Mapping Impacts of Climate Change on the Distributions of Two Endemic Tree Species under Socioeconomic Pathway Scenarios (SSP)
Abstract
1. Introduction
2. Materials and Methods
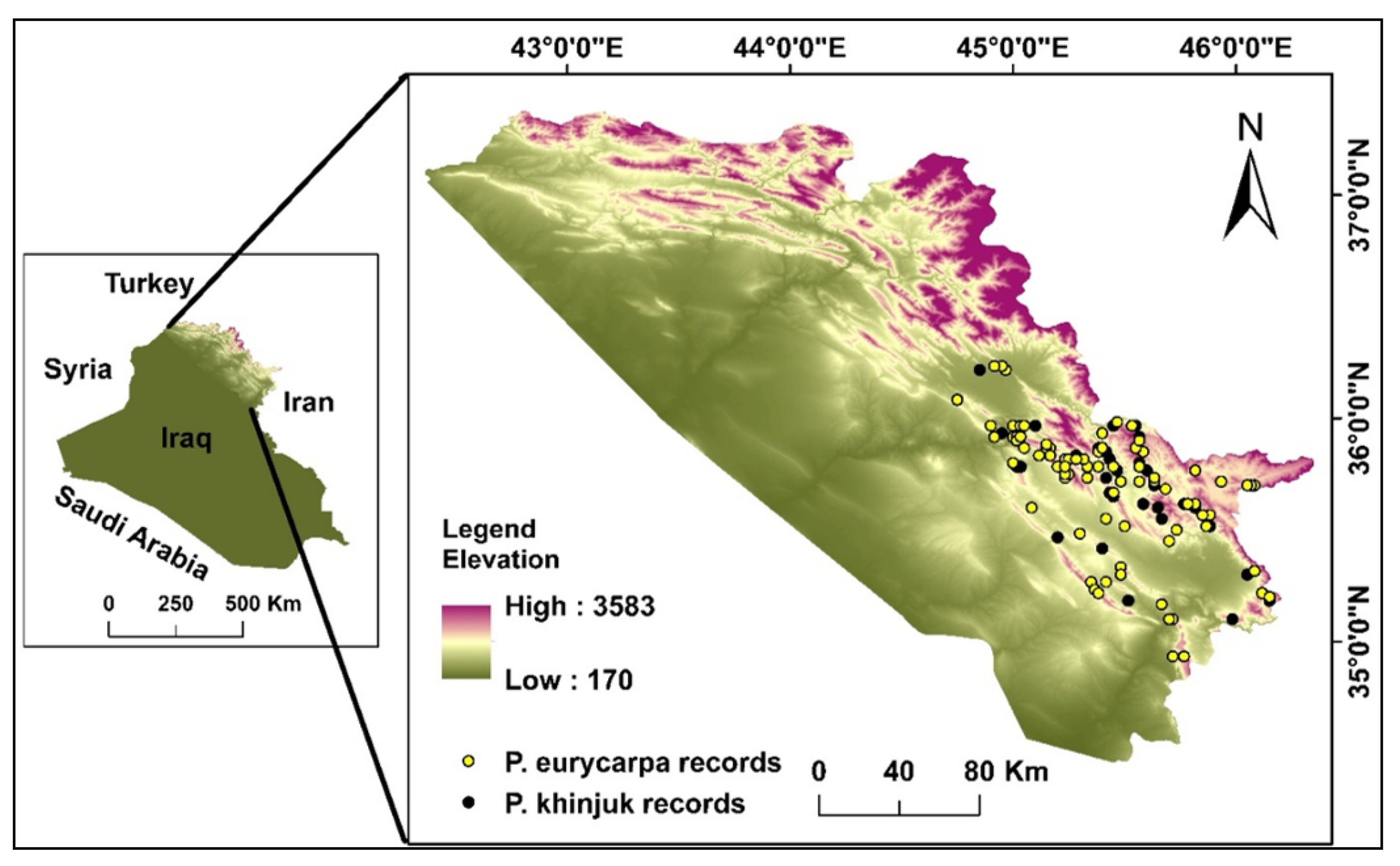
2.1. Study Area
2.2. Ground Data for P. eurycarpa Yalt and P. khinjuk Stock
2.3. Environmental Datasets
2.4. Model Building
2.5. Model Evaluation
2.6. Analysis of the Distribution Change between the Habitat of the Present and Future for the Species
3. Results
3.1. Performance of the Model
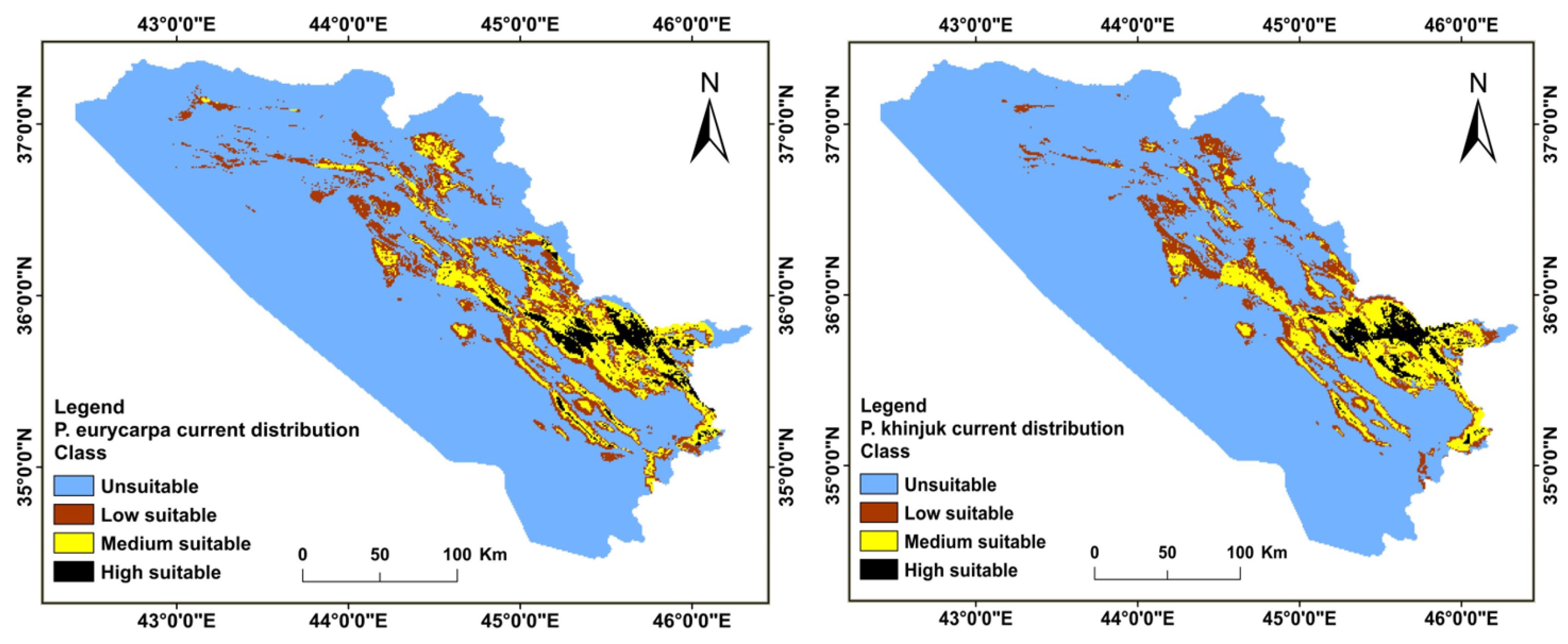
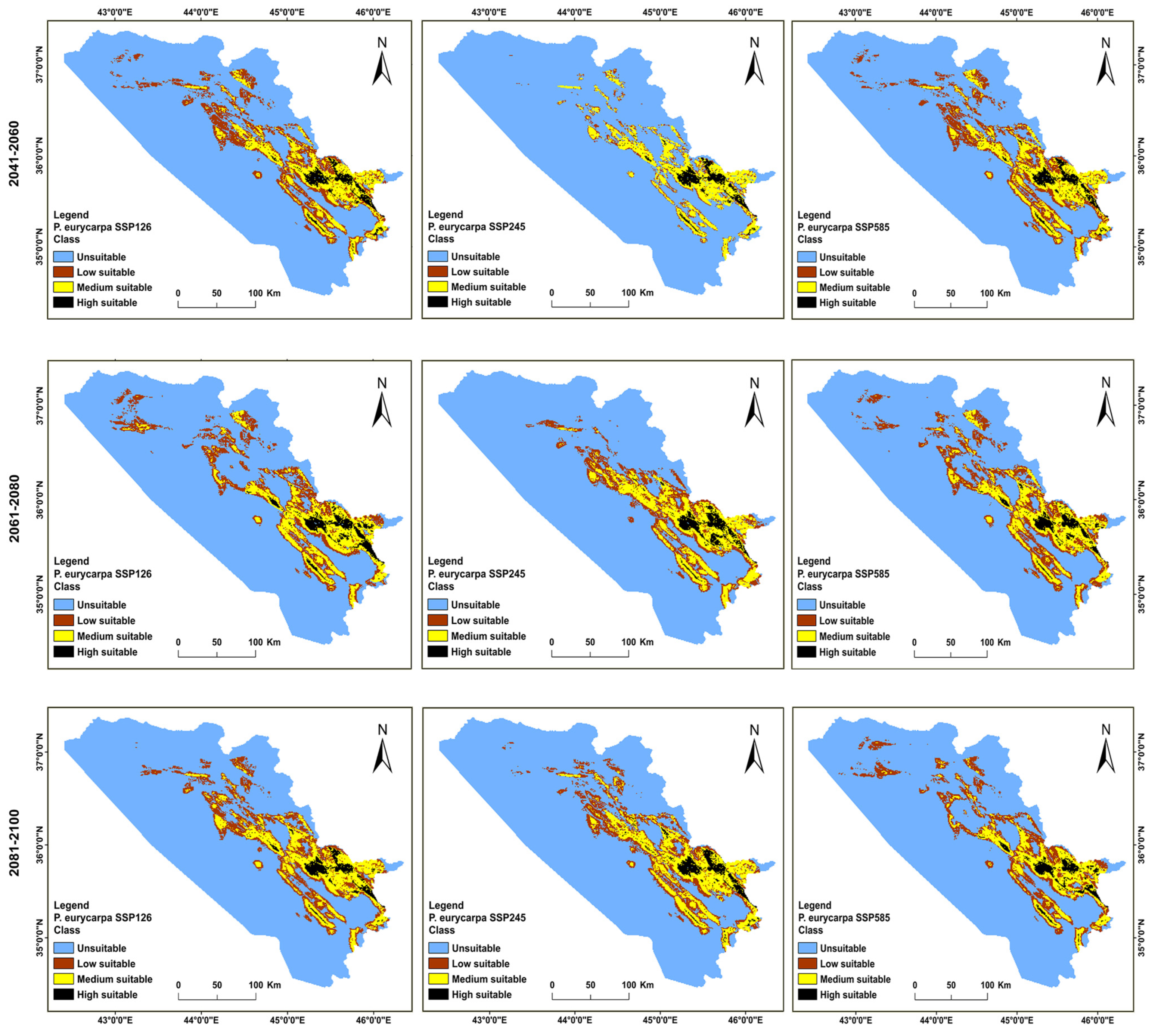
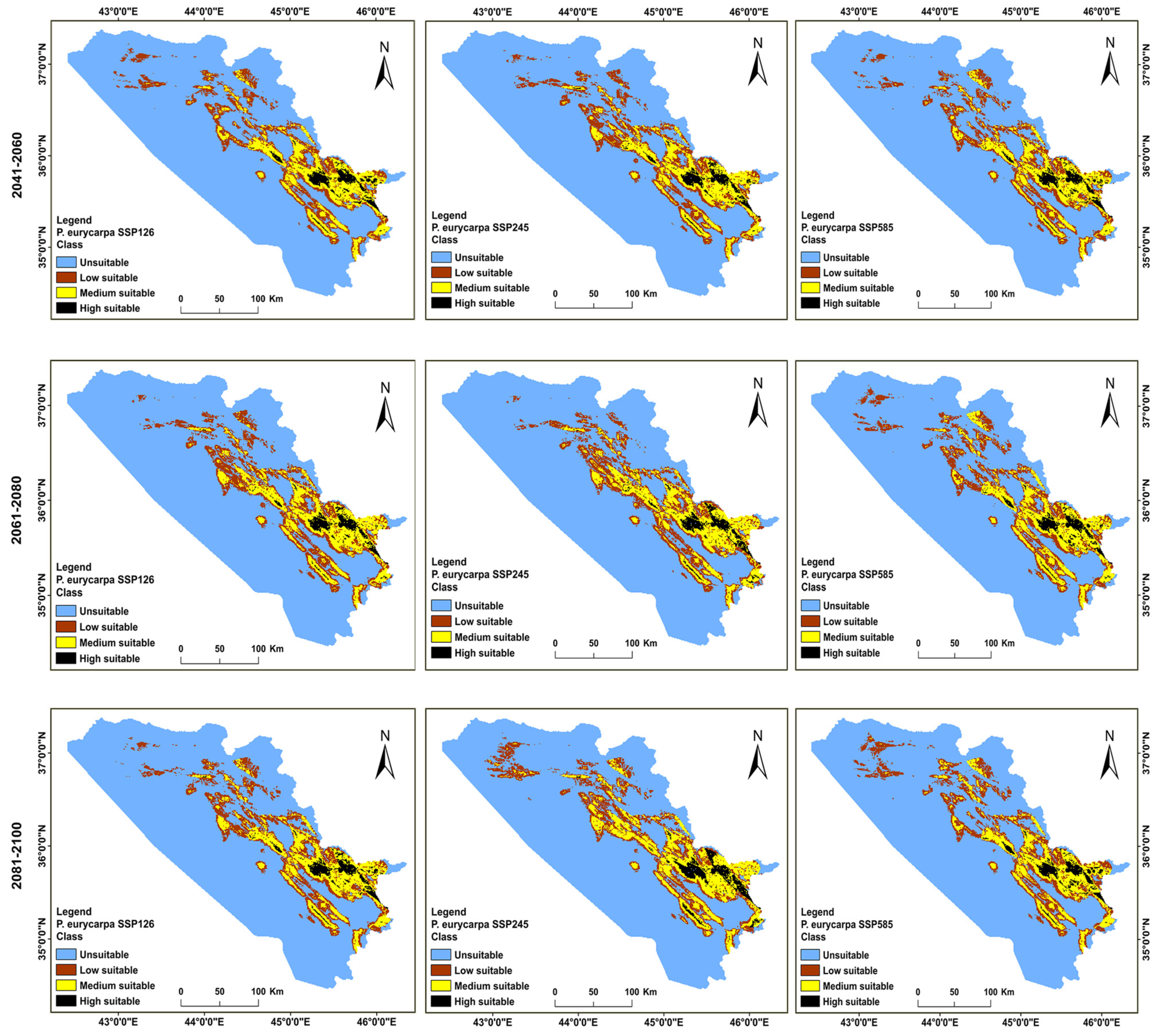
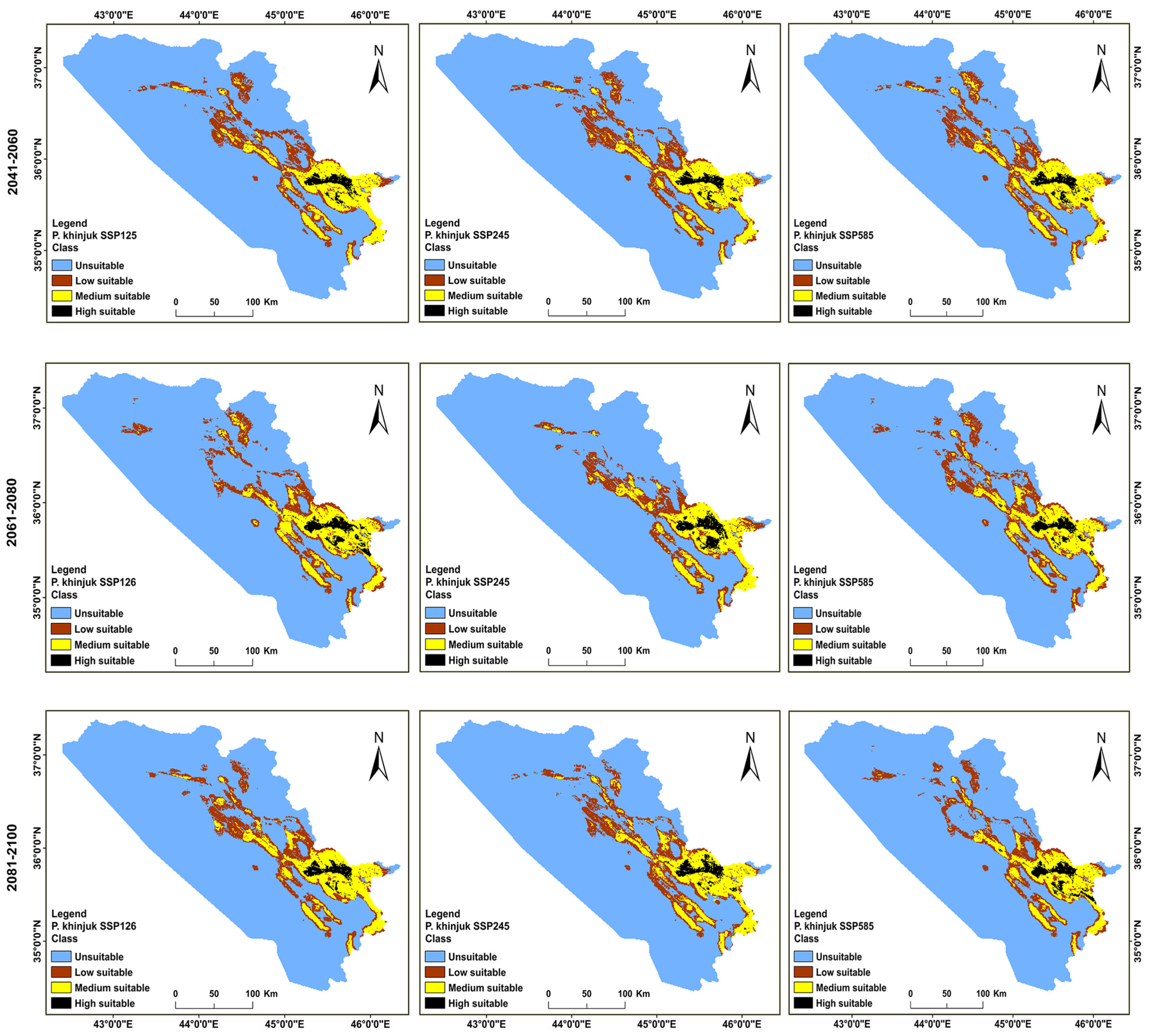
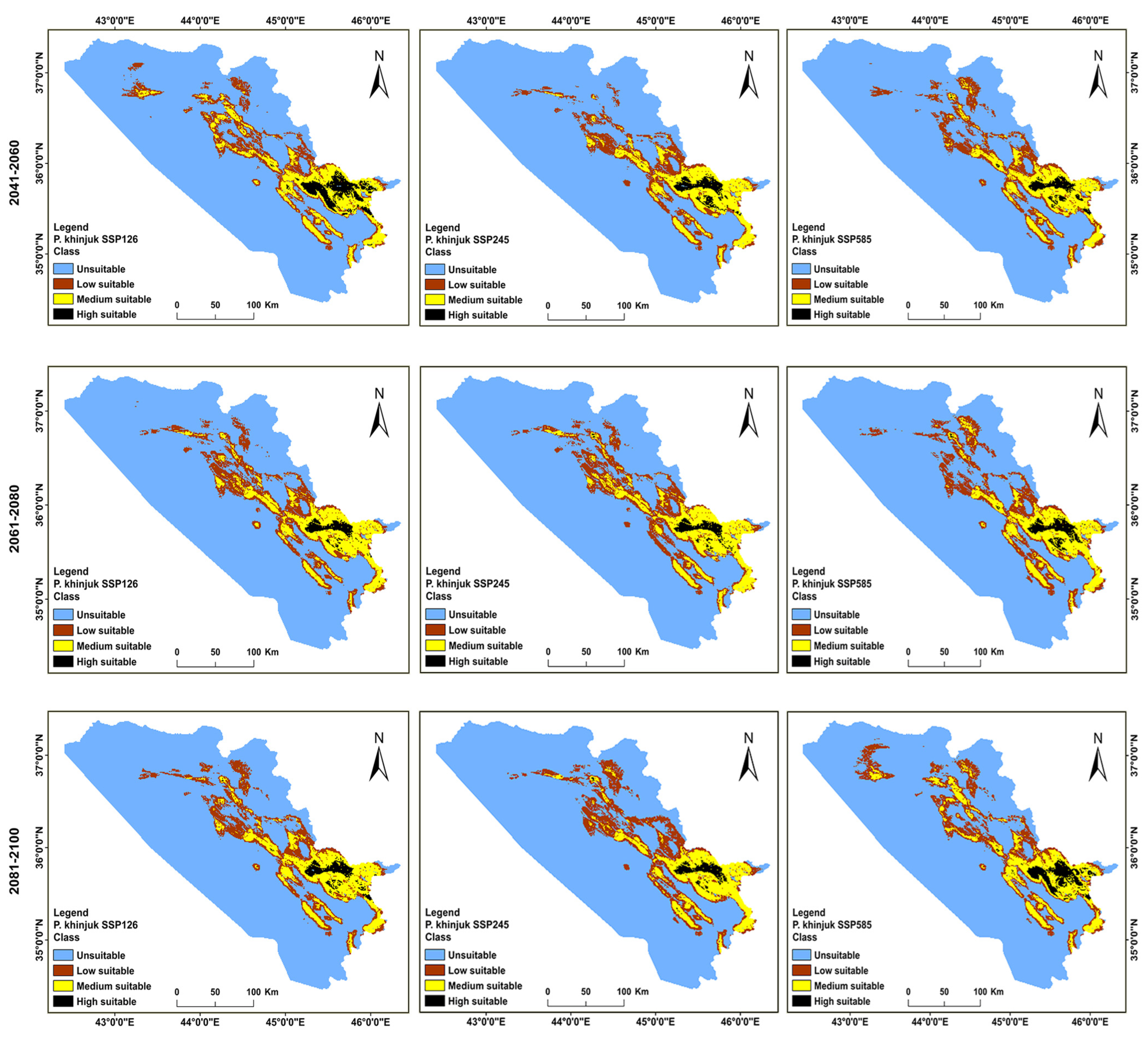
3.2. Distributions of the Habitat in the Present and Future for P. khinjuk and P. eurycarpa
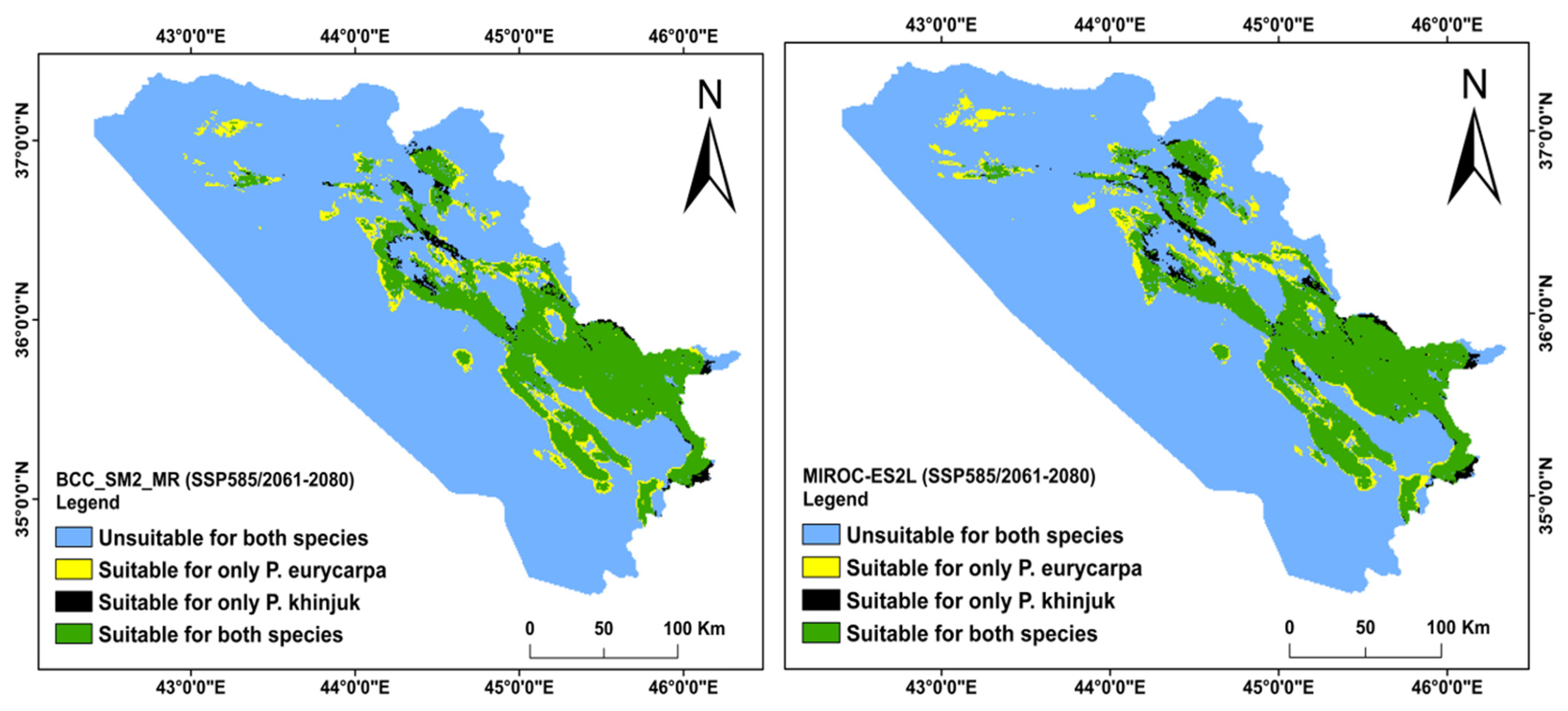
3.3. Analysis of the Distribution Change between the Present and Future Habitats for P. khinjuk and P. eurycarpa
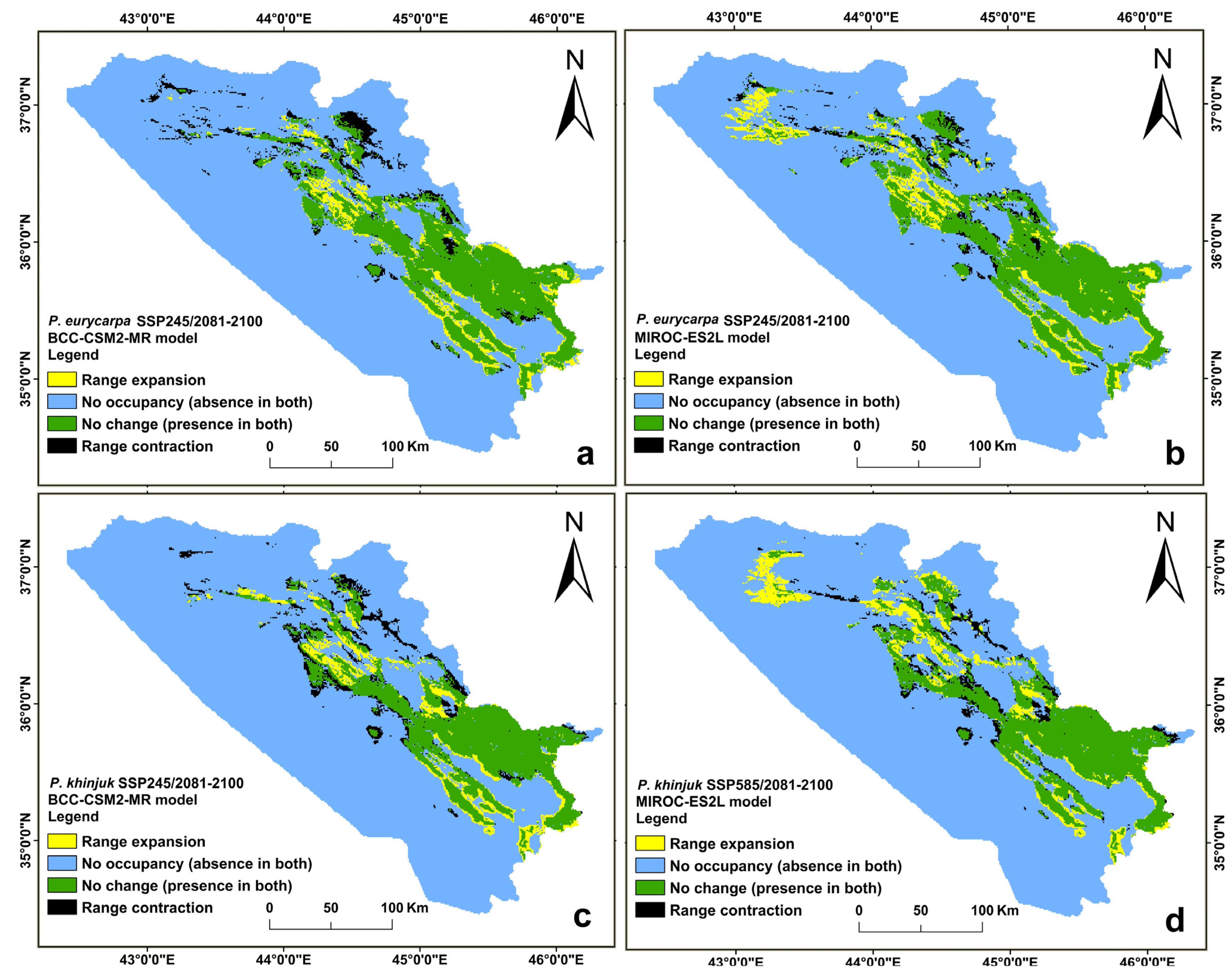
3.4. The Direction and Degree of the Distributional Change for P. eurycarpa and P. khinjuk
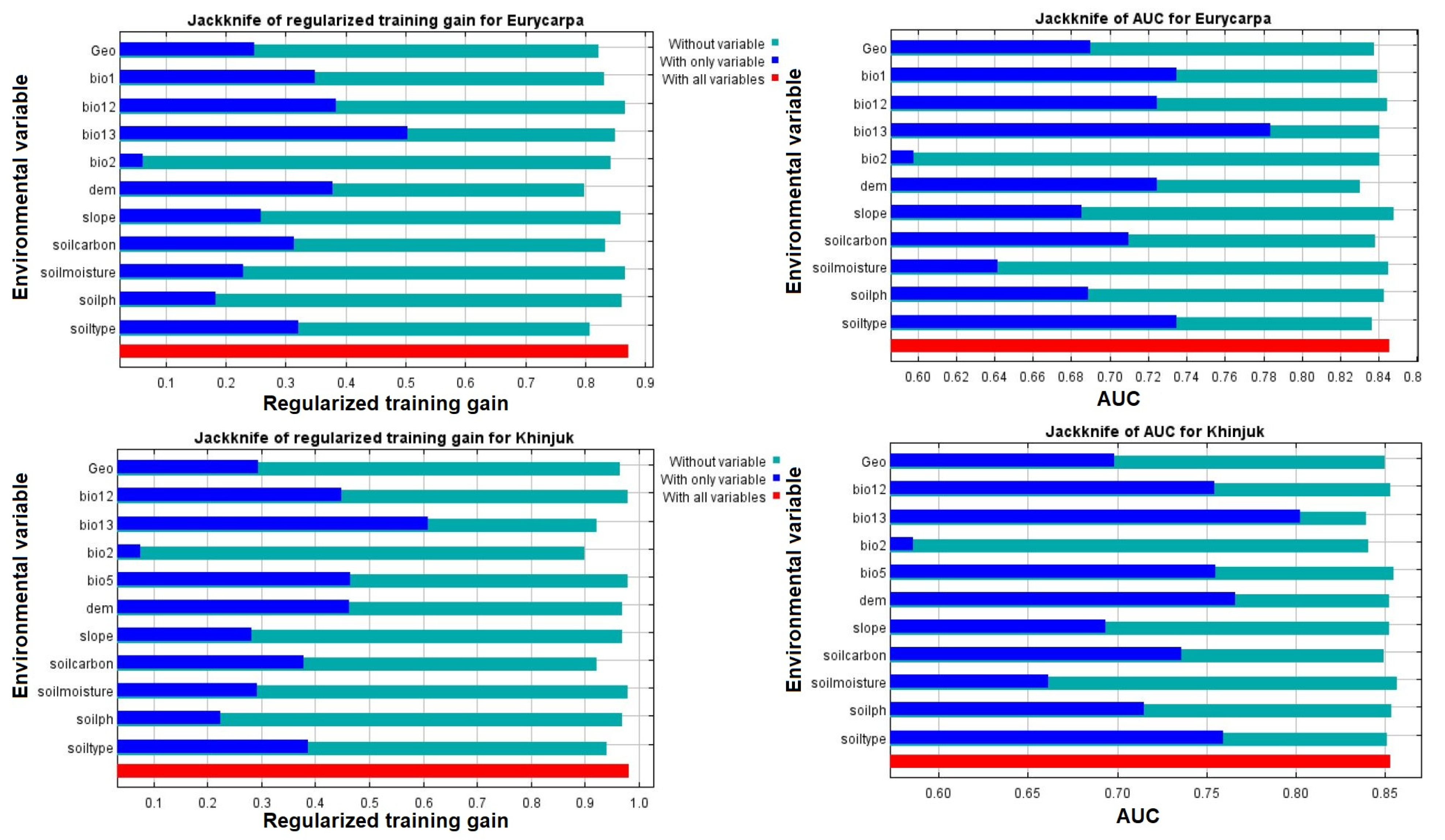
3.5. Environmental Factors’ Relative Relevance and Contribution to the Spread of P. eurycarpa and P. khinjuk
4. Discussion
4.1. Species of Tree Plant Respond Differently to the Scenarios of the Future Climate
4.2. Edaphic and Environmental Variables Contributed to the Expansion and Distribution of the P. khinjuk and P. eurycarpa in the Future Climatic Scenarios
4.3. Implications for Ecological Conservation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Appendix A

| Year | Class | Range Expansion | No Occupancy (Absence in Both) | No Change (Presence in Both) | Range Contraction |
|---|---|---|---|---|---|
| (2041–2060) | SSP 126 area (km2) | 1659 | 38,996 | 9050 | 1392 |
| % | 3.26 | 76.32 | 17.70 | 2.72 | |
| SSP 245 area (km2) | 1724 | 38,931 | 9128 | 1314 | |
| % | 3.38 | 76.20 | 17.86 | 2.56 | |
| SSP 585 area (km2) | 1893 | 38,762 | 9157 | 1285 | |
| % | 3.71 | 75.87 | 17.91 | 2.51 | |
| (2061–2080) | SSP 126 area (km2) | 1978 | 38,677 | 8559 | 1883 |
| % | 3.88 | 75.70 | 16.74 | 3.68 | |
| SSP 245 area (km2) | 1933 | 38,722 | 8057 | 2385 | |
| % | 3.79 | 75.79 | 15.76 | 4.66 | |
| SSP 585 area (km2) | 1718 | 38,937 | 9080 | 1362 | |
| % | 3.37 | 76.21 | 17.76 | 2.66 | |
| (2081–2100) | SSP 126 area (km2) | 1637 | 39,018 | 8993 | 1449 |
| % | 3.21 | 76.37 | 17.59 | 2.83 | |
| SSP 245 area (km2) | 2111 | 38,544 | 8763 | 1679 | |
| % | 4.14 | 75.44 | 17.14 | 3.28 | |
| SSP 585 area (km2) | 1489 | 39,166 | 8278 | 2164 | |
| % | 2.92 | 76.66 | 16.19 | 4.23 |
| Year | Class | Range Expansion | No Occupancy (Absence in Both) | No Change (Presence in Both) | Range Contraction |
|---|---|---|---|---|---|
| (2041–2060) | SSP 126 area (km2) | 1658 | 38,997 | 8916 | 1526 |
| % | 3.25 | 76.33 | 17.44 | 2.98 | |
| SSP 245 area (km2) | 1744 | 38,911 | 9030 | 1412 | |
| % | 3.42 | 76.16 | 17.66 | 2.76 | |
| SSP 585 area (km2) | 1556 | 39,099 | 9135 | 1307 | |
| % | 3.05 | 76.53 | 17.87 | 2.55 | |
| (2061–2080) | SSP 126 area (km2) | 1855 | 38,800 | 9169 | 1273 |
| % | 3.64 | 75.94 | 17.94 | 2.48 | |
| SSP 245 area (km2) | 2045 | 38,610 | 9023 | 1421 | |
| % | 4.01 | 75.57 | 17.65 | 2.77 | |
| SSP 585 area (km2) | 1897 | 38,758 | 9268 | 1174 | |
| % | 3.72 | 75.86 | 18.13 | 2.29 | |
| (2081–2100) | SSP 126 area (km2) | 1753 | 38,901 | 9238 | 1205 |
| % | 3.44 | 76.14 | 18.07 | 2.35 | |
| SSP 245 area (km2) | 2845 | 37,810 | 9283 | 1160 | |
| % | 5.58 | 74 | 18.16 | 2.26 | |
| SSP 585 area (km2) | 2025 | 38,630 | 9185 | 1257 | |
| % | 3.97 | 75.61 | 17.97 | 2.45 |
| Year | Class | Range Expansion | No Occupancy (Absence in Both) | No Change (Presence in Both) | Range Contraction |
|---|---|---|---|---|---|
| (2041–2060) | SSP 126 area (km2) | 2044 | 39,824 | 7536 | 1693 |
| % | 4.01 | 77.95 | 14.74 | 3.31 | |
| SSP 245 area (km2) | 1833 | 40,035 | 7902 | 1327 | |
| % | 3.60 | 78.36 | 15.46 | 2.59 | |
| SSP 585 area (km2) | 1913 | 39,955 | 7771 | 1458 | |
| % | 3.75 | 78.20 | 15.20 | 2.85 | |
| (2061–2080) | SSP 126 area (km2) | 1551 | 40,317 | 7437 | 1792 |
| % | 3.04 | 78.91 | 14.55 | 3.50 | |
| SSP 245 area (km2) | 1762 | 40,106 | 6727 | 2502 | |
| % | 3.46 | 78.50 | 13.16 | 4.89 | |
| SSP 585 area (km2) | 1666 | 40,202 | 7750 | 1479 | |
| % | 3.27 | 78.69 | 15.16 | 2.89 | |
| (2081–2100) | SSP 126 area (km2) | 1786 | 40,082 | 7741 | 1488 |
| % | 3.50 | 78.45 | 15.14 | 2.90 | |
| SSP 245 area (km2) | 2075 | 39,793 | 7544 | 1685 | |
| % | 4.07 | 77.89 | 14.76 | 3.29 | |
| SSP 585 area (km2) | 1431 | 40,437 | 7370 | 1859 | |
| % | 2.81 | 79.15 | 14.42 | 3.63 |
| Year | Class | Range Expansion | No Occupancy (Absence in Both) | No Change (Presence in Both) | Range Contraction |
|---|---|---|---|---|---|
| (2041–2060) | SSP 126 area (km2) | 2241 | 39,627 | 7876 | 1353 |
| % | 4.39 | 77.56 | 15.41 | 2.64 | |
| SSP 245 area (km2) | 1533 | 40,335 | 7511 | 1718 | |
| % | 3.01 | 78.95 | 14.69 | 3.35 | |
| SSP 585 area (km2) | 1780 | 40,088 | 7909 | 1320 | |
| % | 3.49 | 78.46 | 15.47 | 2.58 | |
| (2061–2080) | SSP 126 area (km2) | 1891 | 39,977 | 7902 | 1327 |
| % | 3.71 | 78.25 | 15.46 | 2.59 | |
| SSP 245 area (km2) | 2154 | 39,714 | 7857 | 1372 | |
| % | 4.22 | 77.73 | 15.37 | 2.68 | |
| SSP 585 area (km2) | 2002 | 39,867 | 7985 | 1243 | |
| % | 3.93 | 78.03 | 15.62 | 2.42 | |
| (2081–2100) | SSP 126 area (km2) | 1883 | 39,985 | 8054 | 1175 |
| % | 3.69 | 78.26 | 15.75 | 2.29 | |
| SSP 245 area (km2) | 2445 | 39,423 | 7853 | 1376 | |
| % | 4.79 | 77.16 | 15.36 | 2.68 | |
| SSP 585 area (km2) | 2839 | 39,029 | 7931 | 1298 | |
| % | 5.56 | 76.39 | 15.51 | 2.53 |
| Year | Class | Unsuitable | Low Suitability | Medium Suitability | High Suitability |
|---|---|---|---|---|---|
| Current | Area (km2) | 40,656 | 5032 | 4203 | 1208 |
| % | 79.57 | 9.86 | 8.23 | 2.38 | |
| (2041–2060) | SSP 126 area (km2) | 40,391 | 5270 | 4469 | 969 |
| % | 79.06 | 10.32 | 8.75 | 1.89 | |
| SSP 245 area (km2) | 43,984 | 1538 | 4623 | 954 | |
| % | 86.09 | 3.02 | 9.06 | 1.86 | |
| SSP 585 area (km2) | 40,051 | 5206 | 4902 | 940 | |
| % | 78.39 | 10.20 | 9.60 | 1.83 | |
| (2061–2080) | SSP 126 area (km2) | 40,566 | 4922 | 4496 | 1115 |
| % | 79.40 | 9.64 | 8.81 | 2.17 | |
| SSP 245 area (km2) | 41,109 | 4141 | 4820 | 1029 | |
| % | 80.46 | 8.11 | 9.42 | 2.01 | |
| SSP 585 area (km2) | 40,299 | 5349 | 4555 | 896 | |
| % | 78.88 | 10.48 | 8.92 | 1.75 | |
| (2081–2100) | SSP 126 area (km2) | 40,468 | 5146 | 4589 | 896 |
| % | 79.21 | 10.08 | 8.99 | 1.75 | |
| SSP 245 area (km2) | 40,226 | 4676 | 5163 | 1034 | |
| % | 78.73 | 9.16 | 10.11 | 2.02 | |
| SSP 585 area (km2) | 41,330 | 5000 | 3868 | 901 | |
| % | 80.89 | 9.79 | 7.58 | 1.76 |
| Year | Class | Unsuitable | Low Suitability | Medium Suitability | High Suitability |
|---|---|---|---|---|---|
| Current | Area (km2) | 40,656 | 5032 | 4201 | 1208 |
| % | 79.57 | 9.86 | 8.21 | 2.36 | |
| (2041–2060) | SSP 126 area (km2) | 40,523 | 5220 | 4415 | 939 |
| % | 79.31 | 10.22 | 8.63 | 1.83 | |
| SSP 245 area (km2) | 40,325 | 5347 | 4507 | 918 | |
| % | 78.93 | 10.47 | 8.81 | 1.79 | |
| SSP 585 area (km2) | 40,408 | 5357 | 4427 | 905 | |
| % | 79.09 | 10.49 | 8.66 | 1.76 | |
| (2061–2080) | SSP 126 area (km2) | 40,073 | 5469 | 4703 | 852 |
| % | 78.43 | 10.71 | 9.20 | 1.66 | |
| SSP 245 area (km2) | 40,031 | 5217 | 4859 | 990 | |
| % | 78.35 | 10.22 | 9.50 | 1.93 | |
| SSP 585 area (km2) | 39,932 | 5454 | 4777 | 934 | |
| % | 78.16 | 10.68 | 9.34 | 1.82 | |
| (2081–2100) | SSP 126 area (km2) | 40,107 | 5429 | 4628 | 933 |
| % | 78.50 | 10.63 | 9.05 | 1.82 | |
| SSP 245 area (km2) | 38,974 | 5521 | 5397 | 1205 | |
| % | 76.28 | 10.81 | 10.55 | 2.35 | |
| SSP 585 area (km2) | 39,888 | 5693 | 4547 | 969 | |
| % | 78.07 | 11.15 | 8.89 | 1.89 |
| Year | Class | Unsuitable | Low Suitability | Medium Suitability | High Suitability |
|---|---|---|---|---|---|
| Current | Area (km2) | 41,868 | 4308 | 3771 | 1150 |
| % | 81.95 | 8.44 | 7.37 | 2.24 | |
| (2041–2060) | SSP 126 area (km2) | 41,518 | 4811 | 4153 | 615 |
| % | 81.26 | 9.42 | 8.12 | 1.20 | |
| SSP 245 area (km2) | 41,363 | 5048 | 4084 | 602 | |
| % | 80.96 | 9.89 | 8 | 1.19 | |
| SSP 585 area (km2) | 41,420 | 4808 | 4201 | 668 | |
| % | 81.07 | 9.42 | 8.21 | 1.30 | |
| (2061–2080) | SSP 126 area (km2) | 42,111 | 4269 | 3905 | 812 |
| % | 82.42 | 8.36 | 7.63 | 1.58 | |
| SSP 245 area (km2) | 42,613 | 3854 | 3819 | 811 | |
| % | 83.40 | 7.55 | 7.47 | 1.58 | |
| SSP 585 area (km2) | 41,682 | 4838 | 3942 | 635 | |
| % | 81.58 | 9.48 | 7.71 | 1.23 | |
| (2081–2100) | SSP 126 area (km2) | 41,575 | 4700 | 4211 | 611 |
| % | 81.37 | 9.21 | 8.23 | 1.21 | |
| SSP 245 area (km2) | 41,484 | 4610 | 4315 | 688 | |
| % | 81.19 | 9.03 | 8.44 | 1.34 | |
| SSP 585 area (km2) | 42,298 | 4331 | 3714 | 754 | |
| % | 82.79 | 8.48 | 7.26 | 1.47 |
| Year | Class | Unsuitable | Low Suitability | Medium Suitability | High Suitability |
|---|---|---|---|---|---|
| Current | Area (km2) | 41,868 | 4308 | 3771 | 1150 |
| % | 81.95 | 8.44 | 7.37 | 2.24 | |
| (2041–2060) | SSP 126 area (km2) | 40,984 | 4568 | 4204 | 1341 |
| % | 80.22 | 8.95 | 8.22 | 2.62 | |
| SSP 245 area (km2) | 42,060 | 4159 | 4108 | 770 | |
| % | 82.32 | 8.15 | 8.03 | 1.50 | |
| SSP 585 area (km2) | 41,409 | 4944 | 4038 | 706 | |
| % | 81.05 | 9.68 | 7.89 | 1.37 | |
| (2061–2080) | SSP 126 area (km2) | 41,305 | 4920 | 4306 | 566 |
| % | 80.84 | 9.64 | 8.42 | 1.10 | |
| SSP 245 area (km2) | 41,087 | 4975 | 4445 | 590 | |
| % | 80.42 | 9.74 | 8.69 | 1.15 | |
| SSP 585 area (km2) | 41,110 | 5011 | 4181 | 795 | |
| % | 80.44 | 9.81 | 8.17 | 1.55 | |
| (2081–2100) | SSP 126 area (km2) | 41,163 | 4987 | 4224 | 723 |
| % | 80.57 | 9.77 | 8.26 | 1.41 | |
| SSP 245 area (km2) | 40,800 | 5524 | 4169 | 604 | |
| % | 79.86 | 10.82 | 8.15 | 1.17 | |
| SSP 585 area (km2) | 40,329 | 5407 | 4246 | 1115 | |
| % | 78.93 | 10.59 | 8.30 | 2.17 |
References
- Kozhoridze, G.; Orlovsky, N.; Orlovsky, L.; Blumberg, D.G.; Golan-Goldhirsh, A. Geographic distribution and migration pathways of Pistacia–present, past and future. Ecography 2015, 38, 1141–1154. [Google Scholar] [CrossRef]
- AL-Saghir, M.G.; Porter, D.M. Taxonomic revision of the genus Pistacia L. (Anacardiaceae). Am. J. Plant Sci. 2011, 3, 12–32. [Google Scholar] [CrossRef]
- Guest, E.; Townsend, C. Flora of Iraq; Ministry of Agriculture of the Republic of Iraq: Baghdad, Iraq, 1966.
- Ahmed, H.M. Traditional uses of Kurdish medicinal plant Pistacia atlantica subsp. kurdica Zohary in Ranya, Southern Kurdistan. Genet. Resour. Crop Evol. 2017, 64, 1473–1484. [Google Scholar] [CrossRef]
- Rankou, H.; M’sou, S.; Babahmad, R.A.; Ouhammou, A.; Alifriqui, M.; Martin, G. Pistacia atlantica. In The IUCN Red List of Threatened Species; IUCN red list: Cambridge, UK, 2018. [Google Scholar]
- Khwarahm, N.R. Spatial modeling of land use and land cover change in Sulaimani, Iraq, using multitemporal satellite data. Environ. Monit. Assess. 2021, 193, 148. [Google Scholar] [CrossRef]
- Khwarahm, N.R.; Najmaddin, P.M.; Ararat, K.; Qader, S. Past and future prediction of land cover land use change based on earth observation data by the CA–Markov model: A case study from Duhok governorate, Iraq. Arab. J. Geosci. 2021, 14, 1544. [Google Scholar] [CrossRef]
- Khwarahm, N.R.; Qader, S.; Ararat, K.; Al-Quraishi, A.M.F. Predicting and mapping land cover/land use changes in Erbil/Iraq using CA-Markov synergy model. Earth Sci. Inform. 2021, 14, 393–406. [Google Scholar] [CrossRef]
- Khwarahm, N.R. Modeling forest-shrubland fire susceptibility based on machine learning and geospatial approaches in mountains of Kurdistan Region, Iraq. Arab. J. Geosci. 2022, 15, 1184. [Google Scholar] [CrossRef]
- Nasser, M. Forests and forestry in Iraq: Prospects and limitations. Commonw. For. Rev. 1984, 63, 299–304. [Google Scholar]
- Javanshah, A. Global warming has been affecting some morphological characters of pistachio trees (Pistacia vera L.). Afr. J. Agric. Res. 2010, 5, 3394–3401. [Google Scholar]
- Hama, A.A.; Khwarahm, N.R. Predictive mapping of two endemic oak tree species under climate change scenarios in a semiarid region: Range overlap and implications for conservation. Ecol. Inform. 2023, 73, 101930. [Google Scholar] [CrossRef]
- Radha, K.O.; Khwarahm, N.R. An Integrated Approach to Map the Impact of Climate Change on the Distributions of Crataegus azarolus and Crataegus monogyna in Kurdistan Region, Iraq. Sustainability 2022, 14, 14621. [Google Scholar] [CrossRef]
- Ahmed, Z.B.; Yousfi, M.; Viaene, J.; Dejaegher, B.; Demeyer, K.; Heyden, Y.V. Four Pistacia atlantica subspecies (atlantica, cabulica, kurdica and mutica): A review of their botany, ethnobotany, phytochemistry and pharmacology. J. Ethnopharmacol. 2020, 265, 113329. [Google Scholar] [CrossRef] [PubMed]
- Bozorgi, M.; Memariani, Z.; Mobli, M.; Salehi Surmaghi, M.H.; Shams-Ardekani, M.R.; Rahimi, R. Five Pistacia species (P. vera, P. atlantica, P. terebinthus, P. khinjuk, and P. lentiscus): A review of their traditional uses, phytochemistry, and pharmacology. Sci. World J. 2013, 2013, 219815. [Google Scholar] [CrossRef] [PubMed]
- Hatamnia, A.A.; Abbaspour, N.; Darvishzadeh, R. Antioxidant activity and phenolic profile of different parts of Bene (Pistacia atlantica subsp. kurdica) fruits. Food Chem. 2014, 145, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, M.; Saki, K.; Asadbeygi, M.; Adineh, A.; Saberianpour, S.; Rafieian-Kopaei, M.; Bahmani, F.; Bahmani, E. The effects of nutritional and medicinal mastic herb (Pistacia atlantica). J. Chem. Pharm. Res. 2015, 7, 646–653. [Google Scholar]
- Schulze-Kaysers, N.; Feuereisen, M.; Schieber, A. Phenolic compounds in edible species of the Anacardiaceae family—A review. RSC Adv. 2015, 5, 73301–73314. [Google Scholar] [CrossRef]
- Sharifi, M.S.; Hazell, S.L. GC-MS analysis and antimicrobial activity of the essential oil of the trunk exudates from Pistacia atlantica kurdica. J. Pharm. Sci. Res. 2011, 3, 1364. [Google Scholar]
- Ahmad, S.A.; Askari, A.A. Ethnobotany of the Hawraman region of Kurdistan Iraq. Harv. Pap. Bot. 2015, 20, 85–89. [Google Scholar] [CrossRef]
- Al-Saghir, M.G.; Porter, D.M.; Nilsen, E.T. Leaf anatomy of Pistacia species (Anacardiaceae). J. Biol. Sci. 2006, 6, 242–244. [Google Scholar] [CrossRef]
- Basr Ila, H.; Kafkas, S.; Topaktas, M. Chromosome numbers of four Pistacia (Anacardiaceae) species. J. Hortic. Sci. Biotechnol. 2003, 78, 35–38. [Google Scholar] [CrossRef]
- Parfitt, D.E.; Badenes, M.L. Phylogeny of the genus Pistacia as determined from analysis of the chloroplast genome. Proc. Natl. Acad. Sci. USA 1997, 94, 7987–7992. [Google Scholar] [CrossRef] [PubMed]
- Kafkas, S.; Perl-Treves, R. Morphological and molecular phylogeny of Pistacia species in Turkey. Theor. Appl. Genet. 2001, 102, 908–915. [Google Scholar] [CrossRef]
- Talebi, M.; Kazemi, M.; Sayed-Tabatabaei, B.E. Molecular diversity and phylogenetic relationships of Pistacia vera, Pistacia atlantica subsp. mutica and Pistacia khinjuk using SRAP markers. Biochem. Syst. Ecol. 2012, 44, 179–185. [Google Scholar] [CrossRef]
- Belhadj, S.; Derridj, A.; Aigouy, T.; Gers, C.; Gauquelin, T.; Mevy, J.P. Comparative morphology of leaf epidermis in eight populations of Atlas pistachio (Pistacia atlantica Desf., Anacardiaceae). Microsc. Res. Tech. 2007, 70, 837–846. [Google Scholar] [CrossRef]
- Sawidis, T.; Dafnis, S.; Weryzko-Chmielewska, E. Distribution, development and structure of resin ducts in Pistacia lentiscus var. chia Duhamel. Flora 2000, 195, 83–94. [Google Scholar] [CrossRef]
- Al-Alfy, N.; Moustafa, A.; Alotaibi, M.; Mansour, S. Impact of Climate Change on Pistacia khinjuk as a Medicinal Plant in Egypt and Saudi Arabia. Appl. Sci. Res. Rev. 2019, 6, 3. [Google Scholar]
- Pineda, E.; Lobo, J.M. Assessing the accuracy of species distribution models to predict amphibian species richness patterns. J. Anim. Ecol. 2009, 78, 182–190. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Svenning, J.-C.; Fløjgaard, C.; Marske, K.A.; Nógues-Bravo, D.; Normand, S. Applications of species distribution modeling to paleobiology. Quat. Sci. Rev. 2011, 30, 2930–2947. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Graham, C.H.; Ferrier, S.; Huettman, F.; Moritz, C.; Peterson, A.T. New developments in museum-based informatics and applications in biodiversity analysis. Trends Ecol. Evol. 2004, 19, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Bor, N.L.; Guest, E. Flora of Iraq, Vol. 9. Gramineae. In Flora of Iraq; Ministry of Agriculture and Agrarian Reform: Baghdad, Iraq, 1968. [Google Scholar]
- Malinowski, J.C. Iraq: A Geography; United States Military Academy: West Point, NY, USA, 2002. [Google Scholar]
- Salman, S.A.; Shahid, S.; Ismail, T.; Ahmed, K.; Chung, E.-S.; Wang, X.-J. Characteristics of annual and seasonal trends of rainfall and temperature in Iraq. Asia-Pac. J. Atmos. Sci. 2019, 55, 429–438. [Google Scholar] [CrossRef]
- Sissakian, V.; Jabbar, M.A.; Al-Ansari, N.; Knutsson, S. Development of Gulley Ali Beg Gorge in Rawandooz Area, Northern Iraq. Engineering 2015, 7, 16–30. [Google Scholar] [CrossRef]
- Khwarahm, N.R.; Ararat, K.; Qader, S.; Sabir, D.K. Modeling the distribution of the Near Eastern fire salamander (Salamandra infraimmaculata) and Kurdistan newt (Neurergus derjugini) under current and future climate conditions in Iraq. Ecol. Inform. 2021, 63, 101309. [Google Scholar] [CrossRef]
- Gaznayee, H.A.A.; Al-Quraishi, A.M.F.; Mahdi, K.; Ritsema, C. A Geospatial Approach for Analysis of Drought Impacts on Vegetation Cover and Land Surface Temperature in the Kurdistan Region of Iraq. Water 2022, 14, 927. [Google Scholar] [CrossRef]
- Bhatta, K.P.; Chaudhary, R.P.; Vetaas, O.R. A comparison of systematic versus stratified-random sampling design for gradient analyses: A case study in subalpine Himalaya, Nepal. Phytocoenologia 2012, 42, 191–202. [Google Scholar] [CrossRef]
- Boakes, E.H.; McGowan, P.J.; Fuller, R.A.; Chang-qing, D.; Clark, N.E.; O’Connor, K.; Mace, G.M. Distorted views of biodiversity: Spatial and temporal bias in species occurrence data. PLoS Biol. 2010, 8, e1000385. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making better Maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Brown, J.L. SDM toolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Soberón, J.; Peterson, A.T. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodivers. Inform. 2005, 2, 1–10. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Austin, M.P.; Van Niel, K.P. Improving species distribution models for climate change studies: Variable selection and scale. J. Biogeogr. 2011, 38, 1–8. [Google Scholar] [CrossRef]
- Pradervand, J.-N.; Dubuis, A.; Pellissier, L.; Guisan, A.; Randin, C. Very high resolution environmental predictors in species distribution models: Moving beyond topography? Prog. Phys. Geogr. 2014, 38, 79–96. [Google Scholar] [CrossRef]
- IPCC. The Physical Science Basis: Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; Volume 996, pp. 113–119. [Google Scholar]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. J. R. Meteorol. Soc. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Hanberry, B.B. Global population densities, climate change, and the maximum monthly temperature threshold as a potential tipping point for high urban densities. Ecol. Indic. 2022, 135, 108512. [Google Scholar] [CrossRef]
- Karim, R.; Tan, G.; Ayugi, B.; Babaousmail, H.; Liu, F. Evaluation of historical CMIP6 model simulations of seasonal mean temperature over Pakistan during 1970–2014. Atmosphere 2020, 11, 1005. [Google Scholar] [CrossRef]
- Fan, X.; Duan, Q.; Shen, C.; Wu, Y.; Xing, C. Global surface air temperatures in CMIP6: Historical performance and future changes. Environ. Res. Lett. 2020, 15, 104056. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Syfert, M.M.; Smith, M.J.; Coomes, D.A. The effects of sampling bias and model complexity on the predictive performance of MaxEnt species distribution models. PLoS ONE 2013, 8, e55158. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef] [PubMed]
- Dudík, M.; Phillips, S.J.; Schapire, R.E. Maximum entropy density estimation with generalized regularization and an application to species distribution modeling. J. Mach. Learn. Res. 2007, 8, 1217–1260. [Google Scholar]
- Merow, C.; Smith, M.J.; Silander Jr, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Phillips, S.J. A brief tutorial on Maxent. ATT Res. 2005, 190, 231–259. [Google Scholar]
- Jiménez-Valverde, A.; Lobo, J.M. Threshold criteria for conversion of probability of species presence to either–or presence–absence. Acta Oecologica 2007, 31, 361–369. [Google Scholar] [CrossRef]
- Yang, X.-Q.; Kushwaha, S.; Saran, S.; Xu, J.; Roy, P. Maxent modeling for predicting the potential distribution of medicinal plant, Justicia adhatoda L. in Lesser Himalayan foothills. Ecol. Eng. 2013, 51, 83–87. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, T.; Li, L.; Zhao, Y.; Pei, L.; Zhao, J. Predicting the potential distribution of Polygala tenuifolia Willd. under climate change in China. PLoS ONE 2016, 11, e0163718. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Li, C.; Gao, Y.; Zhao, Z.; Ma, D.; Zhou, R.; Wang, J.; Zhang, Q.; Liu, Q. Potential geographical distribution of Anopheles gambiae worldwide under climate change. J. Biosaf. Biosecur. 2021, 3, 125–130. [Google Scholar] [CrossRef]
- Hoveka, L.N.; van der Bank, M.; Davies, T.J. Winners and losers in a changing climate: How will protected areas conserve red list species under climate change? Divers. Distrib. 2022, 28, 782–792. [Google Scholar] [CrossRef]
- Monzón, J.; Moyer-Horner, L.; Palamar, M.B. Climate change and species range dynamics in protected areas. Bioscience 2011, 61, 752–761. [Google Scholar] [CrossRef]
- Khwarahm, N.R. Mapping current and potential future distributions of the oak tree (Quercus aegilops) in the Kurdistan Region, Iraq. Ecol. Process. 2020, 9, 56. [Google Scholar] [CrossRef]
- Ayebare, S.; Plumptre, A.; Kujirakwinja, D.; Segan, D. Conservation of the endemic species of the Albertine Rift under future climate change. Biol. Conserv. 2018, 220, 67–75. [Google Scholar] [CrossRef]
- Loarie, S.R.; Carter, B.E.; Hayhoe, K.; McMahon, S.; Moe, R.; Knight, C.A.; Ackerly, D.D. Climate change and the future of California’s endemic flora. PLoS ONE 2008, 3, e2502. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, P.C.; McGEOCH, M.A. Rapid range expansion and community reorganization in response to warming. Glob. Chang. Biol. 2008, 14, 2950–2962. [Google Scholar] [CrossRef]
- López-Sánchez, C.A.; Castedo-Dorado, F.; Cámara-Obregón, A.; Barrio-Anta, M. Distribution of Eucalyptus globulus Labill. in northern Spain: Contemporary cover, suitable habitat and potential expansion under climate change. For. Ecol. Manag. 2021, 481, 118723. [Google Scholar] [CrossRef]
- Wang, W.J.; Thompson III, F.R.; He, H.S.; Fraser, J.S.; Dijak, W.D.; Jones-Farrand, T. Climate change and tree harvest interact to affect future tree species distribution changes. J. Ecol. 2019, 107, 1901–1917. [Google Scholar] [CrossRef]
- Setyawan, A.D.; Supriatna, J.; Nisyawati, N.; Nursamsi, I.; Sutarno, S.; Sugiyarto, S.; Sunarto, S.; Pradan, P.; Budiharta, S.; Pitoyo, A.; et al. Projecting expansion range of Selaginella zollingeriana in the Indonesian archipelago under future climate condition. Biodivers. J. Biol. Divers. 2021, 22, 2088–2103. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Štípková, Z.; Kindlmann, P. Role of way of life, latitude, elevation and climate on the richness and distribution of orchid species. Biodivers. Conserv. 2019, 28, 75–96. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, H.; Pan, H.; Shi, W.; Zhao, Y.; Li, S.; Liu, J.; Tao, J. Shifts in potential geographical distribution of Pterocarya stenoptera under climate change scenarios in China. Ecol. Evol. 2020, 10, 4828–4837. [Google Scholar] [CrossRef]
- Nachtergaele, F.O. Classification Systems: FAO☆. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Hao, T.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J.J. A review of evidence about use and performance of species distribution modelling ensembles like BIOMOD. Divers. Distrib. 2019, 25, 839–852. [Google Scholar] [CrossRef]
- Baldwin, R.A. Use of maximum entropy modeling in wildlife research. Entropy 2009, 11, 854–866. [Google Scholar] [CrossRef]
- Elith, J.; Franklin, J. Species distribution modeling. In Encyclopedia of Biodiversity, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 692–705. [Google Scholar]
- Barnhart, P.R.; Gillam, E.H. The impact of sampling method on maximum entropy species distribution modeling for bats. Acta Chiropterologica 2014, 16, 241–248. [Google Scholar] [CrossRef]
- Kearney, M.; Porter, W. Mechanistic niche modelling: Combining physiological and spatial data to predict species’ ranges. Ecol. Lett. 2009, 12, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, L.R.d.A.; Lima, A.P.; Machado, R.B.; Magnusson, W.E. Limitations to the use of species-distribution models for environmental-impact assessments in the Amazon. PLoS ONE 2016, 11, e0146543. [Google Scholar] [CrossRef]
- Miller, J. Species distribution modeling. Geogr. Compass 2010, 4, 490–509. [Google Scholar] [CrossRef]
- Kasagana, V.N.; Karumuri, S.S. Conservation of medicinal plants (past, present & future trends). J. Pharm. Sci. Res. 2011, 3, 1378. [Google Scholar]
- Mahmoodi, S.; Heydari, M.; Ahmadi, K.; Khwarahm, N.R.; Karami, O.; Almasieh, K.; Naderi, B.; Bernard, P.; Mosavi, A. The current and future potential geographical distribution of Nepeta crispa Willd., an endemic, rare and threatened aromatic plant of Iran: Implications for ecological conservation and restoration. Ecol. Indic. 2022, 137, 108752. [Google Scholar] [CrossRef]
- Yadav, P.; Sarma, K.; Dookia, S. The review of biodiversity and conservation study in India using geospatial technology. Int. J. Remote Sens. GIS 2013, 2, 1–10. [Google Scholar]
- Draper, D.; Rosselló-Graell, A.; Garcia, C.; Gomes, C.T.; Sérgio, C.l. Application of GIS in plant conservation programmes in Portugal. Biol. Conserv. 2003, 113, 337–349. [Google Scholar] [CrossRef]


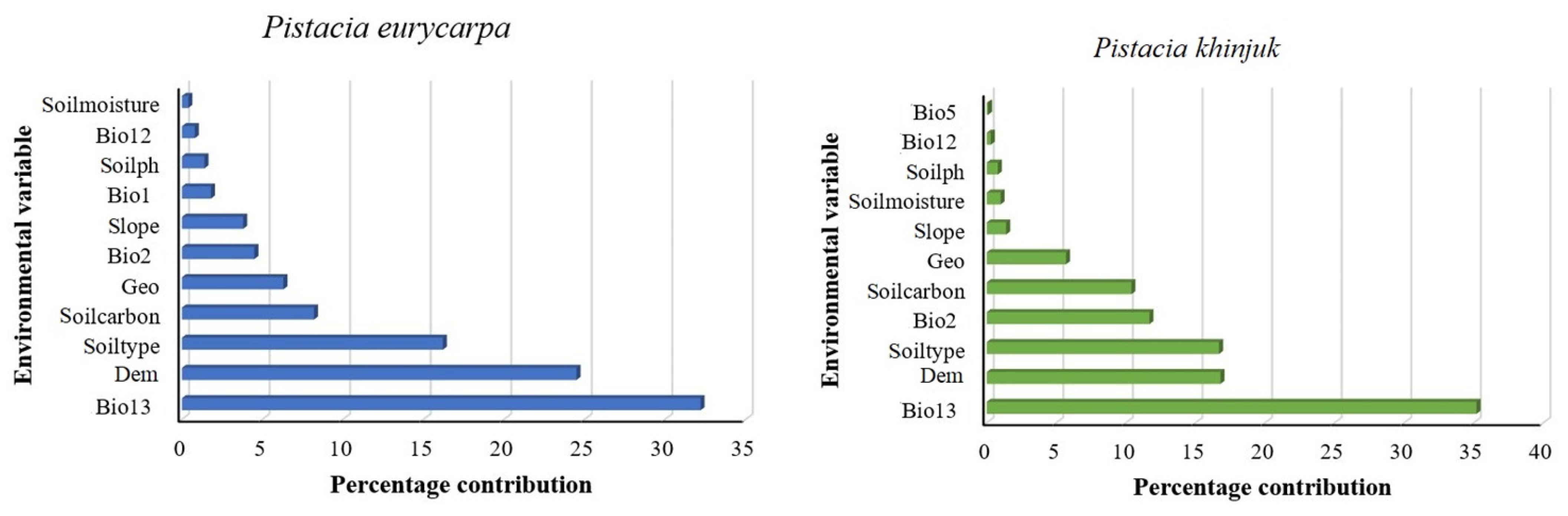
| Variable | Code and Unit | P. eurycarpa | P. khinjuk |
|---|---|---|---|
| Annual mean temperature | Bio1 (°C) | √ | |
| Mean diurnal range | Bio2 (°C) | √ | √ |
| Temperature seasonality | Bio4 (standard deviation × 100) | ||
| Isothermality (BIO2/BIO7) | Bio3 (×100) | ||
| Max temperature of warmest month | Bio5 (°C) | √ | |
| Min temperature of coldest month | Bio6 (°C) | ||
| Temperature annual range | Bio7 (Bio5-Bio6) (°C) | ||
| Mean temperature of wettest quarter | Bio8 (°C) | ||
| Mean temperature of driest quarter | Bio9 (°C) | ||
| Mean temperature of warmest quarter | Bio10 (°C) | ||
| Mean temperature of coldest quarter | Bio11 (°C) | ||
| Annual precipitation | Bio12 mm | √ | √ |
| Precipitation of wettest month | Bio13 mm | √ | √ |
| Precipitation of coldest quarter | Bio19 mm | ||
| Precipitation of warmest quarter | Bio18 mm | ||
| Precipitation of driest quarter | Bio17 mm | ||
| Precipitation of wettest quarter | Bio16 mm | ||
| Precipitation seasonality | Bio15 mm | ||
| Precipitation of driest month | Bio14 mm | ||
| Slope | Slope (degree) | √ | √ |
| Aspect | Aspect (degree) | ||
| Soil type | FAO soil classification | √ | √ |
| Soil carbon | Soil carbon (%) | √ | √ |
| Soil moisture | Soil moisture (mm) | √ | √ |
| Soil pH | Soil (parts hydrogen) | √ | √ |
| DEM | Digital elevation model (m) | √ | √ |
| Geo | √ | √ |
| Precipitation of Wettest Month (Bio13) | Current | P. khinjuk | P. eurycarpa | ||
|---|---|---|---|---|---|
| BCC-CSM2-MR | MIROC-ES2L | BCC-CSM2-MR | MIROC-ES2L | ||
| SSP 245 | SSP 585 | SSP 585 | SSP 245 | ||
| (2041–2060) | (2081–2100) | (2041–2060) | (2081–2100) | ||
| Lowest | 58 | 55 | 56 | 55 | 55 |
| Highest | 200 | 174 | 198 | 181 | 193 |
| Value | Legend | FAOSOIL | Area | Area in % |
|---|---|---|---|---|
| 1 | Lithosol | I-Rc-Xk-c | 5842.2 | 11.4 |
| 3 | Lithosol | I-Be-c | 515.4 | 1.0 |
| 4 | Chromic Luvisols | Lc63-3bc | 11.9 | 0.0 |
| 6 | Calcic Xerosols | Xk5-3ab | 101.1 | 0.2 |
| 7 | Haplic Xerosols | Xh31-3a | 30.7 | 0.1 |
| 9 | Chromic Vertisols | Vc1-3a | 9547 | 18.7 |
| 11 | Haplic Xerosols | Xh33-3a | 29.7 | 0.1 |
| 12 | Chromic Vertisols | Vc50-3ab | 2041 | 4.0 |
| 14 | Lithosol | I-E-bc | 8516 | 16.7 |
| 16 | Lithosol | I-E-Xk-bc | 5331 | 10.4 |
| 18 | Calcic Xerosols | Xk29-ab | 440 | 0.9 |
| 19 | Calcic Xerosols | Xk26-2/3a | 1065 | 2.1 |
| 20 | Gypsic Xerosols | Xy5-a | 1023 | 2.0 |
| 21 | Calcic Xerosols | Xk28-b | 12,464 | 24.4 |
| 22 | Calcic Xerosols | Xk9-2/3a | 586 | 1.1 |
| 23 | Calcaric Fluvisols | Jc1-2a | 462 | 0.9 |
| 24 | Gypsic Yermosols | Yy10-2ab | 259 | 0.5 |
| 25 | Gypsic Yermosols | Yy10-2/3a | 2473 | 4.8 |
| 29 | Calcic Yermosols | Yk34-b | 359 | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
HamadAmin, B.A.; Khwarahm, N.R. Mapping Impacts of Climate Change on the Distributions of Two Endemic Tree Species under Socioeconomic Pathway Scenarios (SSP). Sustainability 2023, 15, 5469. https://doi.org/10.3390/su15065469
HamadAmin BA, Khwarahm NR. Mapping Impacts of Climate Change on the Distributions of Two Endemic Tree Species under Socioeconomic Pathway Scenarios (SSP). Sustainability. 2023; 15(6):5469. https://doi.org/10.3390/su15065469
Chicago/Turabian StyleHamadAmin, Barham A., and Nabaz R. Khwarahm. 2023. "Mapping Impacts of Climate Change on the Distributions of Two Endemic Tree Species under Socioeconomic Pathway Scenarios (SSP)" Sustainability 15, no. 6: 5469. https://doi.org/10.3390/su15065469
APA StyleHamadAmin, B. A., & Khwarahm, N. R. (2023). Mapping Impacts of Climate Change on the Distributions of Two Endemic Tree Species under Socioeconomic Pathway Scenarios (SSP). Sustainability, 15(6), 5469. https://doi.org/10.3390/su15065469






