Food Proteins: Potential Resources
Abstract
:1. Introduction
2. General Characteristics of Plant-Based Protein Sources
| Plant Source | Protein Content, g/100 g | Sources |
|---|---|---|
| Spirulina | 57.47 | [27] |
| Starch | 6.90 | [27] |
| Garlic | 6.36 | [28] |
| Brussels sprouts | 3.38 | [27] |
| Broccoli | 2.82 | [29] |
| Potatos | 2.00 | [27] |
| Dried apricots | 3.50 | [27] |
| Raisins | 3.07 | [27] |
| Semi-dried dates | 2.45 | [27] |
| Fruits * | 1.0–2.0 | [28] |
| Chilli | 13.46 | [27] |
| Fenugreek | 23.00 | [28] |
| Poppy seeds | 17.99 | [28] |
| Sweet pepper | 10.39 | [27] |
| Thyme | 9.11 | [27] |
| Oregano | 9.00 | [29] |
| Herbs ** | 3.0–4.0 | [29] |
3. Dietary Proteins from Algae
4. Dietary Proteins from Insects and Other Sources
| Insect | Moisture, % | Crude fat, % | Crude Protein *, % (Including Chitin Nitrogen) | Other Components, % (e.g., Carbohydrates, Minerals, and Vitamins) | Sources |
|---|---|---|---|---|---|
| T. molitor | 63.5 ± 1.8 | 9.9 ± 1.0 | 19.1 ± 1.3 | 7.5 ± 2.2 | [101] |
| A. diaperinus | 64.5 ± 1.0 | 8.5 ± 0.2 | 20.6 ± 0.1 | 6.4 ± 1.0 | [102] |
| Z. morio | 59.9 ± 5.4 | 16.0 ± 0.7 | 20.7 ± 0.3 | 3.4 ± 5.5 | [103] |
| A. domesticus | 70.8 ± 2.0 | 3.6 ± 0.4 | 21.5 ± 0.5 | 4.1 ± 2.1 | [77] |
| B. dubia | 67.4 ± 2.1 | 7.7 ± 0.1 | 19.3 ± 0.9 | 5.6 ± 2.3 | [76] |
5. Development of the Alternative Protein Market: Advantages and Disadvantages
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joint FAO/WHO/UNU. Expert Consultation on Protein and Amino Acid Requirements in Human Nutrition; World Health Organization: Geneva, Switzerland, 2007. Available online: https://apps.who.int/iris/handle/10665/43411 (accessed on 5 January 2023).
- Application of Risk Analysis to Food Standards Issues; Report of the Joint FAO/WHO Expert Consultation: Geneva, Switzerland, 1995; pp. 13–17.
- Food Consumption in Households in 2018. Report of the Federal Statistics Service of Russia; World Health Organization: Geneva, Switzerland, 1995; Available online: https://www.gks.ru/bgd/regl/b19101/Main.htm (accessed on 5 January 2023).
- Baum, J.I.; Kim, I.Y.; Wolfe, R.R. Protein Consumption and the Elderly: What Is the Optimal Level of Intake? Nutrients 2016, 8, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention (CDC). The State of Aging and Health in America 2013; Centers for Disease Control and Prevention, Ed.; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2013.
- Aragão, C.; Gonçalves, A.T.; Costas, B.; Azeredo, R.; Xavier, M.J.; Engrola, S. Alternative Proteins for Fish Diets: Implications beyond Growth. Animals 2022, 12, 1211. [Google Scholar] [CrossRef]
- World Bank Group. Commodity Markets Outlook, October 2020; World Bank: Washington, DC, USA, 2020; Available online: https://openknowledge.worldbank.org/handle/10986/34621 (accessed on 5 January 2023).
- Romnov, D. Nutritional values, what’s going on with world food prices. Gazeta.ru 2021, 224, 1247–1259. [Google Scholar]
- Molfetta, M.; Celano, G.; Minervini, F. Functional, Nutritional, and Sensory Quality of Mixed Flours-Based Breads as Compared to Durum Wheat Semolina-Based Breads. Foods 2021, 10, 1613. [Google Scholar] [CrossRef]
- Previtali, M.A.; Mastromatteo, M.; Conte, A.; De Vita, P.; Ficco, D.B.; Del Nobile, M.A. Optimization of durum wheat bread from a selenium-rich cultivar fortified with bran. J. Food Sci. Technol. 2016, 53, 1319–1327. [Google Scholar] [CrossRef] [Green Version]
- De Angelis, M.; Minervini, F.; Siragusa, S.; Rizzello, C.G.; Gobbetti, M. Wholemeal wheat flours drive the microbiome and functional features of wheat sourdoughs. Int. J. Food Microbiol. 2019, 302, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Zunjare, R.; Muthusamy, V.; Bhat, J.; Mehta, B.; Sharma, D.; Talukder, M.; Chhabra, R.; Katral, A.; Dutta, S.; Chand, G.; et al. Biofortification of Maize for Nutritional Security. In Biofortification of Staple Crops; Kumar., S., Dikshit., H.K., Mishra., G.P., Singh., A., Eds.; Springer: Singapore, 2022; Volume 6. [Google Scholar] [CrossRef]
- Woodruff, M. The Best Seeds and Nuts for Protein. Available online: https://masonfit.com/best-seeds-nuts-for-protein/ (accessed on 5 January 2023).
- Seeds High in Protein: Which Have The Most? Available online: https://vegfaqs.com/seeds-high-in-protein/ (accessed on 5 January 2023).
- Tu, X.H.; Wu, B.F.; Xie, Y.; Xu, S.L.; Wu, Z.Y.; Lv, X.; Wei, F.; Du, L.Q.; Chen, H. A comprehensive study of raw and roasted macadamia nuts: Lipid profile, physicochemical, nutritional, and sensory properties. Food Sci. Nutr. 2021, 9, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- Margier, M.; Georgé, S.; Hafnaoui, N.; Remond, D.; Nowicki, M.; Du Chaffaut, L.; Amiot, M.-J.; Reboul, E. Nutritional Composition and Bioactive Content of Legumes: Characterization of Pulses Frequently Consumed in France and Effect of the Cooking Method. Nutrients 2018, 10, 1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aremu, M.; Audu, S.; Gav, B. Comparative Review of Crude Protein and Amino Acids of Leguminous Seeds Grown in Nigeria. Int. J. Mol. Sci. 2017, 3, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Nosworthy, M.G.; Medina, G.; Franczyk, A.J.; Neufeld, J.; Appah, P.; Utioh, A.; Frohlich, P.; House, J.D. Effect of Processing on the In Vitro and In Vivo Protein Quality of Beans (Phaseolus vulgaris and Vicia Faba). Nutrients 2018, 10, 671. [Google Scholar] [CrossRef] [Green Version]
- Poutanen, K.S.; Kårlund, A.O.; Gómez-Gallego, C.; Johansson, D.P.; Scheers, N.M.; Marklinder, I.M.; Eriksen, A.K.; Silventoinen, P.C.; Nordlund, E.; Sozer, N.; et al. Grains—A major source of sustainable protein for health. Nutr. Rev. 2022, 80, 1648–1663. [Google Scholar] [CrossRef] [PubMed]
- Kyle, G. State of the Industry Report. Plant-Based Meat, Eggs, and Dairy. 2020. Available online: https://gfi.org/wp-content/uploads/2021/05/COR-SOTIR-Plant-based-meat-eggs-and-dairy-2021-0504-1.pdf (accessed on 26 December 2022).
- Belik, S.N.; Morgul, E.V.; Kryuchkova, V.V.; Avetisyan, Z.E. Products of microbial synthesis in solving protein deficiency. East. Eur. Sci. J. 2016, 7, 122–129. Available online: https://cyberleninka.ru/article/n/produkty-mikrobnogo-sinteza-v-reshenii-problemy-belkovogo-defitsita (accessed on 5 January 2023). (In Russian).
- Gordalina, M.; Pinheiro, H.M.; Mateus, M.; da Fonseca, M.M.R.; Cesário, M.T. Macroalgae as Protein Sources—A Review on Protein Bioactivity, Extraction, Purification and Characterization. Appl. Sci. 2021, 11, 7969. [Google Scholar] [CrossRef]
- Pliego-Cortés, H.; Wijesekara, I.; Lang, M.; Bourgougnon, N.; Bedoux, G. Current Knowledge and Challenges in Extraction, Characterization and Bioactivity of Seaweed Protein and Seaweed-Derived Proteins. Adv. Bot. Res. 2020, 95, 289–326. [Google Scholar] [CrossRef]
- Faye, B.; Webber, H.; Naab, J.B.; MacCarthy, D.S.; Adam, M.; Ewert, F.; Lamers, J.P.A.; Schleussner, C.-F.; Ruane, A.; Gessner, U.; et al. Impacts of 1.5 versus 2.0 °C on cereal yields in the West African Sudan Savanna. Environ. Res. Lett. 2018, 13, 034014. [Google Scholar] [CrossRef]
- Tang, C.; Yang, D.; Liao, H. Edible insects as a food source: A review. Food Prod. Process. Nutr. 2019, 1, 8. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Rodríguez, M.; Barroso, F.G.; Fabrikov, D.; Sánchez-Muros, M.J. In vitro crude protein digestibility of insects: A review. Insects 2022, 13, 682. [Google Scholar] [CrossRef]
- Alcorta, A.; Porta, A.; Tárrega, A.; Alvarez, M.D.; Vaquero, M.P. Foods for Plant-Based Diets: Challenges and Innovations. Foods 2021, 10, 293. [Google Scholar] [CrossRef]
- Grossmann, L.; Weiss, J. Alternative Protein Sources as Technofunctional Food Ingredients. Food Sci. Technol. 2021, 2021, 12. [Google Scholar] [CrossRef]
- Marinangeli, C.P.F.; House, J.D. Potential impact of the digestible indispensable amino acid score as a measure of protein quality on dietary regulations and health. Nutr. Rev. 2017, 75, 658–667. [Google Scholar] [CrossRef] [Green Version]
- Hosseinkhani, N.; McCauley, J.I.; Ralph, P.J. Key challenges for the commercial expansion of ingredients from algae into human food products. Algal. Res. 2022, 64, 102696. [Google Scholar] [CrossRef]
- Boukid, F.; Rosell, C.M.; Rosene, S.; Bover-Cid, S.; Castellari, M. Non-animal proteins as cutting-edge ingredients to reformulate animal-free foodstuffs: Present status and future perspectives. Crit. Rev. Food Sci. Nutr. 2021, 62, 6390–6420. [Google Scholar] [CrossRef] [PubMed]
- López-Pedrouso, M.; Lorenzo, J.M.; Cantalapiedra, J.; Zapata, C.; Franco, J.M.; Franco, D. Aquaculture and by-products: Challenges and opportunities in the use of alternative protein sources and bioactive compounds, Aquaculture and By-Products: Challenges and Opportunities in the Use of Alternative Protein. Curr. Bioact. Compd. 2020, 92, 127–185. [Google Scholar] [CrossRef]
- Mérillon, J.-M.; Ramawat, K.G. Bioactive Molecules in Food: Reference Series in Phytochemistry; Springer Nature: London, UK, 2019. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Wolkers, H.; Barbosa, M.; Kleinegris, D.M.M.; Bosma, R.; Wijffels, R.H. Microalgae: The green gold of the future? In Large-Scale Sustainable Cultivation of Microalgae for the Production of Bulk Commodities; Harmsen., P., Ed.; Propress: Wageningen, the Netherlands, 2011; pp. 1–34. [Google Scholar]
- Christaki, E.; Florou-Paneri, P.; Bonos, E. Microalgae: A novel ingredient innutrition. Int. J. Food Sci. Nutr. 2011, 62, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Becker, W. Microalgae in human and animal nutrition. In Handbook of Microalgal Culture; Richmond, A., Hoboken, N.J., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2004; pp. 312–351. [Google Scholar]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, L.; Hinrichs, J.; Weiss, J. Cultivation and downstream processing of microalgae and cyanobacteria to generate protein-based technofunctional food ingredients. Crit. Rev. Food Sci. Nutr. 2020, 60, 2961–2989. [Google Scholar] [CrossRef]
- Jeurissen, S.M.F.; Buurma-Rethans, E.J.M.; Beukers, M.H.; Jansen-van der Vliet, M.; van Rossum, C.T.M.; Sprong, R.C. Consumption of plant food supplements in the Netherlands. Food Funct. 2018, 9, 179–190. [Google Scholar] [CrossRef] [Green Version]
- van Hunsel, F.P.A.M.; van der Kooi, D.; van de Koppel, S.; Kroes, B.H.; Woerdenbag, H.J. Analysis of Reports on Adverse Drug Reactions Related to Herbal Medicinal Products and Herbal Supplements in the Netherlands Received by the National Pharmacovigilance Centre. Lareb. Drug Saf. 2022, 45, 651–661. [Google Scholar] [CrossRef]
- Bito, T.; Okumura, E.; Fujishima, M.; Watanabe, F. Potential of Chlorella as a Dietary Supplement to Promote Human Health. Nutrients 2020, 12, 2524. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Patelakis, S.J.; Whitney-Lalonde, C.G.; Garrison, L.L.; Wall, C.L.; MacQuarrie, S.P. Nutrient composition and protein quality of microalgae meals produced from the marine prymnesiophyte Pavlova sp. 459 mass-cultivated in enclosed photobioreactors for potential use in salmonid aquafeeds. J. Appl. Phycol. 2019, 32, 239–318. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, C.; Xu, C. Nutritional evaluation of two marine microalgae as feedstock for aquafeed. Aqua. Res. 2020, 51, 946–956. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Mathys, A. Trends in microalgae incorporation into innovativefood products with potential health benefits. Front. Nutr. 2018, 5, 58. [Google Scholar] [CrossRef]
- Rani, K.; Sandal, N.; Sahoo, P.K. A comprehensive review on chlorella-itscomposition, health benefits, market and regulatory scenario. J. Pharm. Innov. 2018, 7, 584–589. [Google Scholar]
- Barrow, C.J.; Shahidi, F. Marine Nutraceuticals and Functional Foods; CRC Press: Boca Raton, FL, USA, 2007; pp. 27–39. [Google Scholar]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Soletto, D.; Binaghi, L.; Lodi, A.; Carvalho, J.C.M.; Converti, A. Batch and fed-batchcultivations of Spirulina platensis using ammonium sulphate and urea asnitrogen sources. Aquaculture 2005, 243, 217–224. [Google Scholar] [CrossRef]
- Yen, A.L. Conservation of Lepidoptera used as human food and medicine. Curr. Opin. Insect. Sci. 2015, 12, 102–108. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Liao, D.; Pu, R.; Cui, Y. Quantifying the effects of spirulina supplementation on plasma lipid and glucose concentrations, body weight, and blood pressure. Diabetes Metab. Syndr. Obes. 2018, 11, 729–742. [Google Scholar] [CrossRef] [Green Version]
- Tanveer, A. Spirulina-Food of the Future. In Innovation, Educational Announcement; BioNatural Healing College: Riverside, CA, USA, 2019; pp. 1–5. [Google Scholar]
- Weilan, S.; Reham, E.; Mostafa, E.-S.; Abdelfatah, A.; Hamed, E. Pharmaceutical applications and consequent environmental impacts of Spirulina (Arthrospira): An overview. Grasas Y Aceites 2019, 70, 292. [Google Scholar] [CrossRef] [Green Version]
- Kirpenko, N.; Leontieva, T. Biotechnological prospects of microalgae. Biotechnol. Acta 2019, 12, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Bendif, E.M.; Probert, I.; Schroeder, D.C.; de Vargas, C. On the description of Tisochrysis lutea gen. nov. sp. nov. and Isochrysis nuda sp. nov. in the Isochrysidales, and the transfer of Dicrateria to the Prymnesiales (Haptophyta). J. Appl. Phycol. 2013, 25, 1763–1776. [Google Scholar] [CrossRef]
- Suresh, P.; Kudre, T.G.; Johny, L.C. Sustainable valorization of seafood processing by-product/discard. In Waste to Wealth; Singhania, R.R., RAgarwal, A., Kumar, R.P., Sukumaran, R.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 111–139. [Google Scholar]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Šimat, V.; Vlahović, J.; Soldo, B.; Mekinić, I.G.; Čagalj, M.; Hamed, I.; Skroza, D. Production and characterization of crude oils from seafood processing by-products. Food Biosci. 2020, 33, 100484. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Heimann, K.; Zhang, W. Protein recovery from underutilised marine bioresources for product development with nutraceutical and pharmaceutical bioactivities. Mar. Drugs 2020, 18, 391. [Google Scholar] [CrossRef]
- Pateiro, M.; Munekata, P.E.; Domínguez, R.; Wang, M.; Barba, F.J.; Bermúdez, R.; Lorenzo, J.M. Nutritional profiling and the value of processing by-products from gilthead sea bream (Sparus aurata). Mar. Drugs 2020, 18, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Schade, S.; Stangl, G.I.; Meier, T. Distinct microalgae species for food—Part 2: Comparative life cycle assessment of microalgae and fish for eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and protein J. Appl. Phycol. 2020, 32, 2997–3013. [Google Scholar] [CrossRef]
- El-Naggar, N.E.A.; Hussein, M.H.; Shaaban-Dessuuki, S.A.; Dalal, S.R. Production, extraction and characterization of Chlorella vulgaris soluble polysaccharides and their applications in AgNPs biosynthesis and biostimulation of plant growth Sci. Rep. 2020, 10, 3011. [Google Scholar] [CrossRef] [Green Version]
- Vernèsa, L.; Abert-Viana, M.; El Maâtaouib, M. Application of ultrasound for green extraction of proteins from spirulina. Mechanism, optimization, modeling, and industrial prospects. Ultrason. Sonochem. 2019, 54, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Xia, Y.; Zeng, Y.; Li, X.; Zhang, Y. Nitrate concentration-shift cultivation to enhance protein content of heterotrophic microalga Chlorella vulgaris: Over-compensation strategy. Bioresour. Technol. 2017, 233, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Waghmare, A.G.; Salve, M.K.; LeBlanc, J.G.; Arya, S.S. Concentration and characterization of microalgae proteins from Chlorella pyrenoidosa Bioresour. Bioprocess 2016, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Chia, S.R.; Chew, K.W.; Zaid, H.F.M.; Chu, D.-T.; Tao, Y.; Show, P.L. Microalgal Protein Extraction from Chlorella vulgaris FSP-E Using Triphasic Partitioning Technique with Sonication. Front. Bioeng. Biotechnol. 2019, 7, 396. [Google Scholar] [CrossRef] [Green Version]
- Chew, K.W.; Chiaa, S.R.; Leeb, S.Y.; Lee, S.Y. Enhanced microalgal protein extraction and purification using sustainable microwave-assisted multiphase partitioning technique Chem. Eng. J. 2019, 367, 1–8. [Google Scholar]
- Andreeva, A.; Budenkova, E.; Babich, O.; Sukhikh, S.; Ulrikh, E.; Ivanova, S.; Prosekov, A.; Dolganyuk, V. Production, Purification, and Study of the Amino Acid Composition of Microalgae Proteins. Molecules 2021, 26, 2767. [Google Scholar] [CrossRef]
- Liceaga, A.M. Processing insects for use in the food and feed industry. Curr. Opin. Insect. Sci. 2021, 48, 32–36. [Google Scholar] [CrossRef]
- Amarender, R.V.; Bhargava, K.; Dossey, A.T.; Gamagedara, S. Lipid and protein extraction from edible insects—Crickets (Gryllidae). LWT 2020, 125, 109222. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.P.; van den Broek, L.A.; Fogliano, V.; Lakemond, C.M. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; van Itterbeeck, J.; Heetkamp, M.J.W.; van den Brand, H.; van Loon, J.J.A. An Exploration on Greenhouse Gas and Ammonia Production by Insect Species Suitable for Animal or Human Consumption. PLoS ONE 2010, 5, e14445. [Google Scholar] [CrossRef] [Green Version]
- Borges, S.; Sousa, P.; Pintado, M. Insects as Food Sources. In Reference Module in Food Science; Elsevier: Amsterdam, the Netherlands, 2023. [Google Scholar] [CrossRef]
- Levi, J.; Martinez, A.; Itzhak, J.-J. The high level of protein content reported in insects for food and feed is overestimated. J. Food Compost. Anal. 2017, 62, 184–188. [Google Scholar] [CrossRef]
- Skotnicka, M.; Karwowska, K.; Kłobukowski, F.; Borkowska, A.; Pieszko, M. Possibilities of the Development of Edible Insect-Based Foods in Europe. Foods 2021, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chen, X.-M.; Zhao, M.; He, Z.; Sun, L.; Wang, C.-Y.; Ding, W.-F. Edible insects in China: Utilization and prospects. Insect Sci. 2017, 25, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Xiang, J.B. Prospect for development and exploitation of insect food. J. Anhui Agric. Sci. 2006, 33, 1728–1729. [Google Scholar]
- van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Zhang, L.; Wang, J.; Wang, W.; Niyati, N.; Guo, Y.; Wang, X. Chinese caterpillar fungus (Ophiocordyceps sinensis) in China: Current distribution, trading, and futures under climate change and overexploitation. Sci. Total Environ. 2021, 755 Pt 1, 142548. [Google Scholar] [CrossRef]
- Gao, R.R.; Hu, Y.T.; Dan, Y.; Hao, L.J.; Liu, X.; Song, J.Y. Chinese herbal medicine resources: Where we stand. Chin. Herb Med. 2019, 12, 3–13. [Google Scholar] [CrossRef]
- Hopping, K.A.; Chignell, S.M.; Lambin, E.F. The demise of caterpillar fungus in the Himalayan region due to climate change and overharvesting. Proc. Natl. Acad. Sci. USA 2018, 115, 11489–11494. [Google Scholar] [CrossRef] [Green Version]
- He, J. Harvest and trade of caterpillar mushroom (Ophiocordyceps sinensis) and the implications for sustainable use in the Tibet Region of Southwest China. J. Ethnopharmacol. 2018, 221, 86–90. [Google Scholar] [CrossRef]
- Shi, J.Y.; Pu, Z.Y.; Yao, J.; Li, Z.W.; Liu, Y.T.; Zheng, H. Research and Development on Nutrition of Butterflies; Science Press: Beijing, China, 2015; pp. 23–37. [Google Scholar]
- Deyrup, S.T.; Stagnitti, N.C.; Perpetua, M.J.; Wong-Deyrup, S.W. Drug Discovery Insights from Medicinal Beetles in Traditional Chinese Medicine. Biomol. Ther. 2021, 29, 105–126. [Google Scholar] [CrossRef]
- Kong, B.H. New Technology of Food Quality and Safety Testing; Science Press: Beijing, China, 2013; pp. 254–345. [Google Scholar]
- Liu, Y.S.; Wang, F.B.; Cui, J.X.; Zhang, L. Recent status and advances on study and utilization of Tenebrio molitor. Environ. Entomol. 2010, 32, 106–114. [Google Scholar]
- Ma, H.; Li, X.; Che, H.; Fan, H.; Liu, Q.; Xia, H. The inhibitory effect of Periplaneta americana L. on hepatocellular carcinoma: Explore the anti-hepatocellular carcinoma active site and its mechanism of action. J. Ethnopharmacol. 2022, 291, 114884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Wu, Z.Q. Effects of forage nutritional components on the feeding and reproduction of the female adults of American cockroach. J. Fujian Agric. For. Univ. 2006, 35, 239–242. [Google Scholar]
- Gao, Y.-Y.; Geng, F.-N.; Chen, S.-M.; Ma, X.-Y. Advances in research on active ingredients and related pharmacology of periplaneta americana Chinese. Chin. J. Exp. Tradit. Med. Formulae 2021, 24, 240–250. [Google Scholar]
- Zhang, X.; Ruan, J.; Ma, Z. Research on history and present situation of medicinal insect resources in China. Chin. J. Bioprocess Eng. 2019, 17, 615–622. [Google Scholar]
- Feng, Y.; Chen, X.M.; Zhao, M. Edible Insects of China; Science Press: Beijing, China, 2016; pp. 38–47. [Google Scholar]
- Yen, A.L. Insects as food and feed in the Asia Pacific region: Current perspectives and future directions. J. Insects Food Feed 2015, 1, 33–55. [Google Scholar] [CrossRef]
- Hanboonsong, Y.; Jamjanya, T.; Durst, P.B. Six-legged Livestock: Edible Insect Farming, Collection and Marketing in Thailand; FAO of the United Nations Regional Office for Asia and the Pacific: Bangkok, Thailand, 2013; pp. 13–16. [Google Scholar]
- Stice, C.; Basu, A. Ocean foods ecosystems for planetary survival in the anthropocene. In World Nutrition Forum: Driving the Protein Economy; Erber, A.G.: Vena, Austria, 2016; pp. 301–320. [Google Scholar]
- Lucakova, S.; Branyikova, I.; Hayes, M. Microalgal proteins and bioactives for food, feed, and other applications. Appl. Sci. 2022, 12, 4402. [Google Scholar] [CrossRef]
- Liu, H.; Tan, B.; Kong, X.; Li, J.; Li, G.; He, L.; Bai, M.; Yin, Y. Dietary Insect Powder Protein Sources Improve Protein Utilization by Regulation on Intestinal Amino Acid-Chemosensing System. Animals 2020, 10, 1590. [Google Scholar] [CrossRef]
- Brogan, E.; Park, Y.-L.; Matak, K.; Jaczynski, J. Characterization of protein in cricket (Acheta domesticus), locust (Locusta migratoria), and silk worm pupae (Bombyx mori) insect powders. LWT 2021, 152, 112314. [Google Scholar] [CrossRef]
- Mishyna, M.; Keppler, J.K.; Chen, J. Techno-functional properties of edible insect proteins and effects of processing, Curr. Opin. Colloid Interface Sci. 2021, 56, 101508. [Google Scholar] [CrossRef]
- Kim, T.-K.; Yong, H.-I.; Chun, H.-H.; Lee, M.-A.; Kim, Y.-B.; Choi, Y.-S. Changes of amino acid composition and protein technical functionality of edible insects by extracting steps J. Asia-Pac. Entomol. 2020, 23, 298–305. [Google Scholar] [CrossRef]
- Azagoh, C.; Ducept, F.; Garcia, R.; Rakotozafy, L.; Cuvelier, M.-E.; Keller, S.; Lewandowski, R.; Mezdour, S. Extraction and physicochemical characterization of Tenebrio molitor proteins Food Res. Int. 2016, 88, 24–31. [Google Scholar] [CrossRef]
- Vorotnikov, V. Bioprotein production is perking up in Russia. Poult. World 2020, 11, 1–6. [Google Scholar]
- Wassie, S.E.; Ali, A.I.M.; Korir, D.; Butterbach-Bahl, K.; Goopy, J.; Merbold, L.; Schlecht, E.; Dickhoefer, U. Effects of feed intake level on efficiency of microbial protein synthesis and nitrogen balance in Boran steers consuming tropical poor-quality forage. Arch. Anim. Nutr. 2019, 73, 140–157. [Google Scholar] [CrossRef] [PubMed]
- Lambert, W.; Rovers, M.; Ensink, J.; Tesseraud, S.; Corrent, E. Interaction between Threonine and Glycine at Low Dietary Crude Protein and the Effect on Production Performance, Meat Quality and Plasma Metabolites in Broiler Chickens; European Symposium on Poultry Nutrition (ESPN): Prague, France, 2015. [Google Scholar]
- Bacterial Protein Synthesis: Definition, Process & Inhibitors. Available online: https://study.com/academy/lesson/bacterial-protein-synthesis-definition-process-inhibitors.html (accessed on 5 January 2023).
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Misurcova, L.; Bunka, F.; Vavra Ambrozova, J.; Machu, L.; Samek, D.; Kracmar, S. Amino acid composition of algal products and its contribution to RDI. Food Chem. 2014, 151, 120–125. [Google Scholar] [CrossRef]
- Wang, Y.; Tibbetts, S.M.; McGinn, P.J. Microalgae as Sources of High-Quality Protein for Human Food and Protein Supplements. Foods 2021, 10, 3002. [Google Scholar] [CrossRef]
- Zhu, H.-G.; Tang, H.-Q.; Cheng, Y.-Q.; Li, Z.-G.; Tong, L.-T. Potential of preparing meat analogue by functional dry and wet pea (Pisum sativum) protein isolate. LWT 2021, 148, 111702. [Google Scholar] [CrossRef]
- Legendre, T. Consumer value-based edible insect market segmentation [edible insect market segmentation. Entomol. Res. 2021, 51, 55–61. [Google Scholar] [CrossRef]
- Legendre, T.S.; Baker, M.A. Legitimizing EdibleInsects for Human Consumption: The Impacts of Trust, Risk–Benefit, and Purchase Activism. J. Hosp. Tour. Res. 2022, 46, 467–489. [Google Scholar] [CrossRef]
- Algae Products Market—Growth, Trends, and Forecast (2023—2028). Available online: https://www.mordorintelligence.com/industry-reports/europe-wheat-protein-market (accessed on 5 January 2023).
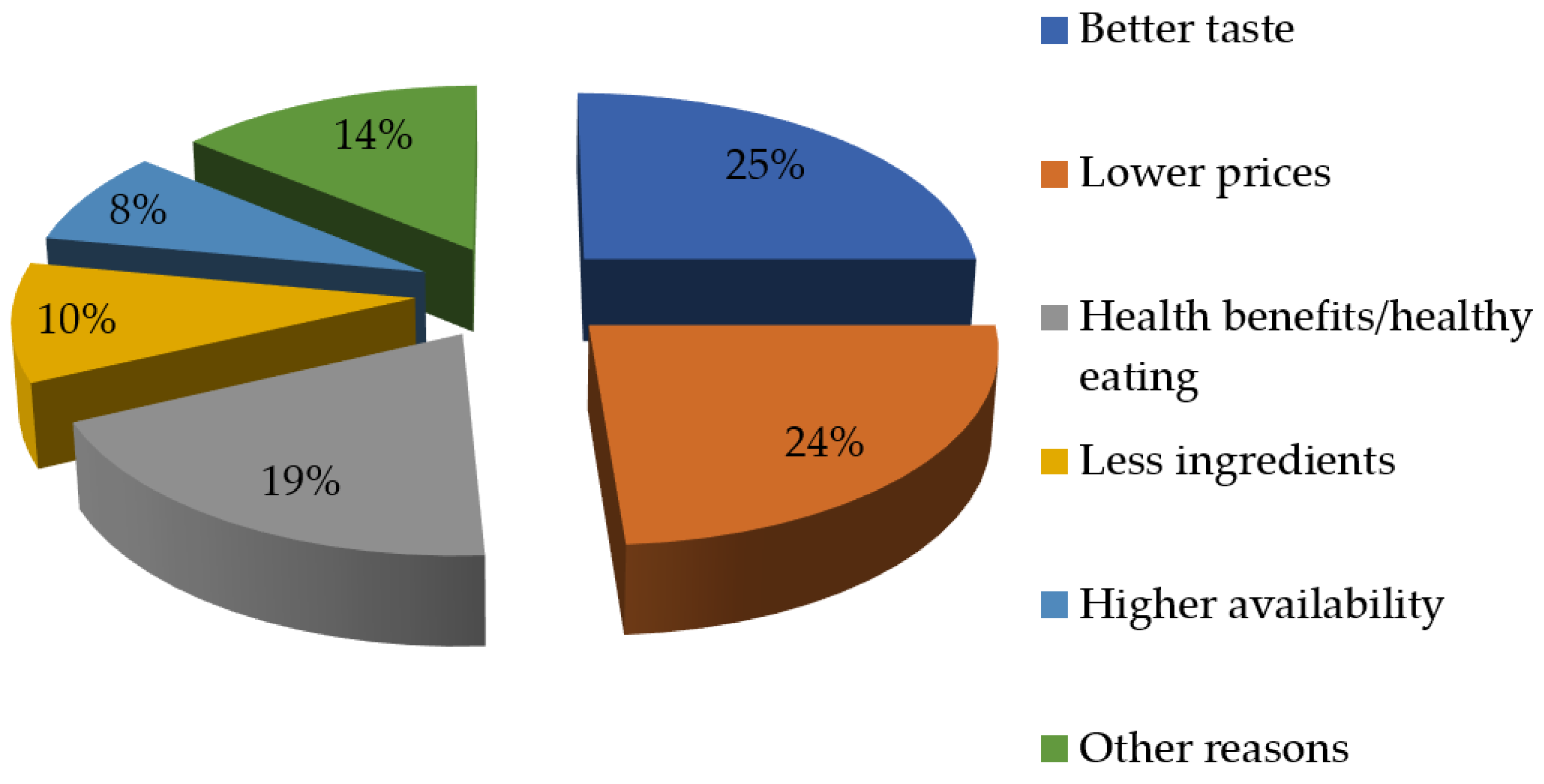
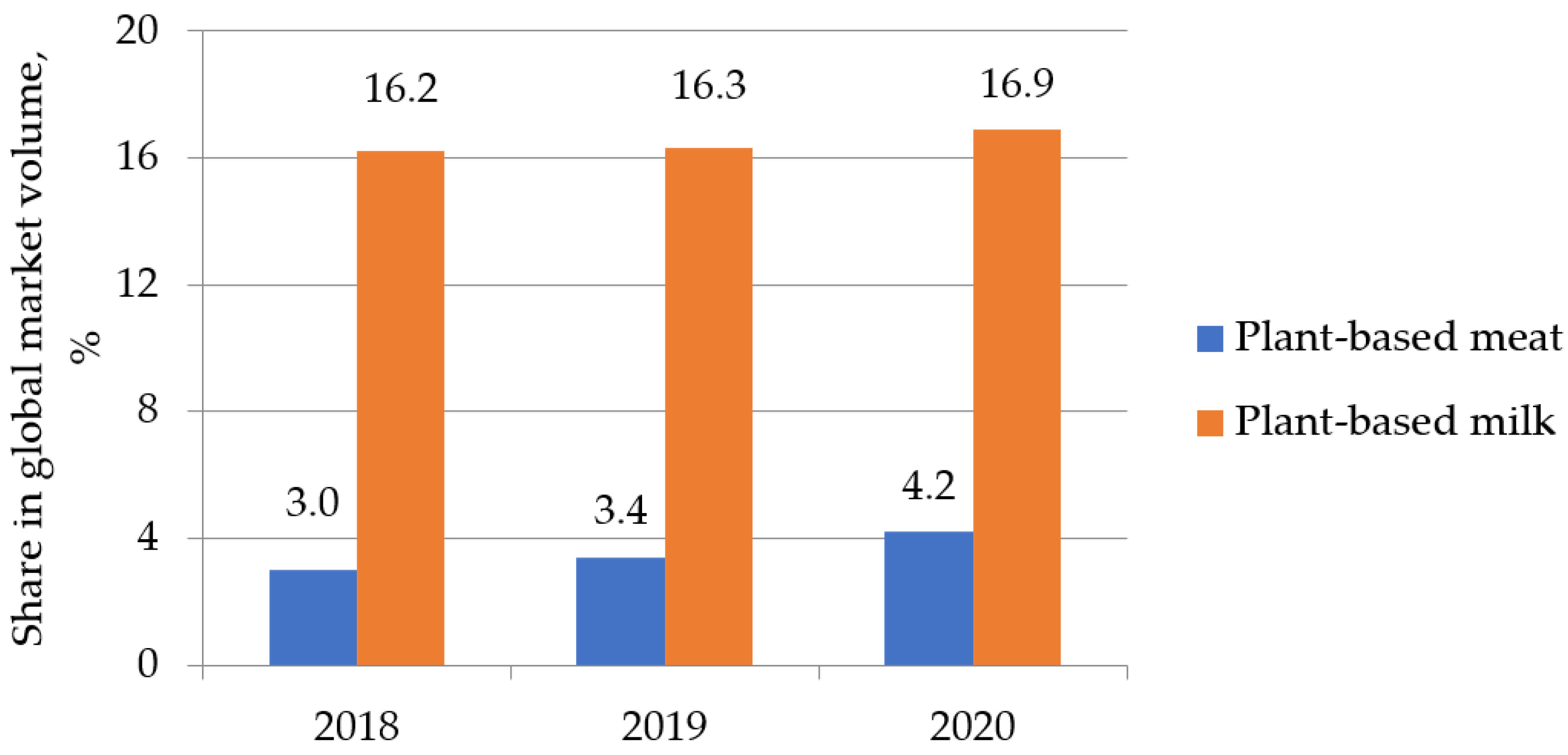
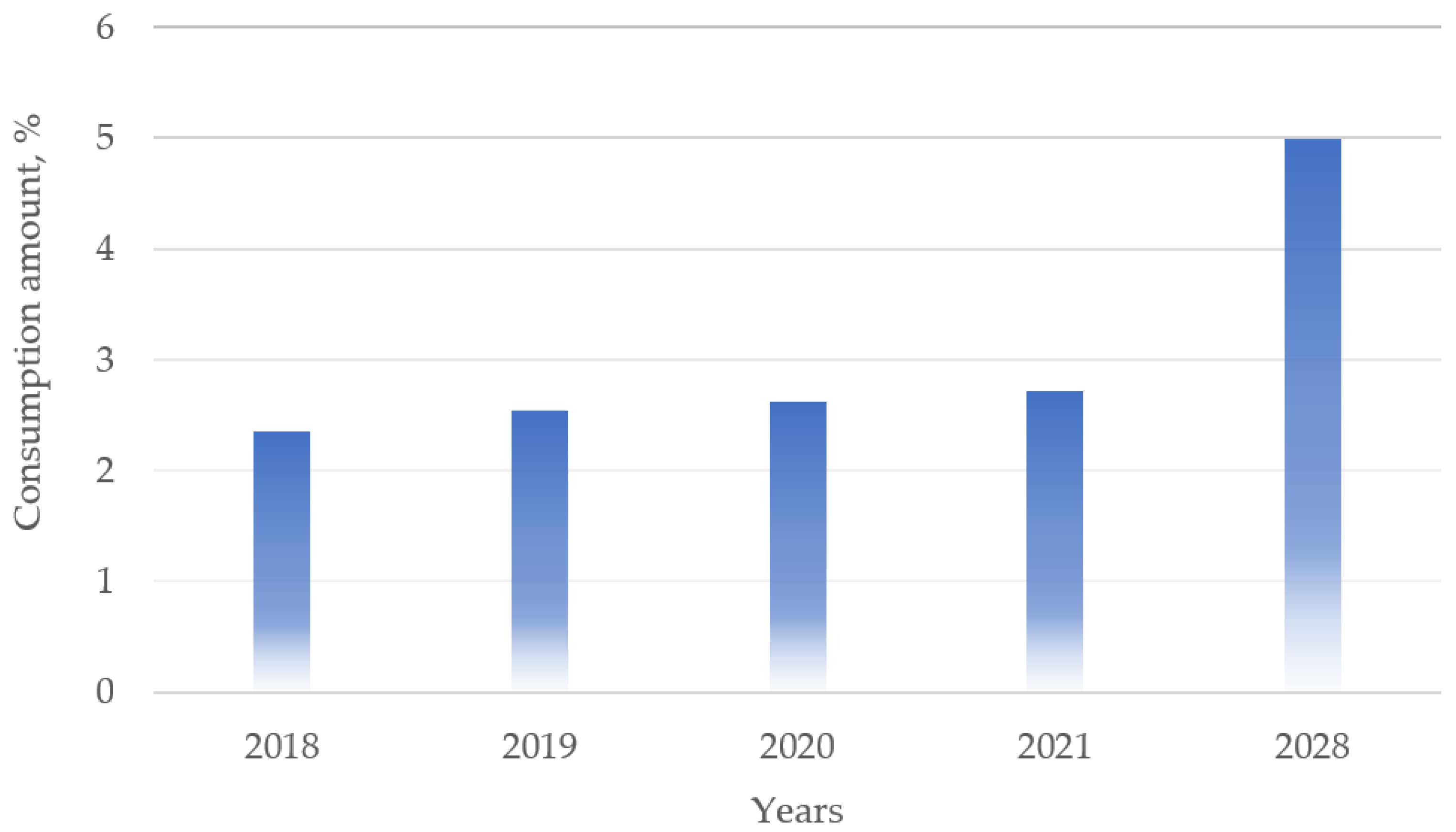
| Essential Amino Acid | g per Body Weight | Daily Consumption in g for a Person Weighing 80 kg | Sources |
|---|---|---|---|
| Histidine (H) | 0.01 | 0.8 | [4] |
| Isoleucine (I) (BCCA *) | 0.02 | 1.6 | [5] |
| Leucine (L) (BCCA) | 0.039 | 3.12 | [5] |
| Lysine (K) | 0.03 | 2.4 | [4] |
| Methionine (M) + Cysteine (C) | 0.015 | 1.2 | [4] |
| Phenylalanine (F) + Tyrosine (Y) | 0.025 | 2.0 | [5] |
| Threonine (T) | 0.015 | 1.2 | [5] |
| Tryptophan (W) | 0.004 | 0.32 | [4] |
| Valine (V) (BCCA) | 0.026 | 2.08 | [4] |
| Food Constituents | Content, % | Sources | ||||
|---|---|---|---|---|---|---|
| Cereals | ||||||
| Wheat | Rye | Oats | Barley | Rice | ||
| Protein | 13.2 | 11.2 | 9.9 | 10.0 | 10.3 | [9,10] |
| Fat | 2.5 | 2.1 | 2.2 | 6.2 | 2.4 | [9] |
| Ash | 2.3 | 1.7 | 1.7 | 3.2 | 2.4 | [11] |
| Monosaccharides | 0.8 | 1.2 | 1.5 | 1.1 | 1.3 | [9] |
| Starch | 54.5 | 54.0 | 54.0 | 65.0 | 48.1 | [11] |
| Fiber | 2.3 | 2.4 | 2.6 | 10.7 | 4.3 | [10] |
| Protein Source | Protein Content, g/100 g | Sources |
|---|---|---|
| Pumpkin seeds | 30.23 | [13] |
| Mustard seeds | 25.80 | [13] |
| Apricot seeds | 25.00 | [13] |
| Sunflower seeds | 20.78 | [14] |
| Sesame seeds | 19.40 | [14] |
| Chia seeds | 15.62 | [13] |
| Pistachios | 20.27 | [13] |
| Almond | 18.60 | [13] |
| Flax seed | 18.29 | [14] |
| Cashew | 18.22 | [13] |
| Hazelnut | 14.95 | [14] |
| Walnut | 15.23 | [14] |
| Pine nuts | 13.69 | [13] |
| Macadamia | 7.91 | [15] |
| Name | Content, % | Sources | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Proteins * | L | V | T | I | K | M | W | F | ||
| Peanuts (all types) | 25.80 | 1.67 | 1.08 | 0.88 | 0.91 | 0.93 | 0.32 | 0.25 | 1.34 | [16] |
| Beans (fava beans) | 26.1 | 1.96 | 1.16 | 0.93 | 1.05 | 1.67 | 0.21 | 1.10 | [17] | |
| Mung bean (mash) | 23.9 | 1.85 | 1.24 | 0.78 | 1.01 | 1.66 | 0.29 | 0.26 | 1.44 | [17] |
| Split peas | 24.55 | 1.76 | 1.16 | 0.87 | 1.77 | 0.25 | 0.28 | 1.13 | [17] | |
| Soy (grains, beans) | 34.9 | 2.67 | 2.09 | 1.39 | 1.81 | 2.09 | 0.52 | 0.45 | 1.61 | [16] |
| Asparagus beans | 24.33 | 1.86 | 1.16 | 0.93 | 0.99 | 1.65 | 0.35 | 0.3 | 1.42 | [16] |
| Tofu | 8.08 | 0.61 | 0.41 | 0.13 | 0.4 | 0.53 | 0.1 | 0.13 | 0.39 | [18] |
| Microalgae | Crude Protein Content *, % | Sources |
|---|---|---|
| Dunaliella salina | 57.0 | [57] |
| Dunaliella tertiolecta | 54.3 | [57] |
| Nannochloropsis occulata | 42.0 | [58] |
| Tetraselmis suecica | 41.4 | [58] |
| Nannochloropsis salina | 40.0 | [59] |
| Chlorella stigmatophora | 39.1 | [60] |
| Isochrysis galbana | 39.2 | [60] |
| Haematococcus pluvialis | 25.0 | [58] |
| Porphyridium cruentum | 35.0 | [58] |
| Phaeodactylym tricornutu | 39.0 | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolganyuk, V.; Sukhikh, S.; Kalashnikova, O.; Ivanova, S.; Kashirskikh, E.; Prosekov, A.; Michaud, P.; Babich, O. Food Proteins: Potential Resources. Sustainability 2023, 15, 5863. https://doi.org/10.3390/su15075863
Dolganyuk V, Sukhikh S, Kalashnikova O, Ivanova S, Kashirskikh E, Prosekov A, Michaud P, Babich O. Food Proteins: Potential Resources. Sustainability. 2023; 15(7):5863. https://doi.org/10.3390/su15075863
Chicago/Turabian StyleDolganyuk, Vyacheslav, Stanislav Sukhikh, Olga Kalashnikova, Svetlana Ivanova, Egor Kashirskikh, Alexander Prosekov, Philippe Michaud, and Olga Babich. 2023. "Food Proteins: Potential Resources" Sustainability 15, no. 7: 5863. https://doi.org/10.3390/su15075863
APA StyleDolganyuk, V., Sukhikh, S., Kalashnikova, O., Ivanova, S., Kashirskikh, E., Prosekov, A., Michaud, P., & Babich, O. (2023). Food Proteins: Potential Resources. Sustainability, 15(7), 5863. https://doi.org/10.3390/su15075863










