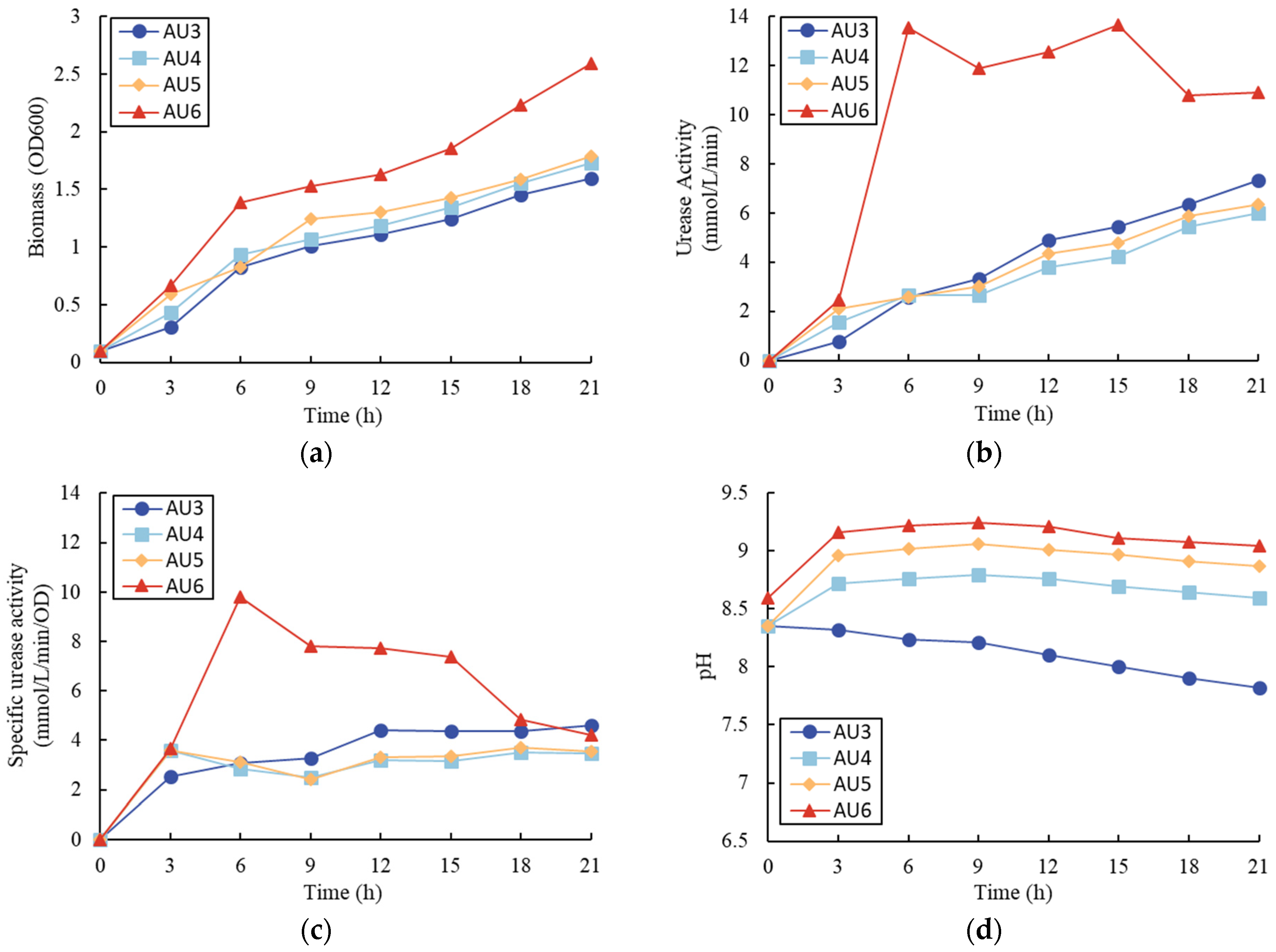
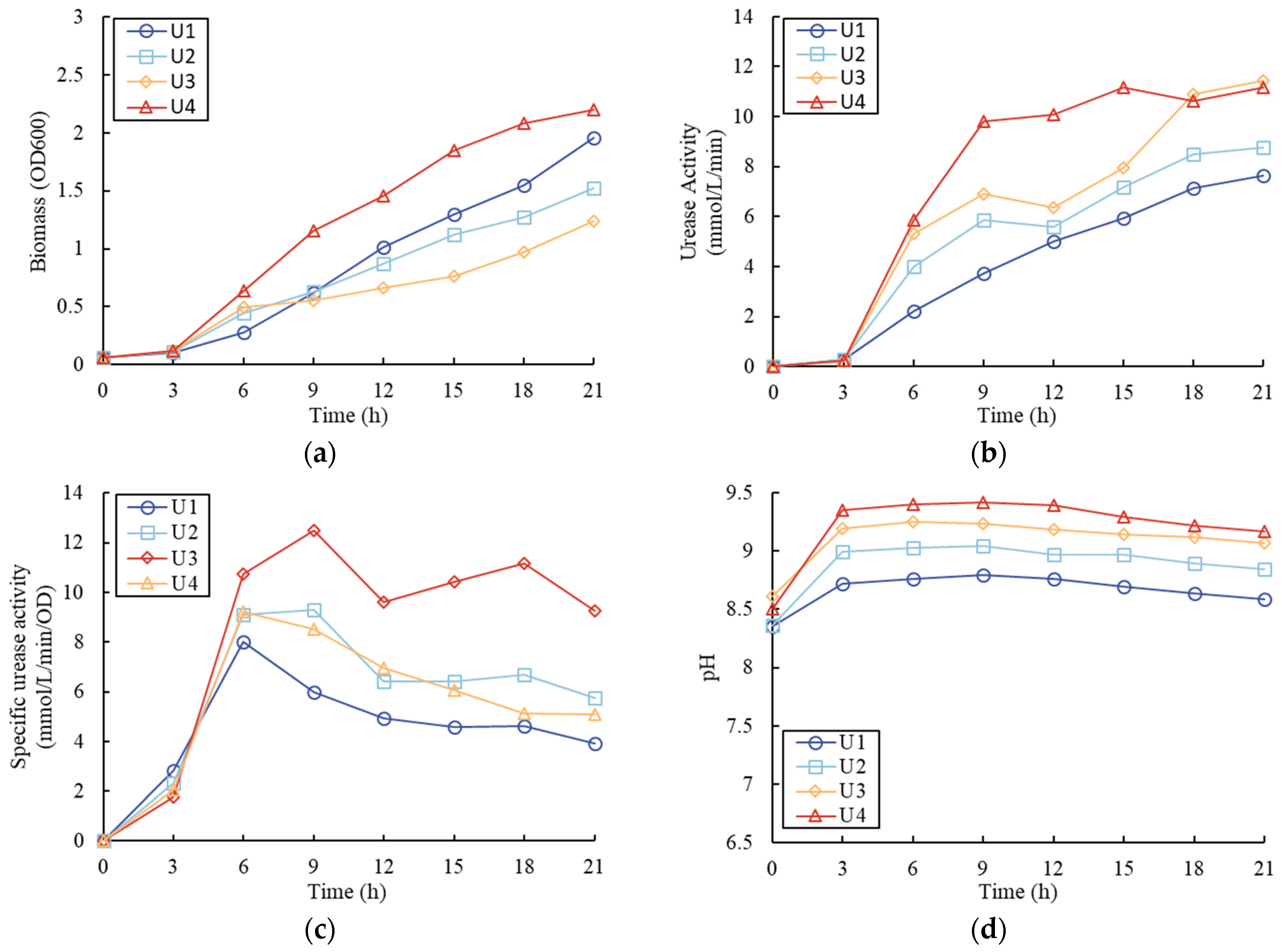

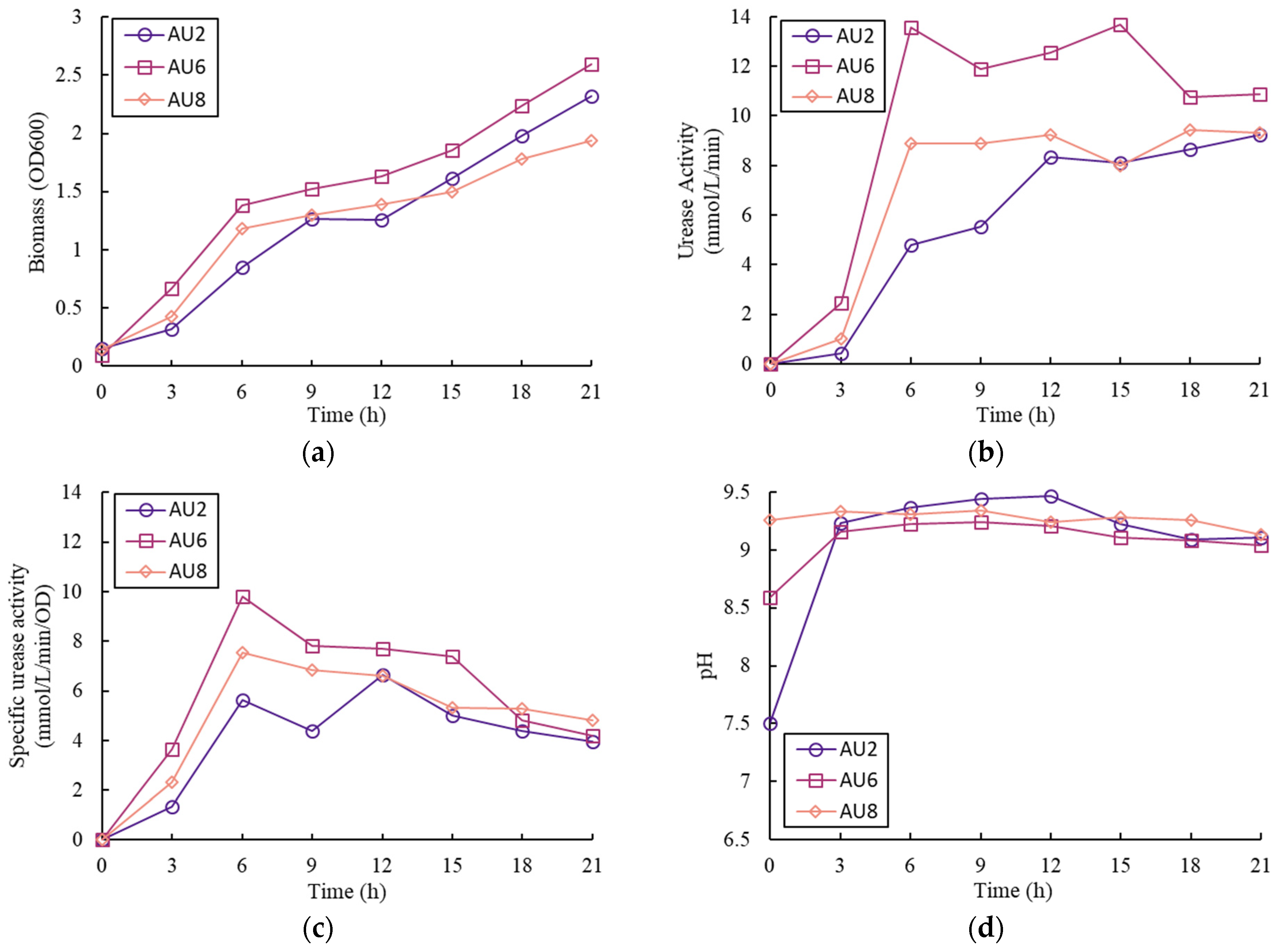
| Samples | NH4Cl (g/L) | Urea (g/L) | Initial pH |
|---|---|---|---|
| AU1 | 10 | / | 7.5 |
| AU2 | / | 10 | 7.5 |
| AU3 | 10 | / | 8.5 |
| AU4 | 6.67 | 3.33 | 8.5 |
| AU5 | 3.33 | 6.67 | 8.5 |
| AU6 | / | 10 | 8.5 |
| AU7 | 10 | / | 9.5 |
| AU8 | / | 10 | 9.5 |
| U1 | / | 3.33 | 8.5 |
| U2 | / | 6.67 | 8.5 |
| U3 | / | 10 | 8.5 |
| U4 | / | 13.33 | 8.5 |
| Substrates (g/L) | Urea (g/L) | Initial pH | Protein Content (g/100 g) |
|---|---|---|---|
| Yeast extract (20) | 10 | 8.5 | 68.1 |
| Industrial-grade peptone (30) | 10 | 8.5 | 59.1 |
| Soybean milk powder (60) | 10 | 8.5 | 16.8 |
| Milk powder (60) | 10 | 8.5 | 18.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hang, L.; Yang, F.; Xu, J.; Zhao, Z.; Xiao, W.; He, J. Experimental Study on the Effective Production of Biocement for Soil Solidification and Wind Erosion Control. Sustainability 2023, 15, 5402. https://doi.org/10.3390/su15065402
Hang L, Yang F, Xu J, Zhao Z, Xiao W, He J. Experimental Study on the Effective Production of Biocement for Soil Solidification and Wind Erosion Control. Sustainability. 2023; 15(6):5402. https://doi.org/10.3390/su15065402
Chicago/Turabian StyleHang, Lei, Feng Yang, Jie Xu, Zihao Zhao, Wei Xiao, and Jia He. 2023. "Experimental Study on the Effective Production of Biocement for Soil Solidification and Wind Erosion Control" Sustainability 15, no. 6: 5402. https://doi.org/10.3390/su15065402
APA StyleHang, L., Yang, F., Xu, J., Zhao, Z., Xiao, W., & He, J. (2023). Experimental Study on the Effective Production of Biocement for Soil Solidification and Wind Erosion Control. Sustainability, 15(6), 5402. https://doi.org/10.3390/su15065402





